Development and Evaluation of Surfactant Nanocapsules for Chemical Enhanced Oil Recovery (EOR) Applications
Abstract
1. Introduction
2. Results
2.1. Nanocapsules Characterization
2.1.1. Size and Morphology of Nanocapsules
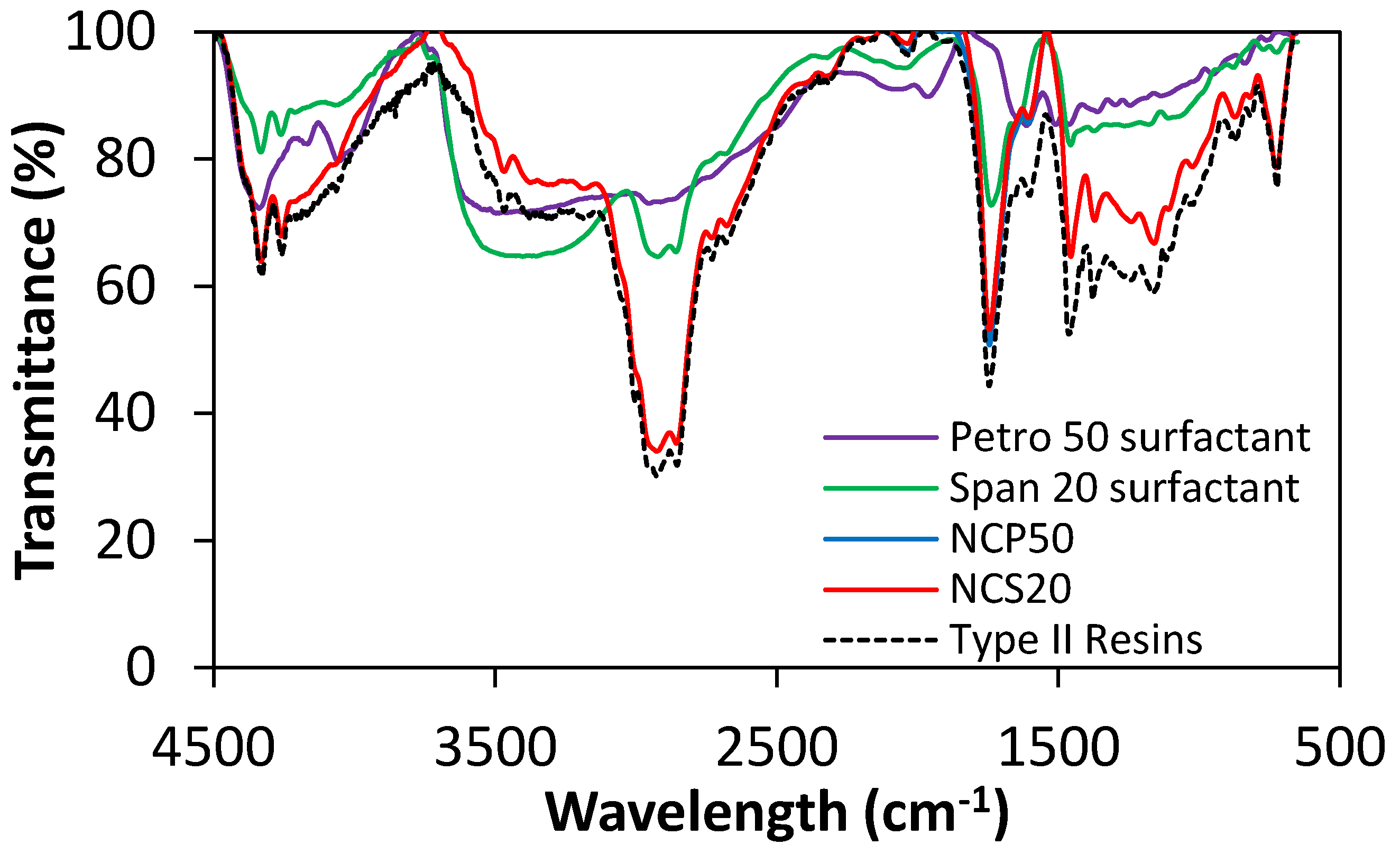
2.1.2. FTIR Measurements
2.1.3. Softening Point, Solvency Tests, and Encapsulation Efficiency
2.2. Adsorption Experiments
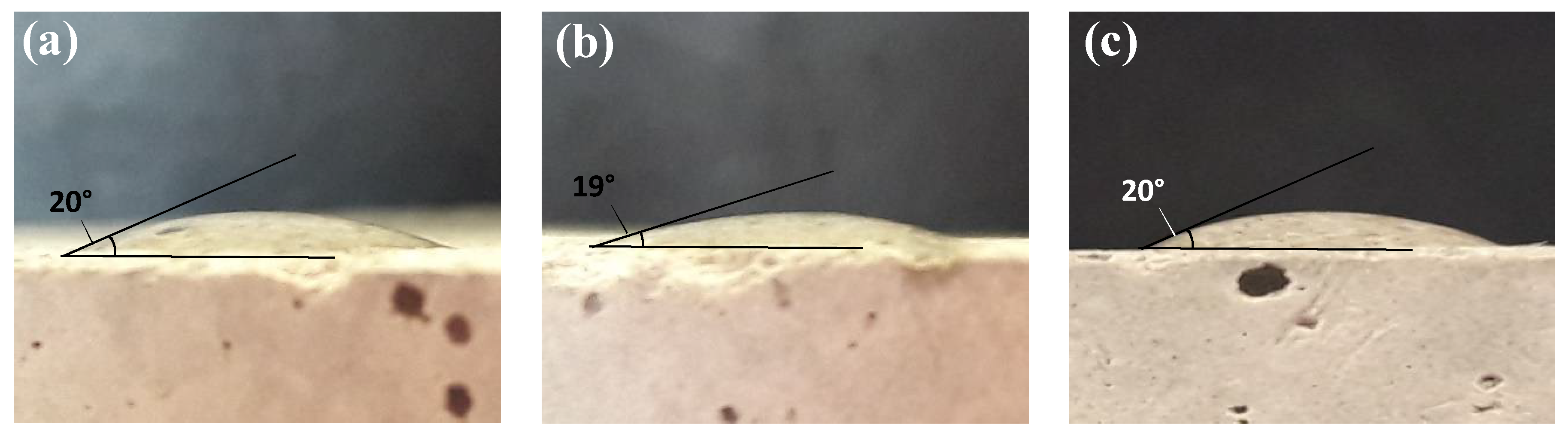
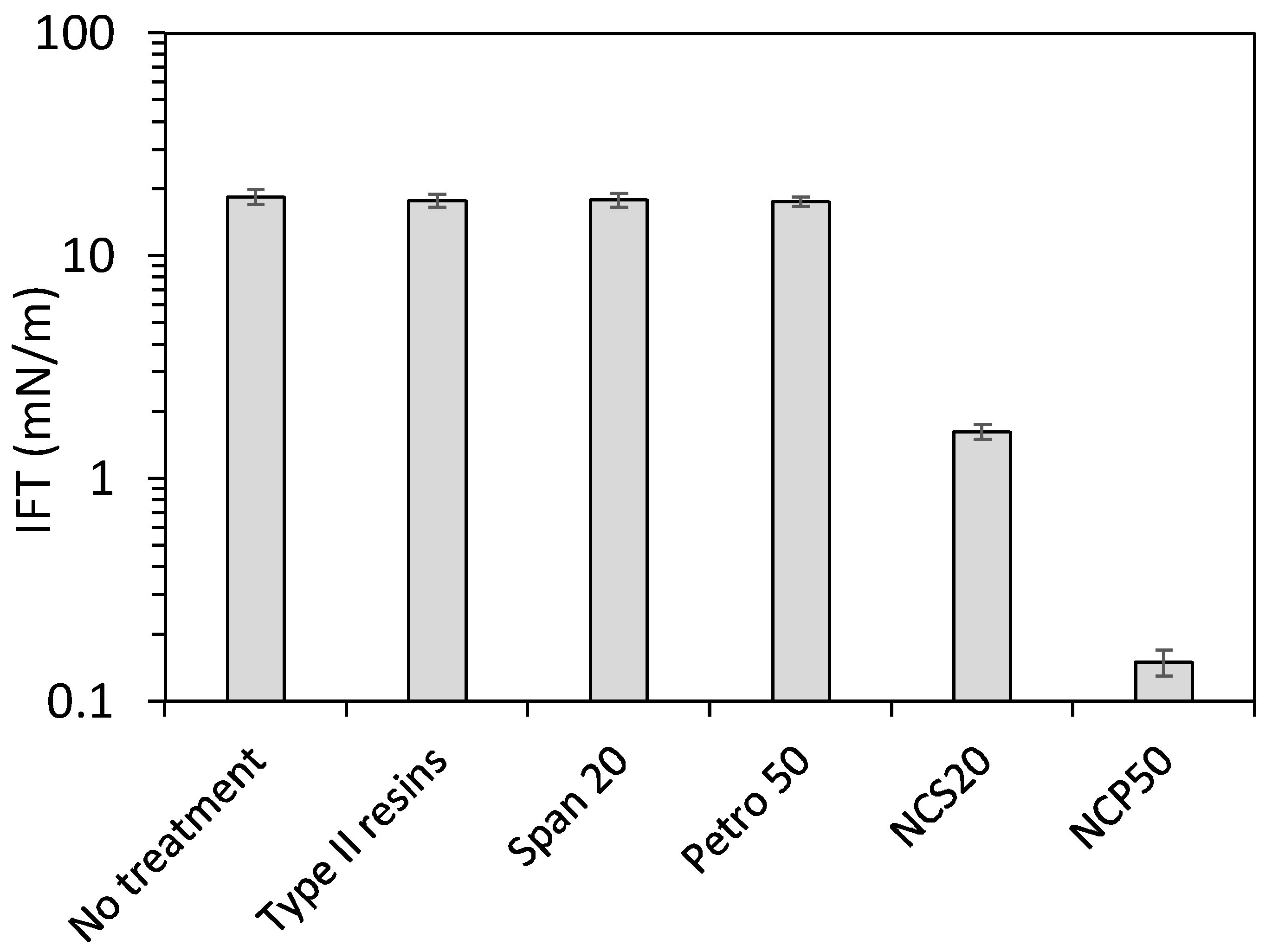
2.3. IFT Measurements
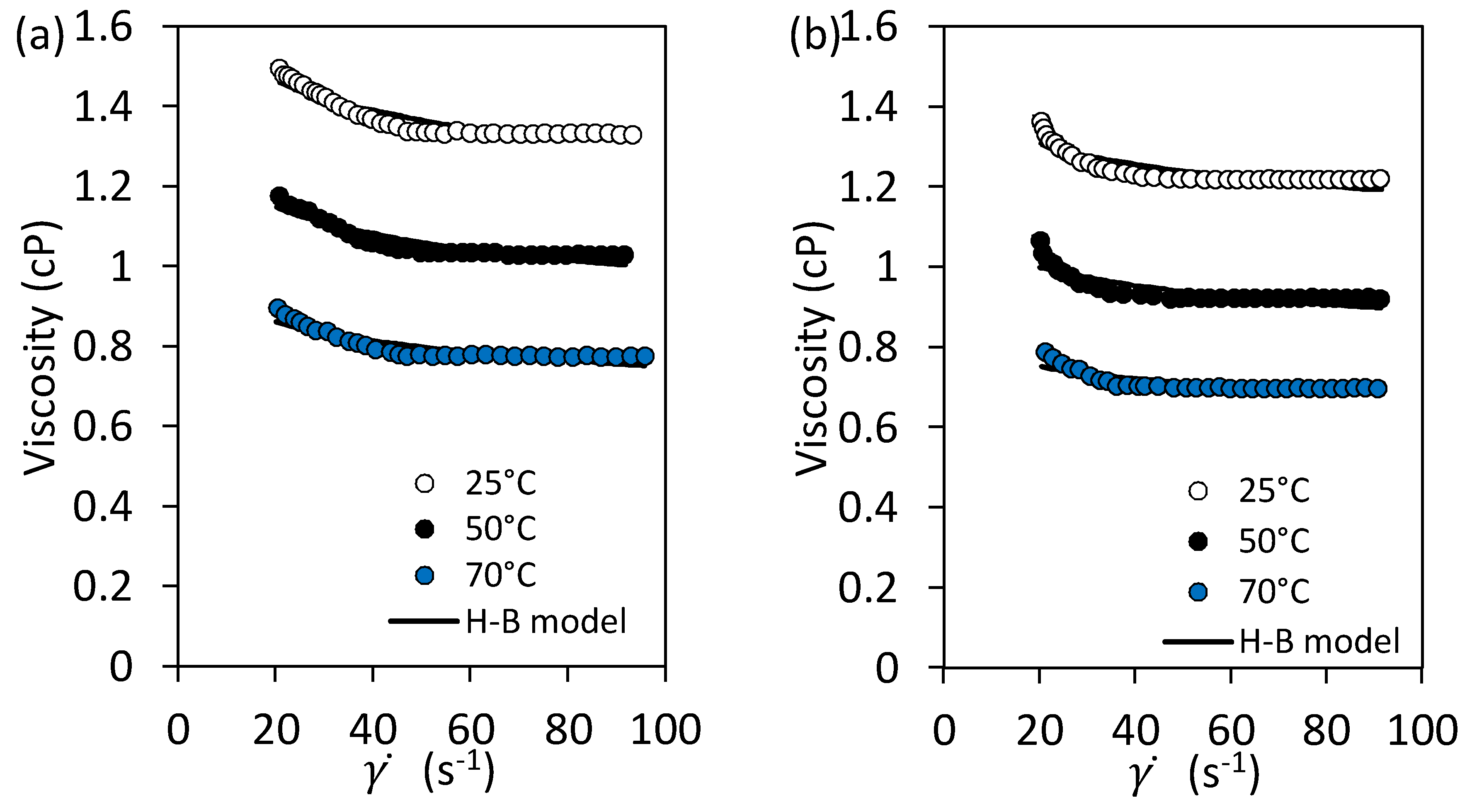
2.4. Rheological Behavior of Nanofluid
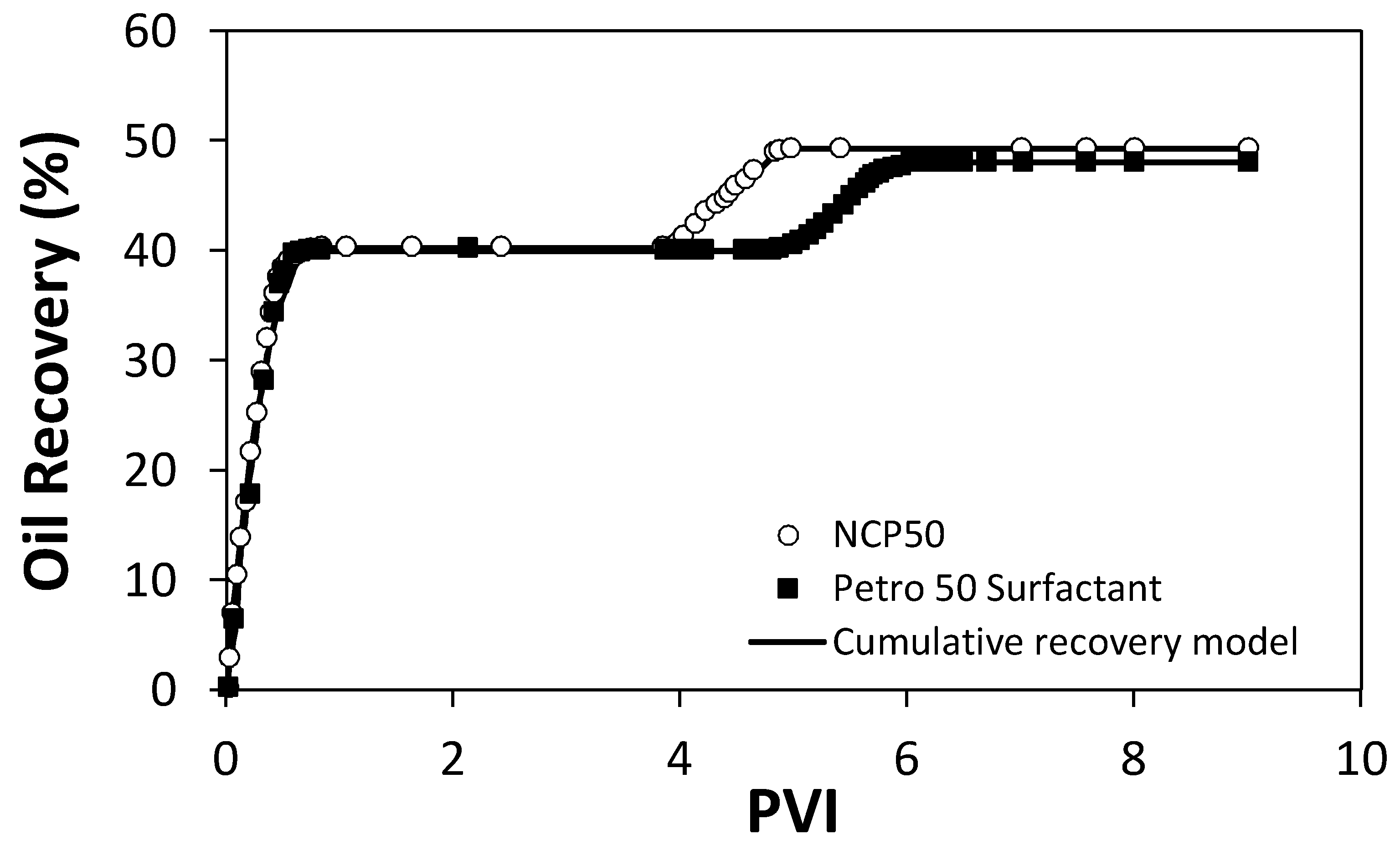
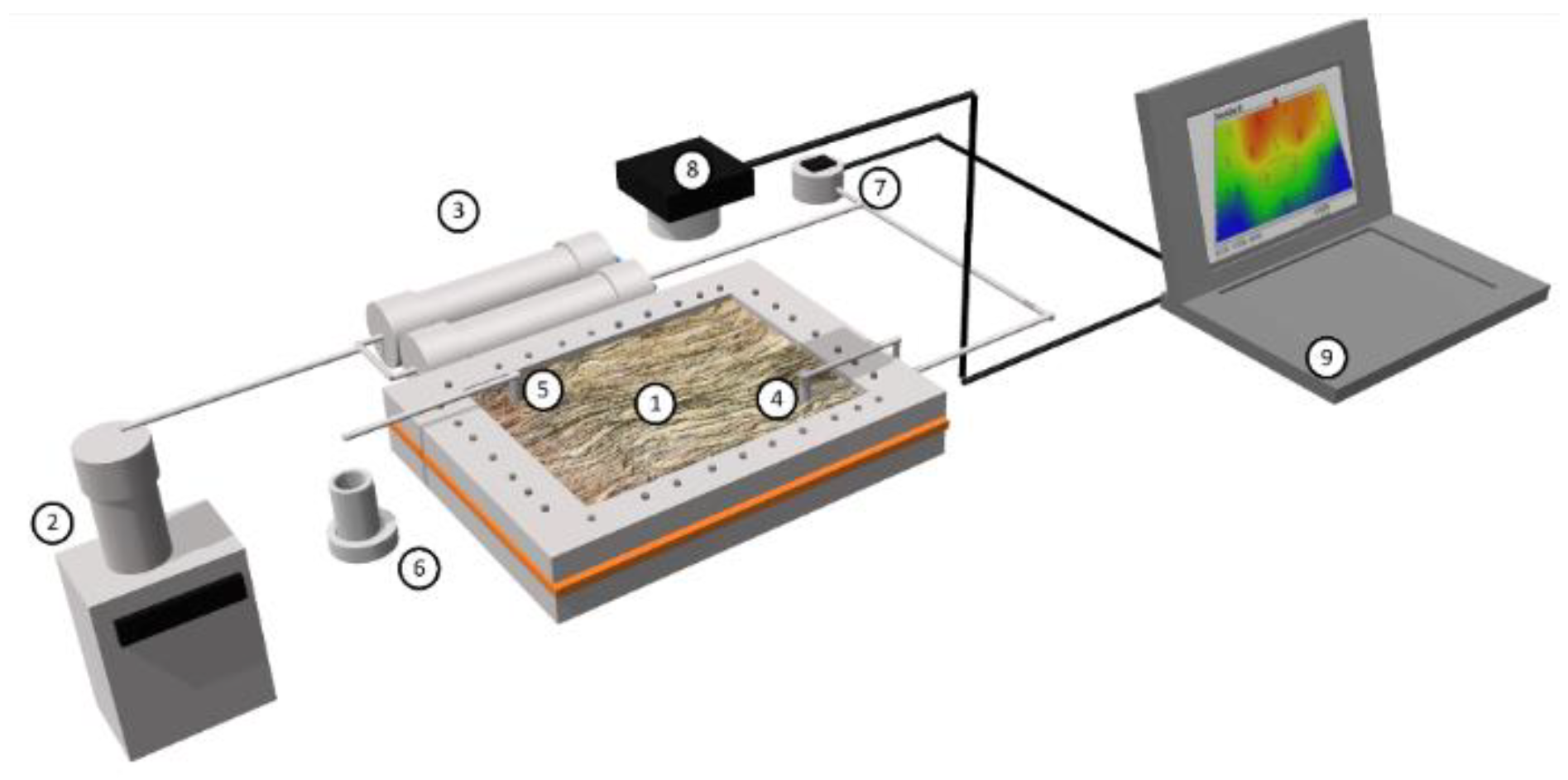
2.5. Displacement Tests in a Quarter Five-Spot Pattern Micromodel
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Isolation of Type II Resins
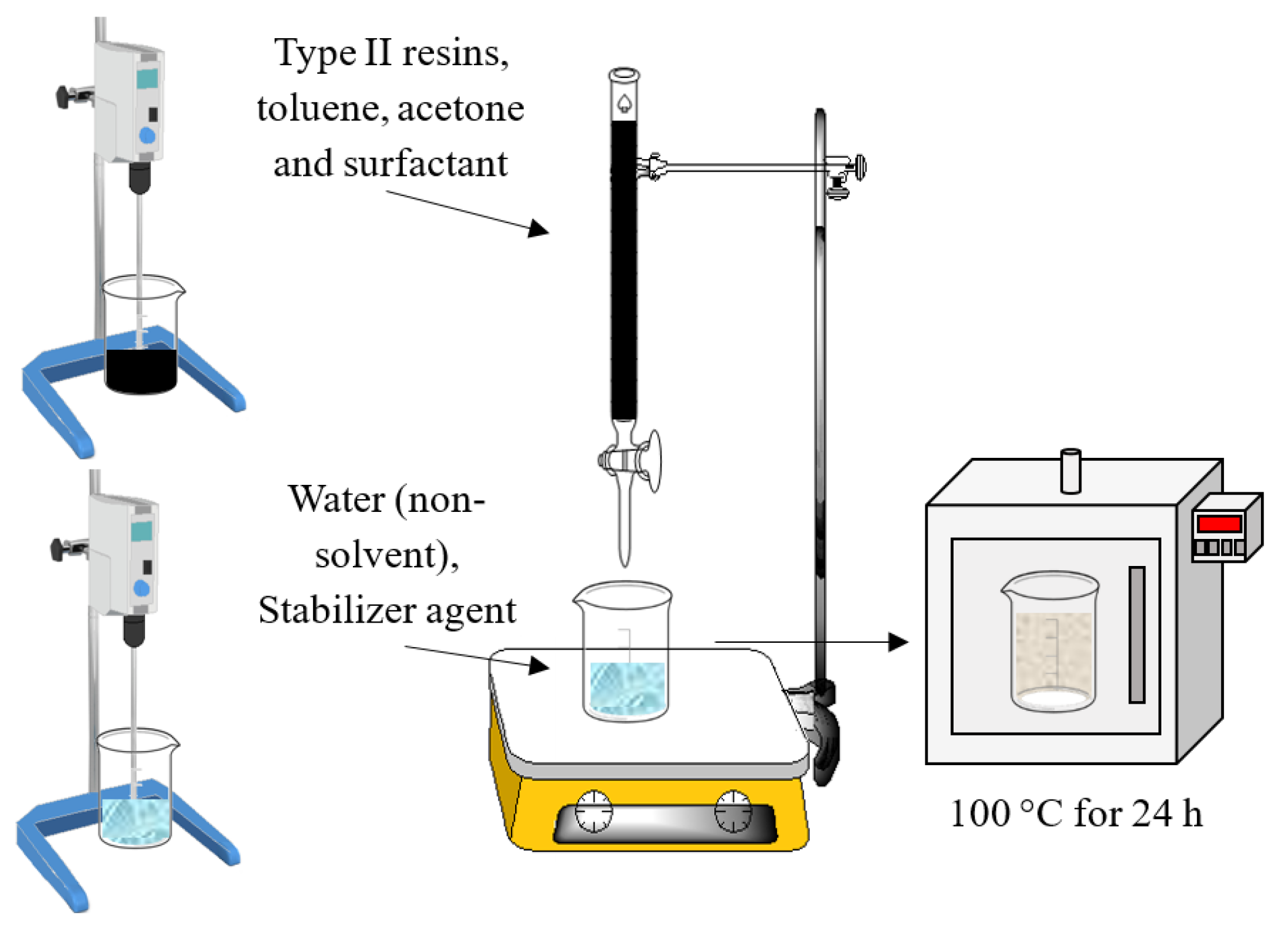
3.2.2. Nanoprecipitation Method for Nanocapsules Preparation
3.2.3. Nanocapsules Characterization
3.2.4. Adsorption Tests
3.2.5. Rheological Behavior of Nanofluid
3.2.6. Interfacial Tension Measurements
3.2.7. Displacement Test in Micromodel
3.3. Modeling
3.3.1. Herschel-Bulkley Rheological Model
3.3.2. Cumulative Recovery Model
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Conti, J.; Holtberg, P.; Diefenderfer, J.; LaRose, A.; Turnure, J.T.; Westfall, L. International Energy Outlook 2016 with Projections to 2040; USDOE Energy Information Administration (EIA), Office of Energy Analysis: Washington, DC, USA, 2016.
- Alvarado, V.; Manrique, E. Enhanced oil recovery: An update review. Energies 2010, 3, 1529–1575. [Google Scholar] [CrossRef]
- Thomas, S. Enhanced oil recovery—An overview. Oil Gas Sci. Technol.-Rev. l'IFP 2008, 63, 9–19. [Google Scholar] [CrossRef]
- De Ferrer, M.P. Inyección de Agua y Gas en Yacimientos Petrolíferos; Ediciones Astro Data SA: Maracaibo, Venezuela, 2001. [Google Scholar]
- Sheng, J. Modern Chemical Enhanced Oil Recovery: Theory and Practice; Gulf Professional Publishing: Houston, TX, USA, 2010. [Google Scholar]
- Sheng, J. Enhanced Oil Recovery Field Case Studies; Gulf Professional Publishing: Houston, TX, USA, 2013. [Google Scholar]
- Negin, C.; Ali, S.; Xie, Q. Most common surfactants employed in chemical enhanced oil recovery. Petroleum 2017, 3, 197–211. [Google Scholar] [CrossRef]
- Sheng, J.J. Status of surfactant EOR technology. Petroleum 2015, 1, 97–105. [Google Scholar] [CrossRef]
- Schramm, L.L.; Stasiuk, E.N.; Marangoni, D.G. Surfactants and their applications. Annu. Rep. Sect. C Phys. Chem. 2003, 99, 3–48. [Google Scholar] [CrossRef]
