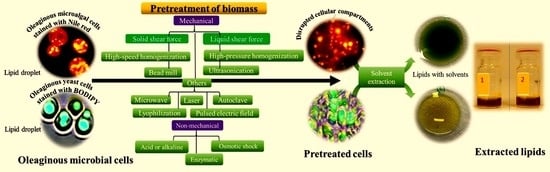
An Overview of Current Pretreatment Methods Used to Improve Lipid Extraction from Oleaginous Microorganisms
Abstract
1. Introduction
2. Microbial Cell Wall and Lipid Composition
3. Conventional Methods for Total Lipid Extraction
3.1. Bligh & Dyer Method
3.2. Folch Method
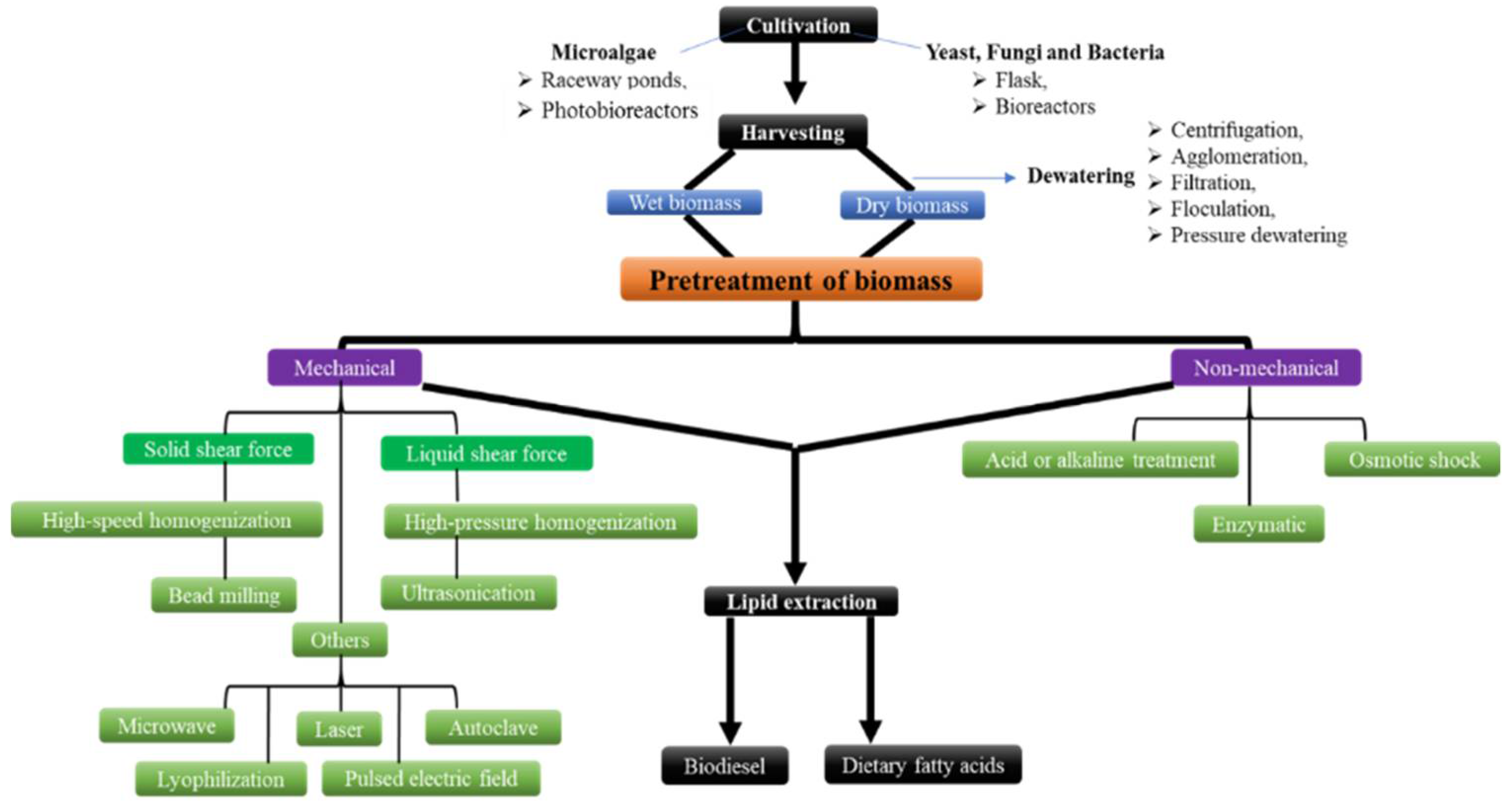
4. Pretreatment of Oleaginous Microbial Biomass to Extract Lipids
4.1. Mechanical Pretreatment Methods
4.1.1. Oil or Expeller Pressing
4.1.2. Bead Milling
4.1.3. High-Pressure Homogenization
4.1.4. High-Speed Shearing Homogenization
4.1.5. Ultrasonication
4.1.6. Microwave Irradiation
4.1.7. Autoclaving
4.1.8. Pulsed Electric Field
4.1.9. Laser
4.1.10. Acid-Catalyzed Hot-Water
4.2. Non-Mechanical Pretreatment Methods
4.2.1. Enzymatic Pretreatment
4.2.2. Other Emerging Methods for the Extraction of Lipids from Oleaginous Microorganisms
5. Conclusions and Recommendations
Author Contributions
Funding
Conflicts of Interest
References
- Patel, A.; Arora, N.; Sartaj, K.; Pruthi, V.; Pruthi, P.A. Sustainable biodiesel production from oleaginous yeasts utilizing hydrolysates of various non-edible lignocellulosic biomasses. Renew. Sustain. Energy Rev. 2016, 62, 836–855. [Google Scholar] [CrossRef]
- Ma, Y.; Gao, Z.; Wang, Q.; Liu, Y. Biodiesels from Microbial Oils: Opportunity and Challenges. Bioresour. Technol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Yellapu, S.K.; Kaur, R.; Kumar, L.R.; Tiwari, B.; Zhang, X.; Tyagi, R.D. Recent developments of downstream processing for microbial lipids and conversion to biodiesel. Bioresour. Technol. 2018, 256, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Chen, X.X.; Xiong, L.; Yang, X.; Chen, X.X.; Ma, L.; Chen, Y. Microbial oil production from corncob acid hydrolysate by oleaginous yeast Trichosporon coremiiforme. Biomass Bioenergy 2013, 49, 273–278. [Google Scholar] [CrossRef]
- Koutinas, A.A.; Chatzifragkou, A.; Kopsahelis, N.; Papanikolaou, S.; Kookos, I.K. Design and techno-economic evaluation of microbial oil production as a renewable resource for biodiesel and oleochemical production. Fuel 2014, 116, 566–577. [Google Scholar] [CrossRef]
- Dourou, M.; Aggeli, D.; Papanikolaou, S.; Aggelis, G. Critical steps in carbon metabolism affecting lipid accumulation and their regulation in oleaginous microorganisms. Appl. Microbiol. Biotechnol. 2018, 102, 2509–2523. [Google Scholar] [CrossRef] [PubMed]
- Angerbauer, C.; Siebenhofer, M.; Mittelbach, M.; Guebitz, G.M.M. Conversion of sewage sludge into lipids by Lipomyces starkeyi for biodiesel production. Bioresour. Technol. 2008, 99, 3051–3056. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Cho, J.M.; Chang, Y.K.; Oh, Y.K. Cell disruption and lipid extraction for microalgal biorefineries: A review. Bioresour. Technol. 2017, 244, 1317–1328. [Google Scholar] [CrossRef] [PubMed]
- Ranjith Kumar, R.; Hanumantha Rao, P.; Arumugam, M. Lipid Extraction Methods from Microalgae: A Comprehensive Review. Front. Energy Res. 2015, 2, 1–9. [Google Scholar] [CrossRef]
- Dong, T.; Knoshaug, E.P.; Pienkos, P.T.; Laurens, L.M.L. Lipid recovery from wet oleaginous microbial biomass for biofuel production: A critical review. Appl. Energy 2016, 177, 879–895. [Google Scholar] [CrossRef]
- Soccol, C.R.; Dalmas Neto, C.J.; Soccol, V.T.; Sydney, E.B.; da Costa, E.S.F.; Medeiros, A.B.P.; de Souza Vandenberghe, L.P. Pilot scale biodiesel production from microbial oil of Rhodosporidium toruloides DEBB 5533 using sugarcane juice: Performance in diesel engine and preliminary economic study. Bioresour. Technol. 2017, 223, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Halim, R.; Danquah, M.K.; Webley, P.A. Extraction of oil from microalgae for biodiesel production: A review. Biotechnol. Adv. 2012, 30, 709–732. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, A.; Patil, C.; Moholkar, V.S. Mechanistic assessment of microalgal lipid extraction. Ind. Eng. Chem. Res. 2010, 49, 2979–2985. [Google Scholar] [CrossRef]
- D’Hondt, E.; Martín-Juárez, J.; Bolado, S.; Kasperoviciene, J.; Koreiviene, J.; Sulcius, S.; Elst, K.; Bastiaens, L. Cell disruption technologies. In Microalgae-Based Biofuels and Bioproducts: From Feedstock Cultivation to End-Products; Elsevier: New York, NY, USA, 2017; pp. 133–154. ISBN 9780081010273. [Google Scholar]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Yoo, G.; Lee, H.; Lim, J.; Kim, K.; Kim, C.W.; Park, M.S.; Yang, J.W. Methods of downstream processing for the production of biodiesel from microalgae. Biotechnol. Adv. 2013, 31, 862–876. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Walker, T.H. Fed-batch fermentation and supercritical fluid extraction of heterotrophic microalgal Chlorella protothecoides lipids. Bioresour. Technol. 2012, 114, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Jeevan Kumar, S.P.; Vijay Kumar, G.; Dash, A.; Scholz, P.; Banerjee, R. Sustainable green solvents and techniques for lipid extraction from microalgae: A review. Algal Res. 2017, 21, 138–147. [Google Scholar] [CrossRef]
- Postma, P.R.; Suarez-Garcia, E.; Safi, C.; Olivieri, G.; Olivieri, G.; Wijffels, R.H.; Wijffels, R.H. Energy efficient bead milling of microalgae: Effect of bead size on disintegration and release of proteins and carbohydrates. Bioresour. Technol. 2017, 224, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Günerken, E.; D’Hondt, E.; Eppink, M.H.M.; Garcia-Gonzalez, L.; Elst, K.; Wijffels, R.H. Cell disruption for microalgae biorefineries. Biotechnol. Adv. 2015, 33, 243–260. [Google Scholar] [CrossRef] [PubMed]
- Demirbaş, A. Production of Biodiesel from Algae Oils. Energy Sources Part A Recover. Util. Environ. Eff. 2008, 31, 163–168. [Google Scholar] [CrossRef]
- Geciova, J.; Bury, D.; Jelen, P. Methods for disruption of microbial cells for potential use in the dairy industry—A review. Int. Dairy J. 2002, 12, 541–553. [Google Scholar] [CrossRef]
