Cobalt(II) Complexes with N,N,N-Scorpionates and Bidentate Ligands: Comparison of Hydrotris(3,5-dimethylpyrazol-1-yl)borate Tp* vs. Phenyltris(4,4-dimethyloxazolin-2-yl)borate ToM to Control the Structural Properties and Reactivities of Cobalt Centers
Abstract
1. Introduction
2. Results and Discussion
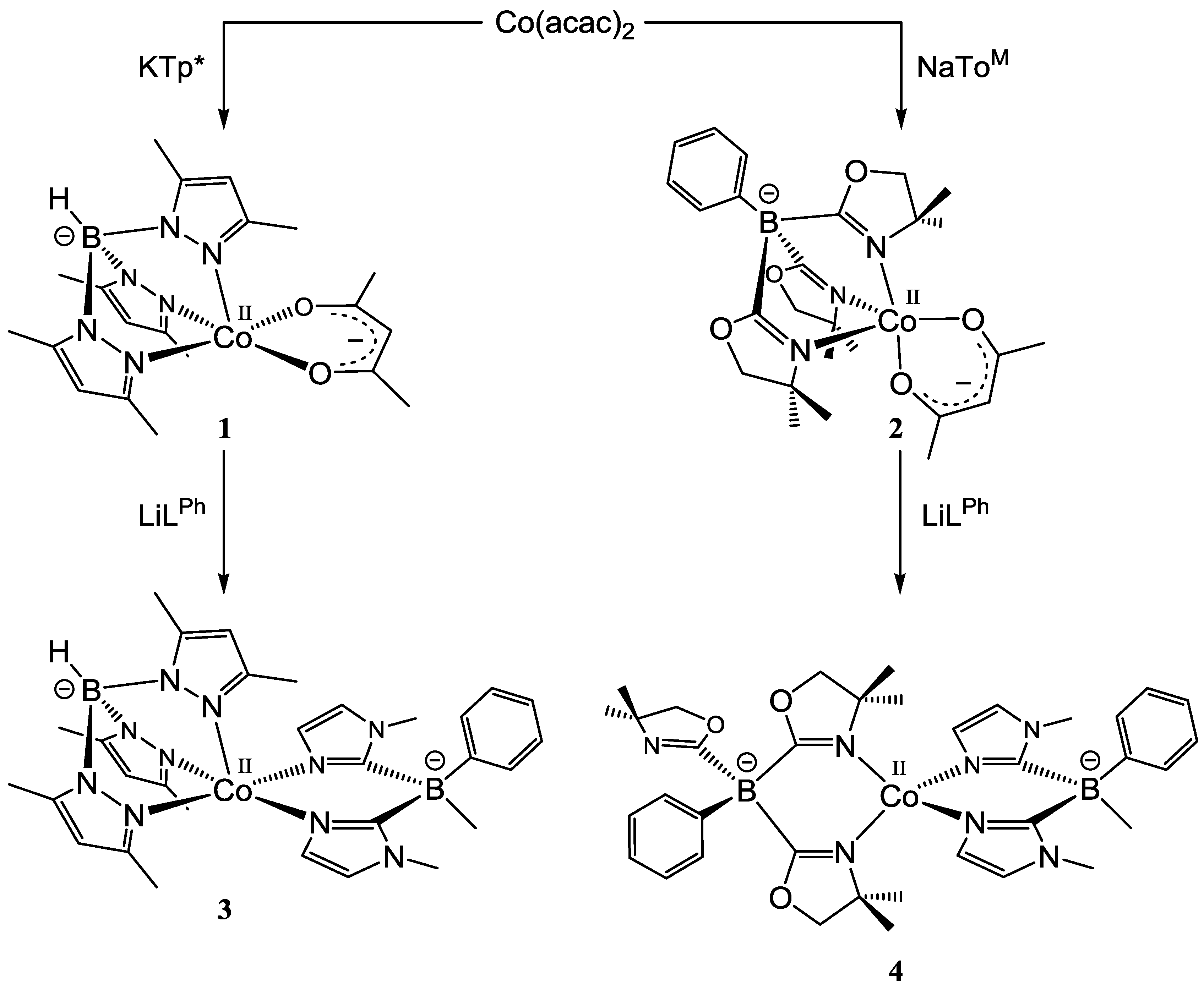
2.1. Synthesis and Characterization of Mixed Ligand Complexes
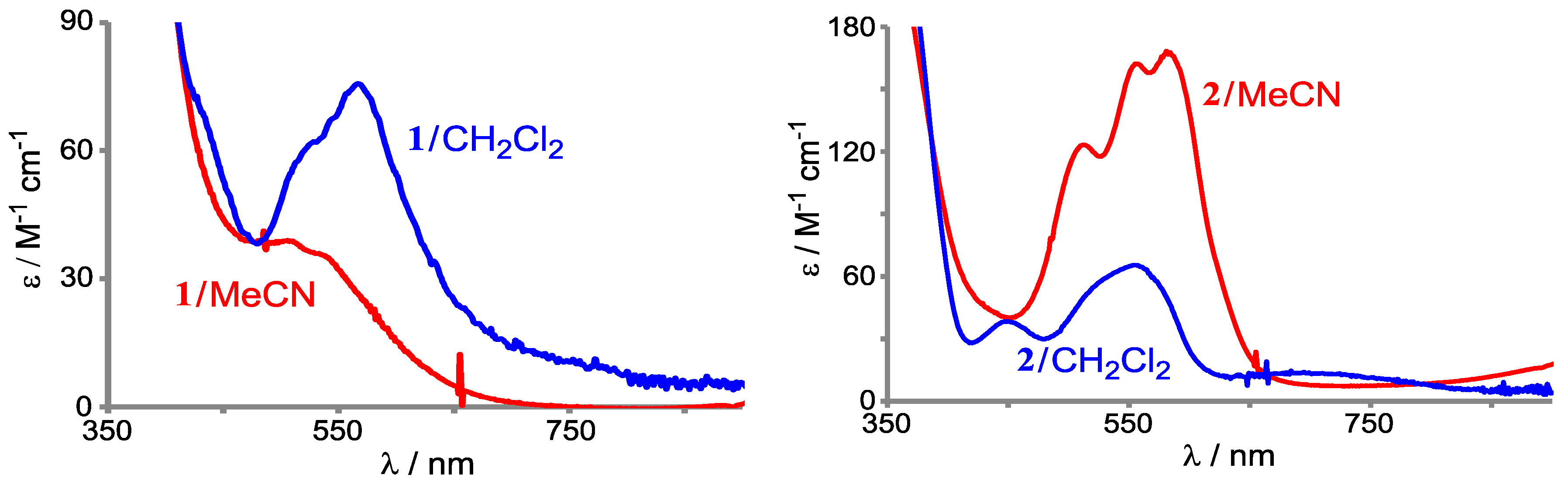
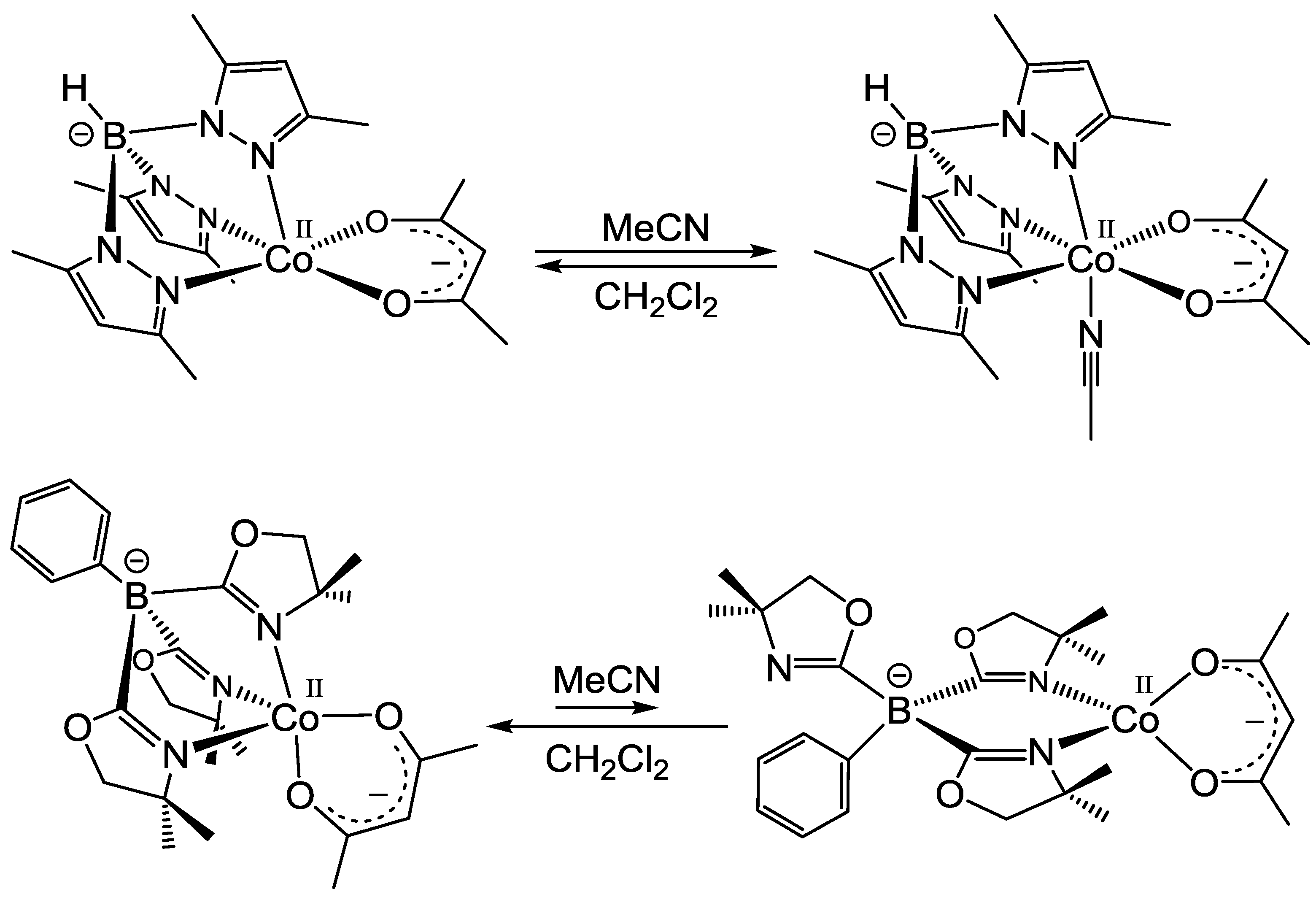
2.1.1. Acetylacetonato Complexes [CoII(Tp*)(acac)] (1) and [CoII(ToM)(acac)] (2)
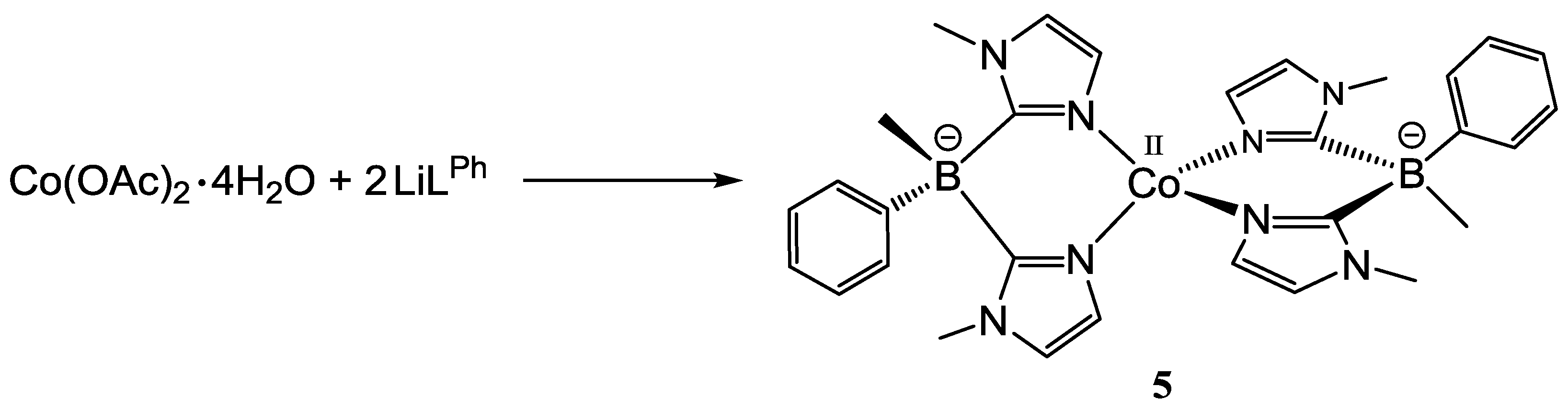
2.1.2. Bis(1-methylimidazol-2-yl)borato Complexes [CoII(Tp*)(LPh)] (3), [CoII(ToM)(LPh)] (4) and [CoII(LPh)2] (5)
2.1.3. Oxidation Potentials
2.2. Oxidation Catalysis
2.2.1. Oxidation of Alkene with TBHP
2.2.2. Oxidation of Alkane with mCPBA
3. Materials and Methods
3.1. General
3.2. Preparation and Characterization of the Cobalt(II) Complexes
3.2.1. [CoII(Tp*)(acac)] (1)
3.2.2. [CoII(ToM)(acac)] (2)
3.2.3. [CoII(ToM)(LPh)] (4)
3.2.4. [CoII(LPh)2] (5)
3.2.5. [CoII(Tp*)2] (6)
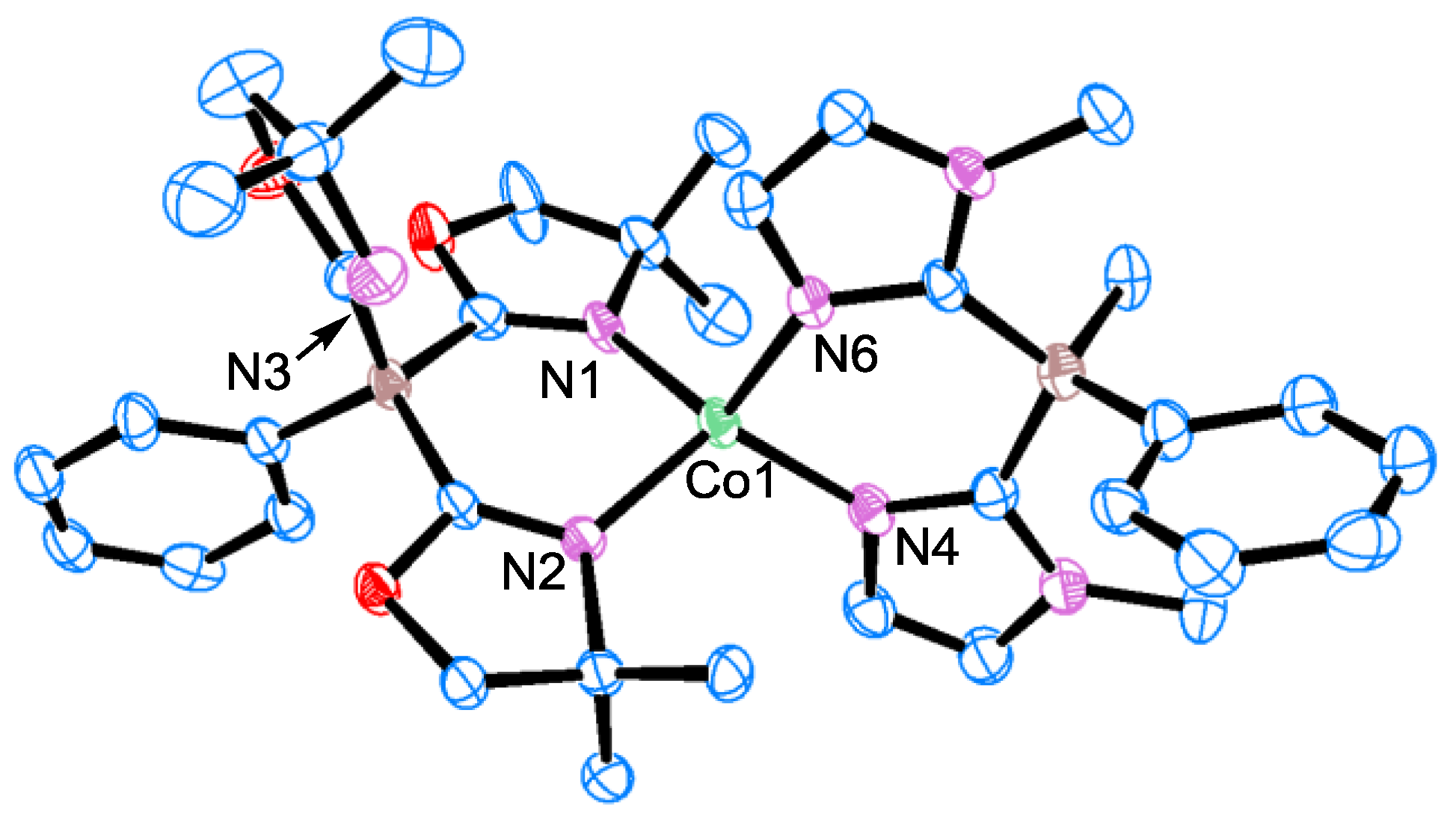
3.3. X-Ray Diffraction Study
3.4. Catalytic Reactions
3.4.1. Cyclohexene Oxidation with TBHP
3.4.2. Cyclohexane Oxidation with mCPBA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Trofimenko, S. Scorpionates—The Coordination Chemistry of Polypyrazolylborate Ligands; Imperial College Press: London, UK, 1999. [Google Scholar]
- Pettinari, C. Scorpionates II: Chelating Borate Ligands; Imperial College Press: London, UK, 2008. [Google Scholar]
