Preparation and Characterization of α-Zinc Molybdate Catalyst: Efficient Sorbent for Methylene Blue and Reduction of 3-Nitrophenol
Abstract
:1. Introduction
2. Experimental
2.1. Preparation of Zinc Molybdate
2.2. Analysis and Characterization of Zinc Molybdate
2.3. 3-Nitrophenol Reduction Test
2.4. Adsorption Test
2.5. Desorption Test
3. Results and Discussion
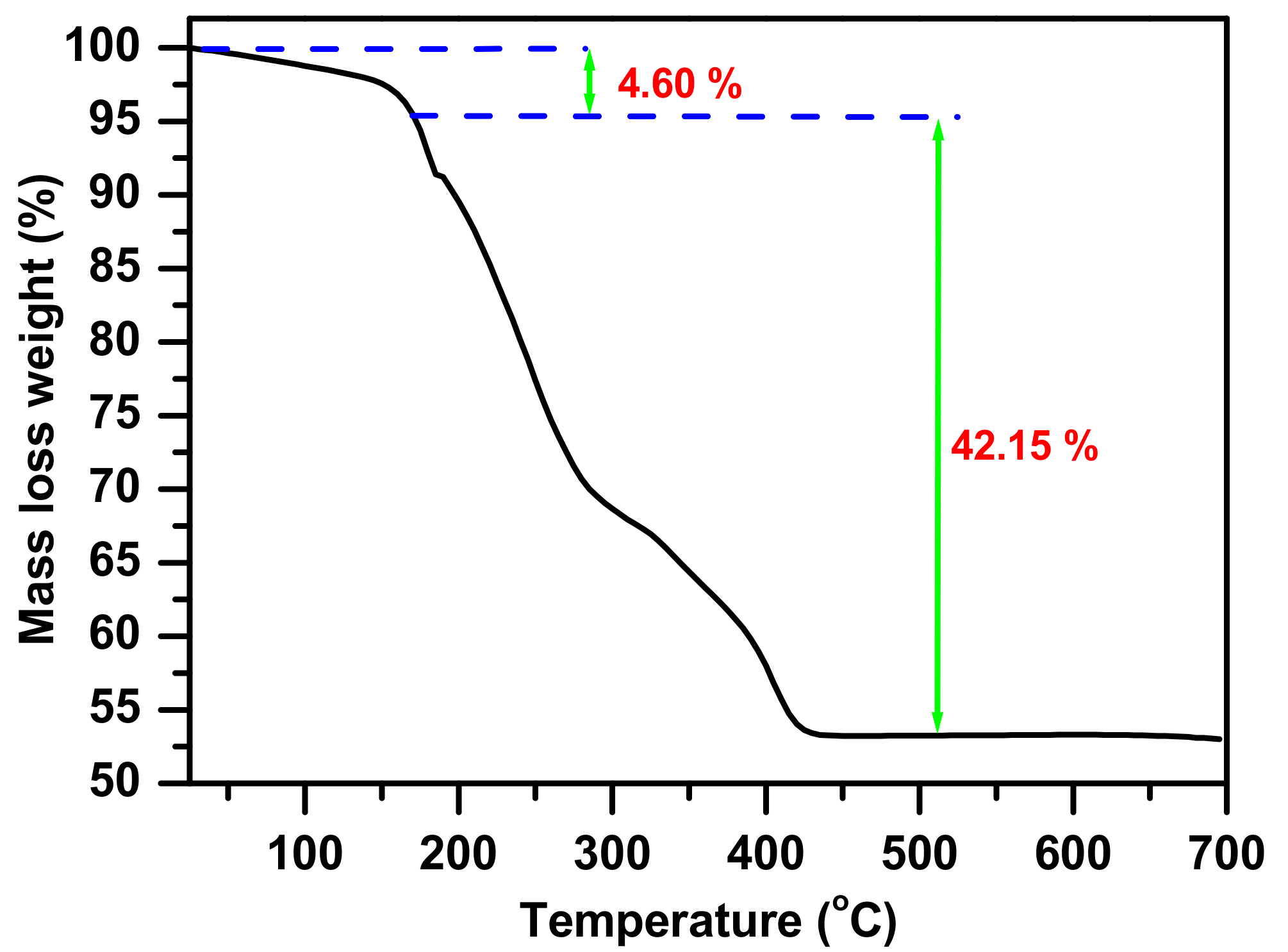
3.1. Complex Identfication and Characterization
3.2. Zinc Molybdate Characterization
3.2.1. X-ray Diffraction
3.2.2. Specific Surface Area Determination
3.2.3. Transmission Electron Microscopy
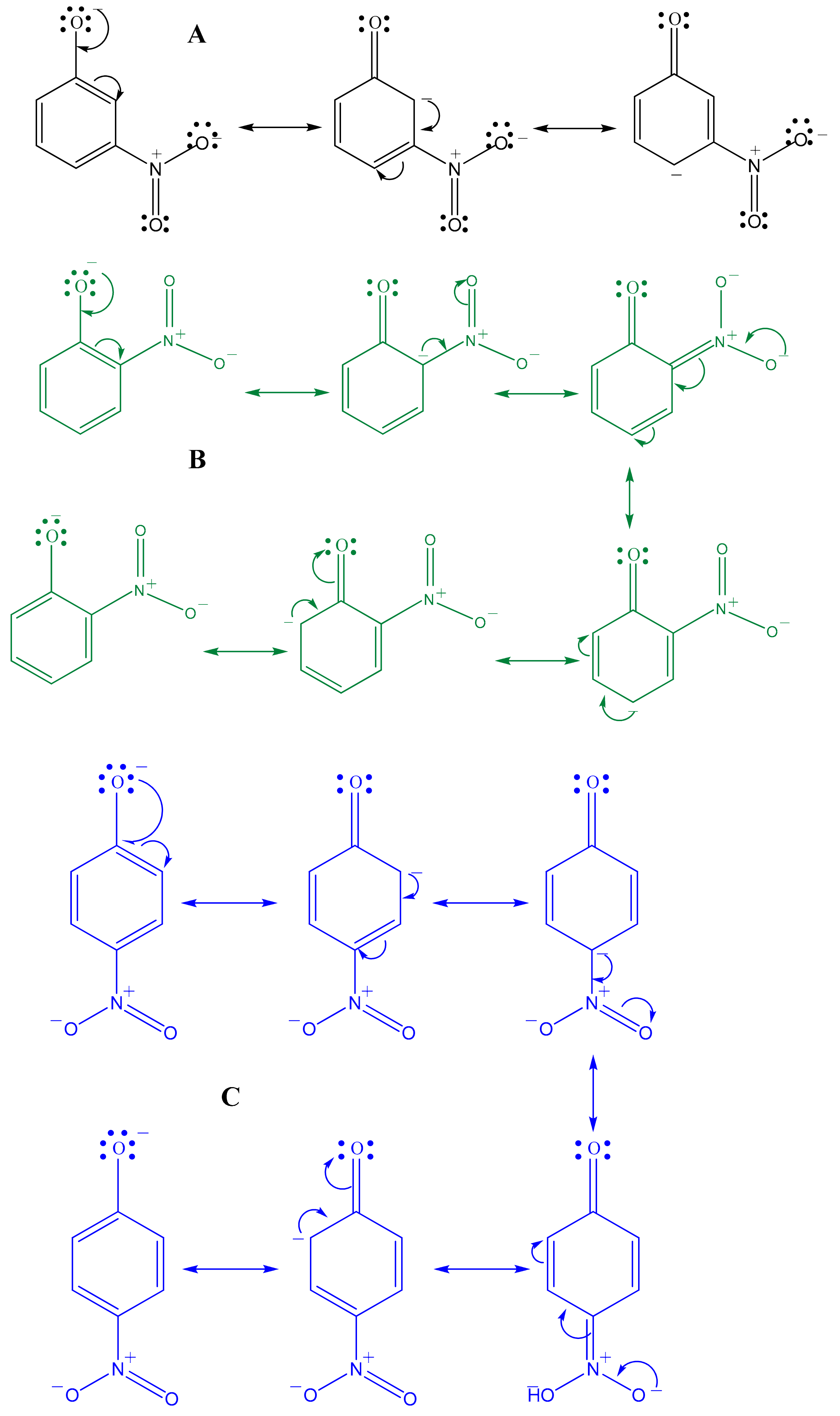
3.3. 3-Nitrophenol Reduction Test
3.4. Zinc Molybdate as Sorbent for Methylene Blue Removal
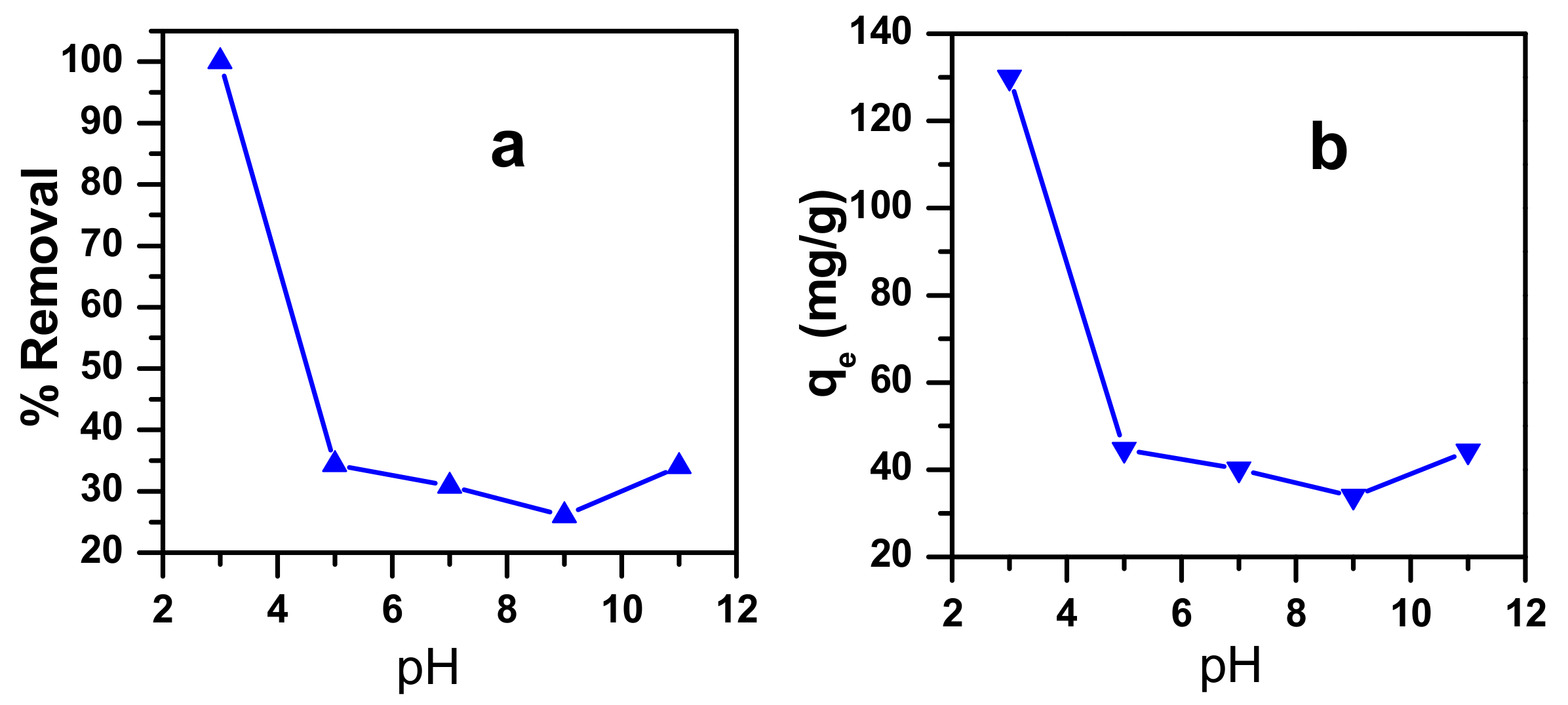
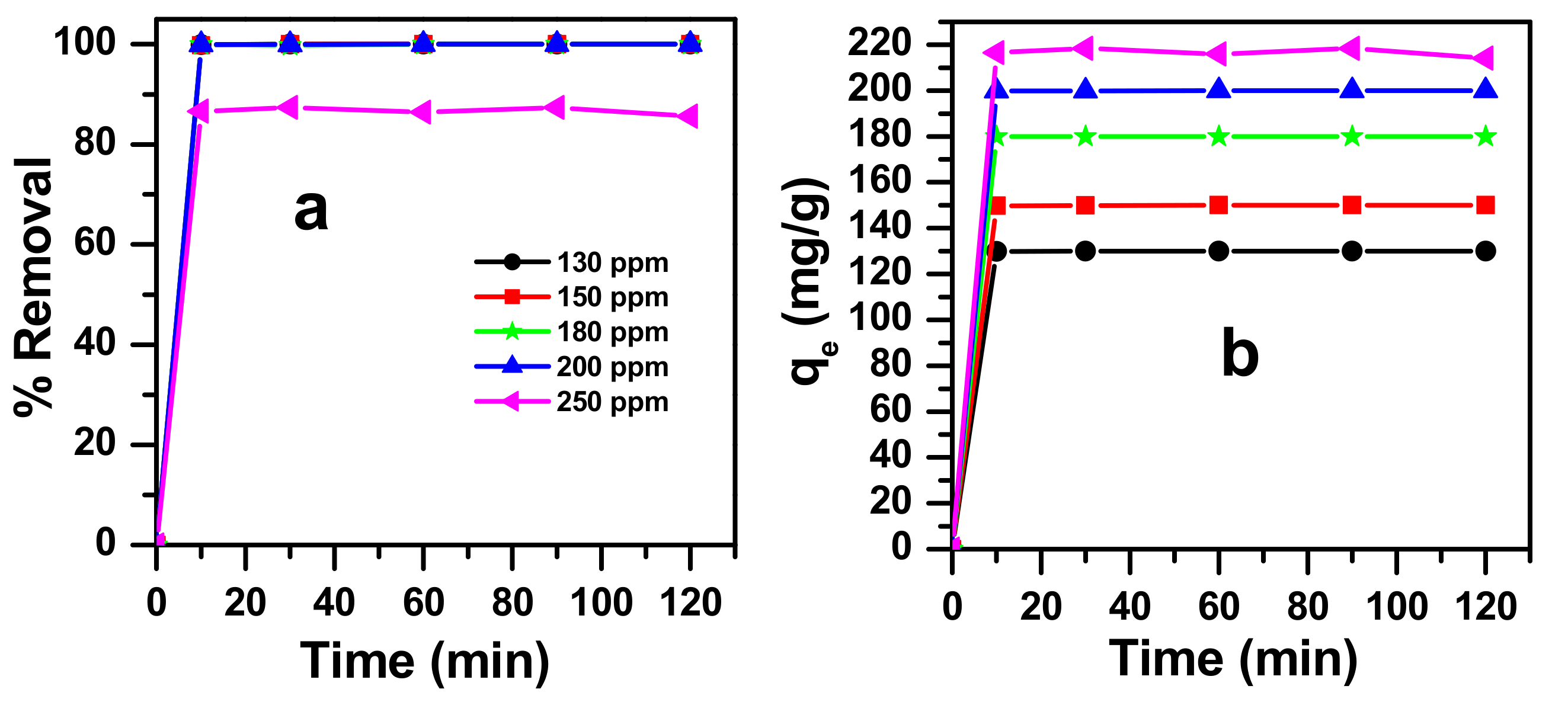
3.4.1. Effect of Initial Dye Concentration
3.4.2. Adsorption Isotherm
3.4.3. Desorption Isotherm
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cornu, L.; Jubera, V.; Demourgues, A.; Salek, G.; Gaudon, M. Luminescence properties and pigment properties of A-doped (Zn,Mg)MoO4 triclinic oxides (with A = Co, Ni, Cu or Mn). Ceram. Int. 2017, 43, 13377–13387. [Google Scholar] [CrossRef]
- Wang, D.; Huang, M.; Zhuang, Y.; Jia, H.-l.; Sun, J.; Guan, M. Phase- and Morphology-Controlled Synthesis of Zinc Molybdate for Excellent Photocatalytic Properties. Eur. J. Inorg. Chem. 2017, 42, 4939–4946. [Google Scholar] [CrossRef]
- Ramezani, M.; Hosseinpour-Mashkani, S.M.; Sobhani-Nasab, A.; Estarki, H.G. Synthesis, characterization, and morphological control of ZnMoO4 nanostructures through precipitation method and its photocatalyst application. J. Mater. Sci. Mater. Electron. 2015, 26, 7588–7594. [Google Scholar] [CrossRef]
- Jiang, Y.-R.; Lee, W.W.; Chen, K.-T.; Wang, M.-C.; Chang, K.-H.; Chen, C.-C. Hydrothermal synthesis of β-ZnMoO4 crystals and their photocatalytic degradation of Victoria Blue R and phenol. J. Taiwan Inst. Chem. Eng. 2014, 45, 207–218. [Google Scholar] [CrossRef]
- Lv, L.; Tong, W.; Zhang, Y.; Su, Y.; Wang, X. Metastable monoclinic ZnMoO4: hydrothermal synthesis, optical properties and photocatalytic performance. J. Nanosci. Nanotechnol. 2011, 11, 9506–9512. [Google Scholar] [CrossRef] [PubMed]
