Tubular and Spherical SiO2 Obtained by Sol Gel Method for Lipase Immobilization and Enzymatic Activity
Abstract
1. Introduction
2. Results and Discussion
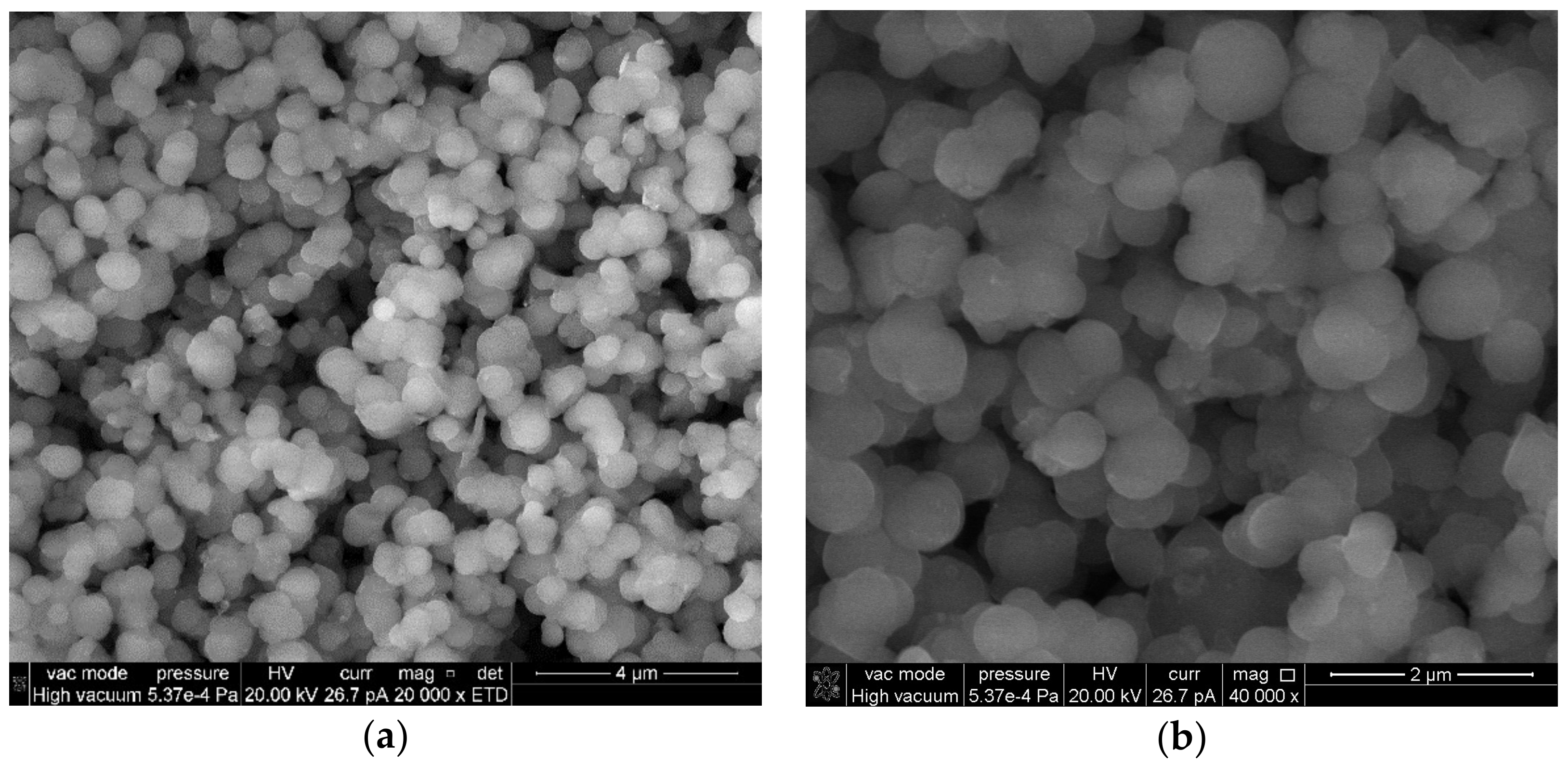
2.1. Scanning Electron Microscopy (SEM)
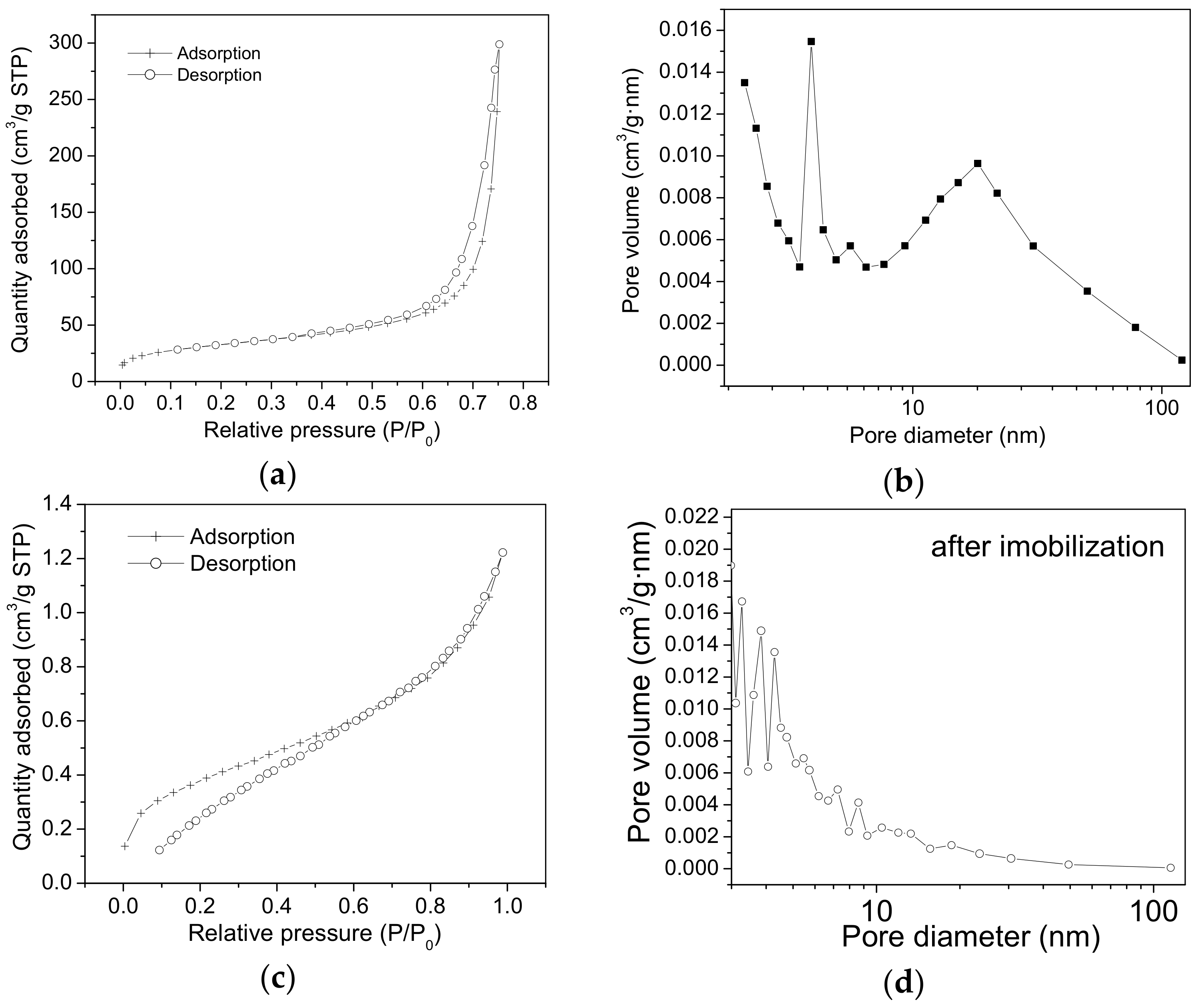
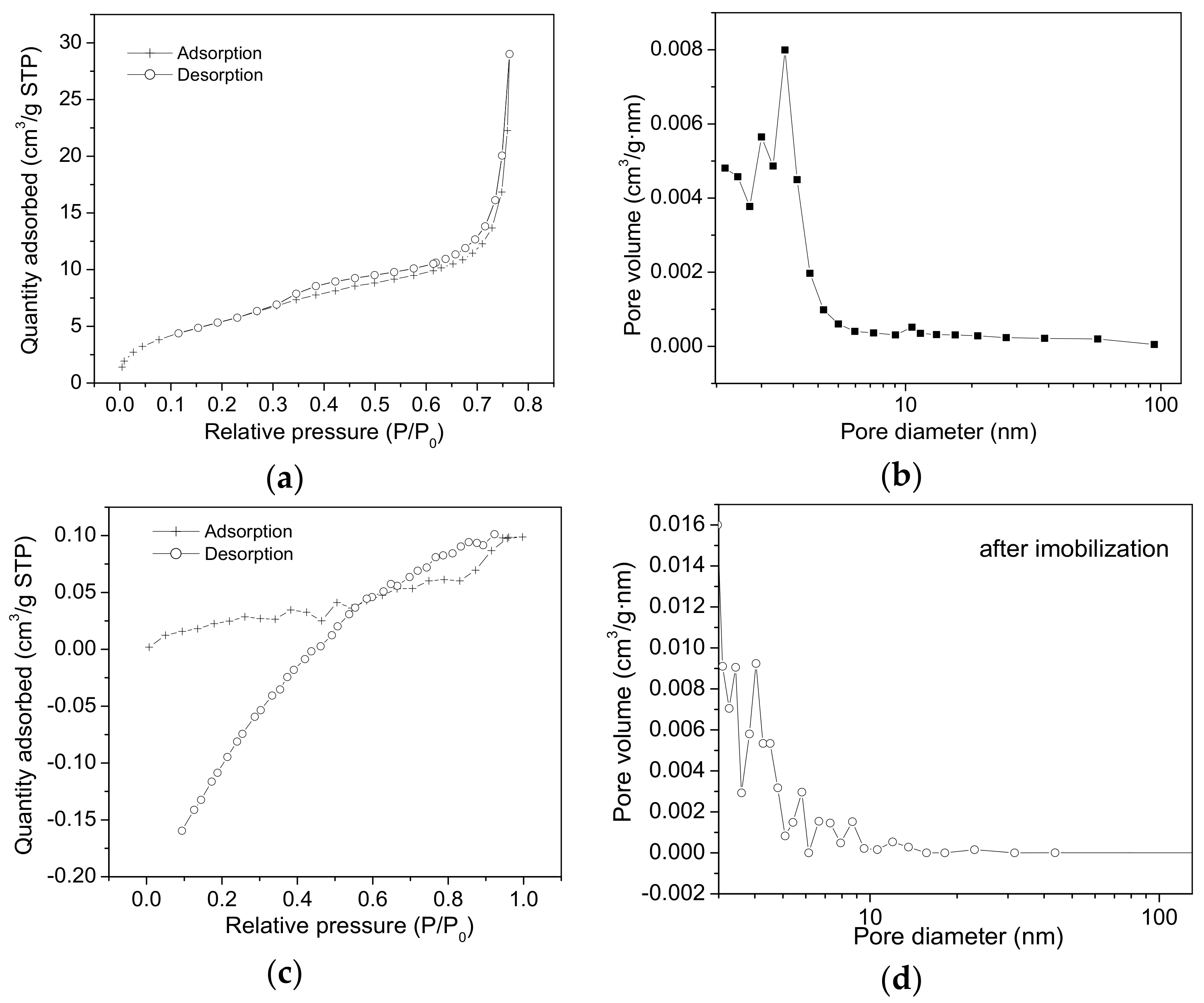
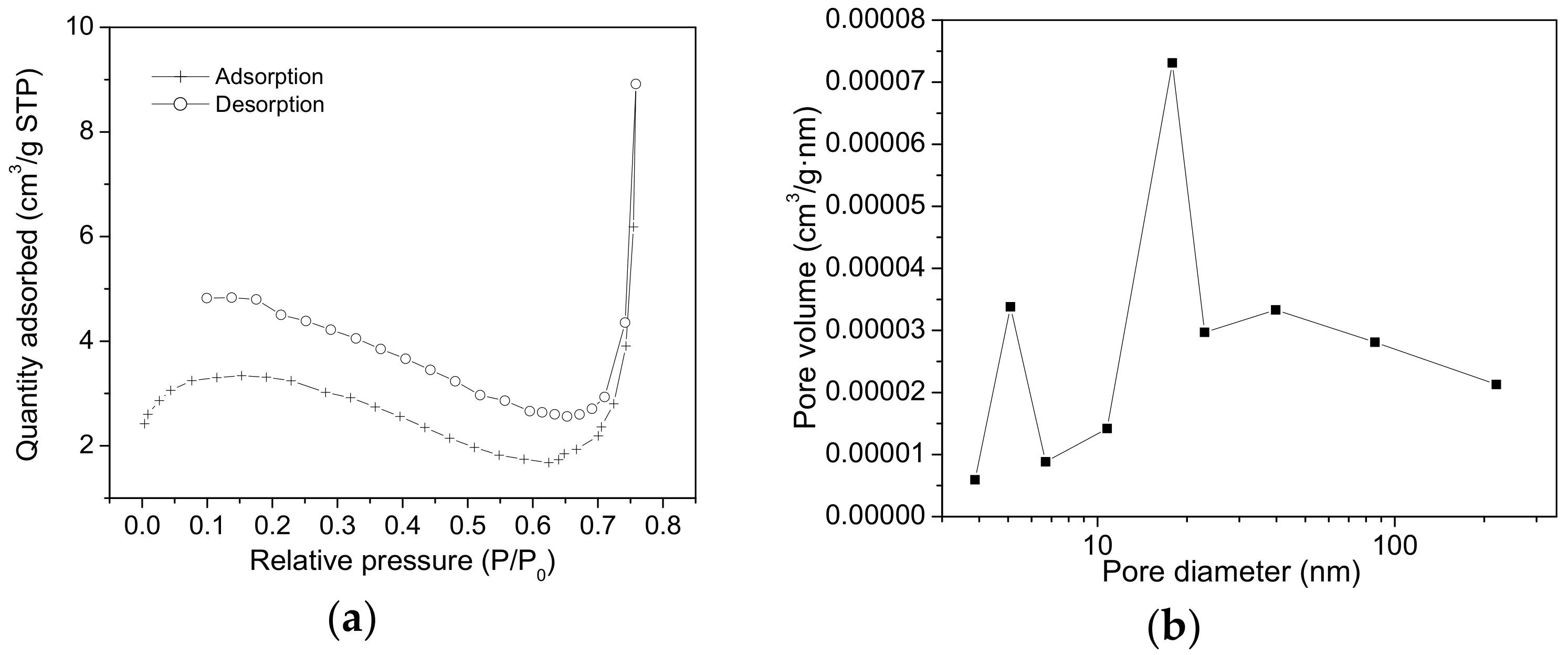
2.2. Nitrogen Sorption Measurements
2.3. Fourier Transformed Infra-Red (FTIR) Spectroscopy
2.4. FTIR-ATR Spectroscopy Performed on Hybrid Materials
2.5. Photoluminescence (PL)
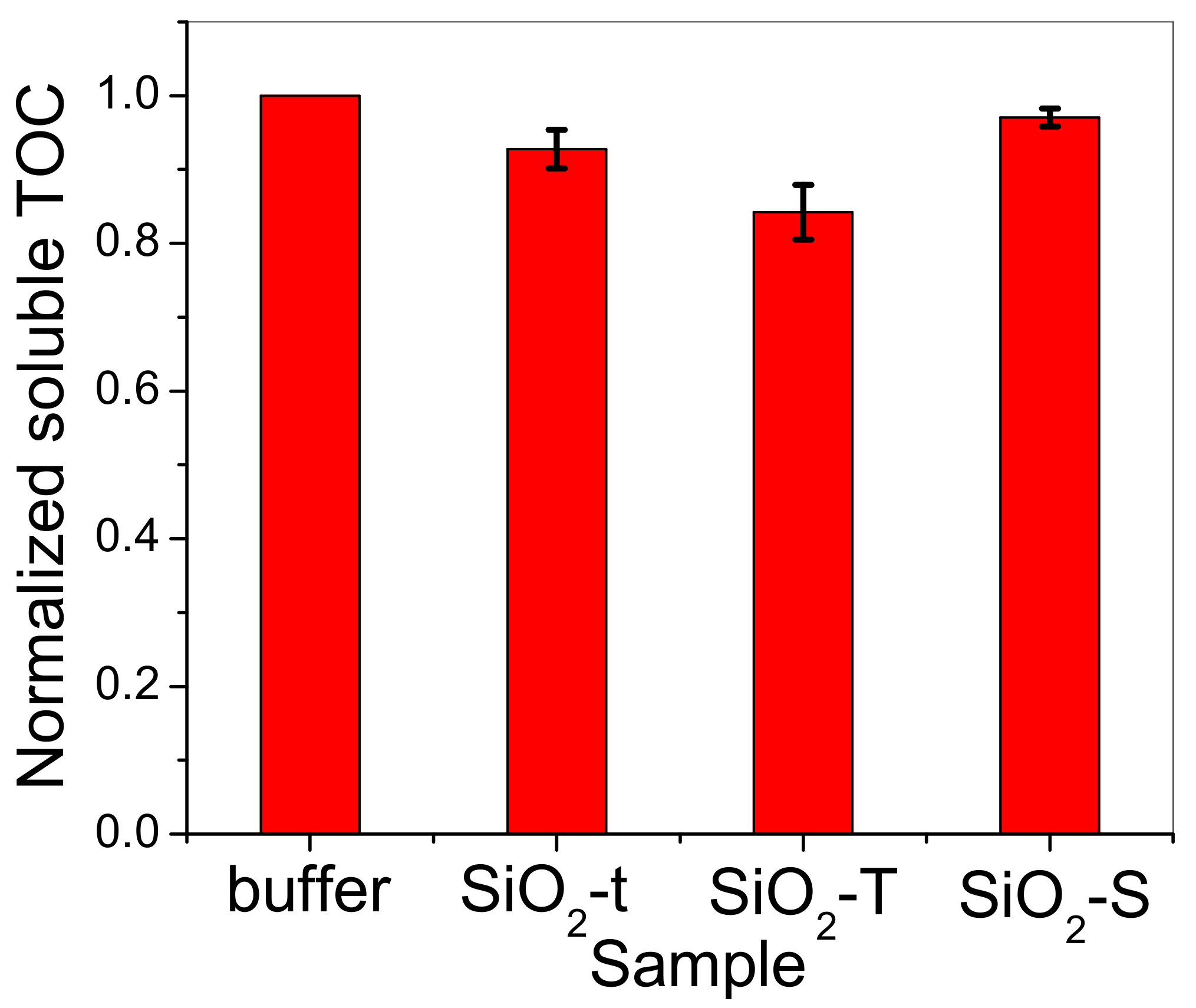
2.6. Total Organic Carbon (TOC)
2.7. The Electro-Kinetic Potential
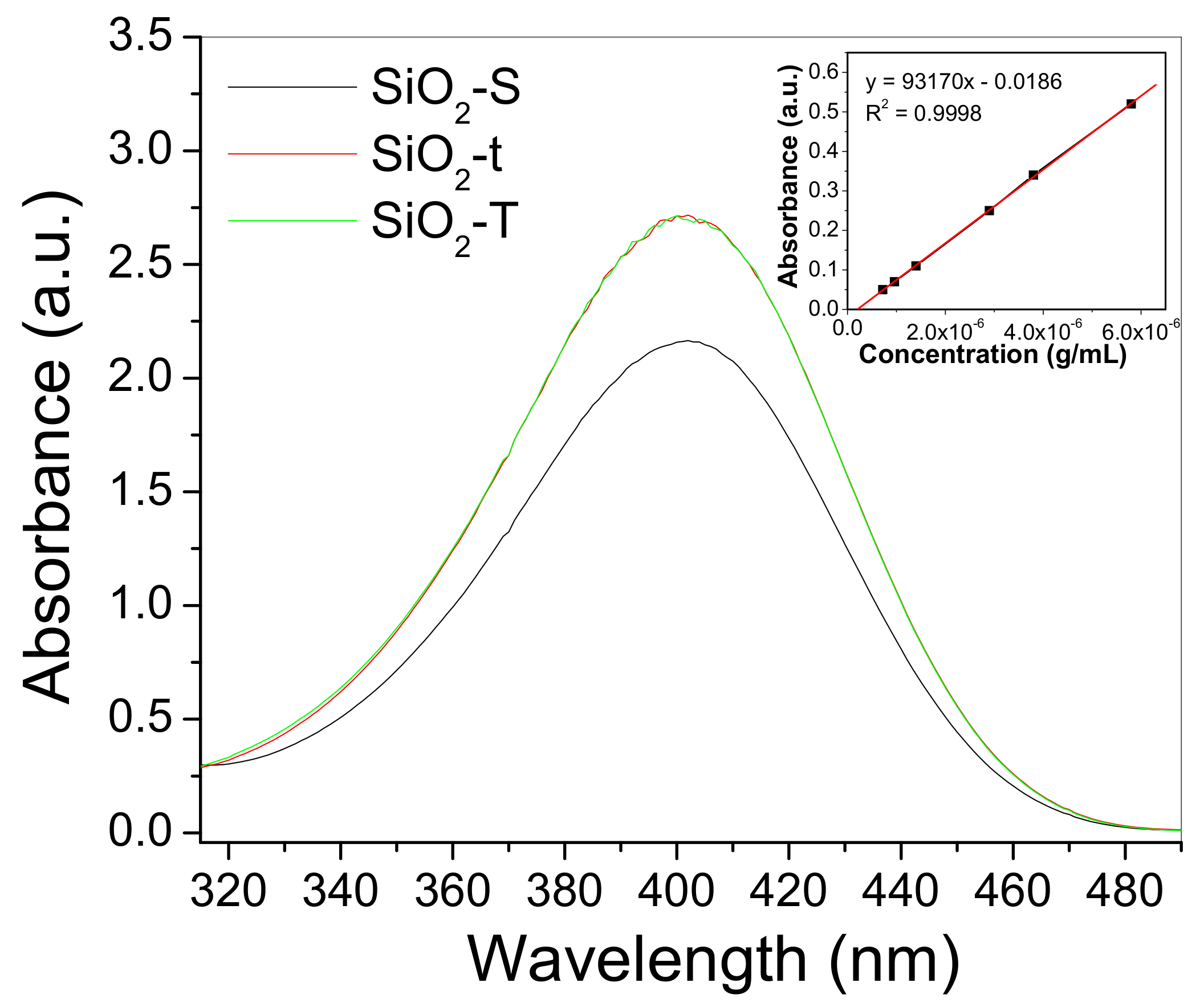
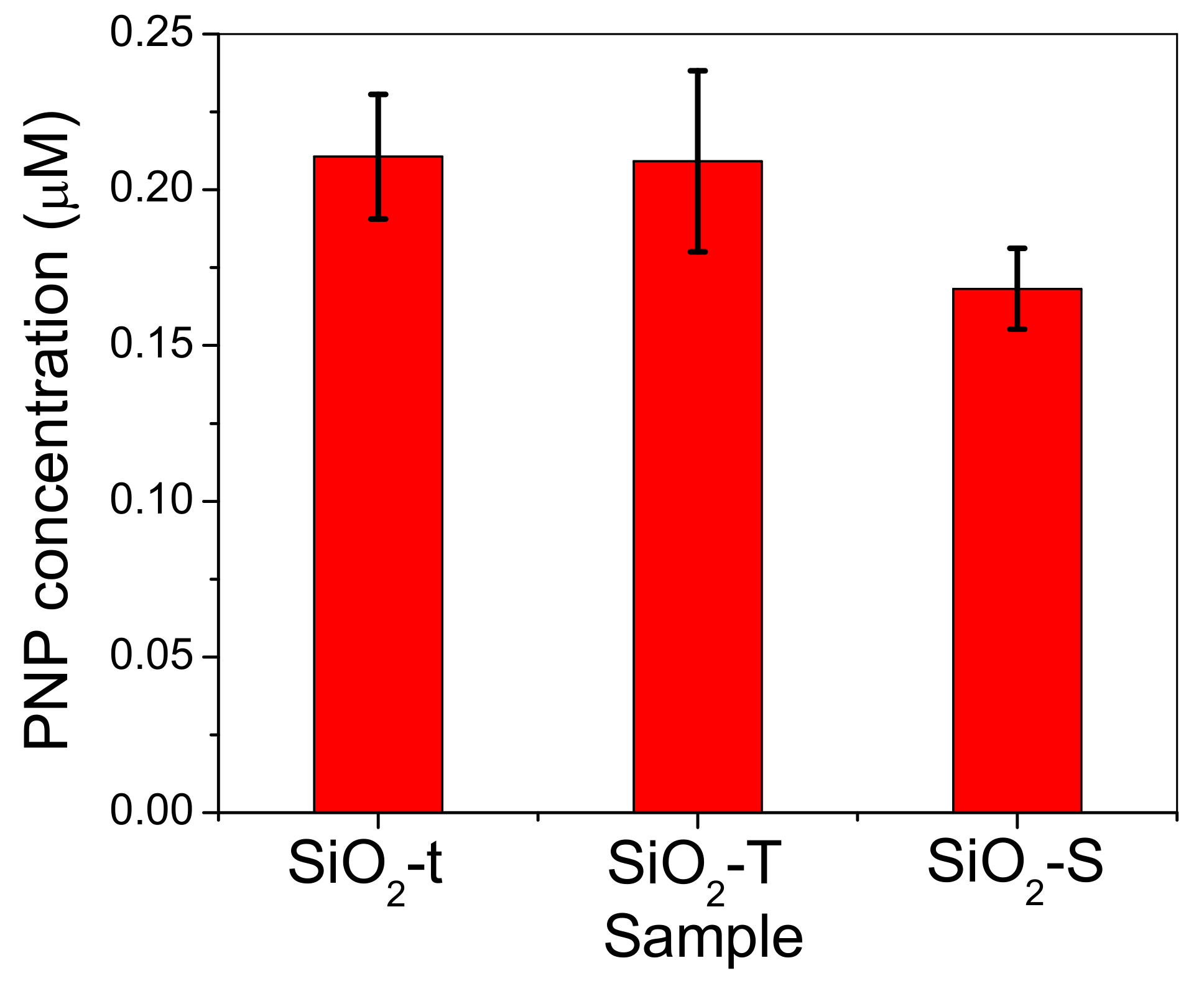
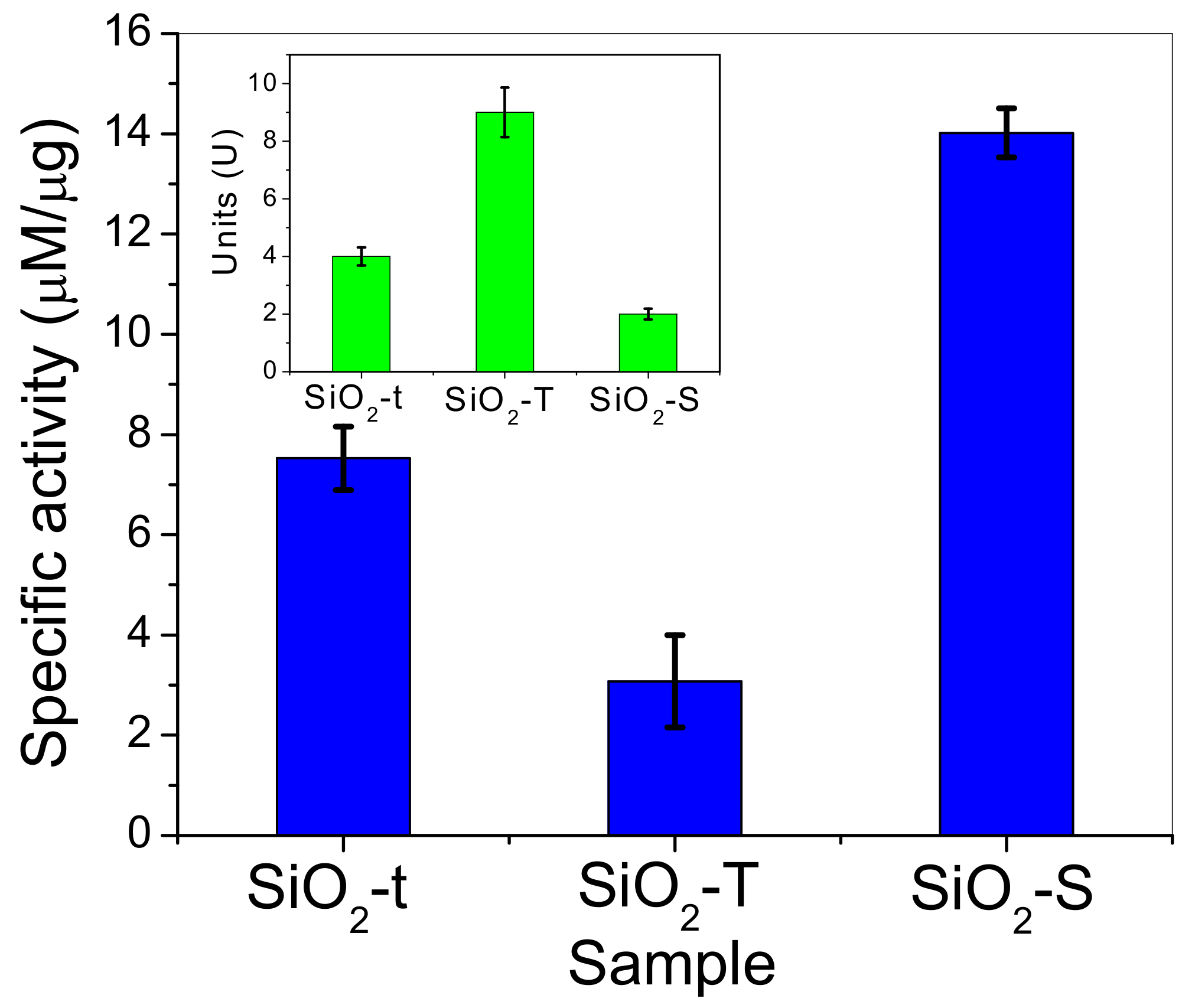
2.8. Catalytic Activity
3. Materials and Methods
3.1. Materials
3.1.1. Synthesis of SiO2 Matrices
3.1.2. The Formation of the Lipase-SiO2 Hybrid Structure
3.1.3. Enzymatic Assays
3.2. Methods
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alarcos, N.; Cohen, B.; Marcin Ziółek, M.; Douhal, A. Photochemistry and Photophysics in Silica-Based Materials: Ultrafast and Single Molecule Spectroscopy Observation. Chem. Rev. 2017, 117, 13639–13720. [Google Scholar] [CrossRef] [PubMed]
- Parvulescu, V.; Anastasescu, C.; Su, B.L. Highly selective oxidation of aromatic hydrocarbons (Styrene, Benzene and Toluene) with H2O2 over Ni, Ni-Cr and Ni-Ru modified MCM-41 catalysts. Stud. Surf. Sci. Catal. 2002, 142, 1213–1220. [Google Scholar]