- Spildo, K.; Sun, L.; Djurhuus, K.; Skauge, A. A strategy for low cost, effective surfactant injection. J. Pet. Sci. Eng. 2014, 117, 8–14. [Google Scholar] [CrossRef]
- Schramm, L.L. Surfactants: Fundamentals and Applications in the Petroleum Industry; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Peru, D.A.; Lorenz, P.B. The Effect of Equilibration Time and Temperature on Drop-Drop Coalescence of Wilmington Crude Oil in a Weakly Alkaline Brine. Chem. Eng. Commun. 1989, 77, 91–114. [Google Scholar] [CrossRef]
- French, T.R.; Burchfield, T.E. Design and optimization of alkaline flooding formulations. In Proceedings of the SPE/DOE Enhanced Oil Recovery Symposium, Tulsa, OK, USA, 22–25 April 1990; Society of Petroleum Engineers: Richardson, TX, USA, 1990. [Google Scholar]
- Zhou, W.; Dong, M.; Liu, Q.; Xiao, H. Experimental investigation of surfactant adsorption on sand and oil-water interface in heavy oil/water/sand systems. In Proceedings of the Canadian International Petroleum Conference, Calgary, AB, Canada, 7–9 June 2005; Petroleum Society of Canada: Calgary, AB, Canada, 2005. [Google Scholar]
- Dai, C.; You, Q.; Wang, Y.; Zhao, F.; Shuler, P.J. Research on reutilization technology of residual polymer in formation after polymer flooding. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 24–28 April 2010; Society of Petroleum Engineers: Richardson, TX, USA, 2010. [Google Scholar]
- Shamsijazeyi, H.; Hirasaki, G.; Verduzco, R. Sacrificial agent for reducing adsorption of anionic surfactants. In Proceedings of the SPE International Symposium on Oilfield Chemistry, The Woodlands, TX, USA, 8–10 April 2013; Society of Petroleum Engineers: Richardson, TX, USA, 2013. [Google Scholar]
- Lv, W.; Bazin, B.; Ma, D.; Liu, Q.; Han, D.; Wu, K. Static and dynamic adsorption of anionic and amphoteric surfactants with and without the presence of alkali. J. Pet. Sci. Eng. 2011, 77, 209–218. [Google Scholar] [CrossRef]
- Krumrine, P.H.; Falcone Jr, J.S.; Campbell, T.C. Surfactant flooding 1: The effect of alkaline additives on IFT, surfactant adsorption, and recovery efficiency. Soc. Pet. Eng. J. 1982, 22, 503–513. [Google Scholar] [CrossRef]
- ShamsiJazeyi, H.; Verduzco, R.; Hirasaki, G.J. Reducing adsorption of anionic surfactant for enhanced oil recovery: Part I. Competitive adsorption mechanism. Colloids Surf. A Physicochem. Eng. Asp. 2014, 453, 162–167. [Google Scholar] [CrossRef]