- Lovisa, L.X.; Oliveira, M.C.; Andres, J.; Gracia, L.; Li, M.S.; Longo, E.; Tranquilin, R.L.; Paskocimas, C.A.; Bomio, M.R.D.; Motta, F.V. Structure, morphology and photoluminescence emissions of ZnMoO4: RE3+ = Tb3+ − Tm3+ − X Eu3+ (x = 1, 1.5, 2, 2.5 and 3 mol %) particles obtained by the sonochemical method. J. Alloys Compd. 2018, 750, 55–70. [Google Scholar] [CrossRef]
- Luitel, H.N.; Chand, R.; Watari, T. ZnMoO4:Er3+, Yb3+ phosphor with controlled morphology and enhanced upconversion through alkali ions doping. Opt. Mater. 2018, 78, 302–311. [Google Scholar] [CrossRef]
- Han, C.L.; Luo, L.; He, J.Q.; Wang, J.X.; Zhang, W. Synthesis and luminescence properties of ZnMoO4:Eu3+, M+(M+ = Li+, Na+ and K+) phosphors. J. Mater. Sci. Mater. Electron. 2017, 28, 4409–4413. [Google Scholar] [CrossRef]
- Chengaiah, T.; Jayasankar, C.K.; Pavani, K.; Sasikala, T.; Moorthy, L.R. Preparation and luminescence characterization of Zn(1-x)MoO4: xDy3+ phosphor for white light-emitting diodes. Opt. Commun. 2014, 312, 233–237. [Google Scholar] [CrossRef]
- Yu, L.; Nogami, M. The synthesis and photoluminescent properties of one-dimensional ZnMoO4:Eu3+ nanocrystals. Mater. Lett. 2010, 64, 1644–1646. [Google Scholar] [CrossRef]
- Zhang, G.K.; Yu, S.J.; Yang, Y.Q.; Jiang, W.; Zhang, S.M.; Huang, B.B. Synthesis, morphology and phase transition of the zinc molybdates ZnMoO4·0.8H2O/α-ZnMoO4/ZnMoO4by hydrothermal method. J. Cryst. Growth 2010, 312, 1866–1874. [Google Scholar] [CrossRef]
- Zhou, L.Y.; Wei, J.S.; Gong, F.Z.; Huang, J.L.; Yi, L.H. A potential red phosphor ZnMoO4:Eu3+ for light-emitting diode application. J. Solid State Chem. 2008, 181, 1337–1341. [Google Scholar] [CrossRef]
- Ryu, J.H.; Koo, S.M.; Yoon, J.W.; Lim, C.S.; Shim, K.B. Synthesis of nanocrystalline MMoO4 (M = Ni, Zn) phosphors via a citrate complex route assisted by microwave irradiation and their photoluminescence. Mater. Lett. 2006, 60, 1702–1705. [Google Scholar] [CrossRef]
- Zazhigalov, V.A.; Sachuk, O.V.; Kopachevska, N.S.; Starchevskyy, V.L.; Sawlowicz, Z. Effect of Ultrasonic Treatment on Formation of Nanodimensional Structures in ZnO-MoO3 System. Theor. Exp. Chem. 2017, 53, 53–59. [Google Scholar] [CrossRef]