- Chemat, F.; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef] [PubMed]
- Boldor, D.; Kanitkar, A.; Terigar, B.G.; Leonardi, C.; Lima, M.; Breitenbeck, G.A. Microwave assisted extraction of biodiesel feedstock from the seeds of invasive chinese tallow tree. Environ. Sci. Technol. 2010, 44, 4019–4025. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.; Yang, F.; Hu, C.; Shen, H.; Zhao, Z.K. Enzyme-assisted extraction of lipids directly from the culture of the oleaginous yeast Rhodosporidium toruloides. Bioresour. Technol. 2012, 111, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Pomraning, K.R.; Wei, S.; Karagiosis, S.A.; Kim, Y.M.; Dohnalkova, A.C.; Arey, B.W.; Bredeweg, E.L.; Orr, G.; Metz, T.O.; Baker, S.E. Comprehensive metabolomic, lipidomic and microscopic profiling of Yarrowia lipolytica during lipid accumulation identifies targets for increased lipogenesis. PLoS ONE 2015, 10, e0123188. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jiang, L.; Zhang, L.; Nie, C.; Pei, H. Lipid productivity in limnetic Chlorella is doubled by seawater added with anaerobically digested effluent from kitchen waste. Biotechnol. Biofuels 2018, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- D’ESPAUX, L.; Mendez-Perez, D.; Li, R.; Keasling, J.D. Synthetic biology for microbial production of lipid-based biofuels. Curr. Opin. Chem. Biol. 2015, 29, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Pravez, M.; Deeba, F.; Pruthi, V.; Singh, R.P.; Pruthi, P.A. Boosting accumulation of neutral lipids in Rhodosporidium kratochvilovae HIMPA1 grown on hemp (Cannabis sativa Linn) seed aqueous extract as feedstock for biodiesel production. Bioresour. Technol. 2014, 165, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Pruthi, V.; Pruthi, P.A. Synchronized nutrient stress conditions trigger the diversion of CDP-DG pathway of phospholipids synthesis towards de novo TAG synthesis in oleaginous yeast escalating biodiesel production. Energy 2017, 139, 962–974. [Google Scholar] [CrossRef]
- Ambat, I.; Srivastava, V.; Sillanpää, M. Recent advancement in biodiesel production methodologies using various feedstock: A review. Renew. Sustain. Energy Rev. 2018, 90, 356–369. [Google Scholar] [CrossRef]
- Bligh, E.; Dyler, W.J. A rapid Method of Total Lipid Extraction and Purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Hussain, J.; Ruan, Z.; Nascimento, I.A.; Liu, Y.; Liao, W. Lipid profiling and corresponding biodiesel quality of Mortierella isabellina using different drying and extraction methods. Bioresour. Technol. 2014, 169, 768–772. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G. A simple method of isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
- Cheirsilp, B.; Kitcha, S. Solid state fermentation by cellulolytic oleaginous fungi for direct conversion of lignocellulosic biomass into lipids: Fed-batch and repeated-batch fermentations. Ind. Crops Prod. 2015, 66, 73–80. [Google Scholar] [CrossRef]
- Kumar, S.; Gupta, N.; Pakshirajan, K. Simultaneous lipid production and dairy wastewater treatment using Rhodococcus opacus in a batch bioreactor for potential biodiesel application. J. Environ. Chem. Eng. 2015, 3, 1630–1636. [Google Scholar] [CrossRef]
- Pedersen, T.A. Lipid Formation in Cryptococcus terricolus. III. Extraction and Purification of Lipids. Acta Chem. Scand. 1962, 16, 374–382. [Google Scholar] [CrossRef]
- Hara, A.; Radin, N.S. Lipid extraction of tissues with a low toxicity solvent. Anal. Biochem. 1978, 90, 420–426. [Google Scholar] [CrossRef]
- Patel, A.; Arora, N.; Pruthi, V.; Pruthi, P.A. A novel rapid ultrasonication-microwave treatment for total lipid extraction from wet oleaginous yeast biomass for sustainable biodiesel production. Ultrason. Sonochem. 2018, in press. [Google Scholar] [CrossRef]
- Johnravindar, D.; Karthikeyan, O.P.; Selvam, A.; Murugesan, K.; Wong, J.W.C. Lipid accumulation potential of oleaginous yeasts: A comparative evaluation using food waste leachate as a substrate. Bioresour. Technol. 2018, 248, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Luo, H.; Mu, T.; Shen, Y.; Yuan, M.; Liu, J. Enhancement of lipid accumulation by oleaginous yeast through phosphorus limitation under high content of ammonia. Bioresour. Technol. 2018, 262, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Manowattana, A.; Techapun, C.; Watanabe, M.; Chaiyaso, T. Bioconversion of biodiesel-derived crude glycerol into lipids and carotenoids by an oleaginous red yeast Sporidiobolus pararoseus KM281507 in an airlift bioreactor. J. Biosci. Bioeng. 2018, 125, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Chaiyaso, T.; Srisuwan, W.; Techapun, C.; Watanabe, M.; Takenaka, S. Direct bioconversion of rice residue from canteen waste into lipids by new amylolytic oleaginous yeast Sporidiobolus pararoseus KX709872. Prep. Biochem. Biotechnol. 2018, 6068, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Selvakumar, P.; Sivashanmugam, P. Study on Lipid Accumulation in Novel Oleaginous Yeast Naganishia liquefaciens NITTS2 Utilizing Pre-digested Municipal Waste Activated Sludge: A Low-cost Feedstock for Biodiesel Production. Appl. Biochem. Biotechnol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Mohan, S.V. Microbial lipid production by Cryptococcus curvatus from vegetable waste hydrolysate. Bioresour. Technol. 2018, 254, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Deeba, F.; Patel, A.; Arora, N.; Pruthi, V.; Pruthi, P.A.; Negi, Y.S. Amaranth seeds (Amaranthus palmeri L.) as novel feedstock for biodiesel production by oleaginous yeast. Environ. Sci. Pollut. Res. 2018, 25, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Bonturi, N.; Matsakas, L.; Nilsson, R.; Christakopoulos, P.; Miranda, E.A.; Berglund, K.A.; Rova, U. Single cell oil producing yeasts Lipomyces starkeyi and Rhodosporidium toruloides: Selection of extraction strategies and biodiesel property prediction. Energies 2015, 8, 5040–5052. [Google Scholar] [CrossRef]
