Synthesis, Antiviral and Cytotoxic Activity of Novel Terpenyl Hybrid Molecules Prepared by Click Chemistry
Abstract
1. Introduction
2. Results and Discussion
2.1. Cytotoxicity Assay
2.2. Antiviral Activity Screening
3. Experimental
3.1. General Procedures
3.2. Preparation of Derivatives
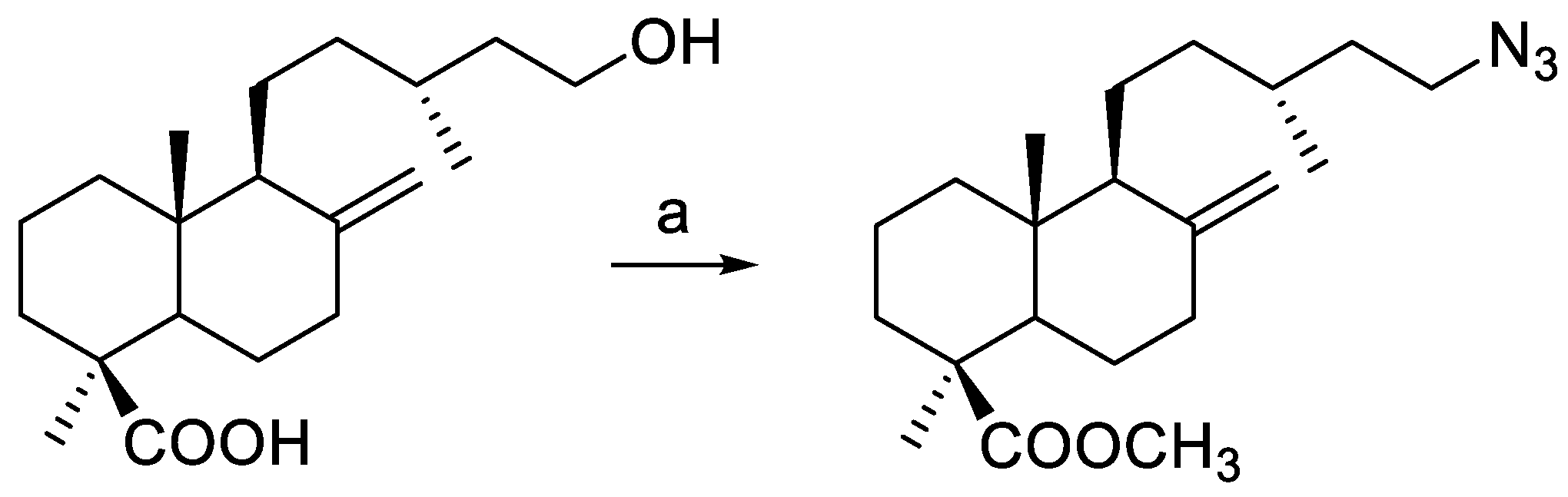
3.2.1. Starting Compounds
3.2.2. Preparation of Terpene-Alkynyl Esters
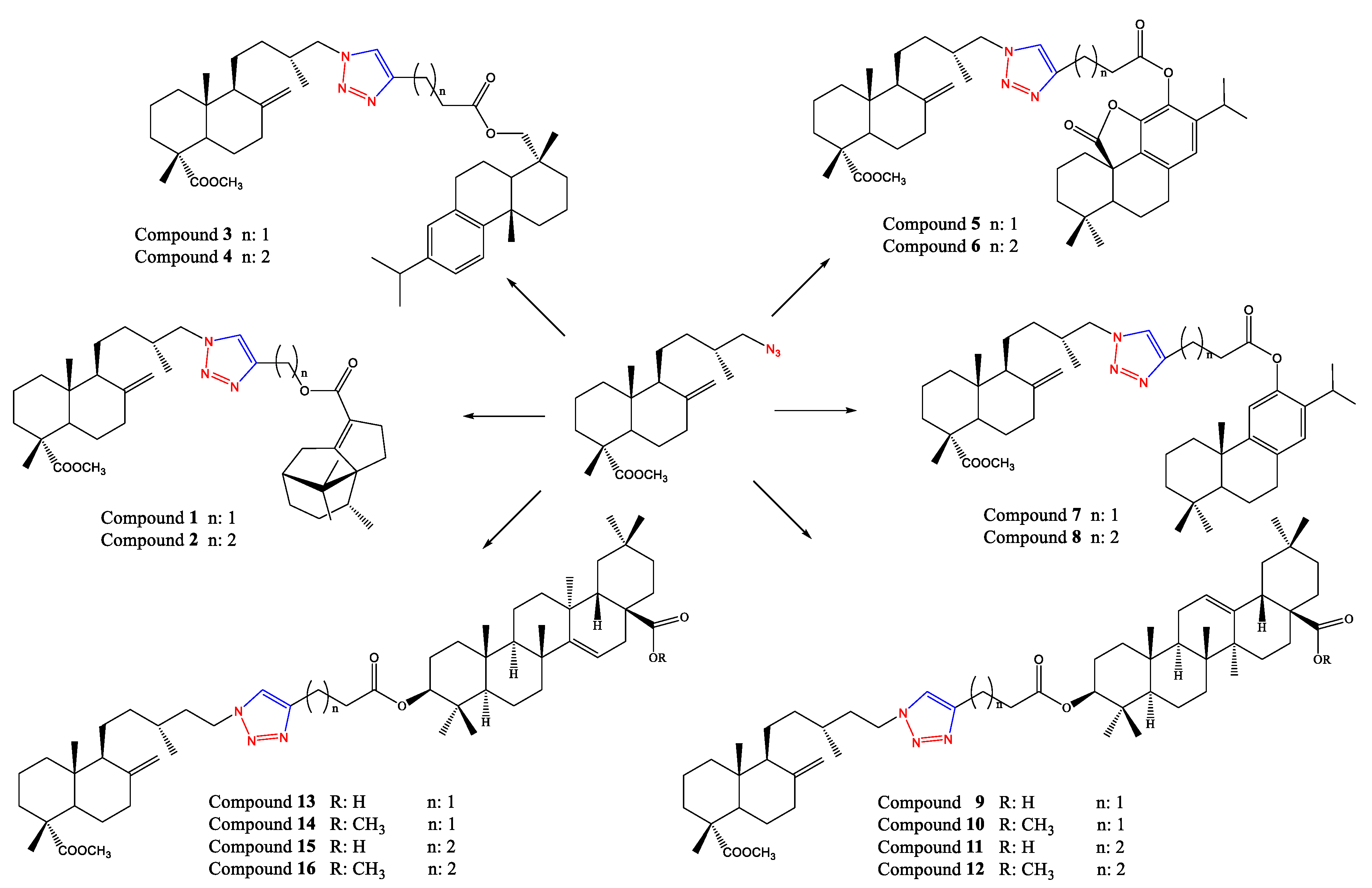
3.2.3. Synthesis of Hybrid Derivatives 1–16
3.3. Treatment Solutions
3.4. Cells and Viruses
3.5. Cytotoxicity Assay
3.6. Cytopathic Effect Assay
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Arthanari, S.; Vanitha, J.; Krishnaswami, V. In vitro antiviral and cytotoxic screening of methanolic extract of Cassia auriculata flowers in HeLa, Vero, CRFK and HEL cell lines. Drug Invent. Today 2013, 5, 28–31. [Google Scholar] [CrossRef]
- Looker, K.J.; Magaret, A.S.; May, M.T.; Turner, K.M.E.; Vickerman, P.; Gottlieb, S.L.; Newman, L.M. Global and regional estimates of prevalent and incident Herpes Simplex Virus type 1 infections in 2012. PLoS ONE 2015, 10, e0140765. [Google Scholar] [CrossRef] [PubMed]
- Medini, F.; Legault, J.; Pichette, A.; Abdelly, C.; Ksouri, R. Antiviral efficacy of Limonium densiflorum against HSV-1 and influenza viruses. S. Afr. J. Bot. 2014, 92, 65–72. [Google Scholar] [CrossRef]
- Agostini, S.; Mancuso, R.; Baglio, F.; Cabinio, M.; Hernis, A.; Saul, A.; Calabrese, E.; Nemni, R.; Clerici, M. Brain, Behavior, and Immunity High avidity HSV-1 antibodies correlate with absence of amnestic mild cognitive impairment conversion to Alzheimer’s disease. Brain Behav. Immun. 2016, 58, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Stránská, R.; Schuurman, R.; Nienhuis, E.; Goedegebuure, I.W.; Polman, M.; Weel, J.F.; Dillen, P.M.W.; Berkhout, R.J.M.; Van Loon, A.M. Survey of acyclovir-resistant herpes simplex virus in The Netherlands: Prevalence and characterization. J. Clin. Virol. 2005, 32, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-D.; Zhang, G.-J.; Qu, J.; Li, Y.-H.; Jiang, J.-D.; Liu, Y.-B.; Ma, S.-G.; Li, Y.; Lv, H.-N.; Yu, S.-S. Diterpenoids and sesquiterpenoids from the roots of Illicium majus. J. Nat. Prod. 2013, 76, 1976–1983. [Google Scholar] [CrossRef] [PubMed]
- González, M.A.; Zaragozá, R.J. Semisynthesis of the antiviral abietane diterpenoid Jiadifenoic acid C from Callitrisic acid (4-Epidehydroabietic acid) isolated from sandarac resin. J. Nat. Prod. 2014, 77, 2114–2117. [Google Scholar] [CrossRef] [PubMed]
