Mahimbrine A, a Novel Isoquinoline Alkaloid Bearing a Benzotropolone Moiety from Mahonia imbricata
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structure Elucidation of Mahimbrine A
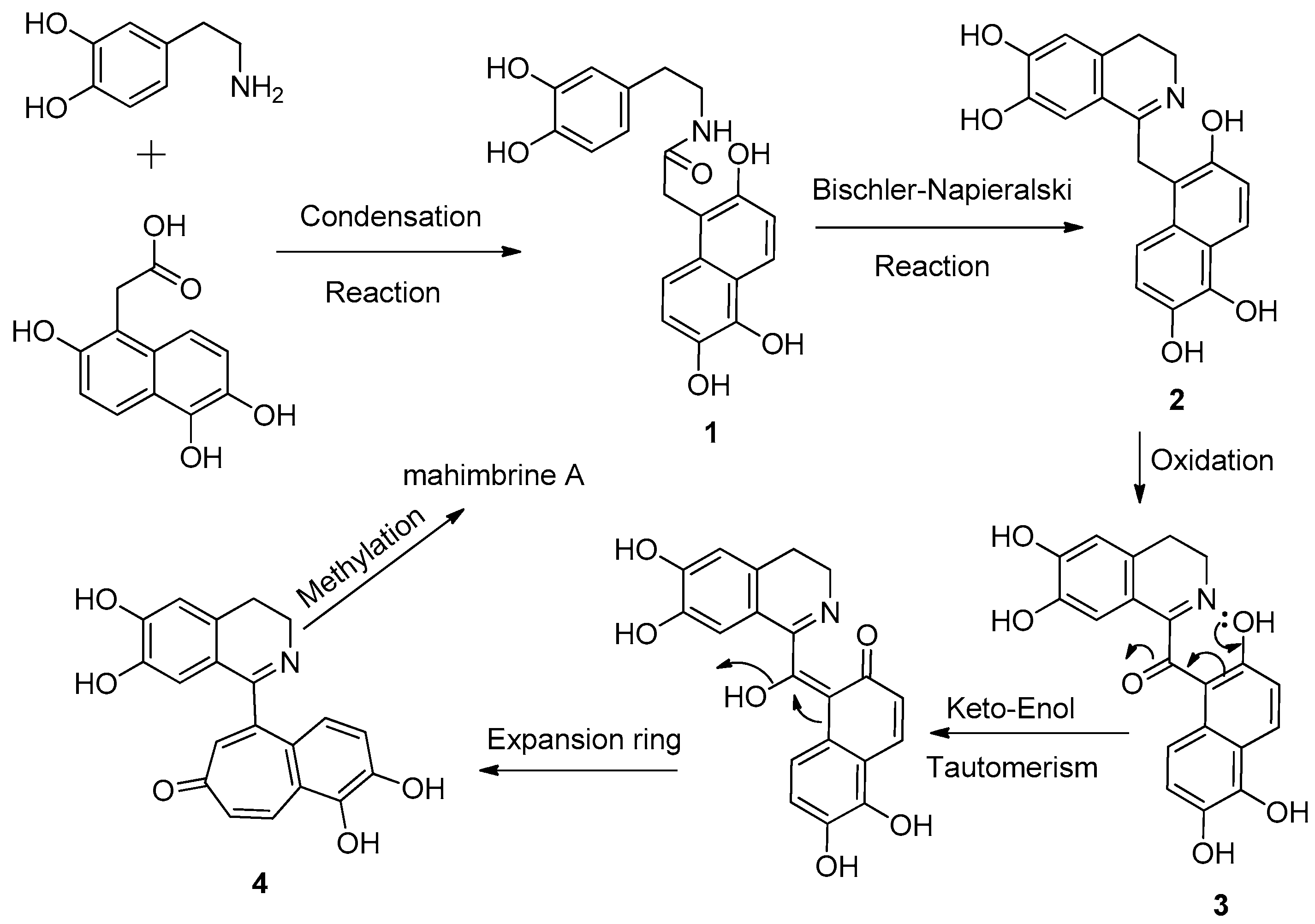
2.2. Plausible Biogenetic Pathway
2.3. Antimicrobial Activity
3. Experimental Section
3.1. General Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Spectral Data
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Ying, J.S.; Cheng, D.Z. Flora of China; Science Press: Beijing, China, 2001; Volume 20, p. 242. [Google Scholar]
- He, J.M.; Mu, Q. The medicinal uses of the genus Mahonia in traditional Chinese medicine: An ethnopharmacological, phytochemical and pharmacological review. J. Ethnopharmacol. 2015, 175, 668–683. [Google Scholar] [CrossRef] [PubMed]
- Müller, K.; Ziereis, K. The antipsoriatic Mahonia aquifolium and its active constituents; I. Pro- and antioxidant properties and inhibition of 5-lipoxygenase. Planta Med. 1994, 60, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Račková, L.; Májeková, M.; Košt’Álová, D.; Štefek, M. Antiradical and antioxidant activities of alkaloids isolated from Mahonia aquifolium. Structural aspects. Bioorg. Med. Chem. 2004, 12, 4709–4715. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.J.; Chia, Y.C.; Wu, Y.C.; Chen, Z.Y. Chemical Constituents from the Stems of Mahonia japonica. J. Chin. Chem. Soc. 2004, 51, 443–446. [Google Scholar] [CrossRef]