- Sanchez, C.; Julián, B.; Belleville, P.; Popall, M. Applications of hybrid organic–inorganic nanocomposites. J. Mater. Chem. 2005, 15, 3559–3592. [Google Scholar] [CrossRef]
- Cao, L. Immobilised enzymes: Science or art? Curr. Opin. Chem. Biol. 2005, 9, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.H.; Fang, M.; Tong, D.S.; Shao, P.; Xu, T.N.; Zhou, C.H. Immobilization of Candida rugosa lipase on hexagonal mesoporous silca and selective estrification in nonaqueous medium. Biochem. Eng. J. 2013, 70, 97–105. [Google Scholar] [CrossRef]
- Tran, D.T.; Chen, C.L.; Chang, J.S. Immobilization of Brukholderia sp. lipase on a ferric nanocomposite for biodiesel production. J. Biotechol. 2012, 158, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Anastasescu, C.; Anastasescu, M.; Zaharescu, M.; Balint, I. Platinum-modified SiO2 with tubular morphology as efficient membrane-type microreactors for mineralization of formic acid. J. Nanopart. Res. 2012, 14, 1198. [Google Scholar] [CrossRef]
- Anastasescu, C.; Zaharescu, M.; Balint, I. Unexpected photocatalytic activity of simple and Platinum modified tubular SiO2 for the oxidation of oxalic acid to CO2. Catal. Lett. 2009, 132, 81–86. [Google Scholar] [CrossRef]
- Tran, D.N.; Balkus, K., Jr. Perspective of recent progress in immobilization of enzymes. ACS Catal. 2011, 1, 956–968. [Google Scholar] [CrossRef]
