Efficient Manipulation of Continuous AFI-Type Aluminophosphate Membranes with Distinctive Microstructures on Macroporous α-Al2O3 Substrates
Abstract
1. Introduction
2. Results
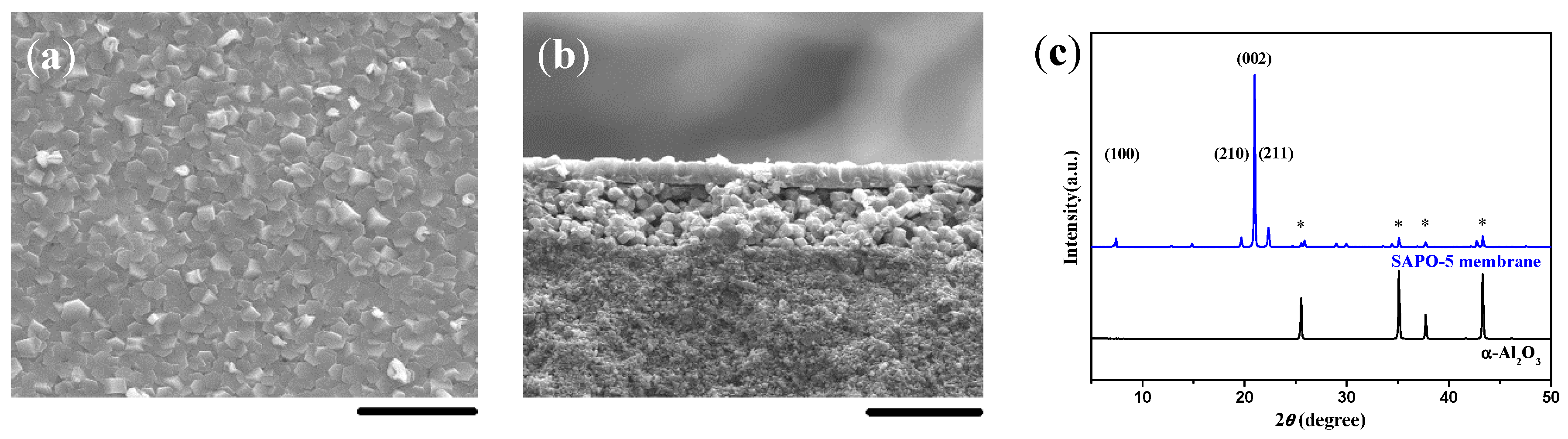
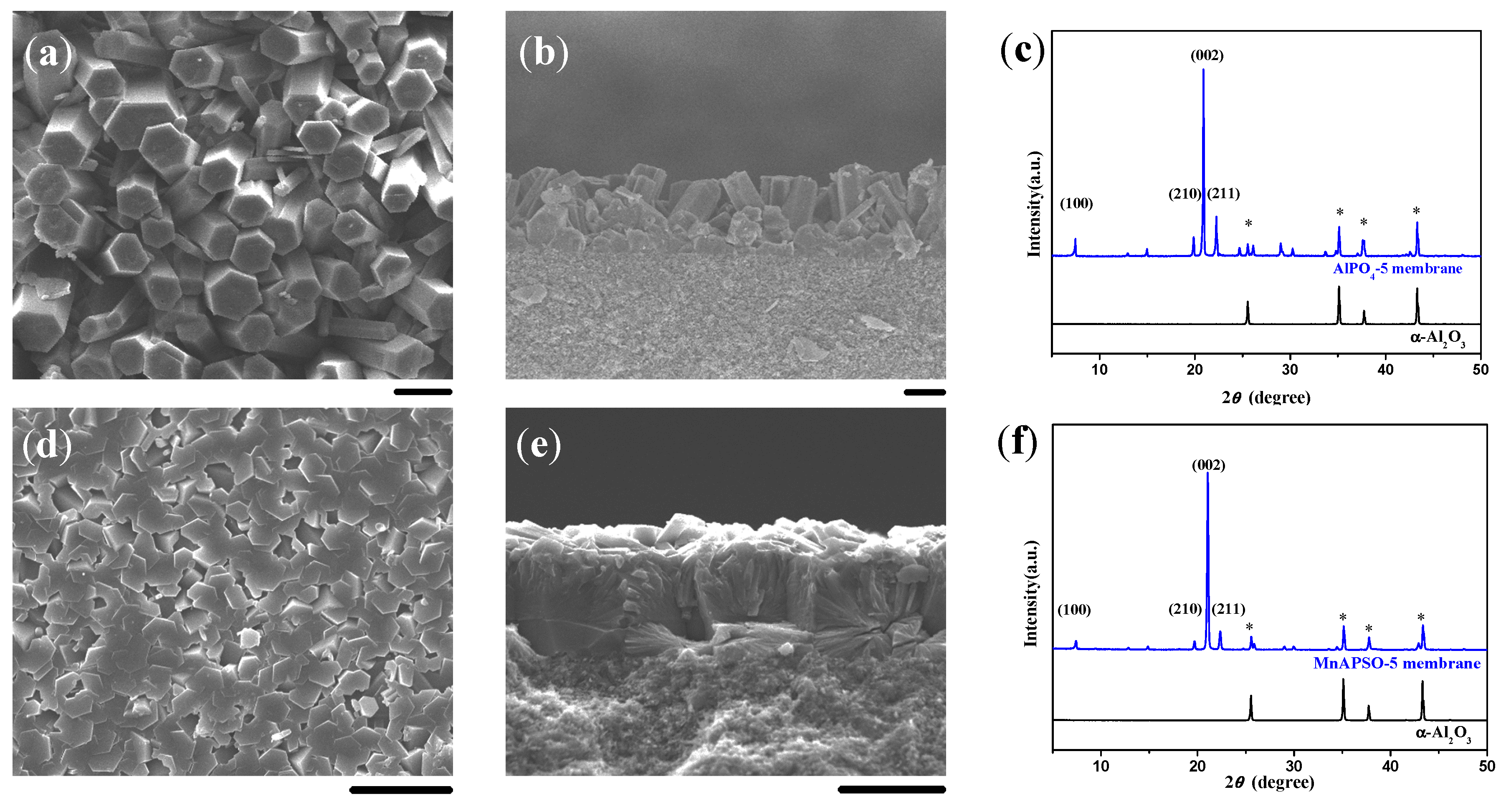
