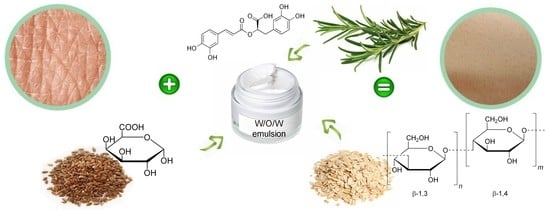
Innovative Natural Ingredients-Based Multiple Emulsions: The Effect on Human Skin Moisture, Sebum Content, Pore Size and Pigmentation
Abstract
1. Introduction
2. Results
3. Materials and Methods
3.1. Materials
3.2. Preparation of Rosemary, Linseed and Oat Extracts
3.3. Preparation of Emulsion
3.4. HPLC Conditions for Determination of Rosmarinic Acid (RA), Ursolic Acid (UA) and Oleanolic Acid (OA)
3.5. Determination of β-glucans
3.6. Determination of Monosaccharides
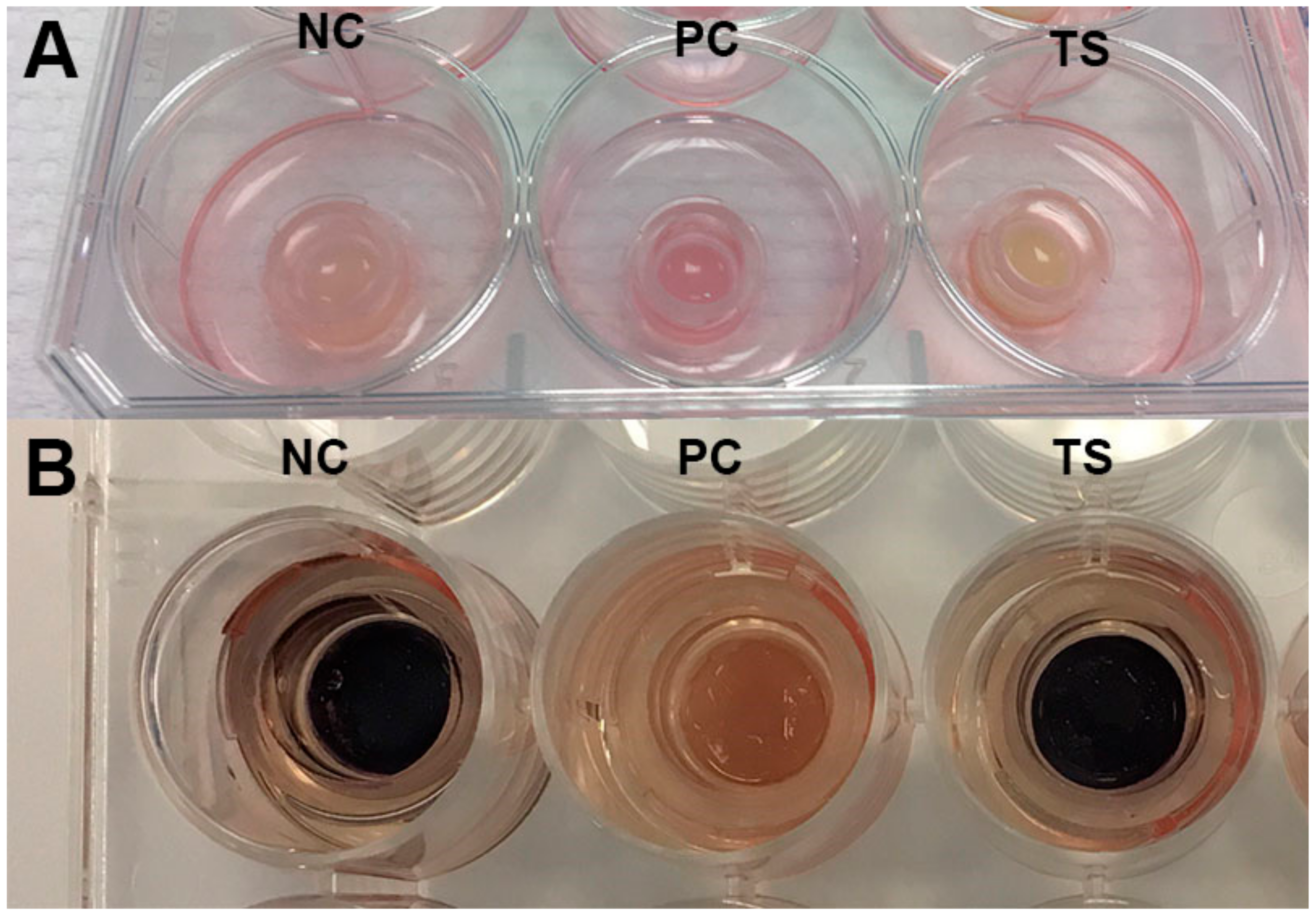
3.7. Skin Irritation, Phototoxicity and Inflammation Determination in Vitro
3.8. The Measurement of Human Skin Moisture, Sebum Content, Pigmentation and Pore Size In Vivo
- The initial data collection: skin moisture, sebum content, pigmentation, pore size (clean skin, without any skin care product applied).