- Tortajada, M.; Ramon, D.; Beltran, D.; Amoros, P. Hierarchical bimodal porous silicas and organosilicas for enzyme immobilization. J. Mater. Chem. 2005, 15, 3859–3868. [Google Scholar] [CrossRef]
- Blanco, R.M.; Terreros, P.; Férnandez-Pérez, M.; Otero, C.; Díaz-González, G. Functionalization of mesoporous silica for lipase immobilization. Characterization of the support and the catalyst. J. Mol. Catal. B Enzym. 2004, 30, 83–93. [Google Scholar] [CrossRef]
- Guisan, J.M.; Sabuquillo, P.P.; Fernandez-Lafuente, R.; Fernandez-Lorente, G.; Mateo, C.; Halling, P.J.; Kennedy, D.; Miyata, E.; Re, D. Preparation of new lipase derivatives with high activity-stability in anhydrous media: Adsorption on hydrophobic supports plus hydrophilization with polyethylenimine. J Mol. Catal. B Enzym. 2001, 11, 817–824. [Google Scholar] [CrossRef]
- Aissaoui, N.; Bergaoui, L.; Boujday, S.; Lambert, J.F.; Méthiever, C.; Landoulsi, J. Enzyme Immobilization on silane-modified surface through short linkers: Fate of interfacial phases and impact on catalytic activity. Langmuir 2014, 30, 4066–4077. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, K.; Phadtare, S.; Vinod, V.P.; Kumar, A.; Rao, M.; Chaudhari, R.V.; Sastry, M. Gold nanoparticles assembled on amine-functionalized Na-Y zeolite: A biocompatible surface for enzyme immobilization. Langmuir 2003, 19, 3858–3863. [Google Scholar] [CrossRef]