- Janiak, C.; Scharmann, T.G.; Günther, W.; Hinrichs, W.; Lentz, D. Copper Coordination Polymers with Infinite Chloride Ion Channels and Different Directions of the Jahn-Teller Distortion, Built from Tris(1,2,4-triazolyl)borate as a Modified Tris(pyrazolyl)borate Ligand. Chem. Ber. 1996, 129, 991–995. [Google Scholar] [CrossRef]
- Fujita, K.; Hikichi, S.; Akita, M.; Moro-oka, Y. Novel monoanionic tripodal ligands: Methylbis(1-methylimidazol-2-yl)(pyrazol-1-yl)borate (= MeB(ImN-Me)2(PzR)]‒). Synthesis and structural characterization of their nickel complexes and carboxylate shift from nickel to boron. J. Chem. Soc. Dalton Trans. 2000, 2, 117–120. [Google Scholar] [CrossRef]
- Dunne, J.F.; Su, J.; Ellern, A.; Sadow, A.D. A New Scorpionate Ligand: Tris(4,4-dimethyl-2-oxazolinyl)borate and Its Zirconium(IV) Complexes. Organometallics 2008, 27, 2399–2401. [Google Scholar] [CrossRef]
- Cui, C.; Lalancette, R.A.; Jäkle, F. The elusive tripodal tris(2-pyridyl)borate ligand: A strongly coordinating tetraarylborate. Chem. Commun. 2012, 48, 6930–6932. [Google Scholar] [CrossRef] [PubMed]
- Ohrenberg, C.; Ge, P.; Schebler, P.; Riordan, C.G.; Yap, G.P.A.; Rheingold, A.L. Synthesis, Molecular Structure, and Physical Properties of [tetrakis((methylthio)methyl)borate]2M (M = Fe, Co, Ni). Inorg. Chem. 1996, 35, 749–754. [Google Scholar] [CrossRef]
- Shapiro, I.R.; Jenkins, D.M.; Thomas, J.C.; Day, M.W.; Peters, J.C. A homoleptic phosphine adduct of Tl(I). Chem. Commun. 2001, 20, 2152–2153. [Google Scholar] [CrossRef]