- Chernyak, D.M.; Danevich, F.A.; Degoda, V.Y.; Dmitruk, I.M.; Ferri, F.; Galashov, E.N.; Giuliani, A.; Ivanov, I.M.; Kobychev, V.V.; Mancuso, M.; et al. Optical, luminescence and thermal properties of radiopure ZnMoO4 crystals used in scintillating bolometers for double beta decay search. Nucl. Instrum. Methods Phys. Res. Sect. A 2013, 729, 856–863. [Google Scholar] [CrossRef]
- Beeman, J.W.; Danevich, F.A.; Degoda, V.Y.; Galashov, E.N.; Giuliani, A.; Ivanov, I.M.; Mancuso, M.; Marnieros, S.; Nones, C.; Pessina, G.; et al. An Improved ZnMoO4 Scintillating Bolometer for the Search for Neutrinoless Double Beta Decay of 100Mo. Low Temp. Phys. 2012, 167, 1021–1028. [Google Scholar] [CrossRef]
- Jeseentharani, V.; Dayalan, A.; Nagaraja, K.S. Co-precipitation synthesis, humidity sensing and photoluminescence properties of nanocrystalline Co2+ substituted zinc(II) molybdate (Zn1-xCoxMoO4; x = 0, 0.3, 0.5, 0.7, 1). Solid State Sci. 2017, 67, 46–58. [Google Scholar] [CrossRef]
- Mardare, C.C.; Tanasic, D.; Rathner, A.; Mueller, N.; Hassel, A.W. Growth inhibition of Escherichia coli by zinc molybdate with different crystalline structures. Phys. Status Solidi A 2016, 213, 1471–1478. [Google Scholar] [CrossRef]
- Luitel, H.N.; Chand, R.; Hamajima, H.; Gaihre, Y.R.; Shingae, T.; Yanagita, T.; Watari, T. Highly efficient NIR to NIR upconversion of ZnMoO4:Tm3+,Yb3+ phosphors and their application in biological imaging of deep tumors. J. Mater. Chem. B 2016, 4, 6192–6199. [Google Scholar] [CrossRef]
- Gao, Y.P.; Huang, K.J.; Zhang, C.X.; Song, S.S.; Wu, X. High-performance symmetric supercapacitor based on flower-like zinc molybdate. J. Alloys Compd. 2018, 731, 1151–1158. [Google Scholar] [CrossRef]
- Yoon, Y.-S.; Fujikawa, N.; Ueda, W.; Moro-oka, Y.; Lee, K.-W. Propane oxidation over various metal molybdate catalysts. Catal. Today 1995, 24, 327–333. [Google Scholar] [CrossRef]
- Maggiore, R.; Galvagno, S.; Bart, J.C.J.; Giannetto, A.; Crisafulli, C.; Toscano, G. Catalytic oxidation of propene over zinc, cadmium and nickel molybdates. Z. Phys. Chem. 1983, 137, 111–118. [Google Scholar] [CrossRef]