- Nakamura, H.; Matsui, Y. Silica gel nanotubes obtained by the sol-gel method. J. Am. Chem. Soc. 1995, 117, 2651–2652. [Google Scholar] [CrossRef]
- Anastasescu, C.; Anastasescu, M.; Teodorescu, V.S.; Gartner, M.; Zaharescu, M. SiO2 nanospheres and tubes obtained by sol gel method. J. Non-Cryst. Solids. 2010, 356, 2634–2640. [Google Scholar] [CrossRef]
- Anastasescu, C.; Zaharescu, M.; Angelescu, D.; Munteanu, C.; Bratan, V.; Spataru, T.; Negrila, C.; Spataru, N.; Balint, I. Defect-related light absorption, photoluminiscence and photocatalytic activity of SiO2 with tubular morphology. Sol. Energy Mater. Sol. Cell 2017, 159, 325–335. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Smit, M.; Dunn, B.; Zink, J.I. Stabilization of creatine kinase encapsulated in silicate sol-gel materials and unusual temperature effects on its activity. Chem. Mater. 2002, 14, 4300–4306. [Google Scholar] [CrossRef]
- Ruscher, C.H.; Bannat, I.; Feldhoff, A.; Ren, L.; Wark, M. SiO2 nanotubes with nanodispersed Pt in the walls. Microporous Mesoporous Mater. 2007, 99, 30–36. [Google Scholar] [CrossRef]
- Anastasescu, C.; Mihaiu, S.; Preda, S.; Zaharescu, M. 1D Oxide Nanostructures Obtained by Sol-Gel and Hydrothermal Methods; Springer: Cham, Switzerland, 2016; ISBN 978-3-319-32988-8. [Google Scholar]
- Lopez, T.; Romero, A.; Gomez, R. Metal-support interaction in Pt/SiO2 catalysts prepared by sol gel method. J. Non-Cryst. Solids 1991, 127, 105–113. [Google Scholar] [CrossRef]