- Lin, Y.; Xie, X.; Yuan, B.; Fu, J.; Liu, L.; Tian, H.; Chen, T.; He, D. Optimization of Enzymatic Cell Disruption for Improving Lipid Extraction from Schizochytrium sp. through Response Surface Methodology. J. Oleo Sci. 2018, 67, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Vandamme, D.; Gheysen, L.; Muylaert, K.; Foubert, I. Impact of harvesting method on total lipid content and extraction efficiency for Phaeodactylum tricornutum. Sep. Purif. Technol. 2018, 194, 362–367. [Google Scholar] [CrossRef]
- Zhang, Y.; Kong, X.; Wang, Z.; Sun, Y.; Zhu, S.; Li, L.; Lv, P. Optimization of enzymatic hydrolysis for effective lipid extraction from microalgae Scenedesmus sp. Renew. Energy 2018, 125, 1049–1057. [Google Scholar] [CrossRef]
- Ellison, C.R.; Overa, S.; Boldor, D. Central composite design parameterization of microalgae/cyanobacteria co-culture pretreatment for enhanced lipid extraction using an external clamp-on ultrasonic transducer. Ultrason. Sonochem. 2018, in press. [Google Scholar] [CrossRef] [PubMed]
- Kwak, M.; Kang, S.G.; Hong, W.K.; Han, J.I.; Chang, Y.K. Simultaneous cell disruption and lipid extraction of wet Aurantiochytrium sp. KRS101 using a high shear mixer. Bioprocess Biosyst. Eng. 2018, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ansari, F.A.; Gupta, S.K.; Nasr, M.; Rawat, I.; Bux, F. Evaluation of various cell drying and disruption techniques for sustainable metabolite extractions from microalgae grown in wastewater: A multivariate approach. J. Clean. Prod. 2018, 182, 634–643. [Google Scholar] [CrossRef]
- Ju, C.; Wang, F.; Huang, Y.; Fang, Y. Selective extraction of neutral lipid from wet algae paste and subsequently hydroconversion into renewable jet fuel. Renew. Energy 2018, 118, 521–526. [Google Scholar] [CrossRef]
- Carvalho, A.K.F.; Bento, H.B.S.; Izário Filho, H.J.; de Castro, H.F. Approaches to convert Mucor circinelloides lipid into biodiesel by enzymatic synthesis assisted by microwave irradiations. Renew. Energy 2018, 125. [Google Scholar] [CrossRef]
- Al-Hawash, A.B.; Li, S.; Zhang, X.; Zhang, X.; Ma, F. Productivity of γ-Linoleic acid by oleaginous fungus Cunninghamella echinulata using a pulsed high magnetic field. Food Biosci. 2018, 21, 1–7. [Google Scholar] [CrossRef]
- Forfang, K.; Zimmermann, B.; Kosa, G.; Kohler, A.; Shapaval, V. FTIR spectroscopy for evaluation and monitoring of lipid extraction efficiency for oleaginous fungi. PLoS ONE 2017, 12, e0170611. [Google Scholar] [CrossRef] [PubMed]
- Kosa, G.; Kohler, A.; Tafintseva, V.; Zimmermann, B.; Forfang, K.; Afseth, N.K.; Tzimorotas, D.; Vuoristo, K.S.; Horn, S.J.; Mounier, J.; et al. Microtiter plate cultivation of oleaginous fungi and monitoring of lipogenesis by high-throughput FTIR spectroscopy. Microb. Cell Fact. 2017, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bagy, M.M.K.; Abd-Alla, M.H.; Morsy, F.M.; Hassan, E.A. Two stage biodiesel and hydrogen production from molasses by oleaginous fungi and Clostridium acetobutylicum ATCC 824. Int. J. Hydrogen Energy 2014, 39, 3185–3197. [Google Scholar] [CrossRef]
- Santala, S.; Efimova, E.; Kivinen, V.; Larjo, A.; Aho, T.; Karp, M.; Santala, V. Improved Triacylglycerol Production in Acinetobacter baylyi ADP1 by Metabolic Engineering. Microb. Cell Fact. 2011, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, Y.; Xia, L. An oleaginous endophyte Bacillus subtilis HB1310 isolated from thin-shelled walnut and its utilization of cotton stalk hydrolysate for lipid production. Biotechnol. Biofuels 2014, 7, 152. [Google Scholar] [CrossRef] [PubMed]
- Carlozzi, P.; Buccioni, A.; Minieri, S.; Pushparaj, B.; Piccardi, R.; Ena, A.; Pintucci, C. Production of bio-fuels (hydrogen and lipids) through a photofermentation process. Bioresour. Technol. 2010, 101, 3115–3120. [Google Scholar] [CrossRef] [PubMed]
- Cea, M.; Sangaletti-Gerhard, N.; Acuña, P.; Fuentes, I.; Jorquera, M.; Godoy, K.; Osses, F.; Navia, R. Screening transesterifiable lipid accumulating bacteria from sewage sludge for biodiesel production. Biotechnol. Rep. 2015, 8, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Goswami, L.; Tejas Namboodiri, M.M.; Vinoth Kumar, R.; Pakshirajan, K.; Pugazhenthi, G. Biodiesel production potential of oleaginous Rhodococcus opacus grown on biomass gasification wastewater. Renew. Energy 2017, 105, 400–406. [Google Scholar] [CrossRef]
- Harun, R.; Singh, M.; Forde, G.M.; Danquah, M.K. Bioprocess engineering of microalgae to produce a variety of consumer products. Renew. Sustain. Energy Rev. 2010, 14, 1037–1047. [Google Scholar] [CrossRef]
- Ramesh, D. Lipid identification and extraction techniques. In Biotechnological Applications of Microalgae: Biodiesel and Value-Added Products; Bux, F., Ed.; CRC Press: Boca Raton, FL, USA, 2013; pp. 89–97. [Google Scholar]
- Topare, N.S.; Raut, S.J.; Renge, V.C.; Khedkar, S.V.; Chavan, Y.P.; Bhagat, S.L. Extraction of oil from algae by solvent extraction and oil expeller method. Int. J. Chem. Sci. 2011, 9, 1746–1750. [Google Scholar]
- Mubarak, M.; Shaija, A.; Suchithra, T.V. A review on the extraction of lipid from microalgae for biodiesel production. Algal Res. 2015, 7, 117–123. [Google Scholar] [CrossRef]
- Johnson, M.B.; Wen, Z. Production of biodiesel fuel from the microalga schizochytrium limacinum by direct transesterification of algal biomass. Energy Fuels 2009, 23, 5179–5183. [Google Scholar] [CrossRef]
- Balasundaram, B.; Skill, S.C.; Llewellyn, C.A. A low energy process for the recovery of bioproducts from cyanobacteria using a ball mill. Biochem. Eng. J. 2012, 69, 48–56. [Google Scholar] [CrossRef]