- Zazhigalov, V.A.; Sachuk, E.V.; Kopachevskaya, N.S.; Bacherikova, I.V.; Wieczorek-Ciurowa, K.; Shcherbakov, S.N. Mechanochemical Synthesis of Nanodispersed Compounds in the ZnO-MoO3 System. Theor. Exp. Chem. 2016, 52, 97–103. [Google Scholar] [CrossRef]
- Verma, N.; Mari, B.; Singh, K.C.; Jindal, J.; Mollar, M.; Yadav, S. Luminescence properties of ZnMoO4:Eu3+:Y3+ materials synthesized by solution combustion synthesis method. AIP Conf. Proc. 2016, 1724, 020130. [Google Scholar] [CrossRef]
- Jain, N.; Singh, B.P.; Singh, R.K.; Singh, J.; Singh, R.A. Enhanced photoluminescence behaviour of Eu3+ activated ZnMoO4 nanophosphors via Tb3+ co-doping for light emitting diode. J. Lumin. 2017, 188, 504–513. [Google Scholar] [CrossRef]
- Lakhlifi, H.; Benchikhi, M.; El Ouatib, R.; Er-Rakho, L.; Guillemet-Fritsch, S.; Durand, B. Synthesis and physicochemical characterization of pigments based on molybdenum <<ZnO-MoO3: Co2+>>. J. Mater. Environ. Sci. 2015, 6, 3465–3469. [Google Scholar]
- Espinosa Bosch, M.; Ruiz Sánchez, A.J.; Sánchez Rojas, F.; Bosch Ojeda, C. Determination of paracetamol: Historical evolution. J. Pharm. Biomed. Anal. 2006, 42, 291–321. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.C.; Luo, X.P.; Wang, X.; Guo, M.; Li, B. Removal of methylene blue from aqueous solution by modified bamboo hydrochar. Ecotoxicol. Environ. Saf. 2018, 157, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Miyah, Y.; Lahrichi, A.; Idrissi, M.; Khalil, A.; Zerrouq, F. Adsorption of methylene blue dye from aqueous solutions onto walnut shells powder: Equilibrium and kinetic studies. Surf. Interfaces 2018, 11, 74–81. [Google Scholar] [CrossRef]
- Low, S.K.; Tan, M.C. Dye adsorption characteristic of ultrasound pre-treated pomelo peel. J. Environ. Chem. Eng. 2018, 6, 3502–3509. [Google Scholar] [CrossRef]
- Mounia, L.; Belkhiri, L.; Bollinger, J.C.; Bouzaza, A.; Assadi, A.; Tirri, A.; Dahmoune, F.; Madani, K.; Remini, H. Removal of Methylene Blue from aqueous solutions by adsorption on Kaolin: Kinetic and equilibrium studies. Appl. Clay. Sci. 2018, 153, 38–45. [Google Scholar] [CrossRef]