- Bey Temsamani, M.; Maeck, M.; El Hassani, I.; Hurwitz, H.D. Fourier Transform Infrared investigation of water states in Aerosol-OT reverse micelles as a function of counterionic nature. J. Phys. Chem. B 1998, 102, 3335–3340. [Google Scholar] [CrossRef]
- Pickup, D.M.; Mountjoy, G.; Wallidge, G.W.; Anderson, R.; Cole, J.M.; Newport, R.J.; Smith, M.E. A structural study of (TiO2)x(SiO2)1−x (x = 0.18, 0.30 and 0.41) xerogels prepared using acetylacetone. J. Mater. Chem. 1999, 9, 1299–1305. [Google Scholar] [CrossRef]
- Orcel, G.; Phalippou, J.; Hench, L.L. Structural changes of silica xerogels during low temperature dehydration. J. Non-Cryst. Solids 1986, 88, 114–130. [Google Scholar] [CrossRef]
- Simonsen, M.E.; Li, Z.; Søgaard, E.G. Influence of the OH groups on the photocatalytic activity and photoinduced hydrophilicity of microwave assisted sol–gel TiO2 film. Appl. Surf. Sci. 2009, 255, 8054–8062. [Google Scholar] [CrossRef]
- Sun, B.; Sun, S.-Q.; Li, T.; Zhang, W.-Q. Preparation and antibacterial activities of Ag-doped SiO2–TiO2 composite films by liquid phase deposition (LPD) method. J. Mater. Sci. 2007, 42, 10085–10089. [Google Scholar] [CrossRef]