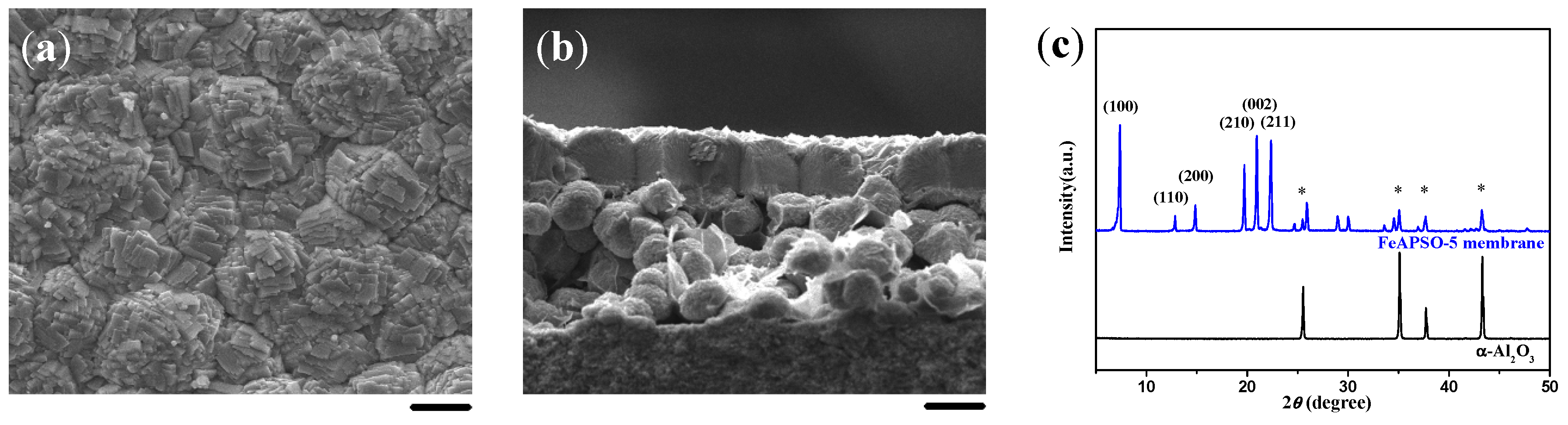
2.1. Double-Layer and c-Oriented AFI Membranes
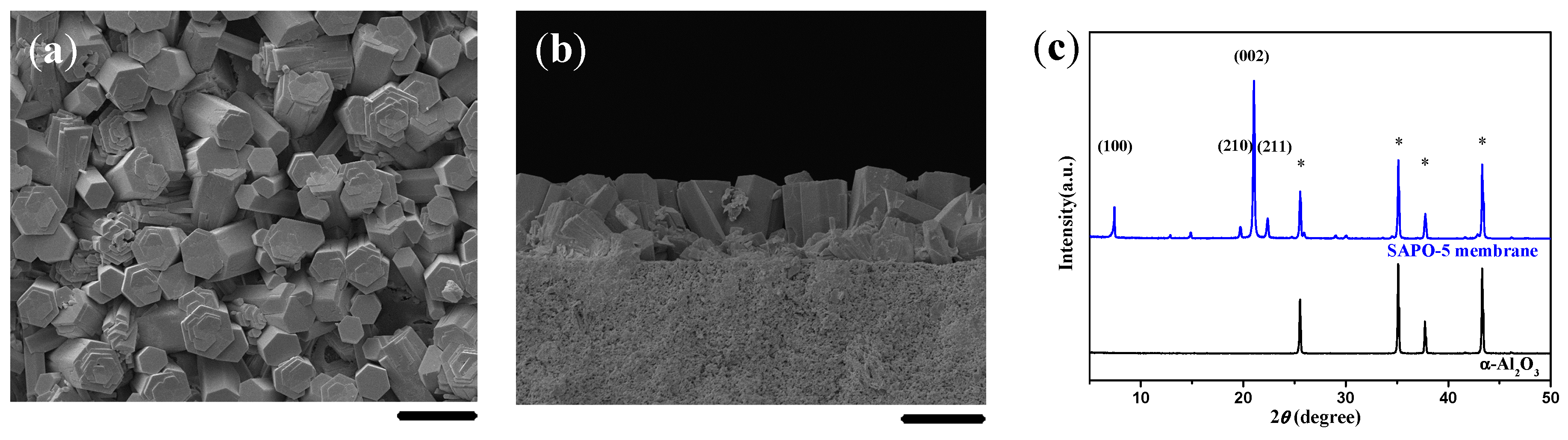