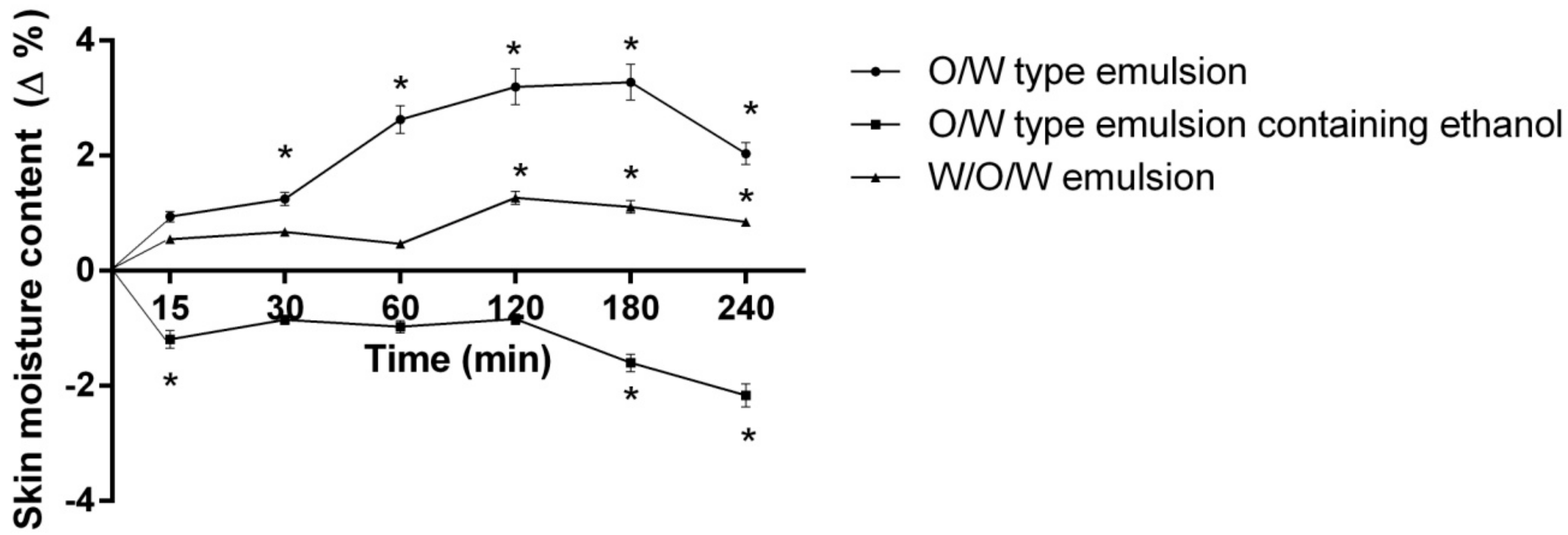
- Double and multiple emulsions application and evaluation of their influence on skin moisture content after 15, 30, 60, 120, 180, 240 min. Emulsions of three different compositions were applied on the inner forearm of human volunteers (Table 2).