- Gustafsson, H.; Thorn, C.; Holmberg, K. A comparison of lipase and trypsin encapsulated in mesoporous materials with varying pore sizes and pH conditions. Colloids Surf. B Biointerfaces 2011, 87, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Jitianu, A.; Crisan, M.; Meghea, A.; Rau, I.; Zaharescu, M. Influence of the silica based matrix on the formation of iron oxide nanoparticles in the Fe2O3-SiO2 system, obtained by sol–gel method. J. Mater. Chem. 2002, 12, 1401–1407. [Google Scholar] [CrossRef]
- Stuart, B.H. Infrared Spectroscopy: Fundamentals and Applications; John Wiley & Sons, Ltd.: Chichester, West Sussex, UK, 2004; ISBN 9780470854273. [Google Scholar]
- Shikha, S.; Thakur, K.G.; Bhattacharyya, M.S. Facile fabrication of lipase to amine functionalized gold nanoparticles to enhance stability and activity. RSC Adv. 2017, 7, 42845–42855. [Google Scholar] [CrossRef]
- Gustafsson, H.; Johansson, E.M.; Barrabino, A.; Odén, M.; Holmberg, K. Immobilization of lipase from Mucor miehei and Rhizopus oryzae into mesoporous silica—The effect of varied particle size and morphology. Colloids Surf. B Biointerfaces 2012, 100, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.; Byrne, T.; Woelfel, K.J.; Meany, J.E.; Spyridis, G.T.; Pocker, Y. The Hydrolysis of p-nitrophenyl acetate: A versatile reaction to study enzyme kinetics. J. Chem. Educ. 1994, 71, 715. [Google Scholar] [CrossRef]
- Buncel, E.; Cannes, C.; Chatrousse, A.-P.; Terrier, F. Reactions of oximate α-nucleophiles with esters: evidence from solvation effects for substantial decoupling of desolvation and bond formation. J. Am. Chem. Soc. 2002, 124, 8766–8767. [Google Scholar] [CrossRef] [PubMed]