- Bunge, F.; Pietzsch, M.; Müller, R.; Syldatk, C. Mechanical disruption of Arthrobacter sp. DSM 3747 in stirred ball mills for the release of hydantoin-cleaving enzymes. Chem. Eng. Sci. 1992, 47, 225–232. [Google Scholar] [CrossRef]
- Postma, P.R.; Miron, T.L.; Olivieri, G.; Barbosa, M.J.; Wijffels, R.H.; Eppink, M.H.M. Mild disintegration of the green microalgae Chlorella vulgaris using bead milling. Bioresour. Technol. 2015, 184, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Montalescot, V.; Rinaldi, T.; Touchard, R.; Jubeau, S.; Frappart, M.; Jaouen, P.; Bourseau, P.; Marchal, L. Optimization of bead milling parameters for the cell disruption of microalgae: Process modeling and application to Porphyridium cruentum and Nannochloropsis oculata. Bioresour. Technol. 2015, 196, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Doucha, J.; Lívanský, K. Influence of processing parameters on disintegration of Chlorella cells in various types of homogenizers. Appl. Microbiol. Biotechnol. 2008, 81, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Meullemiestre, A.; Breil, C.; Abert-Vian, M.; Chemat, F. Microwave, ultrasound, thermal treatments, and bead milling as intensification techniques for extraction of lipids from oleaginous Yarrowia lipolytica yeast for a biojetfuel application. Bioresour. Technol. 2016, 211, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Samarasinghe, N. Effect of High Pressure Homogenization on Aqueous Phase Solvent Extraction of Lipids from Nannochloris Oculata Microalgae. J. Energy Nat. Resour. 2012, 1. [Google Scholar] [CrossRef]
- Coccaro, N.; Ferrari, G.; Donsì, F. Understanding the break-up phenomena in an orifice-valve high pressure homogenizer using spherical bacterial cells (Lactococcus lactis) as a model disruption indicator. J. Food Eng. 2018. [Google Scholar] [CrossRef]
- Ekpeni, L.E.N.; Benyounis, K.Y.; Nkem-Ekpeni, F.F.; Stokes, J.; Olabi, A.G. Underlying factors to consider in improving energy yield from biomass source through yeast use on high-pressure homogenizer (hph). Energy 2015, 81, 74–83. [Google Scholar] [CrossRef]
- Shene, C.; Monsalve, M.T.; Vergara, D.; Lienqueo, M.E.; Rubilar, M. High pressure homogenization of Nannochloropsis oculata for the extraction of intracellular components: Effect of process conditions and culture age. Eur. J. Lipid Sci. Technol. 2016, 118, 631–639. [Google Scholar] [CrossRef]
- Halim, R.; Rupasinghe, T.W.T.; Tull, D.L.; Webley, P.A. Mechanical cell disruption for lipid extraction from microalgal biomass. Bioresour. Technol. 2013, 140, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Donsì, F.; Annunziata, M.; Ferrari, G. Microbial inactivation by high pressure homogenization: Effect of the disruption valve geometry. J. Food Eng. 2013, 115, 362–370. [Google Scholar] [CrossRef]
- Floury, J.; Legrand, J.; Desrumaux, A. Analysis of a new type of high pressure homogeniser. Part B. study of droplet break-up and recoalescence phenomena. Chem. Eng. Sci. 2004, 59, 1285–1294. [Google Scholar] [CrossRef]
- Lee, L.; Norton, I.T. Comparing droplet breakup for a high-pressure valve homogeniser and a Microfluidizer for the potential production of food-grade nanoemulsions. J. Food Eng. 2013, 114, 158–163. [Google Scholar] [CrossRef]
- Olmstead, I.L.D.; Kentish, S.E.; Scales, P.J.; Martin, G.J.O. Low solvent, low temperature method for extracting biodiesel lipids from concentrated microalgal biomass. Bioresour. Technol. 2013, 148, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Hua, X.; Xu, S.; Wang, M.; Chen, Y.; Yang, H.; Yang, R. Effects of high-speed homogenization and high-pressure homogenization on structure of tomato residue fibers. Food Chem. 2017, 232, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Deng, J.; Liu, X.; He, P.; He, L.; Zhang, F.; Linhardt, R.J.; Sun, P. Structure and conformation of α-glucan extracted from Agaricus blazei Murill by high-speed shearing homogenization. Int. J. Biol. Macromol. 2018, 113, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Araujo, G.S.; Matos, L.J.B.L.; Fernandes, J.O.; Cartaxo, S.J.M.; Gonçalves, L.R.B.; Fernandes, F.A.N.; Farias, W.R.L. Extraction of lipids from microalgae by ultrasound application: Prospection of the optimal extraction method. Ultrason. Sonochem. 2013, 20, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Šoštarič, M.; Klinar, D.; Bricelj, M.; Golob, J.; Berovič, M.; Likozar, B. Growth, lipid extraction and thermal degradation of the microalga Chlorella vulgaris. New Biotechnol. 2012, 29, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Yoo, C.; Jun, S.-Y.; Ahn, C.-Y.; Oh, H.-M. Comparison of several methods for effective lipid extraction from microalgae. Bioresour. Technol. 2010, 101, S75–S77. [Google Scholar] [CrossRef] [PubMed]
- Wiyarno, B.; Yunus, R.M.; Mel, M. Extraction of Algae Oil from Nannocloropsis sp.: A Study of Soxhlet and Ultrasonic-Assisted Extractions. J. Appl. Sci. 2011, 11, 3607–3612. [Google Scholar] [CrossRef]
- Cravotto, G.; Boffa, L.; Mantegna, S.; Perego, P.; Avogadro, M.; Cintas, P. Improved extraction of vegetable oils under high-intensity ultrasound and/or microwaves. Ultrason. Sonochem. 2008, 15, 898–902. [Google Scholar] [CrossRef] [PubMed]
- Mecozzi, M.; Amici, M.; Romanelli, G.; Pietrantonio, E.; Deluca, A. Ultrasound extraction and thin layer chromatography-flame ionization detection analysis of the lipid fraction in marine mucilage samples. J. Chromatogr. A 2002, 963, 363–373. [Google Scholar] [CrossRef]