- Bentahar, S.; Dbik, A.; El Khomri, M.; El Messaoudi, N.; Lacherai, A. Removal of a cationic dye from aqueous solution by natural clay. Groundw. Sustain. Dev. 2018, 6, 255–262. [Google Scholar] [CrossRef]
- Kang, S.; Zhao, Y.; Wang, W.; Zhang, T.; Chen, T.; Yi, H.; Rao, F.; Song, S. Removal of methylene blue from water with montmorillonite nanosheets/chitosan hydrogels as adsorbent. Appl. Surf. Sci. 2018, 448, 203–211. [Google Scholar] [CrossRef]
- Sadeghzadeh-Attar, A. Efficient photocatalytic degradation of methylene blue dye by SnO2 nanotubes synthesized at different calcination temperatures. Sol. Energy Mater. Sol. Cells 2018, 183, 16–24. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, G.; Liu, J.; Wang, T.; Wang, X.; Liu, B.; Liu, Y.; Huo, Q.; Chu, Z. Synthesis of hierarchical hollow sodium titanate microspheres and their application for selective removal of organic dyes. J. Colloid Interface Sci. 2018, 528, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Oliva, J.; Martinez, A.I.; Oliva, A.I.; Garcia, C.R.; Martinez-Luevanos, C.; Garcia-Lobato, M.; Ochoa-Valiente, M.; Berlanga, A. Flexible graphene composites for removal of methylene blue dye-contaminant from water. Appl. Surf. Sci. 2018, 436, 739–746. [Google Scholar] [CrossRef]
- Bayat, M.; Javanbakht, V.; Esmaili, J. Synthesis of zeolite/nickel ferrite/sodium alginate bionanocomposite via a co-precipitation technique for efficient removal of water-soluble methylene blue dye. Int. J. Biol. Macromol. 2018, 116, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Kanakaraju, D.; Shahdad, N.R.M.; Lim, Y.C.; Pace, A. Magnetic hybrid TiO2/Alg/FeNPs triads for the efficient removal of methylene blue from water. Sustain. Chem. Pharm. 2018, 8, 50–62. [Google Scholar] [CrossRef]
- Oudghiri-Hassani, H. Synthesis, characterization and catalytic performance of iron molybdate Fe2(MoO4)3 nanoparticles. Catal. Commun. 2015, 60, 19–22. [Google Scholar] [CrossRef]
- Abboudi, M.; Messali, M.; Kadiri, N.; Ben Ali, A.; Moran, E. Synthesis of CuO, La2O3, and La2CuO4 by the Thermal-Decomposition of Oxalates Precursors Using a New Method. Synth. React. Inorg. Met. Org. Nano Met. Chem. 2011, 41, 683–688. [Google Scholar] [CrossRef]