- Umezawa, H.; Aoyagi, T.; Hazato, T.; Uotani, K.; Kojima, F.; Hamada, M.; Tacheuchi, T. Esterastin, an inhibitor of esterase, produced by Actinomycetes. J. Antibiot. 1978, 31, 639–641. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Bromberg, L.; Hatton, T.A.; Rutledge, G.C. Catalytic hydrolysis of p-nitrophenyl acetate by electrospun polyacrylamidoxime nanofibers. Polymer 2007, 48, 4675–4682. [Google Scholar] [CrossRef]
- O’Brien, R.W.; Ward, D.N. The electrophoresis of a spheroid with a thin double layer. J. Colloid Interface Sci. 1988, 121, 402–413. [Google Scholar] [CrossRef]
Sample Availability: Samples are available from the authors at their location. |


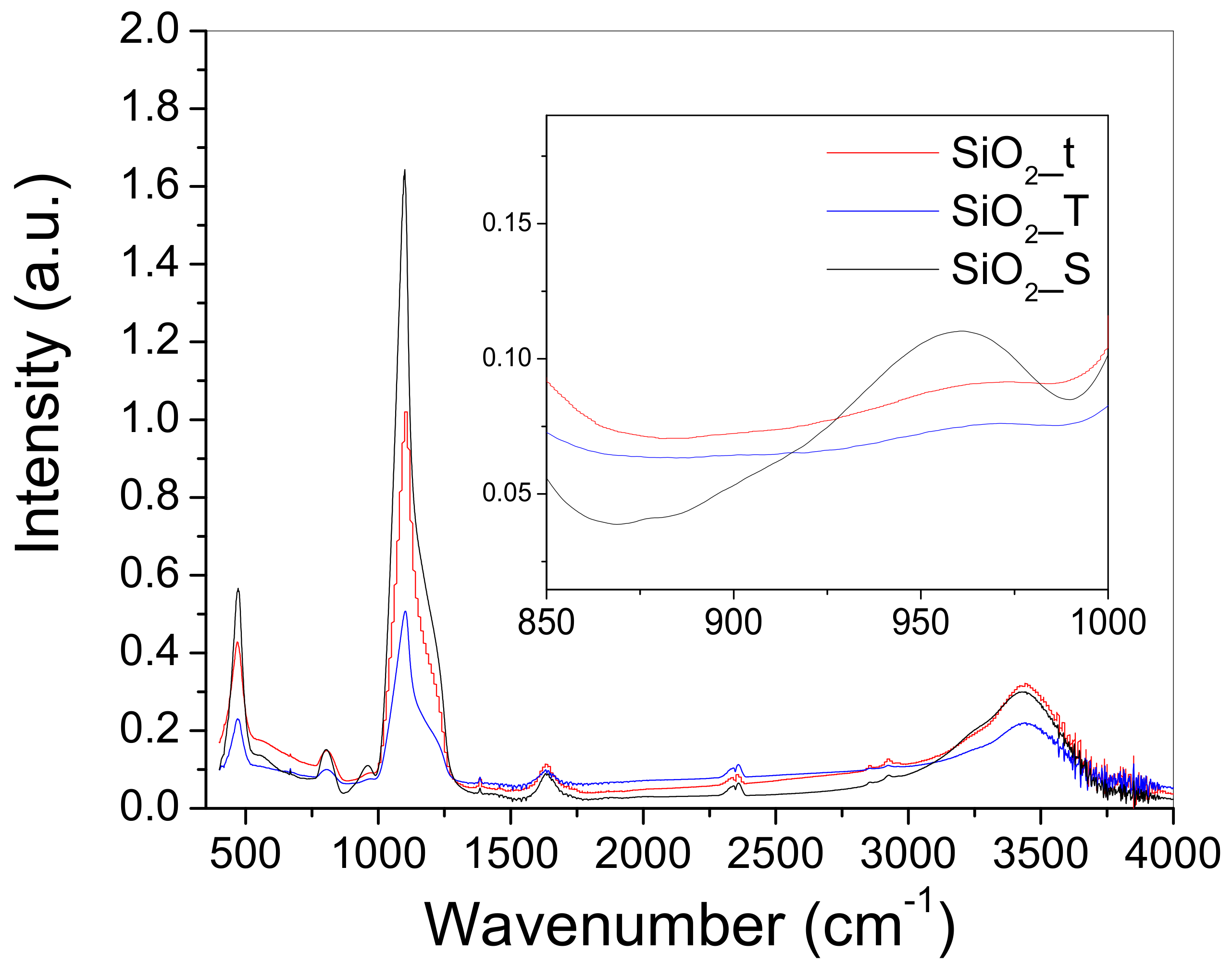




| Wavenumber (cm−1) | Assignation |
|---|---|
| 1124 | Si-O stretching in SiO2 [16] |
| ~1200 (shoulder) | asymmetric vibration of Si-O-Si [19] |
| ~980–960 (shoulder) | silanol groups (Si-OH) [21] |
| 847 | νs(Si-O-Si) [20] |
| 484 | δ(Si-O-Si) [20] |
| 1650 | H2O [20,28] |
| 3350–3600 | structural hydroxyls and free OH groups [22,28] |
| Identified Band (cm−1) | Assignment |
|---|---|
| 3271 | N-H (γ) |
| 2930 | C-H (γ) |
| 1635 | Amide I, N-H def., (δ) |
| 1521 | Amide II, N-H stretch., (γ) |
| 1245 | Amide III, C-N stretch., (γ) |
| 1060 | C-O-C (γ) |
| 1395 | C-OH bending |