- Xiao, S.J.; Lei, X.X.; Xia, B.; Xiao, H.P.; He, D.H.; Fang, D.M.; Qi, H.Y.; Chen, F.; Ding, L.S.; Zhou, Y. Two novel norlignans from Gymnotheca chinensis. Tetrahedron Lett. 2014, 55, 2869–2871. [Google Scholar] [CrossRef]
- Xiao, S.J.; Lei, X.X.; Xia, B.; Xu, D.Q.; Xiao, H.P.; Xu, H.X.; Chen, F.; Ding, L.S.; Zhou, Y. Two novel polycyclic spiro lignans from Gymnotheca involucrate. Tetrahedron Lett. 2014, 55, 5949–5951. [Google Scholar] [CrossRef]
- He, D.H.; Ding, L.S.; Xu, H.X.; Lei, X.X.; Xiao, H.P.; Zhou, Y. Gymnothelignans A–O: Conformation and absolute configuration analyses of lignans bearing tetrahydrofuran from Gymnotheca chinensis. J. Org. Chem. 2012, 77, 8435–8443. [Google Scholar] [CrossRef] [PubMed]
- Kuroyanagi, M.; Shirota, O.; Sekita, S.; Nakane, T. Transannular cyclization of (4S,5S)-germacrone-4, 5-epoxide into guaiane and secoguaiane-type sesquiterpenes. Nat. Prod. Comm. 2012, 7, 441–446. [Google Scholar]
- Tam, E.K.W.; Liu, L.Y.; Chen, A.Q. 2-Furanylboronic acid as an effective catalyst for the direct amidation of carboxylic acids at room temperature. Eur. J. Org. Chem. 2015, 2015, 1100–1107. [Google Scholar] [CrossRef]