- Fränkel, R.; Kernbach, U.; Bakola-Christianopoulou, M.; Plaia, U.; Suter, M.; Ponikwar, W.; Nöth, H.; Moinet, C.; Fehlhammer, W.P. Homoleptic carbene complexes Part VIII. Hexacarbene complexes. J. Organomet. Chem. 2001, 617–618, 530–545. [Google Scholar] [CrossRef]
- Reinig, R.R.; Mukherjee, D.; Weinstein, Z.B.; Xie, W.; Albright, T.; Baird, B.; Gray, T.S.; Ellern, A.; Miller, G.J.; Winter, A.H.; et al. Synthesis and Oxidation Catalysis of [Tris(oxazolinyl)borato]cobalt(II) Scorpionates. Eur. J. Inorg. Chem. 2016, 15–16, 2486–2494. [Google Scholar] [CrossRef]
- Reinig, R.R.; Fought, E.L.; Ellern, A.; Windus, T.L.; Sadow, A.D. Rapid and ordered carbonylation and oxygenation of a cobalt(II) methyl. Chem. Commun. 2017, 53, 11020–11023. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Mukherjee, D.; Ellern, A.; Sadow, A.D.; Geiger, W.E. Anodic electrochemistry of Mn and Re tricarbonyl complexes of tris(oxazolinyl)phenyl borate ligands: Comparison to tris(pyrazolyl) borate complexes. New J. Chem. 2011, 35, 2169–2178. [Google Scholar] [CrossRef]
- Xu, S.; Magoon, Y.; Reinig, R.R.; Schmidt, B.M.; Ellern, A.; Sadow, A.D. Organometallic Complexes of Bulky, Optically Active, C3-Symmetric Tris(4S-isopropyl-5,5-dimethyl-2-oxazolinyl)phenylborate (ToP*). Organometallics 2015, 34, 3508–3519. [Google Scholar] [CrossRef]
- Takayama, T.; Nakazawa, J.; Hikichi, S. A pseudo tetrahedral nickel(II) complex with a tridentate oxazoline-based scorpionate ligand: Chlorido[tris(4,4-dimethyloxazolin-2-yl)phenylborato]nickel(II). Acta Crystllogr. 2016, C72, 842–845. [Google Scholar]