- Prabakaran, P.; Ravindran, A.D. A comparative study on effective cell disruption methods for lipid extraction from microalgae. Lett. Appl. Microbiol. 2011, 53, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Ríos, S.D.; Castañeda, J.; Torras, C.; Farriol, X.; Salvadó, J. Lipid extraction methods from microalgal biomass harvested by two different paths: Screening studies toward biodiesel production. Bioresour. Technol. 2013, 133, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yan, S.; Tyagi, R.D.; Drogui, P.; Surampalli, R.Y. Ultrasonication assisted lipid extraction from oleaginous microorganisms. Bioresour. Technol. 2014, 158, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Joyce, E.M.; Mason, T.J. Evaluation of the mechanisms of the effect of ultrasound on Microcystis aeruginosa at different ultrasonic frequencies. Water Res. 2012, 46, 2851–2858. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yuan, W.; Jiang, X.; Jing, Y.; Wang, Z. Disruption of microalgal cells using high-frequency focused ultrasound. Bioresour. Technol. 2014, 153, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Palou, R. Microwave-assisted synthesis using ionic liquids. Mol. Divers. 2010, 14, 3–25. [Google Scholar] [CrossRef] [PubMed]
- Budarin, V.L.; Shuttleworth, P.S.; De Bruyn, M.; Farmer, T.J.; Gronnow, M.J.; Pfaltzgraff, L.; Macquarrie, D.J.; Clark, J.H. The potential of microwave technology for the recovery, synthesis and manufacturing of chemicals from bio-wastes. Catal. Today 2015, 239, 80–89. [Google Scholar] [CrossRef]
- Wahlen, B.D.; Willis, R.M.; Seefeldt, L.C. Biodiesel production by simultaneous extraction and conversion of total lipids from microalgae, cyanobacteria, and wild mixed-cultures. Bioresour. Technol. 2011, 102, 2724–2730. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Guerra, E.; Gude, V.G.; Mondala, A.; Holmes, W.; Hernandez, R. Extractive-transesterification of algal lipids under microwave irradiation with hexane as solvent. Bioresour. Technol. 2014, 156, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Teo, C.L.; Idris, A. Enhancing the various solvent extraction method via microwave irradiation for extraction of lipids from marine microalgae in biodiesel production. Bioresour. Technol. 2014, 171, 477–481, Ahead of Print. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Yin, J.; Gao, Z.; Huang, H.; Ji, X.; Dou, C. Disruption of chlorella vulgaris cells for the release of biodiesel-producing lipids: A comparison of grinding, ultrasonication, bead milling, enzymatic lysis, and microwaves. Appl. Biochem. Biotechnol. 2011, 164, 1215–1224. [Google Scholar] [CrossRef] [PubMed]
- Mendes-Pinto, M.M.; Raposo, M.F.J.; Bowen, J.; Young, A.J.; Morais, R. Evaluation of different cell disruption process on encysted cells of Haematococcus pluvialis. J. Appl. Phycol. 2001, 13, 19–24. [Google Scholar] [CrossRef]
- Rakesh, S.; Dhar, D.W.; Prasanna, R.; Saxena, A.K.; Saha, S.; Shukla, M.; Sharma, K. Cell disruption methods for improving lipid extraction efficiency in unicellular microalgae. Eng. Life Sci. 2015, 15, 443–447. [Google Scholar] [CrossRef]
- Florentino de Souza Silva, A.P.; Costa, M.C.; Colzi Lopes, A.; Fares Abdala Neto, E.; Carrhá Leitão, R.; Mota, C.R.; Bezerra dos Santos, A. Comparison of pretreatment methods for total lipids extraction from mixed microalgae. Renew. Energy 2014, 63, 762–766. [Google Scholar] [CrossRef]
- Tsong, T.Y. Electroporation of cell membranes. Minireview. Biophys. J. 1991, 60, 297–306. [Google Scholar] [CrossRef]
- Weaver, J.C.; Harrison, G.I.; Bliss, J.G.; Mourant, J.R.; Powell, K.T. Electroporation: High frequency of occurrence of a transient high-permeability state in erythrocytes and intact yeast. FEBS Lett. 1988, 229, 30–34. [Google Scholar] [CrossRef]
- Eing, C.; Goettel, M.; Straessner, R.; Gusbeth, C.; Frey, W. Pulsed electric field treatment of microalgae—Benefits for microalgae biomass processing. IEEE Trans. Plasma Sci. 2013, 41, 2901–2907. [Google Scholar] [CrossRef]
- Sheng, J.; Vannela, R.; Rittmann, B.E. Evaluation of cell-disruption effects of pulsed-electric-field treatment of Synechocystis PCC 6803. Environ. Sci. Technol. 2011, 45, 3795–3802. [Google Scholar] [CrossRef] [PubMed]
- Zbinden, M.D.A.; Sturm, B.S.M.; Nord, R.D.; Carey, W.J.; Moore, D.; Shinogle, H.; Stagg-Williams, S.M. Pulsed electric field (PEF) as an intensification pretreatment for greener solvent lipid extraction from microalgae. Biotechnol. Bioeng. 2013, 110, 1605–1615. [Google Scholar] [CrossRef] [PubMed]
- Byreddy, A.R.; Gupta, A.; Barrow, C.J.; Puri, M. Comparison of cell disruption methods for improving lipid extraction from thraustochytrid strains. Mar. Drugs 2015, 13, 5111–5127. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Sun, J.; Huang, Y.; Feng, J.; Zhou, J.; Cen, K. Dynamic microstructures and fractal characterization of cell wall disruption for microwave irradiation-assisted lipid extraction from wet microalgae. Bioresour. Technol. 2013, 150, 67–72. [Google Scholar] [CrossRef] [PubMed]
- McMillan, J.R.; Watson, I.A.; Ali, M.; Jaafar, W. Evaluation and comparison of algal cell disruption methods: Microwave, waterbath, blender, ultrasonic and laser treatment. Appl. Energy 2013, 103, 128–134. [Google Scholar] [CrossRef]
- Yu, Q.; Zhuang, X.; Lv, S.; He, M.; Zhang, Y.; Yuan, Z.; Qi, W.; Wang, Q.; Wang, W.; Tan, X. Liquid hot water pretreatment of sugarcane bagasse and its comparison with chemical pretreatment methods for the sugar recovery and structural changes. Bioresour. Technol. 2013, 129, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Nam, B.; Choi, S.A.; Oh, Y.K.; Lee, J.S. Effects of anionic surfactant on extraction of free fatty acid from Chlorella vulgaris. Bioresour. Technol. 2014, 166, 620–624. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-Y.; Oh, Y.-K.; Lee, J.-S.; Lee, K.; Jeong, M.-J.; Choi, S.-A. Acid-catalyzed hot-water extraction of lipids from Chlorella vulgaris. Bioresour. Technol. 2014, 153, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.A.; Jung, J.Y.; Kim, K.; Lee, J.S.; Kwon, J.H.; Kim, S.W.; Yang, J.W.; Park, J.Y. Acid-catalyzed hot-water extraction of docosahexaenoic acid (DHA)-rich lipids from Aurantiochytrium sp. KRS101. Bioresour. Technol. 2014, 161, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.K.; Lewis, D.M.; Ashman, P.J. Disruption of microalgal cells for the extraction of lipids for biofuels: Processes and specific energy requirements. Biomass Bioenergy 2012, 46, 89–101. [Google Scholar] [CrossRef]