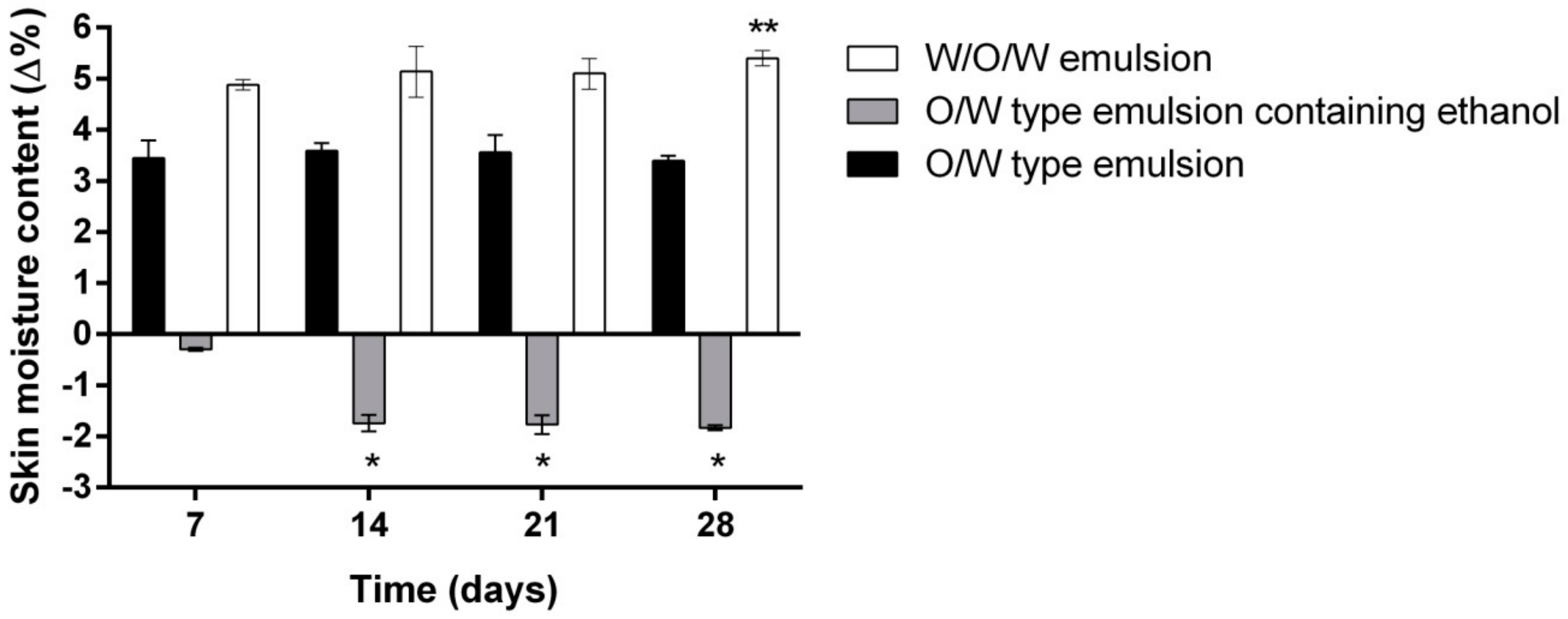
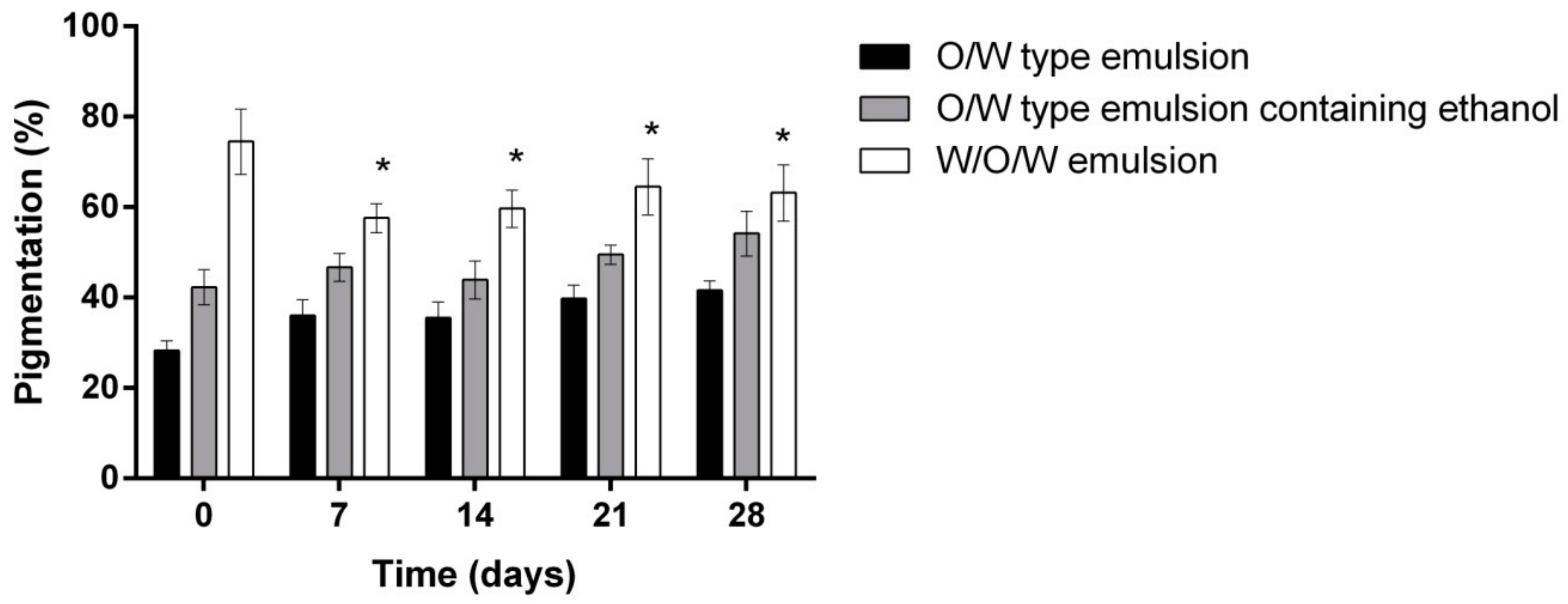
- Evaluation of the influence of three different emulsion compositions on the skin moisture, sebum content, pigmentation and pore size after 7, 14, 21 and 28 days of daily use evaluation.
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Henriksen, L.; Simonsen, J.; Haerskjold, A.; Linder, M.; Kieler, H.; Thomsen, S.F.; Stensballe, L.G. Incidence rates of atopic dermatitis, asthma, and allergic rhinoconjunctivitis in Danish and Swedish children. J. Allergy Clin. Immunol. 2015, 136, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-P.; Woerdenbag, H.J. Traditional Chinese herbal medicine. Pharm. World Sci. 1995, 17, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Choochote, W.; Chaiyasit, D.; Kanjanapothi, D.; Rattanachanpichai, E.; Jitpakdi, A.; Tuetun, B.; Pitasawat, B. Chemical composition and anti-mosquito potential of rhizome extract and volatile oil derived from Curcuma aromatica against Aedes aegypti (Diptera: Culicidae). J. Vector. Ecol. 2005, 30, 302. [Google Scholar] [PubMed]
- Astier, C.; Benchad, Y.; Moneret-Vautrin, D.A.; Bihain, B.; Kanny, G. Anaphylaxis to argan oil. Allergy 2010, 65, 662–663. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, K.J.; Yiannias, J.A. Contact dermatitis to cosmetics, fragrances, and botanicals. Dermatol. Ther. 2004, 17, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Kiken, D.A.; Cohen, D.E. Contact dermatitis to botanical extracts. Am. J. Contact Dermat. 2002, 13, 148–152. [Google Scholar] [PubMed]
- Pigatto, P.; Bigardi, A.; Caputo, R.; Angelini, G.; Foti, C.; Grandolfo, M.; Rizer, R.L. An evaluation of the allergic contact dermatitis potential of colloidal grain suspensions. Am. J. Contact Derm. 1997, 8, 207–209. [Google Scholar]
- Yamakawa, Y.; Ohsuna, H.; Aihara, M.; Tsubaki, K.; Ikezawa, Z. Contact urticaria from rice. Contact Derm. 2001, 44, 91–93. [Google Scholar] [CrossRef] [PubMed]
- Amaro, C.; Goossens, A. Immunological occupational contact urticaria and contact dermatitis from proteins: A review. Contact Derm. 2008, 58, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Cizauskaite, U.; Ivanauskas, L.; Jakštas, V.; Marksiene, R.; Jonaitiene, L.; Bernatoniene, J. Rosmarinus officinalis L. extract and some of its active ingredients as potential emulsion stabilizers: A new approach to the formation of multiple (W/O/W) emulsion. Pharm. Dev. Technol. 2016, 21, 716–724. [Google Scholar] [PubMed]