- ShamsiJazeyi, H.; Verduzco, R.; Hirasaki, G.J. Reducing adsorption of anionic surfactant for enhanced oil recovery: Part II. Applied aspects. Colloids Surf. A Physicochem. Eng. Asp. 2014, 453, 168–175. [Google Scholar] [CrossRef]
- Wanli, K.; Yi, L.; Baoyan, Q.; Guangzhi, L.; Zhenyu, Y.; Jichun, H. Interactions between alkali/surfactant/polymer and their effects on emulsion stability. Colloids Surf. A Physicochem. Eng. Asp. 2000, 175, 243–247. [Google Scholar] [CrossRef]
- Bryan, J.L.; Mai, A.T.; Kantzas, A. Investigation into the processes responsible for heavy oil recovery by alkali-surfactant flooding. In Proceedings of the SPE Symposium on Improved Oil Recovery, Tulsa, OK, USA, 20–23 April 2008; Society of Petroleum Engineers: Richardson, TX, USA, 2008. [Google Scholar]
- Ahmadi, M.-A.; Ahmad, Z.; Phung, L.T.K.; Kashiwao, T.; Bahadori, A. Experimental investigation the effect of nanoparticles on micellization behavior of a surfactant: Application to EOR. Pet. Sci. Technol. 2016, 34, 1055–1061. [Google Scholar] [CrossRef]
- Rahimi, K.; Adibifard, M. Experimental study of the nanoparticles effect on surfactant absorption and oil recovery in one of the Iranian oil reservoirs. Pet. Sci. Technol. 2015, 33, 79–85. [Google Scholar] [CrossRef]
- Zargartalebi, M.; Kharrat, R.; Barati, N. Enhancement of surfactant flooding performance by the use of silica nanoparticles. Fuel 2015, 143, 21–27. [Google Scholar] [CrossRef]
- Moghadam, T.F.; Azizian, S.; Wettig, S. Synergistic behaviour of ZnO nanoparticles and gemini surfactants on the dynamic and equilibrium oil/water interfacial tension. Phys. Chem. Chem. Phys. 2015, 17, 7122–7129. [Google Scholar] [CrossRef] [PubMed]
- Mohajeri, M.; Hemmati, M.; Shekarabi, A.S. An experimental study on using a nanosurfactant in an EOR process of heavy oil in a fractured micromodel. J. Pet. Sci. Eng. 2015, 126, 162–173. [Google Scholar] [CrossRef]
- Bagrezaie, M.A.; Pourafshary, P. Improvement of surfactant flooding performance by application of nanoparticles in sandstone reservoirs. J. Jpn. Pet. Inst. 2015, 58, 97–102. [Google Scholar] [CrossRef]
- Cheraghian, G.; Kiani, S.; Nassar, N.N.; Alexander, S.; Barron, A.R. Silica Nanoparticle Enhancement in the Efficiency of Surfactant Flooding of Heavy Oil in a Glass Micromodel. Ind. Eng. Chem. Res. 2017, 56, 8528–8534. [Google Scholar] [CrossRef]