- Arnó, M.; Betancur-Galvis, L.; Bueno-Sanchez, J.G.; González, M.A.; Zaragozá, R.J. Synthesis and antiviral activity of scopadulcic acids analogues. Tetrahedron 2003, 59, 6455–6464. [Google Scholar] [CrossRef]
- Oliveira, C.S.; Salvador, M.J.; de Carvalho, J.E.; Santos, É.P.; Barison, A.; Stefanello, M.É.A. Cytotoxic abietane-derivative diterpenoids of Salvia lachnostachys. Phytochem. Lett. 2016, 17, 140–143. [Google Scholar] [CrossRef]
- Vila-Luna, S.E.; Moo-Puc, R.E.; Torres-Tapia, L.W.; Peraza-Sánchez, S.R. New metabolites with cytotoxic and antiproliferative activities isolated from Bonellia macrocarpa. Phytochem. Lett. 2017, 19, 121–125. [Google Scholar] [CrossRef]
- Pertino, M.; Schmeda-Hirschmann, G.; Rodríguez, J.A.; Theoduloz, C. Gastroprotective effect and cytotoxicity of terpenes from the Paraguayan crude drug “yagua rova” (Jatropha isabelli). J. Ethnopharmacol. 2007, 111, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Pertino, M.; Rodríguez, J.A.; Theoduloz, C.; Razmilic, I.; Schmeda-Hirschmann, G. Gastroprotective activity and cytotoxic effect of cyperenoic acid derivatives. J. Pharm. Pharmacol. 2006, 58, 1507–1513. [Google Scholar] [CrossRef] [PubMed]
- Pertino, M.; Theoduloz, C.; Butassi, E.; Zacchino, S.; Schmeda-Hirschmann, G. Synthesis, antiproliferative and antifungal activities of 1,2,3-triazole-substituted carnosic acid and carnosol derivatives. Molecules 2015, 20, 8666–8686. [Google Scholar] [CrossRef] [PubMed]
- Pertino, M.; Schmeda-Hirschmann, G.; Rodríguez, J.A.; Theoduloz, C. Gastroprotective effect and cytotoxicity of semisynthetic jatropholone derivatives. Planta Med. 2007, 73, 1095–1100. [Google Scholar] [CrossRef] [PubMed]
- Theoduloz, C.; Rodríguez, J.A.; Pertino, M.; Schmeda-Hirschmann, G. Antiproliferative activity of the diterpenes jatrophone and jatropholone and their derivatives. Planta Med. 2009, 75, 1520–1522. [Google Scholar] [CrossRef] [PubMed]
- Pertino, M.W.; Theoduloz, C.; Bastías, M.; Schmeda-Hirschmann, G. Dimeric labdane diterpenes: Synthesis and antiproliferative effects. Molecules 2013, 18, 5936–5953. [Google Scholar] [CrossRef] [PubMed]
- Pertino, M.W.; Theoduloz, C.; Palenzuela, J.A.; Afonso, M.; Yesilada, E.; Monsalve, F.; González, P.; Droguett, D.; Schmeda-hirschmann, G. Synthesis and pharmacological activity of diterpenylnaphthoquinone derivatives. Molecules 2011, 16, 8614–8628. [Google Scholar] [CrossRef] [PubMed]