- Cizauskaite, U.; Marksiene, R.; Viliene, V.; Gruzauskas, R.; Bernatoniene, J. New strategy of multiple emulsion formation based on the interactions between polymeric emulsifier and natural ingredients. Colloids Surf. A Physicochem. Eng. Asp. 2017, 515, 22–33. [Google Scholar] [CrossRef]
- Khan, A.Y.; Talegaonkar, S.; Iqbal, Z.; Ahmed, F.J.; Khar, R.K. Multiple emulsions: An overview. Curr. Drug Deliv. 2006, 3, 429–443. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Kumar, M.S.; Mahadevan, N. Multiple emulsions: A review. Int. J. Recent Adv. Pharm. Res. 2012, 2, 9–19. [Google Scholar]
- Bouyer, E.; Mekhloufi, G.; Rosilio, V.; Grossiord, J.-L.; Agnely, F. Proteins, polysaccharides, and their complexes used as stabilizers for emulsions: Alternatives to synthetic surfactants in the pharmaceutical field? Int. J. Pharm. 2012, 436, 359–378. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Díaz, C.; Rubilar, M.; Morales, E.; Medina, C.; Acevedo, F.; Marqués, A.M.; Shene, C. Naturally occurring protein–polysaccharide complexes from linseed (Linum usitatissimum) as bioemulsifiers. Eur. J. Lipid Sci. Technol. 2016, 118, 165–174. [Google Scholar] [CrossRef]
- Fuchs, S.M.; Schliemann-Willers, S.; Fischer, T.W.; Elsner, P. Protective Effects of Different Marigold (Calendula officinalis L.) and Rosemary Cream Preparations against Sodium-Lauryl-Sulfate-Induced Irritant Contact Dermatitis. Skin Pharmacol. Phys. 2005, 18, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Stingni, L.; Lapomarda, V.; Lisi, P. Occupational hand dermatitis in hospital environments. Contact Derm. 1995, 33, 172–176. [Google Scholar] [CrossRef]
- Rasul, A.; Akhtar, N. Formulation and in vivo evaluation for anti-aging effects of an emulsion containing basil extract using non-invasive biophysical techniques. DARU: J. Fac. Pharm. Tehran Univ. Med. Sci. 2011, 19, 344. [Google Scholar]
- Montenegro, L.; Pasquinucci, L.; Zappalà, A.; Chiechio, S.; Turnaturi, R.; Parenti, C. Rosemary Essential Oil-Loaded Lipid Nanoparticles: In Vivo Topical Activity from Gel Vehicles. Pharmaceutics 2017, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Han, J.; Lee, C.S.; Heung Soo, B.; Lim, K.M.; Ha, H. Carnosic acid, a phenolic diterpene from rosemary, prevents UV-induced expression of matrix metalloproteinases in human skin fibroblasts and keratinocytes. Exp. Dermatol. 2013, 22, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Vie, K.; Cours-Darne, S.; Vienne, M.; Boyer, F.; Fabre, B.; Dupuy, P. Modulating effects of oatmeal extracts in the sodium lauryl sulfate skin irritancy model. Skin Pharmacol. Phys. 2002, 15, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Michelle Garay, M. Colloidal Oatmeal (Avena Sativa) Improves Skin Barrier Through Multi-Therapy Activity. J. Drugs Dermatol. 2016, 15, 684–690. [Google Scholar]