- Pei, H.; Zhang, G.; Ge, J.; Zhang, J.; Zhang, Q.; Fu, L. Investigation of nanoparticle and surfactant stabilized emulsion to enhance oil recovery in waterflooded heavy oil reservoirs. In Proceedings of the SPE Canada Heavy Oil Technical Conference, Calgary, AB, Canada, 9–11 June 2015; Society of Petroleum Engineers: Richardson, TX, USA, 2015. [Google Scholar]
- Le, N.Y.T.; Pham, D.K.; Le, K.H.; Nguyen, P.T. Design and screening of synergistic blends of SiO2 nanoparticles and surfactants for enhanced oil recovery in high-temperature reservoirs. Adv. Nat. Sci. Nanosci. Nanotechnol. 2011, 2, 035013. [Google Scholar] [CrossRef]
- Zendehboudi, S.; Ahmadi, M.A.; Rajabzadeh, A.R.; Mahinpey, N.; Chatzis, I. Experimental study on adsorption of a new surfactant onto carbonate reservoir samples—Application to EOR. Can. J. Chem. Eng. 2013, 91, 1439–1449. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, W.; Dai, C.; Huang, Y.; Li, H.; Zhao, M.; He, L.; Jiao, B. Reducing surfactant adsorption on rock by silica nanoparticles for enhanced oil recovery. J. Pet. Sci. Eng. 2017, 153, 283–287. [Google Scholar] [CrossRef]
- Park, S.; Lee, E.S.; Sulaiman, W.R.W. Adsorption behaviors of surfactants for chemical flooding in enhanced oil recovery. J. Ind. Eng. Chem. 2015, 21, 1239–1245. [Google Scholar] [CrossRef]
- Quintanar-Guerrero, D.; Allémann, E.; Doelker, E.; Fessi, H. Preparation and characterization of nanocapsules from preformed polymers by a new process based on emulsification-diffusion technique. Pharm. Res. 1998, 15, 1056–1062. [Google Scholar] [CrossRef] [PubMed]
- Speight, J. Petroleum Asphaltenes-Part 1: Asphaltenes, resins and the structure of petroleum. Oil Gas Sci. Technol. 2004, 59, 467–477. [Google Scholar] [CrossRef]
- Badawy, A.M.E.; Luxton, T.P.; Silva, R.G.; Scheckel, K.G.; Suidan, M.T.; Tolaymat, T.M. Impact of environmental conditions (pH, ionic strength, and electrolyte type) on the surface charge and aggregation of silver nanoparticles suspensions. Environ. Sci. Technol. 2010, 44, 1260–1266. [Google Scholar] [CrossRef] [PubMed]
- Guinebretière, S.; Briançon, S.; Fessi, H.; Teodorescu, V.; Blanchin, M. Nanocapsules of biodegradable polymers: Preparation and characterization by direct high resolution electron microscopy. Mater. Sci. Eng. C 2002, 21, 137–142. [Google Scholar] [CrossRef]
- Kohori, F.; Yokoyama, M.; Sakai, K.; Okano, T. Process design for efficient and controlled drug incorporation into polymeric micelle carrier systems. J. Control. Release 2002, 78, 155–163. [Google Scholar] [CrossRef]