- Ferreira, A.F.; Dias, A.P.S.; Silva, C.M.; Costa, M. Effect of low frequency ultrasound on microalgae solvent extraction: Analysis of products, energy consumption and emissions. Algal Res. 2016, 14, 9–16. [Google Scholar] [CrossRef]
- Keris-Sen, U.D.; Sen, U.; Soydemir, G.; Gurol, M.D. An investigation of ultrasound effect on microalgal cell integrity and lipid extraction efficiency. Bioresour. Technol. 2014, 152, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Popoola, T.O.S.; Yangomodou, O.D. Extraction, properties and utilization potentials of cassava seed oil. Biotechnology 2006, 5, 38–41. [Google Scholar] [CrossRef][Green Version]
- Gogate, P.R.; Kabadi, A.M. A review of applications of cavitation in biochemical engineering/biotechnology. Biochem. Eng. J. 2009, 44, 60–72. [Google Scholar] [CrossRef]
- Yusaf, T.; Al-Juboori, R.A. Alternative methods of microorganism disruption for agricultural applications. Appl. Energy 2014, 114, 909–923. [Google Scholar] [CrossRef]
- Follows, M.; Hetherington, P.J.; Dunnill, P.; Lilly, M.D. Release of enzymes from bakers’ yeast by disruption in an industrial homogenizer. Biotechnol. Bioeng. 1971, 13, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Currie, J.A.; Dunnill, P.; Lilly, M.D. Release of protein from Bakers’ yeast (Saccharomyces cerevisiae) by disruption in an industrial agitator mill. Biotechnol. Bioeng. 1972, 14, 725–736. [Google Scholar] [CrossRef]
- Safi, C.; Camy, S.; Frances, C.; Varela, M.M.; Badia, E.C.; Pontalier, P.Y.; Vaca-Garcia, C. Extraction of lipids and pigments of Chlorella vulgaris by supercritical carbon dioxide: Influence of bead milling on extraction performance. J. Appl. Phycol. 2014, 26, 1711–1718. [Google Scholar] [CrossRef]
- Clavijo Rivera, E.; Montalescot, V.; Viau, M.; Drouin, D.; Bourseau, P.; Frappart, M.; Monteux, C.; Couallier, E. Mechanical cell disruption of Parachlorella kessleri microalgae: Impact on lipid fraction composition. Bioresour. Technol. 2018, 256, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Huang, R.; Li, T.; Zhou, J.; Cen, K. Biodiesel from wet microalgae: Extraction with hexane after the microwave-assisted transesterification of lipids. Bioresour. Technol. 2014, 170, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Nadar, S.S.; Rao, P.; Rathod, V.K. Enzyme assisted extraction of biomolecules as an approach to novel extraction technology: A review. Food Res. Int. 2018, 108, 309–330. [Google Scholar] [CrossRef] [PubMed]
- Sowbhagya, H.B.; Srinivas, P.; Krishnamurthy, N. Effect of enzymes on extraction of volatiles from celery seeds. Food Chem. 2010, 120, 230–234. [Google Scholar] [CrossRef]
- Puri, M.; Sharma, D.; Barrow, C.J. Enzyme-assisted extraction of bioactives from plants. Trends Biotechnol. 2012, 30, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Zuorro, A.; Maffei, G.; Lavecchia, R. Optimization of enzyme-assisted lipid extraction from Nannochloropsis microalgae. J. Taiwan Inst. Chem. Eng. 2016, 67, 106–114. [Google Scholar] [CrossRef]
- Latif, S.; Anwar, F. Physicochemical studies of hemp (Cannabis sativa) seed oil using enzyme-assisted cold-pressing. Eur. J. Lipid Sci. Technol. 2009, 111, 1042–1048. [Google Scholar] [CrossRef]
- Shankar, D.; Agrawal, Y.C.; Sarkar, B.C.; Singh, B.P.N. Enzymatic hydrolysis in conjunction with conventional pretreatments to soybean for enhanced oil availability and recovery. JAOCS J. Am. Oil Chem. Soc. 1997, 74, 1543–1547. [Google Scholar] [CrossRef]
- Cho, H.S.; Oh, Y.K.; Park, S.C.; Lee, J.W.; Park, J.Y. Effects of enzymatic hydrolysis on lipid extraction from Chlorella vulgaris. Renew. Energy 2013, 54, 156–160. [Google Scholar] [CrossRef]
- Maffei, G.; Bracciale, M.P.; Broggi, A.; Zuorro, A.; Santarelli, M.L.; Lavecchia, R. Effect of an enzymatic treatment with cellulase and mannanase on the structural properties of Nannochloropsis microalgae. Bioresour. Technol. 2018, 249, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Harun, R.; Danquah, M.K. Enzymatic hydrolysis of microalgal biomass for bioethanol production. Chem. Eng. J. 2011, 168, 1079–1084. [Google Scholar] [CrossRef]
- Sathish, A.; Sims, R.C. Biodiesel from mixed culture algae via a wet lipid extraction procedure. Bioresour. Technol. 2012, 118, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Kostić, M.D.; Joković, N.M.; Stamenković, O.S.; Rajković, K.M.; Milić, P.S.; Veljković, V.B. Optimization of hempseed oil extraction by n-hexane. Ind. Crops Prod. 2013, 48, 133–143. [Google Scholar] [CrossRef]
- Sawangkeaw, R.; Ngamprasertsith, S. A review of lipid-based biomasses as feedstocks for biofuels production. Renew. Sustain. Energy Rev. 2013, 25, 97–108. [Google Scholar] [CrossRef]
- Kumar, D.; Singh, B.; Korstad, J. Utilization of lignocellulosic biomass by oleaginous yeast and bacteria for production of biodiesel and renewable diesel. Renew. Sustain. Energy Rev. 2017, 73, 654–671. [Google Scholar] [CrossRef]
- Bai, X.; Ghasemi Naghdi, F.; Ye, L.; Lant, P.; Pratt, S. Enhanced lipid extraction from algae using free nitrous acid pretreatment. Bioresour. Technol. 2014, 159, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Boyd, A.R.; Champagne, P.; McGinn, P.J.; MacDougall, K.M.; Melanson, J.E.; Jessop, P.G. Switchable hydrophilicity solvents for lipid extraction from microalgae for biofuel production. Bioresour. Technol. 2012, 118, 628–632. [Google Scholar] [CrossRef] [PubMed]