- Criquet, M.; Roure, R.; Dayan, L.; Nollent, V.; Bertin, C. Safety and efficacy of personal care products containing colloidal oatmeal. Clin. Cosmet. Investig. Dermatol. 2012, 5, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.; Sharma, V.; Upadhyay, N.; Gill, S.; Sihag, M. Flax and flaxseed oil: An ancient medicine & modern functional food. J. Food Sci. Technol. 2014, 51, 1633–1653. [Google Scholar] [PubMed]
- Péterszegi, G.; Isnard, N.; Robert, A.; Robert, L. Studies on skin aging. Preparation and properties of fucose-rich oligo-and polysaccharides. Effect on fibroblast proliferation and survival. Biomed. Pharmacother. 2003, 57, 187–194. [Google Scholar] [CrossRef]
- Cizauskaite, U.; Marksa, M.; Bernatoniene, J. The optimization of technological processes, stability and microbiological evaluation of innovative natural ingredients-based multiple emulsion. Pharm. Dev. Technol. 2018, 23, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Coecke, S.; Pelkonen, O.; Leite, S.B.; Bernauer, U.; Bessems, J.G.; Bois, F.Y.; Gundert-Remy, U.; Loizou, G.; Testai, E.; Zaldívar, J.-M. Toxicokinetics as a key to the integrated toxicity risk assessment based primarily on non-animal approaches. Toxicol. In Vitro 2013, 27, 1570–1577. [Google Scholar] [CrossRef] [PubMed]
- Liebsch, M.; Barrabas, C.; Traue, D.; Spielmann, H. Development of a new in vitro test for dermal phototoxicity using a model of reconstituted human epidermis. Altex 1997, 14, 165–174. [Google Scholar] [PubMed]
- Kandárová, H.; Liebsch, M.; Genschow, E.; Gerner, I.; Traue, D.; Slawik, B.; Spielmann, H. Optimisation of the EpiDerm test protocol for the upcoming ECVAM validation study on in vitro skin irritation tests. Altex 2004, 21, 107–114. [Google Scholar] [PubMed]
- Buzek, J.; Ask, B. Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products. Off. J. Eur. Union L 2009, 342, 59–209. [Google Scholar]
- Vecino, X.; Cruz, J.M.; Moldes, A.B.; Rodrigues, L.R. Biosurfactants in cosmetic formulations: Trends and challenges. Crit. Rev. Biotechnol. 2017, 37, 911–923. [Google Scholar] [CrossRef] [PubMed]
- Evy, P. Contact sensitization from Compositae-containing herbal remedies and cosmetics. Contact Derm. 2002, 47, 189–198. [Google Scholar]
- Bárány, E.; Lindberg, M.; Lodén, M. Unexpected skin barrier influence from nonionic emulsifiers. Int. J. Pharm. 2000, 195, 189–195. [Google Scholar] [CrossRef]
- Guin, J.D. Use of consumer product ingredients for patch testing. Dermatitis 2005, 16, 71–77. [Google Scholar] [PubMed]