- Lozano, M.M.; Franco, C.A.; Acevedo, S.A.; Nassar, N.N.; Cortés, F.B. Effects of resin I on the catalytic oxidation of n-C 7 asphaltenes in the presence of silica-based nanoparticles. RSC Adv. 2016, 6, 74630–74642. [Google Scholar] [CrossRef]
- Quintero, K.; López, L.; De Lima, L. Espectroscopía infrarroja con transformadas de Fourier-Reflectancia total atenuada (IRTF/RTA) aplicada a la caracterización de crudos y su relación con la gravedad API. Rev. Fac. Ing. Univ. Cent. Venezuela 2014, 29, 93–102. [Google Scholar]
- Chang, C.-L.; Fogler, H.S. Stabilization of asphaltenes in aliphatic solvents using alkylbenzene-derived amphiphiles. 1. Effect of the chemical structure of amphiphiles on asphaltene stabilization. Langmuir 1994, 10, 1749–1757. [Google Scholar] [CrossRef]
- Franco, C.A.; Cortés, F.B.; Nassar, N.N. Adsorptive removal of oil spill from oil-in-fresh water emulsions by hydrophobic alumina nanoparticles functionalized with petroleum vacuum residue. J. Colloid Interface Sci. 2014, 425, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Vlachos, N.; Skopelitis, Y.; Psaroudaki, M.; Konstantinidou, V.; Chatzilazarou, A.; Tegou, E. Applications of Fourier transform-infrared spectroscopy to edible oils. Anal. Chim. Acta 2006, 573, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.; Abdelaziz, A.E.; Samy, A.M.; Kassem, A.A. Screening study for formulation variables in preparation of diclofenac sodium niosomes using plackett–burman design. Az. J. Pharm. Sci. 2008, 38. [Google Scholar]
- ASTM International. E28-14 Standard Test Methods for Softening Point of Resins Derived from Pine Chemicals and Hydrocarbons, by Ring-and-Ball Apparatus; ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar]
- Reig, F.B.; Adelantado, J.G.; Moreno, M.M. FTIR quantitative analysis of calcium carbonate (calcite) and silica (quartz) mixtures using the constant ratio method. Application to geological samples. Talanta 2002, 58, 811–821. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Z.; Qiao, W.; Cheng, L. Dynamic interfacial behavior between crude oil and octylmethylnaphthalene sulfonate surfactant flooding systems. Colloids Surf. A Physicochem. Eng. Asp. 2005, 259, 71–80. [Google Scholar] [CrossRef]
- Bortolotti, V.; Macini, P.; Srisuriyachai, F. Laboratory Evaluation of Alkali and Alkali-Surfactant-Polymer Flooding Combined with Intermittent Flow in Carbonatic Rocks. In Proceedings of the Asia Pacific Oil and Gas Conference & Exhibition, Jakarta, Indonesia, 4–6 August 2009; Society of Petroleum Engineers: Richardson, TX, USA, 2009. [Google Scholar]