- Messali, M.; Al Wadaani, F.; Oudghiri-Hassani, H.; Rakass, S.; Al Amri, S.; Benaissa, M.; Abboudi, M. Preparation, characterization and photocatalytic activity of hexagonal ZnO nanoparticles. Mater. Lett. 2014, 128, 187–190. [Google Scholar] [CrossRef]
- Oudghiri-Hassani, H.; Rakass, S.; Wadaani, F.T.; Al-ghamdi, K.; Omer, A.; Messali, M.; Abboudi, M. Synthesis, characterization and photocatalytic activity of α-Bi2O3 nanoparticles. J. Taibah Univ. Sci. 2015, 9, 508–512. [Google Scholar] [CrossRef]
- Abboudi, M.; Oudghiri-Hassani, H.; Wadaani, F.; Rakass, S.; Al Ghamdi, A.; Messali, M. Enhanced catalytic reduction of para-nitrophenol using α-MoO3 molybdenum oxide nanorods and stacked nanoplates as catalysts prepared from different precursors. J. Taibah Univ. Sci. 2018, 12, 133–137. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds Part. B: Applications in Coordination, Organometallic, and Bioinorganic Chemistry, 6th ed.; Wiley: New York, NY, USA, 2009; pp. 152–165. ISBN 978-0-471-74493-1. [Google Scholar]
- Ng, K.Y.S.; Zhou, X.; Gulari, E. Spectroscopic characterization of molybdenum oxalate in solution and on alumina. J. Phys. Chem. 1985, 89, 2477–2481. [Google Scholar] [CrossRef]
- Ramis, G.; Yi, L.; Busca, G. Ammonia activation over catalysts for the selective catalytic reduction of NOx and the selective catalytic oxidation of NH3. An FT-IR study. Catal. Today 1996, 28, 373–380. [Google Scholar] [CrossRef]
- Wen, N.; Brooker, M.H. Ammonium Carbonate, Ammonium Bicarbonate, and Ammonium Carbamate Equilibria: A Raman Study. J. Phys. Chem. 1995, 99, 359–368. [Google Scholar] [CrossRef]
- Rivenet, M.; Roussel, P.; Abraham, F. One-dimensional inorganic arrangement in the bismuth oxalate hydroxide Bi(C2O4)OH. J. Solid State Chem. 2008, 181, 2586–2590. [Google Scholar] [CrossRef]
- Shaheen, W.M. Thermal behaviour of pure and binary basic nickel carbonate and ammonium molybdate systems. Mater. Lett. 2002, 52, 272–282. [Google Scholar] [CrossRef]
- Angermann, A.; Topfer, J. Synthesis of nanocrystalline Mn–Zn ferrite powders through thermolysis of mixed oxalates. Ceram. Int. 2011, 37, 995–1002. [Google Scholar] [CrossRef]