- Viegas-Junior, C.; Danuello, A.; Bolzani, S.; Barreiro, E.J.; Alberto, C.; Fraga, M. Molecular hybridization: A useful tool in the design of new drug prototypes. Curr. Med. Chem. 2007, 14, 1829–1852. [Google Scholar] [CrossRef] [PubMed]
- Shaveta, M.S.; Singh, P. Hybrid molecules: The privileged scaffolds for various pharmaceuticals. Eur. J. Med. Chem. 2016, 124, 500–536. [Google Scholar] [CrossRef] [PubMed]
- Belluti, F.; Fontana, G.; Bo, L.D.; Carenini, N.; Giommarelli, C.; Zunino, F. Design, synthesis and anticancer activities of stilbene-coumarin hybrid compounds: Identification of novel proapoptotic agents. Bioorganic Med. Chem. 2010, 18, 3543–3550. [Google Scholar] [CrossRef] [PubMed]
- Sashidhara, K.V.; Kumar, M.; Modukuri, R.K.; Sonkar, R.; Bhatia, G.; Khanna, A.K.; Rai, S.; Shukla, R. Synthesis and anti-inflammatory activity of novel biscoumarin–chalcone hybrids. Bioorg. Med. Chem. Lett. 2011, 21, 4480–4484. [Google Scholar] [CrossRef] [PubMed]
- Sum, T.H.; Sum, T.J.; Galloway, W.R.J.D.; Collins, S.; Twigg, D.G.; Hollfelder, F.; Spring, D.R. Combinatorial synthesis of structurally diverse triazole-bridged flavonoid dimers and trimers. Molecules 2016, 21, 1230. [Google Scholar] [CrossRef] [PubMed]
- Tron, G.C.; Pirali, T.; Billington, R.A.; Canonico, P.L.; Sorba, G.; Genazzani, A.A. Click chemistry reactions in medicinal chemistry: Applications of the 1,3-dipolar cycloaddition between azides and alkynes. Med. Res. Rev. 2008, 28, 278–308. [Google Scholar] [CrossRef] [PubMed]
- Kategaonkar, A.H.; Shinde, P.V.; Kategaonkar, A.H.; Pasale, S.K.; Shingate, B.B.; Shingare, M.S. Synthesis and biological evaluation of new 2-chloro-3-((4-phenyl-1H-1,2,3-triazol-1-yl)methyl)quinoline derivatives via click chemistry approach. Eur. J. Med. Chem. 2010, 45, 3142–3146. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekhar, M.; Nayak, V.L.; Ramakrishna, S.; Mallavadhani, U.V. Novel triazole hybrids of myrrhanone C, a natural polypodane triterpene: Synthesis, cytotoxic activity and cell based studies. Eur. J. Med. Chem. 2016, 114, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Anand, A.; Naik, R.J.; Revankar, H.M.; Kulkarni, M.V.; Dixit, S.R.; Joshi, S.D. A click chemistry approach for the synthesis of mono and bis aryloxy linked coumarinyl triazoles as anti-tubercular agents. Eur. J. Med. Chem. 2015, 105, 194–207. [Google Scholar] [CrossRef] [PubMed]