- Guzei, I.A.; Wendt, M. An improved method for the computation of ligand steric effects based on solid angles. Dalton Trans. 2006, 33, 3991–3999. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Hikichi, S.; Akita, M.; Moro-oka, Y. Synthesis and structural characterization of a new-class of organoborato ligands containing imidazolyl functional groups [MeB(ImN-Me)2(X)]‒. J. Chem. Soc. Dalton Trans. 2000, 8, 1255–1260. [Google Scholar] [CrossRef]
- Hikichi, S.; Fujita, K.; Manabe, Y.; Akita, M.; Nakazawa, J.; Komatsuzaki, H. Coordination property of organoborate ligands: Steric hindrance around distal boron center direct the conformation of dialkylbis(imidazolyl)borate scaffold. Eur. J. Inorg. Chem. 2010, 35, 5529–5537. [Google Scholar] [CrossRef]
- Oddon, F.; Chiba, Y.; Nakazawa, J.; Ohta, T.; Ogura, T.; Hikichi, S. Characterization of Mononuclear Non-heme Iron(III)-Superoxo Complex with a Five-Azole Ligand Set. Angew. Chem. Int. Ed. 2015, 54, 7336–7339. [Google Scholar] [CrossRef] [PubMed]
- Ando, K.; Nakazawa, J.; Hikichi, S. Synthesis, Characterization and Aerobic Alcohol Oxidation Catalysis of Palladium(II) Complexes with a Bis(imidazolyl)borate Ligand. Eur. J. Inorg. Chem. 2016, 15–16, 2603–2608. [Google Scholar] [CrossRef]
- Myers, W.K.; Duesler, E.D.; Tierney, D.L. Integrated Paramagnetic Resonance of High-Spin Co(II) in Axial Symmetry: Chemical Separation of Dipolar and Contact Electron—Nuclear Couplings. Inorg. Chem. 2008, 47, 6701–6710. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, J.C.; Domaille, P.J.; Thompson, J.S.; Trofimenko, S. Steric Effects in Polypyrazolylborates: Mixed Complexes M(HB(3-isopropyl-4-bromopyrazolyl)3)L. Inorg. Chem. 1990, 29, 4429–4437. [Google Scholar] [CrossRef]