- Fagerlund, G. Determination of specific surface by BET method. Mater. Constr. 1973, 6, 239–245. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar]
- Goyal, A.; Bansal, S.; Singhal, S. Facile reduction of nitrophenols: Comparative catalytic efficiency of MFe2O4 (M = Ni, Cu, Zn) nano ferrites. Int. J. Hydrog. Energy 2014, 39, 4895–4908. [Google Scholar] [CrossRef]
- Hassani, H.O.; Al Wadaani, F.T. Preparation, Characterization and Catalytic Activity of Nickel Molybdate (NiMoO4) Nanoparticles. Molecules 2018, 23, 273. [Google Scholar] [CrossRef] [PubMed]
- Nandanwar, S.U.; Chakraborty, M. Synthesis of Colloidal CuO/γ-Al2O3 by Microemulsion and Its Catalytic Reduction of Aromatic Nitro Compounds. Chin. J. Catal. 2012, 33, 1532–1541. [Google Scholar] [CrossRef]
- Al-Wadaani, F.; Omer, A.; Abboudi, M.; Hassani, H.O.; Rakass, S.; Messali, M.; Benaissa, M. High Catalytic Efficiency of Nanostructured β-CoMoO4 in the Reduction of the Ortho-, Meta- and Para-Nitrophenol Isomers. Molecules 2018, 23, 364. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; He, G.; Zhang, L.; Sun, X.; Wang, X. Hydrogenation of nitrophenols catalyzed by carbon black-supported nickel nanoparticles under mild conditions. Appl. Catal. B Environ. 2016, 180, 408–415. [Google Scholar] [CrossRef]
- Holbrook, K.A.; Twist, P.J. Hydrolysis of the Borohydride Ion catalysed by Metal-Boron Alloys. J. Chem. Soc. A Inorg. Phys. Theor. 1971, 890–894. [Google Scholar] [CrossRef]
- Mahida, V.P.; Pate, M.P. Removal of some most hazardous cationic dyes using novel poly (NIPAAm/AA/N-allylisatin) nanohydrogel. Arab. J. Chem. 2016, 9, 430–442. [Google Scholar] [CrossRef]
- Du, Q.; Sun, J.; Li, Y.; Yang, X.; Wang, X.; Wang, Z.; Xia, L. Highly enhanced adsorption of congo red onto graphene oxide/chitosan fibers by wet-chemical etching off silica nanoparticles. Chem. Eng. J. 2014, 245, 99–106. [Google Scholar] [CrossRef]
- Dawood, S.; Sen, T.K. Removal of anionic dye Congo red from aqueous solution by raw pine and acid-treated pine cone powder as adsorbent: Equilibrium, thermodynamic, kinetics, mechanism and process design. Water Res. 2012, 46, 1933–1946. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds zinc molybdate (α-ZnMoO4) are available from the authors. |