- Pete, U.D.; Zade, C.M.; Bhosale, J.D.; Tupe, S.G.; Chaudhary, P.M.; Dikundwar, A.G.; Bendre, R.S. Hybrid molecules of carvacrol and benzoyl urea/thiourea with potential applications in agriculture and medicine. Bioorganic Med. Chem. Lett. 2012, 22, 5550–5554. [Google Scholar] [CrossRef] [PubMed]
- Schmeda-Hirschmann, G.; Pertino, M.W.; Rodriguez, J.A.; Monsalve, F.; Droguett, D.; Theoduloz, C. Synthesis, gastroprotective effect and cytotoxicity of new amino acid diterpene monoamides and diamides. Molecules 2010, 15, 7378–7394. [Google Scholar] [CrossRef] [PubMed]
- Marenin, K.S.; Gatilov, Y.V.; Agafontsev, A.M.; Tkachev, A.V. Stereoselectivity of formation of monoterpene—Amino acids hybrid molecules in the reaction of monoterpene nitroso chlorides with α-amino acid derivatives. Steroids 2017, 117, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Theoduloz, C.; Bravo, I.; Pertino, M.; Valenzuela, D.; Schmeda-Hirschmann, G. Potential gastroprotective effect of novel cyperenoic acid/quinone derivatives in human cell cultures. Planta Med 2012, 78, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Theoduloz, C.; Delporte, C.; Valenzuela-Barra, G.; Silva, X.; Cádiz, S.; Bustamante, F.; Pertino, M.W.; Schmeda-Hirschmann, G. Topical anti-inflammatory activity of new hybrid molecules of terpenes and synthetic drugs. Molecules 2015, 20, 11219–11235. [Google Scholar] [CrossRef] [PubMed]
- Pawełczyk, A.; Olender, D.; Sowa-Kasprzak, K.; Zaprutko, L. Hybrid compounds strategy in the synthesis of oleanolic acid skeleton-NSAID derivatives. Molecules 2016, 21, 420. [Google Scholar] [CrossRef] [PubMed]
- Pertino, M.W.; Verdugo, V.; Theoduloz, C.; Schmeda-hirschmann, G. Synthesis and antiproliferative activity of some novel triazole derivatives from dehydroabietic acid. Molecules 2014, 19, 2523–2535. [Google Scholar] [CrossRef] [PubMed]
- Pertino, M.W.; Lopez, C.; Theoduloz, C.; Schmeda-hirschmann, G. 1,2,3-Triazole-substituted oleanolic acid derivatives: Synthesis and antiproliferative activity. Molecules 2013, 18, 7661–7674. [Google Scholar] [CrossRef] [PubMed]
- Pertino, M.W.; Theoduloz, C.; Rodríguez, J.A.; Yáñez, T.; Lazo, V.; Schmeda-Hirschmann, G. Gastroprotective effect of carnosic acid gamma-lactone derivatives. J. Nat. Prod. 2010, 73, 639–643. [Google Scholar] [CrossRef] [PubMed]