| 650–600 | C-C bending |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anastasescu, C.; Preda, S.; Rusu, A.; Culita, D.; Plavan, G.; Strungaru, S.; Calderon-Moreno, J.M.; Munteanu, C.; Gifu, C.; Enache, M.; et al. Tubular and Spherical SiO2 Obtained by Sol Gel Method for Lipase Immobilization and Enzymatic Activity. Molecules 2018, 23, 1362. https://doi.org/10.3390/molecules23061362
Anastasescu C, Preda S, Rusu A, Culita D, Plavan G, Strungaru S, Calderon-Moreno JM, Munteanu C, Gifu C, Enache M, et al. Tubular and Spherical SiO2 Obtained by Sol Gel Method for Lipase Immobilization and Enzymatic Activity. Molecules. 2018; 23(6):1362. https://doi.org/10.3390/molecules23061362
Chicago/Turabian StyleAnastasescu, Crina, Silviu Preda, Adriana Rusu, Dana Culita, Gabriel Plavan, Stefan Strungaru, Jose Maria Calderon-Moreno, Cornel Munteanu, Catalina Gifu, Mirela Enache, and et al. 2018. "Tubular and Spherical SiO2 Obtained by Sol Gel Method for Lipase Immobilization and Enzymatic Activity" Molecules 23, no. 6: 1362. https://doi.org/10.3390/molecules23061362
APA StyleAnastasescu, C., Preda, S., Rusu, A., Culita, D., Plavan, G., Strungaru, S., Calderon-Moreno, J. M., Munteanu, C., Gifu, C., Enache, M., Socoteanu, R., Angelescu, D., Anastasescu, M., Gartner, M., Balint, I., & Zaharescu, M. (2018). Tubular and Spherical SiO2 Obtained by Sol Gel Method for Lipase Immobilization and Enzymatic Activity. Molecules, 23(6), 1362. https://doi.org/10.3390/molecules23061362