- Kumar, S.; Mandal, A. Rheological properties and performance evaluation of synthesized anionic polymeric surfactant for its application in enhanced oil recovery. Polymer 2017, 120, 30–42. [Google Scholar] [CrossRef]
- Macosko, C.W. Rheology: Principles, Measurements, and Applications; Wiley-VCH: Weinheim, Germany, 1994. [Google Scholar]
- Taborda, E.A.; Franco, C.A.; Lopera, S.H.; Alvarado, V.; Cortés, F.B. Effect of nanoparticles/nanofluids on the rheology of heavy crude oil and its mobility on porous media at reservoir conditions. Fuel 2016, 184, 222–232. [Google Scholar] [CrossRef]
- Nassar, N.N. Asphaltene adsorption onto alumina nanoparticles: Kinetics and thermodynamic studies. Energy Fuels 2010, 24, 4116–4122. [Google Scholar] [CrossRef]
- Franco, C.A.; Lozano, M.M.; Acevedo, S.; Nassar, N.N.; Cortés, F.B. Effects of resin I on asphaltene adsorption onto nanoparticles: A novel method for obtaining asphaltenes/resin isotherms. Energy Fuels 2015, 30, 264–272. [Google Scholar] [CrossRef]
- Navarro, L.; Álvarez, M.; Grosso, J.-L.; Navarro, U. Separación y caracterización de resinas y asfaltenos provenientes del crudo Castilla. Evaluación de su interacción molecular. CT&F-Cienc. Tecnol. Futuro 2004, 2, 53–67. [Google Scholar]
- Fessi, H.; Puisieux, F.; Devissaguet, J.P.; Ammoury, N.; Benita, S. Nanocapsule formation by interfacial polymer deposition following solvent displacement. Int. J. Pharm. 1989, 55, R1–R4. [Google Scholar] [CrossRef]
- Miller, C.C. The Stokes-Einstein law for diffusion in solution. Proc. R. Soc. Lond. Ser. A 1924, 106, 724–749. [Google Scholar] [CrossRef]
- Zabala, R.; Mora, E.; Botero, O.; Cespedes, C.; Guarin, L.; Franco, C.; Cortes, F.; Patino, J.; Ospina, N. Nano-technology for asphaltenes inhibition in Cupiagua South Wells. In Proceedings of the IPTC 2014: International Petroleum Technology Conference, Doha, Qatar, 19–22 January 2014. [Google Scholar]
- Acevedo, S.; Castro, A.; Vásquez, E.; Marcano, F.; Ranaudo, M.A.A. Investigation of physical chemistry properties of asphaltenes using solubility parameters of asphaltenes and their fractions A1 and A2. Energy Fuels 2010, 24, 5921–5933. [Google Scholar] [CrossRef]
- Rohlf, F. tpsDig 2.17, Digitize Landmarks and Outlines; Department of Ecology and Evolution, State University of New York at Stony Brook: Stony Brook, NY, USA, 2013. [Google Scholar]