- Areche, C.; Rodríguez, J.A.; Razmilic, I.; Yáñez, T.; Theoduloz, C.; Schmeda-Hirschmann, G. Gastroprotective and cytotoxic effect of semisynthetic ferruginol derivatives. Pharm. Pharmacol. 2006, 59, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.; Theoduloz, C.; Schmeda-Hirschmann, G.; Razmilic, I.; Yáñez, T.; Rodríguez, J.A. Gastroprotective and ulcer-healing activity of oleanolic acid derivatives: In vitro-in vivo relationships. Life Sci. 2006, 79, 1349–1356. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1–16 are available from the authors. |
| Compound | CC50 (μM) | |||
|---|---|---|---|---|
| Vero | HEP-2 | C6 | Raw 264.7 | |
| 1 | 623.9 ± 5 | 229.7 ± 1.8 | >1000 | >1000 |
| 2 | 216.2 ± 2 | 48.1 ± 1.1 | 354.9 ± 2.5 | 300 ± 1.9 |
| 3 | 667.5 ± 4.2 | 411.8 ± 2.1 | >1000 | >1000 |
| 4 | >1000 | >1000 | >1000 | >1000 |
| 7 | 680 ± 4.8 | 700 ± 5.8 | 552.5 ± 4 | 552.5 ± 3.9 |
| 8 | >1000 | >1000 | >1000 | >1000 |
| Compound | CC50 a (μM) | EC50 (μM) | SI | ||||
|---|---|---|---|---|---|---|---|
| KOS | Field | B2006 | KOS | Field | B2006 | ||
| 2 | 216.2 ± 2 | 118.4 ± 2.3 | 157.9 ± 1.9 | 226.3 ± 2.1 | 1.8 | 1.3 | 0.9 |
| 3 | 667.5 ± 4.2 | 107.9 ± 1.2 | 140.2 ± 1.2 | 122.1 ± 1.2 | 6.2 | 4.76 | 5.5 |
| 4 | >1000 | 109 ± 1.0 | 137.5 ± 1.9 | 110 ± 1.0 | >9.2 | >7.2 | >9 |
| 5 | >1000 | 105.1 ± 1.1 | 140.3 ± 2.1 | 112.1 ± 1.1 | >9.5 | >7.1 | >8.9 |
| 6 | >1000 | 96.2 ± 1.2 | 137.8 ± 1.4 | 120 ± 1.5 | >10.4 | >7.3 | >8.3 |
| 8 | >1000 | 99.3 ± 1.5 | 132.4 ± 1.1 | 105.9 ± 1.9 | >10 | >7.6 | >9.4 |
| ACV b | >1000 | 8.2 ± 1.0 | 73 ± 1.0 | 50 ± 2.0 | >121.9 | >13.7 | >20 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pertino, M.W.; Petrera, E.; Alché, L.E.; Schmeda-Hirschmann, G. Synthesis, Antiviral and Cytotoxic Activity of Novel Terpenyl Hybrid Molecules Prepared by Click Chemistry. Molecules 2018, 23, 1343. https://doi.org/10.3390/molecules23061343
Pertino MW, Petrera E, Alché LE, Schmeda-Hirschmann G. Synthesis, Antiviral and Cytotoxic Activity of Novel Terpenyl Hybrid Molecules Prepared by Click Chemistry. Molecules. 2018; 23(6):1343. https://doi.org/10.3390/molecules23061343
Chicago/Turabian StylePertino, Mariano Walter, Erina Petrera, Laura Edith Alché, and Guillermo Schmeda-Hirschmann. 2018. "Synthesis, Antiviral and Cytotoxic Activity of Novel Terpenyl Hybrid Molecules Prepared by Click Chemistry" Molecules 23, no. 6: 1343. https://doi.org/10.3390/molecules23061343
APA StylePertino, M. W., Petrera, E., Alché, L. E., & Schmeda-Hirschmann, G. (2018). Synthesis, Antiviral and Cytotoxic Activity of Novel Terpenyl Hybrid Molecules Prepared by Click Chemistry. Molecules, 23(6), 1343. https://doi.org/10.3390/molecules23061343