- Nimgirawath, S.; Lorpitthaya, R.; Wanbanjob, A.; Taechowisan, T.; Shen, Y.M. Total synthesis and the biological activities of (+/−)-norannuradhapurine. Molecules 2008, 14, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Daniel, L.C.; Paresh, M.T.; Matthew, F.B.; Badawi, M.M. Chiral auxiliary mediated pictet-spengler reactions: Asymmetric syntheses of (−)-laudanosine, (+)-glaucine and (−)-xylopinine. Tetrahedron 1997, 53, 16327–16340. [Google Scholar]
- Xin, M.; Bugg, T.D.H. Biomimetic formation of 2-tropolones by dioxygenase-catalysed ring expansion of substituted 2,4-cyclohexadienones. Chembiochem 2010, 11, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.D.; Zhang, Y.Y.; Gao, D.M.; Zhang, H.M. Antibacterial and anti-inflammatory activities of extract and fractions from Pyrrosia petiolosa (Christ et Bar.) Ching. J. Ethnopharmacol. 2014, 155, 1300–1305. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compound mahimbrine A are available from the authors. |
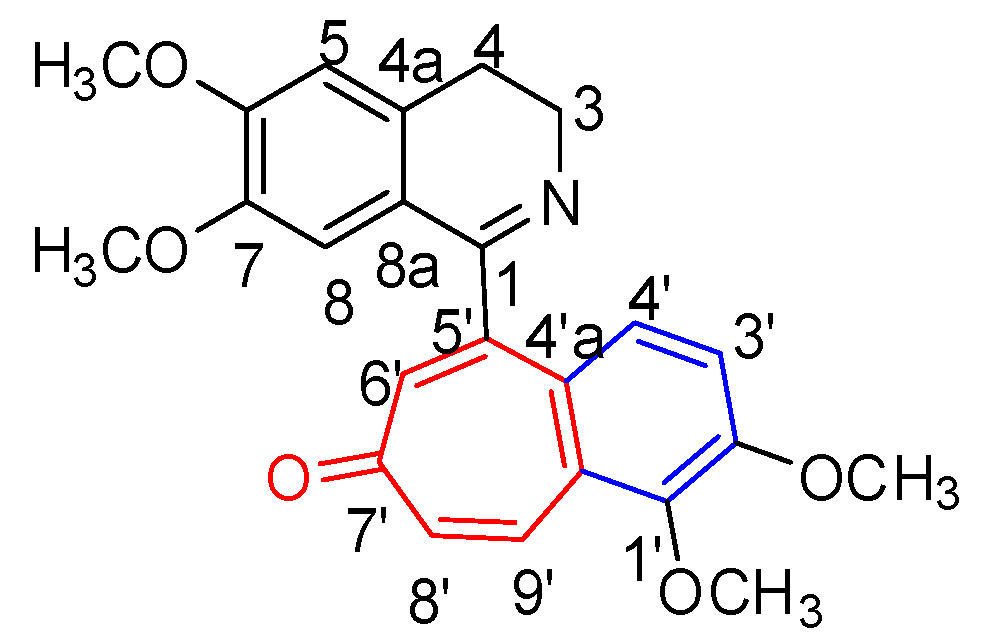
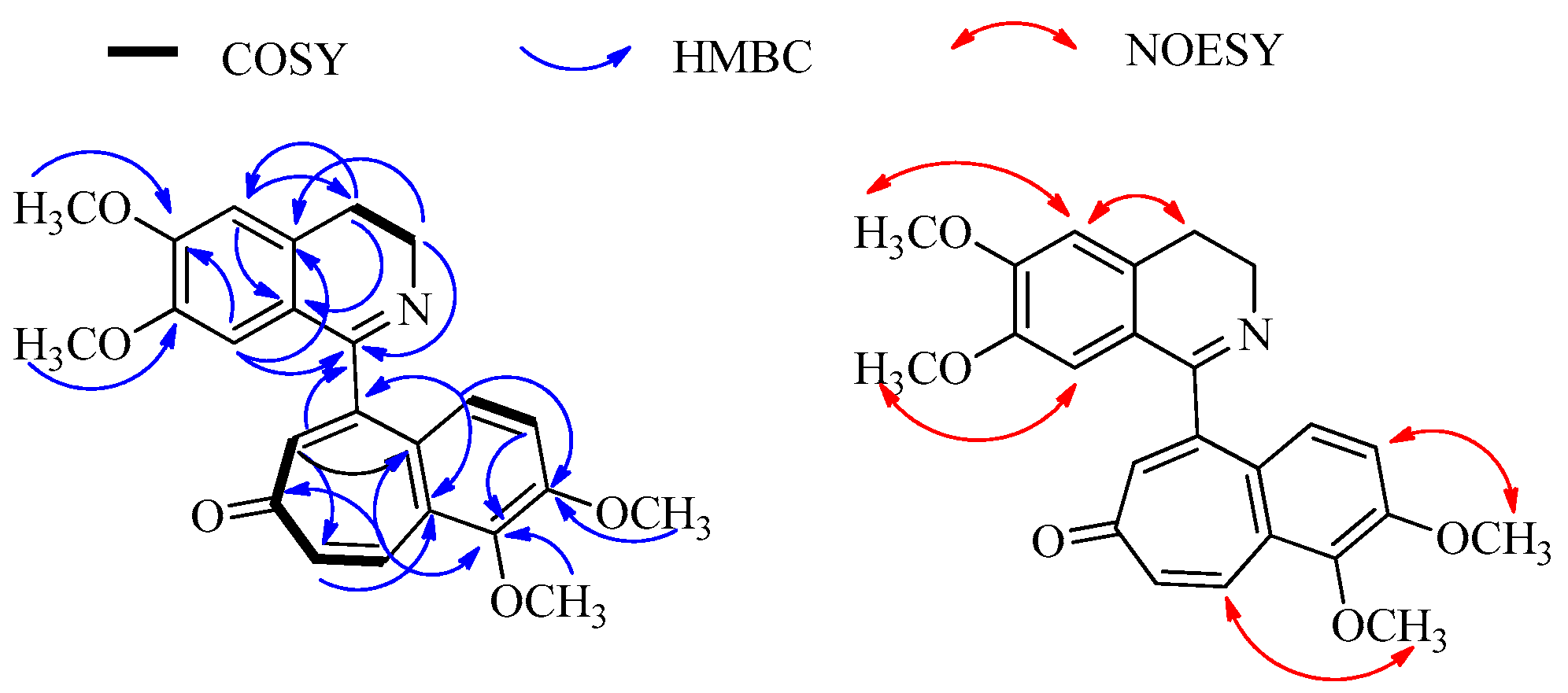
| Position | δH | δC | Position | δH | δC |
|---|---|---|---|---|---|
| 1 | 168.4 | 1′ | 148.4 | ||
| 3 | 4.03, 3.90, m | 47.9 | 2′ | 153.7 | |
| 4 | 2.91, m | 25.8 | 3′ | 7.04, d (9.1) | 114.5 |
| 4a | 131.4 | 4′ | 7.28, d (9.1) | 128.8 | |
| 5 | 6.78, s | 110.6 | 4′a | 129.2 | |