- Tornier, C.; Rosdy, M.; Maibach, H.I. In vitro skin irritation testing on reconstituted human epidermis: Reproducibility for 50 chemicals tested with two protocols. Toxicol. In Vitro 2006, 20, 401–416. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.; Vecino, X.; Ferreira, D.; Cruz, J.M.; Moldes, A.B.; Rodrigues, L.R. Novel cosmetic formulations containing a biosurfactant from Lactobacillus paracasei. Coll. Surf. B Biointerfaces 2017, 155, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Yapar, E.A.; İNAL, Ö.; Erdal, M.S. Design and in vivo evaluation of emulgel formulations including green tea extract and rose oil. Acta Pharm. 2013, 63, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Otto, A.; Du Plessis, J.; Wiechers, J.W. Formulation effects of topical emulsions on transdermal and dermal delivery. Int. J. Cosmet. Sci. 2009, 31, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Pershing, L.K.; Lambert, L.D.; Knutson, K. Mechanism of ethanol-enhanced estradiol permeation across human skin in vivo. Pharm. Res. 1990, 7, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Pecquet, C.; Pradalier, A.; Dry, J. Allergic contact dermatitis from ethanol in a transdermal estradiol patch. Contact Derm. 1992, 27, 275. [Google Scholar] [CrossRef] [PubMed]
- Pietsch, H. Hand antiseptics: Rubs versus scrubs, alcoholic solutions versus alcoholic gels. J. Hosp. Infect. 2001, 48, S33–S36. [Google Scholar] [CrossRef]
- Flament, F.; Francois, G.; Qiu, H.; Ye, C.; Hanaya, T.; Batisse, D.; Cointereau-Chardon, S.; Seixas, M.D.G.; Dal Belo, S.E.; Bazin, R. Facial skin pores: A multiethnic study. Clin. Cosmet. Investig. Dermatol 2015, 8, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Roh, M.; Han, M.; Kim, D.; Chung, K. Sebum output as a factor contributing to the size of facial pores. Br. J. Dermatol. 2006, 155, 890–894. [Google Scholar] [CrossRef] [PubMed]
- Duffy, J.A.; Znaiden, A.P. Composition and method for visibly reducing the size of skin pores. U.S. Patent 5,472,699, 2 December 1995. [Google Scholar]
- Kramer, A.; Bernig, T.; Kampf, G. Clinical double-blind trial on the dermal tolerance and user acceptability of six alcohol-based hand disinfectants for hygienic hand disinfection. J. Hosp. Infect. 2002, 51, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Yazan, Y. Formulation and in vivo evaluation of a cosmetic multiple emulsion containing vitamin C and wheat protein. Pak. J. Pharm. Sci. 2008, 21, 45–50. [Google Scholar] [PubMed]