- Kremer-Aach, A.; Kläui, W.; Bell, R.; Strerath, A.; Wunderlich, H.; Mootz, D. Cobalt as a Probe for Zinc in Metalloenzyme Model Compounds? A Comparison of Spectroscopic Features and Coordination Geometry of Four- and Five-Coordinate Complexes. Crystal and Molecular Structures of [Co(η3-TpPh)(η2-TpPh)], [(η3-TpPh)Zn(anthranilate)], and [(η3-TpPh)M(η2-acac)] (TpPh = Hydrotris(3-phenylpyrazol-1-yl)borate, acac = Pentane-2,4-dionate, and M = Zn, Co). Inorg. Chem. 1997, 36, 1552–1563. [Google Scholar] [PubMed]
- Rheingold, A.L.; Liable-Sands, L.M.; Golen, J.M.; Yap, G.P.A.; Trofimenko, S. The coordination chemistry of the hydrotris(3-diphenylmethylpyrazol-1-yl)borate (TpCHPh2) ligand. Dalton Trans. 2004, 4, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Harding, D.J.; Harding, P.; Daengngern, R.; Yimklan, S.; Adams, H. Synthesis and characterization of redox-active tris(pyrazolyl)borate cobalt complexes. Dalton Trans. 2009, 8, 1314–1320. [Google Scholar] [CrossRef]
- Hikichi, S.; Sasakura, Y.; Yoshizawa, M.; Ohzu, Y.; Moro-oka, Y.; Akita, M. Selective Synthesis, Characterization, and Configurational Flexibility of the Coordinatively Unsaturated Metal Center of Half-sandwich Type Complexes with the Less-Hindered Hydrotris(3,5-dimethyl-4-X-1-pyrazolyl)borate Ligands [TpMe2,XMII(κ2-O,O-L)] (M = Ni, Co; L = NO3, OAc; X = Me, H, Br). Bull. Chem. Soc. Jpn. 2002, 75, 1255–1262. [Google Scholar]
- Nishiura, T.; Chiba, Y.; Nakazawa, J.; Hikichi, S. Tuning of O2 Binding Affinity of Cobalt(II) Centers by Changing Structural and Electronic Properties of Distal Substituent Groups on Azole-based Chelating Ligands. Manuscript in preparation.