| Catalyst | Type | Concentration of NP (mol/L) | Reaction Time (min) | References |
|---|---|---|---|---|
| ZnMoO4 | Nanoparticles | 2 × 10−4 | 1 for 3-NP | This work |
| Fe2(MoO4)3 | Nanoparticles | 2 × 10−4 | 6 for 3-NP | [39] |
| CuFe2O4 | Nanoparticles | 3.6 × 10−5 | 5 for 3-NP | [53] |
| NiFe2O4 | Nanoparticles | 3.6 × 10−5 | 36 for 3-NP | [53] |
| NiMoO4 | Nanoparticles | 2 × 10−4 | 3 for 3-NP | [54] |
| CuO/γAl2O3 | Nanocomposites | 2.9 × 10−5 | 20 for 3-NP | [55] |
| CoMoO4 | Nanoparticles | 2 × 10−4 | 1 for 3-NP | [56] |
| Ni/C black | Nanocomposites | 5.0 × 10−4 | 15 for 3-NP | [57] |
| Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|
| qm (mg/g) | KL (L/mg) | R2 | Range RL | qF (mg1-1/n/L1/n/g) | 1/n | R2 |
| 217.86 | 13.80 | 1 | 0.0003–0.0006 | 215.10 | 0.21 | 0.97 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oudghiri-Hassani, H.; Rakass, S.; Abboudi, M.; Mohmoud, A.; Al Wadaani, F. Preparation and Characterization of α-Zinc Molybdate Catalyst: Efficient Sorbent for Methylene Blue and Reduction of 3-Nitrophenol. Molecules 2018, 23, 1462. https://doi.org/10.3390/molecules23061462
Oudghiri-Hassani H, Rakass S, Abboudi M, Mohmoud A, Al Wadaani F. Preparation and Characterization of α-Zinc Molybdate Catalyst: Efficient Sorbent for Methylene Blue and Reduction of 3-Nitrophenol. Molecules. 2018; 23(6):1462. https://doi.org/10.3390/molecules23061462
Chicago/Turabian StyleOudghiri-Hassani, Hicham, Souad Rakass, Mostafa Abboudi, Ahmed Mohmoud, and Fahd Al Wadaani. 2018. "Preparation and Characterization of α-Zinc Molybdate Catalyst: Efficient Sorbent for Methylene Blue and Reduction of 3-Nitrophenol" Molecules 23, no. 6: 1462. https://doi.org/10.3390/molecules23061462
APA StyleOudghiri-Hassani, H., Rakass, S., Abboudi, M., Mohmoud, A., & Al Wadaani, F. (2018). Preparation and Characterization of α-Zinc Molybdate Catalyst: Efficient Sorbent for Methylene Blue and Reduction of 3-Nitrophenol. Molecules, 23(6), 1462. https://doi.org/10.3390/molecules23061462