- Dejoye Tanzi, C.; Abert Vian, M.; Chemat, F. New procedure for extraction of algal lipids from wet biomass: A green clean and scalable process. Bioresour. Technol. 2013, 134, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Schuur, B.; Samorì, C.; Tagliavini, E.; Brilman, D.W.F. Secondary amines as switchable solvents for lipid extraction from non-broken microalgae. Bioresour. Technol. 2013, 149, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Choi, Y.K.; Park, J.; Lee, S.; Yang, Y.H.; Kim, H.J.; Park, T.J.; Hwan Kim, Y.; Lee, S.H. Ionic liquid-mediated extraction of lipids from algal biomass. Bioresour. Technol. 2012, 109, 312–315. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Huh, Y.S.; Farooq, W.; Chung, J.; Han, J.I.; Shin, H.J.; Jeong, S.H.; Lee, J.S.; Oh, Y.K.; Park, J.Y. Lipid extractions from docosahexaenoic acid (DHA)-rich and oleaginous Chlorella sp. biomasses by organic-nanoclays. Bioresour. Technol. 2013, 137, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Steriti, A.; Rossi, R.; Concas, A.; Cao, G. A novel cell disruption technique to enhance lipid extraction from microalgae. Bioresour. Technol. 2014, 164, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.J.; Lee, O.K.; Lee, E.Y. Dimethyl carbonate-mediated lipid extraction and lipase-catalyzed in situ transesterification for simultaneous preparation of fatty acid methyl esters and glycerol carbonate from Chlorella sp. KR-1 biomass. Bioresour. Technol. 2014, 158, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Godwin, C.M.; Hietala, D.C.; Lashaway, A.R.; Narwani, A.; Savage, P.E.; Cardinale, B.J. Algal polycultures enhance coproduct recycling from hydrothermal liquefaction. Bioresour. Technol. 2017, 224, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Hietala, D.C.; Koss, C.K.; Narwani, A.; Lashaway, A.R.; Godwin, C.M.; Cardinale, B.J.; Savage, P.E. Influence of biodiversity, biochemical composition, and species identity on the quality of biomass and biocrude oil produced via hydrothermal liquefaction. Algal Res. 2017, 26, 203–214. [Google Scholar] [CrossRef]
- Xu, D.; Savage, P.E. Effect of temperature, water loading, and Ru/C catalyst on water-insoluble and water-soluble biocrude fractions from hydrothermal liquefaction of algae. Bioresour. Technol. 2017, 239, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, J.D.; Savage, P.E. Modeling the effects of microalga biochemical content on the kinetics and biocrude yields from hydrothermal liquefaction. Bioresour. Technol. 2017, 239, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Hietala, D.C.; Faeth, J.L.; Savage, P.E. A quantitative kinetic model for the fast and isothermal hydrothermal liquefaction of Nannochloropsis sp. Bioresour. Technol. 2016, 214, 102–111. [Google Scholar] [CrossRef] [PubMed]
| Oleaginous Micro-Organism | Lipid Extraction Method | Pretreatment of Cells | Lipid Content (%, w/w) | References |
|---|---|---|---|---|
| Oleaginous yeasts | ||||
| Rhodosporidium kratochvilovae HIMPA1 | Bligh & Dyer method | Ultrasonication at 40 Hz for 5 min | 59.7 | [39] |
| Organic-solvent n-hexane | Acid-catalyzed hot-water treatment | 61.9 | ||
| Organic-solvent n-hexane | Microwave irradiation | 67.4 | ||
| Organic-solvent n-hexane | Rapid ultrasonication-microwave treatment | 70.1 | ||
| Cryptococcus curvatus (DSM 70022) | Solvent extraction (chloroform-methanol; 2:1, v/v) | Dried biomass, Acid-catalyzed hot-water treatment. (2 mL of 3 M HCl and then digested at 60 °C for 2 h), Sonication for 30 s at 30 kHz | NA | [40] |
| 46 | ||||
| Rhodotorula glutinis (DSM 10134) | ||||
| 48.9 | ||||
| Yarrowia lipolytica (DSM 8218) | ||||
| C. curvatus MUCL 29819 | Solvent extraction (chloroform-methanol; 1:1, v/v) | Dried yeast cells, Bead milling (glass beads, diameter 0.5 mm) | 30.3 | [41] |
| Sporidiobolus pararoseus KM281507 | Bligh & Dyer method | Vortexed with glass beads, sonicated at 70 Hz for 30 min | 30.7 | [42] |
| S. pararoseus KX709872 | Bligh & Dyer method | Vortexed with glass beads for 30 min in the presence of 100 ppm ascorbic acid and sonicated for 30 min in ultrasonication bath | 56.6 | [43] |
| Naganishia liquefaciens NITTS2 | Solvent extraction (chloroform-methanol; 1:1, v/v) | Ultrasonication at 20 kHz for 20 min at 40 °C | 55.8 | [44] |
| C. curvatus MTCC 2698 | Bligh & Dyer method | Sonication at 40 kHz for 2 min | 28.3 | [45] |
| Cryptococcus vishniaccii | Bligh & Dyer method | Sonication at 20 kHz for 5 min | 52.3 | [46] |
| Rhodosporidium toruloides and Lipomyces starkeyi | Bligh & Dyer method | Acid (2 mol/L of HCl) | 25 and 34 | [47] |
| None | 23 and 7 | |||
| Folch method | Acid (2 mol/L of HCl) | 34 and 48 | ||
| Enzymatic | 31 and 37 | |||
| None | 42 and 47 | |||
| Oleaginous microalgae | ||||
| Schizochytrium sp. ATCC20888 | Soxhlet extraction | Enzymatic lysis with alkaline protease | 63 | [48] |
| Chlorella vulgaris/Cyanobacteria leptolyngbya | Solvent extraction with hexane or chloroform-methanol (1:1, v/v) | Sonicated in an ultrasonic reactor with a clamp-on transducer | 16 | [48] |
| Phaeodactylum tricornutum | Solvent extraction (chloroform-methanol; 1:1, v/v) | Lyophilization | 47 | [49] |
| Scenedesmus sp. | Solvent extraction (chloroform methanol; 1:1, v/v) | Enzymatic treatment with cellulase, xylanase and pectinase | 86.4 (lipid recovery) | [50] |
| Tetraselmis sp. KCTC12429BP | Solvent extraction with mixture of hexane and polar solvents (ethanol, isopropanol, methanol, tetrahydrofuran, acetone, acetonitrile) | Lyophilization | 5.5 with Chloroform-methanol, 5.2 with hexane-methanol | [51] |
| Aurantiochytrium sp. KRS101 | Solvent extraction with chloroform, chloroform-methanol (2:1, v/v), hexane, hexane-isopropanol (3:2, v/v), methanol and ethanol | High shear mixer (HSM) | High non-esterifiable lipids with chloroform-methanol and esterifiable lipids with chloroform | [52] |