| 6 | 151.8 | 5′ | 148.8 | ||
| 7 | 147.9 | 6′ | 6.74, d (2.7) | 133.5 | |
| 8 | 6.57, s | 110.6 | 7′ | 188.4 | |
| 8a | 121.9 | 8′ | 6.87, dd (13.0, 2.7) | 134.9 | |
| 6-OCH3 | 3.94, s | 56.2 | 9′ | 8.20, d (13.0) | 133.6 |
| 7-OCH3 | 3.60, s | 56.2 | 9′a | 131.5 | |
| 1′-OCH3 | 3.93, s | 61.8 | |||
| 2′-OCH3 | 3.93, s | 56.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.-S.; Deng, Y.; Fu, S.-B.; Guo, D.-L.; Xiao, S.-J. Mahimbrine A, a Novel Isoquinoline Alkaloid Bearing a Benzotropolone Moiety from Mahonia imbricata. Molecules 2018, 23, 1539. https://doi.org/10.3390/molecules23071539
Zhang M-S, Deng Y, Fu S-B, Guo D-L, Xiao S-J. Mahimbrine A, a Novel Isoquinoline Alkaloid Bearing a Benzotropolone Moiety from Mahonia imbricata. Molecules. 2018; 23(7):1539. https://doi.org/10.3390/molecules23071539
Chicago/Turabian StyleZhang, Mao-Sheng, Yan Deng, Shao-Bin Fu, Da-Le Guo, and Shi-Ji Xiao. 2018. "Mahimbrine A, a Novel Isoquinoline Alkaloid Bearing a Benzotropolone Moiety from Mahonia imbricata" Molecules 23, no. 7: 1539. https://doi.org/10.3390/molecules23071539
APA StyleZhang, M.-S., Deng, Y., Fu, S.-B., Guo, D.-L., & Xiao, S.-J. (2018). Mahimbrine A, a Novel Isoquinoline Alkaloid Bearing a Benzotropolone Moiety from Mahonia imbricata. Molecules, 23(7), 1539. https://doi.org/10.3390/molecules23071539