- Guzmán, J.D.; Betancur, S.; Carrasco-Marín, F.; Franco, C.A.; Nassar, N.N.; Cortés, F.B. Importance of the Adsorption Method Used for Obtaining the Nanoparticle Dosage for Asphaltene-Related Treatments. Energy Fuels 2016, 30, 2052–2059. [Google Scholar] [CrossRef]
- Jurado, J.; Alcázar, A.; Muñiz-Valencia, R.; Ceballos-Magaña, S.; Raposo, F. Some practical considerations for linearity assessment of calibration curves as function of concentration levels according to the fitness-for-purpose approach. Talanta 2017, 172, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Lamour, G.; Hamraoui, A.; Buvailo, A.; Xing, Y.; Keuleyan, S.; Prakash, V.; Eftekhari-Bafrooei, A.; Borguet, E. Contact angle measurements using a simplified experimental setup. J. Chem. Educ. 2010, 87, 1403–1407. [Google Scholar] [CrossRef]
- Giraldo, L.J.; Giraldo, M.A.; Llanos, S.; Maya, G.; Zabala, R.; Nassar, N.N.; Franco, C.A.; Alvarado, V.; Cortés, F.B. The effects of SiO2 nanoparticles on the thermal stability and rheological behavior of hydrolyzed polyacrylamide based polymeric solutions. J. Pet. Sci. Eng. 2017, 159, 841–852. [Google Scholar] [CrossRef]
- Betancur, S.; Carmona, J.C.; Nassar, N.N.; Franco, C.A.; Cortés, F.B. Role of Particle Size and Surface Acidity of Silica Gel Nanoparticles in Inhibition of Formation Damage by Asphaltene in Oil Reservoirs. Ind. Eng. Chem. Res. 2016, 55, 6122–6132. [Google Scholar] [CrossRef]
- Cardona, L.; Arias-Madrid, D.; Cortés, F.B.; Lopera, S.H.; Franco, C.A. Heavy Oil Upgrading and Enhanced Recovery in a Steam Injection Process Assisted by NiO-and PdO-Functionalized SiO2 Nanoparticulated Catalysts. Catalysts 2018, 8, 132. [Google Scholar] [CrossRef]
- Nik, W.W.; Ani, F.; Masjuki, H.; Giap, S.E. Rheology of bio-edible oils according to several rheological models and its potential as hydraulic fluid. Ind. Crops Prod. 2005, 22, 249–255. [Google Scholar] [CrossRef]
- Sarpkaya, T. Flow of non-Newtonian fluids in a magnetic field. AIChE J. 1961, 7, 324–328. [Google Scholar] [CrossRef]
- Shao, S.; Lo, E.Y. Incompressible SPH method for simulating Newtonian and non-Newtonian flows with a free surface. Adv. Water Resour. 2003, 26, 787–800. [Google Scholar] [CrossRef]
- Ahmed, T. Reservoir Engineering Handbook; Gulf Professional Publishing: Houston, TX, USA, 2006. [Google Scholar]