| Scenedesmus obliquus | Solvent (chloroform-methanol; 2:1, v/v) | Drying of biomass by sun, freeze, and oven followed by microwave, sonication, autoclaving, osmotic shock (10% NaCl) | Highest lipid content of 25.4% was obtained after freeze-drying followed by microwave digestion | [53] |
| Scendesmus dimorphus | Solvent extraction with ethanol (6 mL/g dry algae), Fractionation with (ethanol: hexane: water; 1:1:1, v/v/v) | Extraction autoclave equipped with condenser, mechanical stirring and thermocouple | Oil extraction by fractional method gave neutral lipid (97) with polar lipids (2) | [54] |
| Oleaginous fungus | ||||
| Mucor circinelloides URM 4182 | Solvent extraction With ethanol (96%) | Microwave irradiation at 60 °C for 30 min | 31.2 | [55] |
| Cunninghamella echinulata | Soxhlet extraction with diethyl ether anhydrous at 50 °C | Dried biomass ground in a laboratory blender | 22.2 | [56] |
| M. circinelloides VI04473 and Mortierella alpina UBOCC-A-112046 | Folch method, Bligh & Dyer method | Acid hydrolysis with 2 mL 3 N HCl (incubation of the sample at 80 °C for 1 h), bead beating and homogenization (4.0 m/s for 60 s) | NA | [57] |
| M. circinelloides VI 04473, Umbelopsis isabellina UBOCC-A-101350 and Penicillium glabrum FRR 419 | Lewis extraction | Freeze-dried, biomass, glass beads in high-speed benchtop homogenizer at 6.5 m/s, for 1 min cycle length and 6 cycles | Highest lipid content was obtained from U. isabellina at 30 °C | [58] |
| Alternaria alternata, Cladosporium cladosporioides, Epicoccum nigrum, Fusarium oxysporum, Aspergillus parasiticus and Emericella nidulans var. lata | Folch method | NA | Highest lipid content (40.8) from A. alternata | [59] |
| Aspergillus tubingensis TSIP9 | Folch method | Slurry of biomass and chloroform-methanol sonicated for 30 min | 39.5 mg per gram dry substrate (gds) | [35] |
| Oleaginous bacteria | ||||
| Acinetobacter baylyi ADP1 | Bligh & Dyer method | Freeze-dried cells, vortexed | 1.6 with wild strain, 12.4 with genetically modified strain | [60] |
| Rhodococcus opacus | Folch method | Homogenized with chloroform-methanol (2:1, v/v), followed by shaking | 71 with synthetic medium | [36] |
| Bacillus subtilis HB1310 | Bligh & Dyer method | 4 M HCl, incubation at 80 °C for 1 h | 39.8 | [61] |
| Rhodopseudomonas palustris (strain 42OL) | Solvent extraction methanol-chloroform (1:2, v/v) | Grinding of freeze-dried bacterial cells in a mortar with sand | 22 to 39 | [62] |
| Bacillus sp. V10 | Bligh & Dyer method | Freeze-drying of the cells | 7.4 | [63] |
| R. opacus | Folch method | Homogenized with chloroform-methanol (2:1 v/v), followed by shaking | 65.8 | [64] |
| Pretreatment Methods | Mode of Action | Energy Consumption | Scale-Up Possibility | Advantages | Disadvantages | References |
|---|---|---|---|---|---|---|
| Ultrasonication | Cavitation, acoustic streaming and liquid shear stress | Medium/low | Yes/no | Less processing time, lower solvent consumption, greater penetration of solvent into cellular compartment | High power consumption, difficult to scale up | [23,91,92,96,97,120,121] |
| Oil/expeller press | Mechanical compaction and shear forces | High | Yes | Easy process, no solvent | Large amount of sample required, slow process, unsuitable for samples with high moisture content | [67,122] |
| High-speed homogenization | Cavitation and shear forces | High/medium | Yes | Simple process, effective, short contact time | High energy consumption, increased temperature during operation | [20,85,86] |
| High-pressure homogenization | Cavitation and shear forces | High/medium | Yes | Solvent-free, simple process, effective, short contact time | High maintenance cost, less efficient with filamentous microorganisms, no residual effect | [22,123,124,125] |
| Bead milling | Mechanical compaction and shear forces | High/medium | Yes | Solvent-free, suitable for samples with high moisture content | Low efficiency with rigid cells, depending on various parameters such as bead size and agitation, no residual effect | [19,72,73,75,103,124,126,127,128] |
| Microwave irradiation | Temperature increase, molecular energy increase | High/medium | Yes/no | Eco-friendly, reduced processing time and solvent consumption | Filtration or centrifugation is necessary to remove the solid residue, unsuitable for non-polar or volatile compounds | [78,95,106,107,128,129,130] |
| Pulsed electric field treatment | Pore formation due to electric waves | High | Yes/no | Relatively simple, high energetic efficiency, relatively fast | High maintenance costs, high temperature, dependence on medium composition, decomposition of fragile compounds | [20,56,107,108,109,110,111] |
| Enzymatic treatment | Specific enzyme-substrate interaction | Low | Yes | Simple, high energetic efficiency | Long processing time and high capital cost | [22,25,71,123,125,130,131,132,133] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, A.; Mikes, F.; Matsakas, L. An Overview of Current Pretreatment Methods Used to Improve Lipid Extraction from Oleaginous Microorganisms. Molecules 2018, 23, 1562. https://doi.org/10.3390/molecules23071562
Patel A, Mikes F, Matsakas L. An Overview of Current Pretreatment Methods Used to Improve Lipid Extraction from Oleaginous Microorganisms. Molecules. 2018; 23(7):1562. https://doi.org/10.3390/molecules23071562
Chicago/Turabian StylePatel, Alok, Fabio Mikes, and Leonidas Matsakas. 2018. "An Overview of Current Pretreatment Methods Used to Improve Lipid Extraction from Oleaginous Microorganisms" Molecules 23, no. 7: 1562. https://doi.org/10.3390/molecules23071562
APA StylePatel, A., Mikes, F., & Matsakas, L. (2018). An Overview of Current Pretreatment Methods Used to Improve Lipid Extraction from Oleaginous Microorganisms. Molecules, 23(7), 1562. https://doi.org/10.3390/molecules23071562