- Tran, T.-N. T.; Schulman, J.; Fisher, D.E. UV and pigmentation: Molecular mechanisms and social controversies. Pigment Cell. Melanoma Res. 2008, 21, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Friedmann, P.S.; Gilchrest, B.A. Ultraviolet radiation directly induces pigment production by cultured human melanocytes. J. Cell. Physiol. 1987, 133, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Nobile, V.; Michelotti, A.; Cestone, E.; Caturla, N.; Castillo, J.; Benavente-García, O.; Pérez-Sánchez, A.; Micol, V. Skin photoprotective and antiageing effects of a combination of rosemary (Rosmarinus officinalis) and grapefruit (Citrus paradisi) polyphenols. Food Nutr. Res. 2016, 60, 31871. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.; Pierrard, C.; Lejeune, F.; Hilaire, P.; Breton, L.; Bernerd, F. Photoprotective effect of a water-soluble extract of Rosmarinus officinalis L. against UV-induced matrix metalloproteinase-1 in human dermal fibroblasts and reconstructed skin. Eur. J. Dermatol. 2008, 18, 128–135. [Google Scholar] [PubMed]
- Sánchez-Campillo, M.; Gabaldon, J.; Castillo, J.; Benavente-García, O.; Del Baño, M.; Alcaraz, M.; Vicente, V.; Alvarez, N.; Lozano, J. Rosmarinic acid, a photo-protective agent against UV and other ionizing radiations. Food Chem. Toxicol. 2009, 47, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Suggs, A.; Oyetakin-White, P.; Baron, D.E. Effect of botanicals on inflammation and skin aging: Analyzing the evidence. Inflamm. Allergy Drug Targets 2014, 13, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Offord, E.A.; Gautier, J.-C.; Avanti, O.; Scaletta, C.; Runge, F.; Krämer, K.; Applegate, L.A. Photoprotective potential of lycopene, β-carotene, vitamin E, vitamin C and carnosic acid in UVA-irradiated human skin fibroblasts. Free Radic. Biol. Med. 2002, 32, 1293–1303. [Google Scholar] [CrossRef]
- McCleary, B.V.; Codd, R. Measurement of (1→3),(1→4)-β-d-glucan in barley and oats: A streamlined enzymic procedure. J. Sci. Food Agric. 1991, 55, 303–312. [Google Scholar] [CrossRef]
- Emaga, T.H.; Rabetafika, N.; Blecker, C.S.; Paquot, M. Kinetics of the hydrolysis of polysaccharide galacturonic acid and neutral sugars chains from flaxseed mucilage. Biotechnology 2012, 16, 139. [Google Scholar]
- Spielmann, H.; Müller, L.; Averbeck, D.; Balls, M.; Brendler-Schwaab, S.; Castell, J.V.; Curren, R.; De Silva, O.; Gibbs, N.; Liebsch, M. The second ECVAM workshop on phototoxicity testing. The report and recommendations of ECVAM workshop. Altern. Lab. Anim. 2000, 42, 777–814. [Google Scholar]
- Jones, P.A.; Lovell, W.W.; King, A.V.; Earl, L.K. In vitro testing for phototoxic potential using the EpiDerm 3-D reconstructed human skin model. Toxicol. Mech. Methods 2001, 11, 1–19. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