- Gorun, S.M.; Hu, Z.; Stibrany, R.T.; Carpenter, G. Synthesis and molecular structures and oxidation catalysis of mixed alkyl, fluoroalkyl pyrazolylborate metal complexes. Inorg. Chim. Acta 2000, 297, 383–388. [Google Scholar] [CrossRef]
- Nakamizu, A.; Kasai, T.; Nakazawa, J.; Hikichi, S. Immobilization of a Boron Center-Functionalized Scorpionate Ligand on Mesoporous Silica Supports for Heterogeneous Tp-Based Catalysts. ACS Omega 2017, 2, 1025–1030. [Google Scholar] [CrossRef]
- Chavez, F.A.; Mascharak, P.K. Co(III)—Alkylperoxo Complexes: Syntheses, Structure—Reactivity Correlations, and Use in the Oxidation of Hydrocarbons. Acc. Chem. Res. 2000, 33, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Chavez, F.A.; Briones, J.A.; Olmstead, M.M.; Mascharak, P.K. Syntheses and Stuctures of Alkyl Peroxo Adducts of β-Diketonate Cobalt(III) Complexes and Their Role in Oxidation of Hydrocarbons and Olefin Epoxidation. Inorg. Chem. 1999, 38, 1603–1608. [Google Scholar] [CrossRef]
- Nowotny, M.; Pedersen, L.N.; Hanefeld, U.; Maschmeyer, T. Increasing the Ketone Selectivity of the Cobalt-Catalyzed Radical Chain Oxidation of Cyclohexane. Chem. Eur. J. 2002, 8, 3724–3731. [Google Scholar] [CrossRef]
- Sehlotho, N.; Nyokong, T. Catalytic activity of iron and cobalt phthalocyanine complexes towards the oxidation of cyclohexene using tert-butylhydroperoxide and chloroperoxybenzoic acid. J. Mol. Catal. A Chem. 2004, 209, 51–57. [Google Scholar] [CrossRef]
- Salavati-Niasari, M.; Babazadeh-Arani, H. Cyclohexene oxidation with tert-butylhydroperoxide and hydrogen peroxide catalyzed by new square-planar manganese(II), cobalt(II), nickel(II) and copper(II) bis(2-mercaptoanil)benzil complexes supported on alumina. J. Mol. Catal. A Chem. 2007, 274, 58–64. [Google Scholar] [CrossRef]
- Tonigold, M.; Lu, Y.; Bredenkötter, B.; Rieger, B.; Bahnmüller, S.; Hitzbleck, J.; Langstein, G.; Volkmer, D. Heterogeneous Catalytic Oxidation by MFU-1: A Cobalt(II)-Containing Metal—Organic Framework. Angew. Chem. Int. Ed. 2009, 48, 7546–7550. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-C.; Ding, F.-W.; Ma, J.-P.; Liu, Q.-K.; Cheng, J.-Y.; Dong, Y.-B. Co(II)-MOF: A Highly Efficient Organic Oxidation Catalyst with Open Metal Sites. Inorg. Chem. 2015, 54, 10865–10872. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Qi, L.; Li, Z. Cobalt–Catalyzed Alkylation–Peroxidation of Alkenes with 1,3-Dicarbonyl Compounds and T-Hydro. Asian J. Org. Chem. 2017, 6, 313–321. [Google Scholar] [CrossRef]
- Nam, W.; Kim, I.; Kim, Y.; Kim, C. Biomimetic alkane hydroxylation by cobalt(III) porphyrin complex and m-chloroperbenzoic acid. Chem. Commun. 2001, 14, 1262–1263. [Google Scholar] [CrossRef]
- Nam, W.; Ryu, J.Y.; Kim, I.; Kim, C. Stereoselective alkane hydroxylations by metal salts and m-chloroperbenzoic acid. Tetrahedron Lett. 2002, 43, 5487–5490. [Google Scholar] [CrossRef]
- Nagataki, T.; Tachi, Y.; Itoh, S. NiII(TPA) as an efficient catalyst for alkane hydroxylation with m-CPBA. Chem. Commun. 2006, 38, 4016–4018. [Google Scholar] [CrossRef] [PubMed]