- Montoya, T.; Coral, D.; Franco, C.A.; Nassar, N.N.; Cortés, F.B. A Novel Solid–Liquid Equilibrium Model for Describing the Adsorption of Associating Asphaltene Molecules onto Solid Surfaces Based on the “Chemical Theory”. Energy Fuels 2014, 28, 4963–4975. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds employed in this study for preparing the nanocapsules are available from the authors. |






| Sample | Temperature (°C) | H-B Model Parameters | ||||
|---|---|---|---|---|---|---|
| KH (Pa∙Sn) | nH | μ∞,γ (cP) | R2 | RSME% | ||
| NCS20 | 25 | 3.15 | 0.9922 | 1.12 | 0.96 | 6.76 |
| 50 | 2.38 | 0.945 | 0.903 | 0.95 | 7.64 | |
| 70 | 2.13 | 0.9722 | 0.65 | 0.94 | 9.12 | |
| NCP50 | 25 | 2.73 | 0.9572 | 1.03 | 0.87 | 8.73 |
| 50 | 2.15 | 0.964 | 0.81 | 0.91 | 9.73 | |
| 70 | 2.03 | 0.9812 | 0.55 | 0.93 | 8.76 | |
| Step | Cumulative Recovery Model Parameters | |||
|---|---|---|---|---|
| q0 (mL/min) | Di × 10−2 (min−1) | R2 | RSME% | |
| Water | 3.10 | 6.83 | 0.99 | 1.2 |
| Petro 50 surfactant | 0.17 | 0.14 | 0.99 | 3.6 |
| NCP50 | 0.23 | 0.05 | 0.98 | 7.8 |
| Surfactant | Type | Color | Solubility | HLB | MW (g/mol) |
|---|---|---|---|---|---|
| Span 20 | Sorbitan monolaurate | Yellow to yellow-green | Water-insoluble | 8.6 | 346.47 |
| Petro 50 | Oleic acid ethyl ester | Colorless | More lipid-soluble | 10.5 | ~305 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cortés, F.B.; Lozano, M.; Santamaria, O.; Betancur Marquez, S.; Zapata, K.; Ospina, N.; Franco, C.A. Development and Evaluation of Surfactant Nanocapsules for Chemical Enhanced Oil Recovery (EOR) Applications. Molecules 2018, 23, 1523. https://doi.org/10.3390/molecules23071523
Cortés FB, Lozano M, Santamaria O, Betancur Marquez S, Zapata K, Ospina N, Franco CA. Development and Evaluation of Surfactant Nanocapsules for Chemical Enhanced Oil Recovery (EOR) Applications. Molecules. 2018; 23(7):1523. https://doi.org/10.3390/molecules23071523
Chicago/Turabian StyleCortés, Farid B., Mónica Lozano, Oveimar Santamaria, Stefania Betancur Marquez, Karol Zapata, Natalia Ospina, and Camilo A. Franco. 2018. "Development and Evaluation of Surfactant Nanocapsules for Chemical Enhanced Oil Recovery (EOR) Applications" Molecules 23, no. 7: 1523. https://doi.org/10.3390/molecules23071523
APA StyleCortés, F. B., Lozano, M., Santamaria, O., Betancur Marquez, S., Zapata, K., Ospina, N., & Franco, C. A. (2018). Development and Evaluation of Surfactant Nanocapsules for Chemical Enhanced Oil Recovery (EOR) Applications. Molecules, 23(7), 1523. https://doi.org/10.3390/molecules23071523