| Origin of Active Ingredients | Active Ingredients Found in W/O/W Emulsion | Amount (mg/g ± SD) |
|---|---|---|
| Rosemary extract | Rosmarinic acid (RA) | 0.047 ± 0.001 |
| Oleanolic acid (OA) | 0.028 ± 0.001 | |
| Ursolic acid (UA) | 0.053 ± 0.001 | |
| Linseed and oat extracts | l-rhamnose | 0.795 ± 0.001 |
| l-(+)-arabinose | 269.810 ± 0.005 | |
| d-(+)-xylose | 4.80 ± 0.01 | |
| d-(+)-galacturonic acid | 3.02 ± 0.02 | |
| Oat extract | β-glucan | 1.70 ± 0.01 |
| Emulsion Phase | Investigated Samples | |||||
|---|---|---|---|---|---|---|
| O/W Type Emulsion | (%) | O/W Type Emulsion Containing Ethanol | (%) | W/O/W Emulsion | (%) | |
| Lipophilic phase | Olive oil | 20 | Olive oil | 20 | Olive oil | 20 |
| Viscoptima SE | 0.5 | Viscoptima SE | 0.5 | Viscoptima SE | 0.5 | |
| Hydrophilic phase | Distilled water | up to 100 | Ethanol (90%) | 7.5 | Ethanolic rosemary extract | 7.5 |
| Linseed extract | 24.18 | |||||
| Distilled water | up to 100 | Oat extract | 44.03 | |||
| Distilled water | up to 100 | |||||
| Time (days) | Investigated Samples | ||
|---|---|---|---|
| O/W Type Emulsion | O/W type Emulsion Containing Ethanol | W/O/W Type Emulsion | |
| Pore Size (mm) | |||
| 0 | 0.0326 ± 0.0035 | 0.0302 ± 0.0011 | 0.0335 ± 0.0032 |
| 7 | 0.0340 ± 0.0033 | 0.0324 ± 0.0013 * | 0.0329 ± 0.0025 |
| 14 | 0.0352 ± 0.0024 | 0.0356 ± 0.0024 * | 0.0324 ± 0.0031 |
| 21 | 0.0341 ± 0.0031 | 0.0355 ± 0.0035 * | 0.032 1± 0.0012 |
| 28 | 0.0350 ± 0.0011 | 0.0350 ± 0.0034 * | 0.0319 ± 0.0029 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cizauskaite, U.; Bernatoniene, J. Innovative Natural Ingredients-Based Multiple Emulsions: The Effect on Human Skin Moisture, Sebum Content, Pore Size and Pigmentation. Molecules 2018, 23, 1428. https://doi.org/10.3390/molecules23061428
Cizauskaite U, Bernatoniene J. Innovative Natural Ingredients-Based Multiple Emulsions: The Effect on Human Skin Moisture, Sebum Content, Pore Size and Pigmentation. Molecules. 2018; 23(6):1428. https://doi.org/10.3390/molecules23061428
Chicago/Turabian StyleCizauskaite, Ugne, and Jurga Bernatoniene. 2018. "Innovative Natural Ingredients-Based Multiple Emulsions: The Effect on Human Skin Moisture, Sebum Content, Pore Size and Pigmentation" Molecules 23, no. 6: 1428. https://doi.org/10.3390/molecules23061428
APA StyleCizauskaite, U., & Bernatoniene, J. (2018). Innovative Natural Ingredients-Based Multiple Emulsions: The Effect on Human Skin Moisture, Sebum Content, Pore Size and Pigmentation. Molecules, 23(6), 1428. https://doi.org/10.3390/molecules23061428