- Tordin, E.; List, M.; Monkowius, U.; Schindler, S.; Knör, G. Synthesis and characterisation of cobalt, nickel and copper complexes with tripodal 4N ligands as novel catalysts for the homogeneous partial oxidation of alkanes. Inorg. Chim. Acta 2013, 402, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, J.; Yata, A.; Hori, T.; Stack, T.D.P.; Naruta, Y.; Hikichi, S. Catalytic Alkane Oxidation by Homogeneous and Silica Supported Cobalt Complex Catalysts with a Triazolyl-group Containing Tetradentate Ligand. Chem. Lett. 2013, 42, 1197–1199. [Google Scholar] [CrossRef]
- Nakazawa, J.; Doi, Y.; Hikichi, S. Alkane oxidation reactivity of homogeneous and heterogeneous metal complex catalysts with mesoporous silica-immobilized (2-pyridylmethyl)amine type ligands. Mol. Catal. 2017, 443, 14–24. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Loginov, D.A.; Shul’pina, L.S.; Ikonnikov, N.S.; Idrisov, V.O.; Vinogradov, M.M.; Osipov, S.N.; Nelyubina, Y.V.; Tyubaeva, P.M. Stereoselective Alkane Oxidation with meta-Chloroperoxybenzoic Acid (MCPBA) Catalyzed by Organometallic Cobalt Complexes. Molecules 2016, 21, 1593. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, E.C.; Choi, H.; Wang, K.; Chiou, G.; Doyle, M.P. Allylic Oxidations Catalyzed by Dirhodium Caprolactamate via Aqueous tert-Butyl Hydroperoxide: The Role of the tert-Butylperoxy Radical. J. Org. Chem. 2009, 74, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Hikichi, S.; Komatsuzaki, H.; Akita, M.; Moro-oka, Y. Aliphatic C-H Bond Oxygenation by the CoIIOOX Species with the Hindered Hydrotris(pyrazolyl)borate Ligand (X = Co(II), Alkyl, H). J. Am. Chem. Soc. 1998, 120, 4699–4710. [Google Scholar] [CrossRef]
- Hikichi, S.; Hanaue, K.; Fujimura, T.; Okuda, H.; Nakazawa, J.; Ohzu, Y.; Kobayashi, C.; Akita, M. Characterization of Nickel(II)-Acylperoxo Species Relevant to Catalytic Alkane Hydroxylation by Nickel Complex with mCPBA. Dalton Trans. 2013, 42, 3346–3356. [Google Scholar] [CrossRef] [PubMed]
- Rigaku. CrystalClear; Rigaku Corporation: Tokyo, Japan, 2008. [Google Scholar]
- Altomare, A.; Cascarano, G.; Giacovazzo, C.; Guagliardi, A.; Burla, M.C.; Polidori, G.; Camalli, M. SIR92—A program for automatic solution of crystal structures by direct methods. J. Appl. Cryst. 1994, 27, 435. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. WinGX suite for small-molecule single-crystal crystallography. J. Appl. Cryst. 1999, 32, 837–838. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Cryst. 2012, 45, 849–854. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

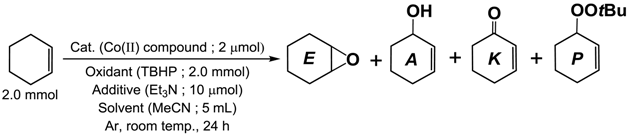
| Catalyst (Cat.) | Products/μmol | TON *1 | |||
|---|---|---|---|---|---|
| E | A | K | P | ||
| [CoII(Tp*)(acac)] (1) | 0.5 | 6.4 | 20.4 | 52.7 | 50.2 |
| [CoII(ToM)(acac)] (2) | 1.3 | 12.1 | 49.4 | 85.2 | 98.7 |
| [CoII(Tp*)(LPh)] (3) | 0.7 | 1.9 | 12.1 | 13.3 | 20.0 |
| [CoII(ToM)(LPh)] (4) | 1.1 | 5.0 | 24.7 | 73.9 | 64.7 |
| [CoII(LPh)2] (5) | 0.7 | 6.9 | 27.5 | 23.0 | 42.8 |
| [CoII(Tp*)2] (6) | 0.7 | 5.8 | 28.1 | 70.2 | 66.4 |
| none | 0.0 | 0.4 | 4.6 | 2.7 | − |
| CoII(acac)2·2H2O | 1.2 | 7.7 | 21.9 | 187.7 | 120.2 |
| CoII(OAc)2·4H2O | 1.1 | 10.0 | 27.0 | 195.0 | 130.1 |
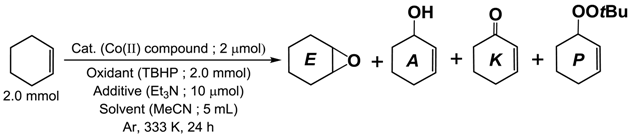
| Catalyst (Cat.) | Products/μmol | TON *1 | |||
|---|---|---|---|---|---|
| E | A | K | P | ||
| [CoII(Tp*)(acac)] (1) | 3.0 | 11.9 | 32.8 | 345.2 | 212.9 |
| [CoII(ToM)(acac)] (2) | 6.4 | 5.6 | 49.5 | 702.6 | 406.8 |
| [CoII(Tp*)(LPh)] (3) | 1.7 | 4.0 | 33.6 | 117.7 | 95.3 |
| [CoII(ToM)(LPh)] (4) | 4.9 | 5.0 | 56.1 | 693.2 | 407.7 |
| [CoII(LPh)2] (5) | 1.4 | 6.4 | 27.0 | 105.3 | 83.5 |
| [CoII(Tp*)2] (6) | 3.6 | 3.6 | 36.1 | 612.4 | 345.9 |
| none | 1.1 | 5.9 | 26.8 | 95.2 | − |
| CoII(acac)2·2H2O | 6.4 | 10.2 | 84.2 | 871.1 | 528.1 |
| CoII(OAc)2·4H2O | 6.3 | 28.1 | 118.5 | 735.6 | 503.5 |
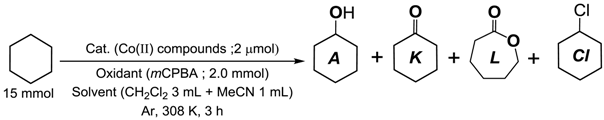
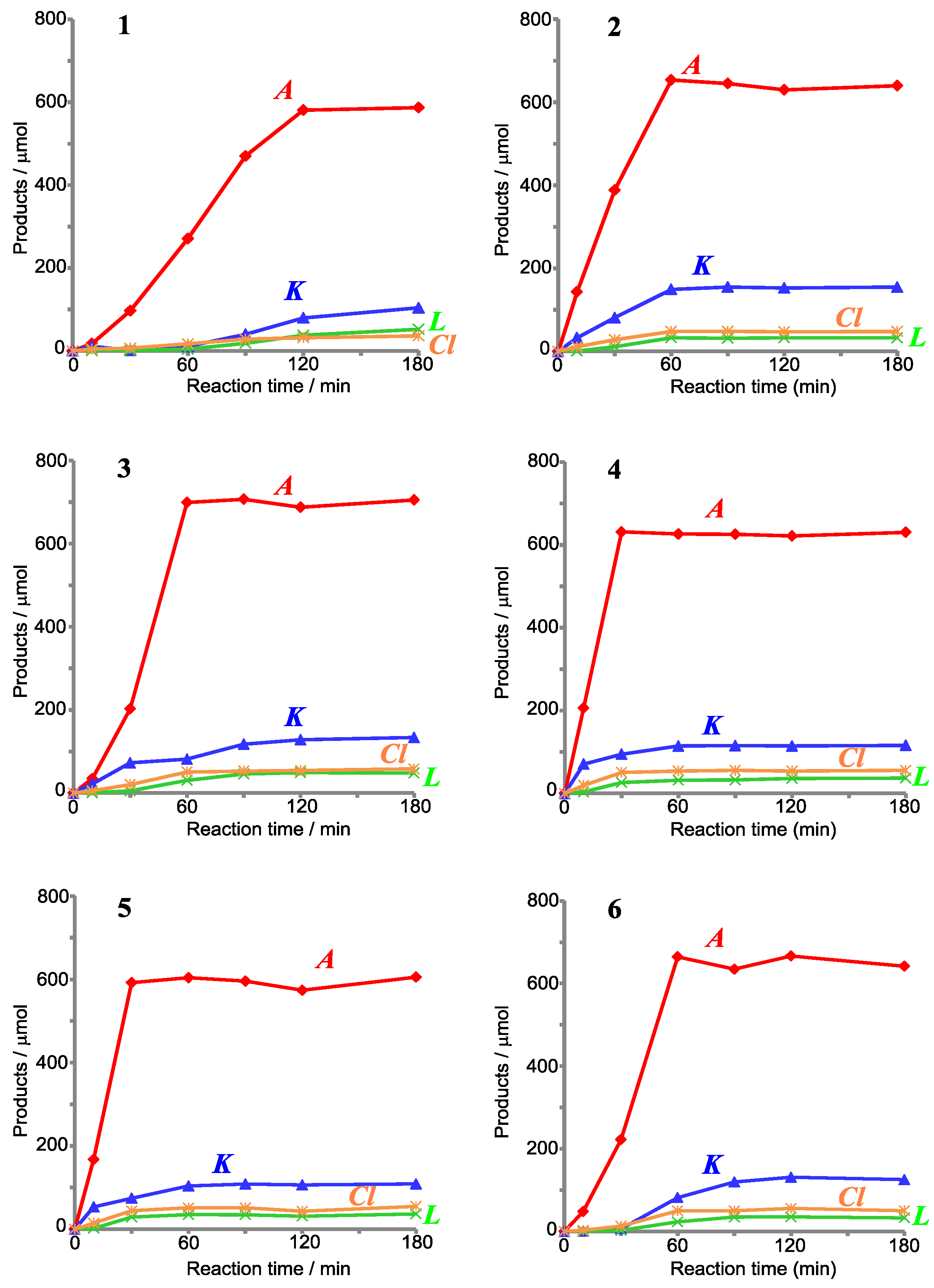
| Catalyst (Cat.) | Products/μmol | TON *1 | A/(K + L) *2 | |||
|---|---|---|---|---|---|---|
| A | K | L | Cl | |||
| [CoII(Tp*)(acac)] (1) | 587.2 | 104.1 | 52.4 | 36.2 | 468.1 | 3.8 |
| [CoII(ToM)(acac)] (2) | 640.0 | 154.8 | 32.5 | 47.8 | 531.2 | 3.4 |
| [CoII(Tp*)(LPh)] (3) | 705.2 | 133.6 | 48.1 | 58.1 | 563.3 | 3.9 |
| [CoII(ToM)(LPh)] (4) | 605.4 | 108.8 | 36.7 | 54.8 | 475.6 | 4.2 |
| [CoII(LPh)2] (5) | 630.3 | 115.9 | 37.0 | 55.5 | 495.8 | 4.1 |
| [CoII(Tp*)2] (6) | 641.8 | 125.4 | 32.9 | 50.3 | 504.3 | 4.1 |
| none | 69.4 | 0.5 | 2.1 | 3.8 | − | 26.6 |
| CoII(acac)2·2H2O | 456.2 | 146.6 | 18.4 | 26.7 | 406.5 | 2.8 |
| CoII(OAc)2·4H2O | 609.9 | 181.3 | 33.2 | 33.1 | 535.9 | 2.8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishiura, T.; Uramoto, T.; Takiyama, Y.; Nakazawa, J.; Hikichi, S. Cobalt(II) Complexes with N,N,N-Scorpionates and Bidentate Ligands: Comparison of Hydrotris(3,5-dimethylpyrazol-1-yl)borate Tp* vs. Phenyltris(4,4-dimethyloxazolin-2-yl)borate ToM to Control the Structural Properties and Reactivities of Cobalt Centers. Molecules 2018, 23, 1466. https://doi.org/10.3390/molecules23061466
Nishiura T, Uramoto T, Takiyama Y, Nakazawa J, Hikichi S. Cobalt(II) Complexes with N,N,N-Scorpionates and Bidentate Ligands: Comparison of Hydrotris(3,5-dimethylpyrazol-1-yl)borate Tp* vs. Phenyltris(4,4-dimethyloxazolin-2-yl)borate ToM to Control the Structural Properties and Reactivities of Cobalt Centers. Molecules. 2018; 23(6):1466. https://doi.org/10.3390/molecules23061466
Chicago/Turabian StyleNishiura, Toshiki, Takahiro Uramoto, Yuichiro Takiyama, Jun Nakazawa, and Shiro Hikichi. 2018. "Cobalt(II) Complexes with N,N,N-Scorpionates and Bidentate Ligands: Comparison of Hydrotris(3,5-dimethylpyrazol-1-yl)borate Tp* vs. Phenyltris(4,4-dimethyloxazolin-2-yl)borate ToM to Control the Structural Properties and Reactivities of Cobalt Centers" Molecules 23, no. 6: 1466. https://doi.org/10.3390/molecules23061466
APA StyleNishiura, T., Uramoto, T., Takiyama, Y., Nakazawa, J., & Hikichi, S. (2018). Cobalt(II) Complexes with N,N,N-Scorpionates and Bidentate Ligands: Comparison of Hydrotris(3,5-dimethylpyrazol-1-yl)borate Tp* vs. Phenyltris(4,4-dimethyloxazolin-2-yl)borate ToM to Control the Structural Properties and Reactivities of Cobalt Centers. Molecules, 23(6), 1466. https://doi.org/10.3390/molecules23061466