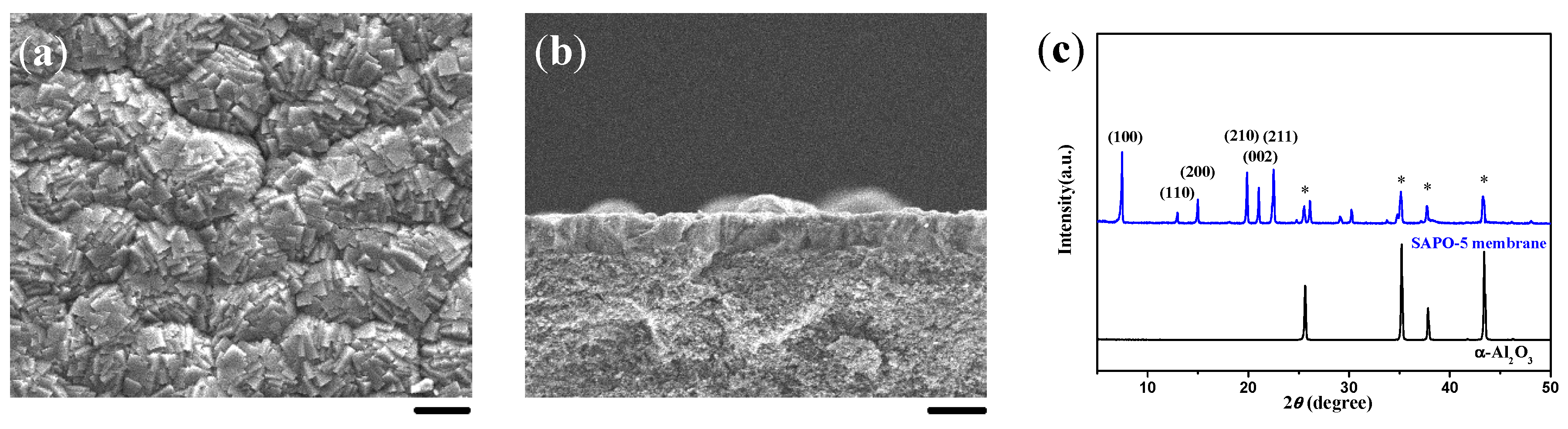
2.2. Single-Layer and c-Oriented AFI Membranes
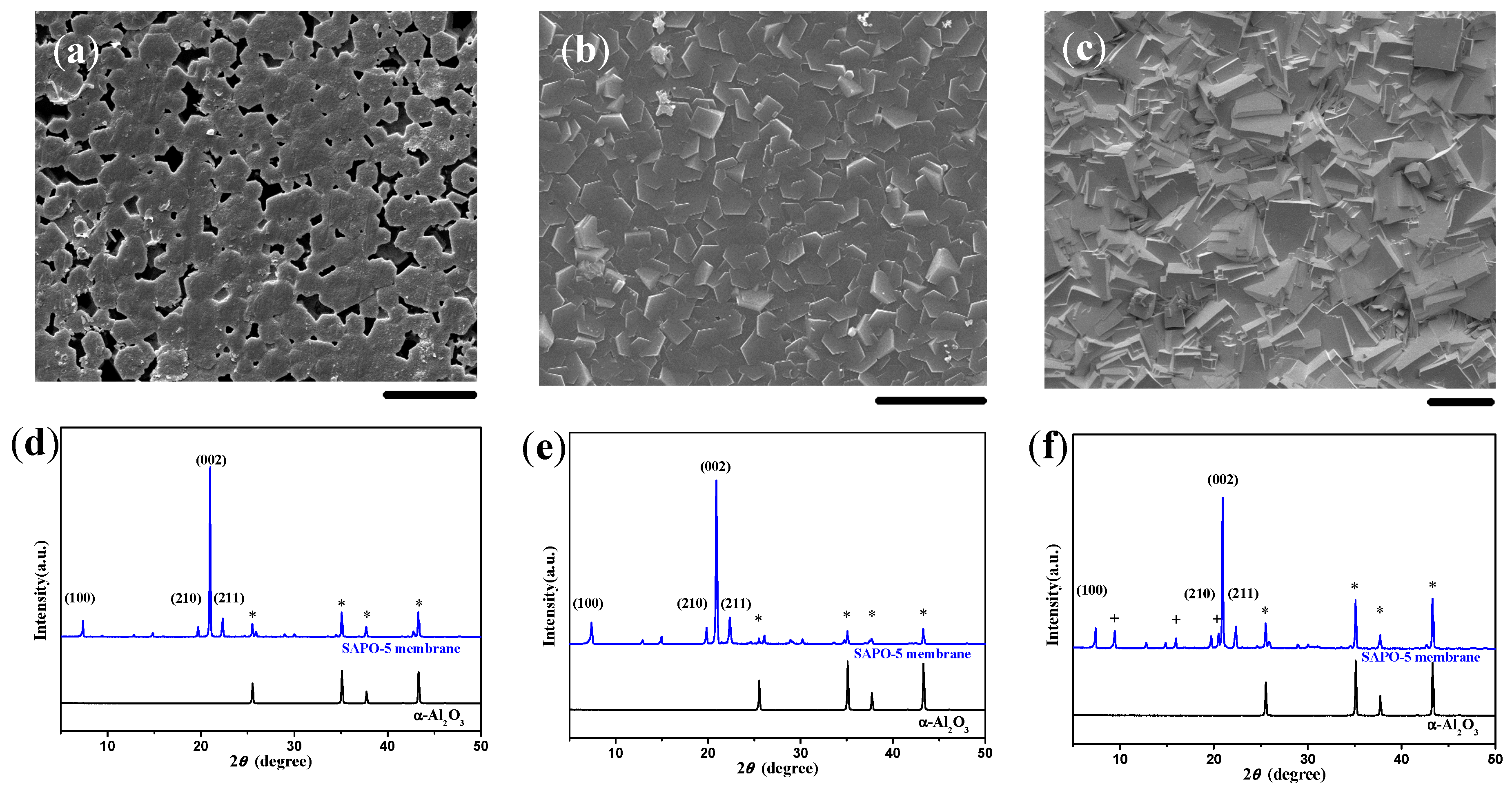
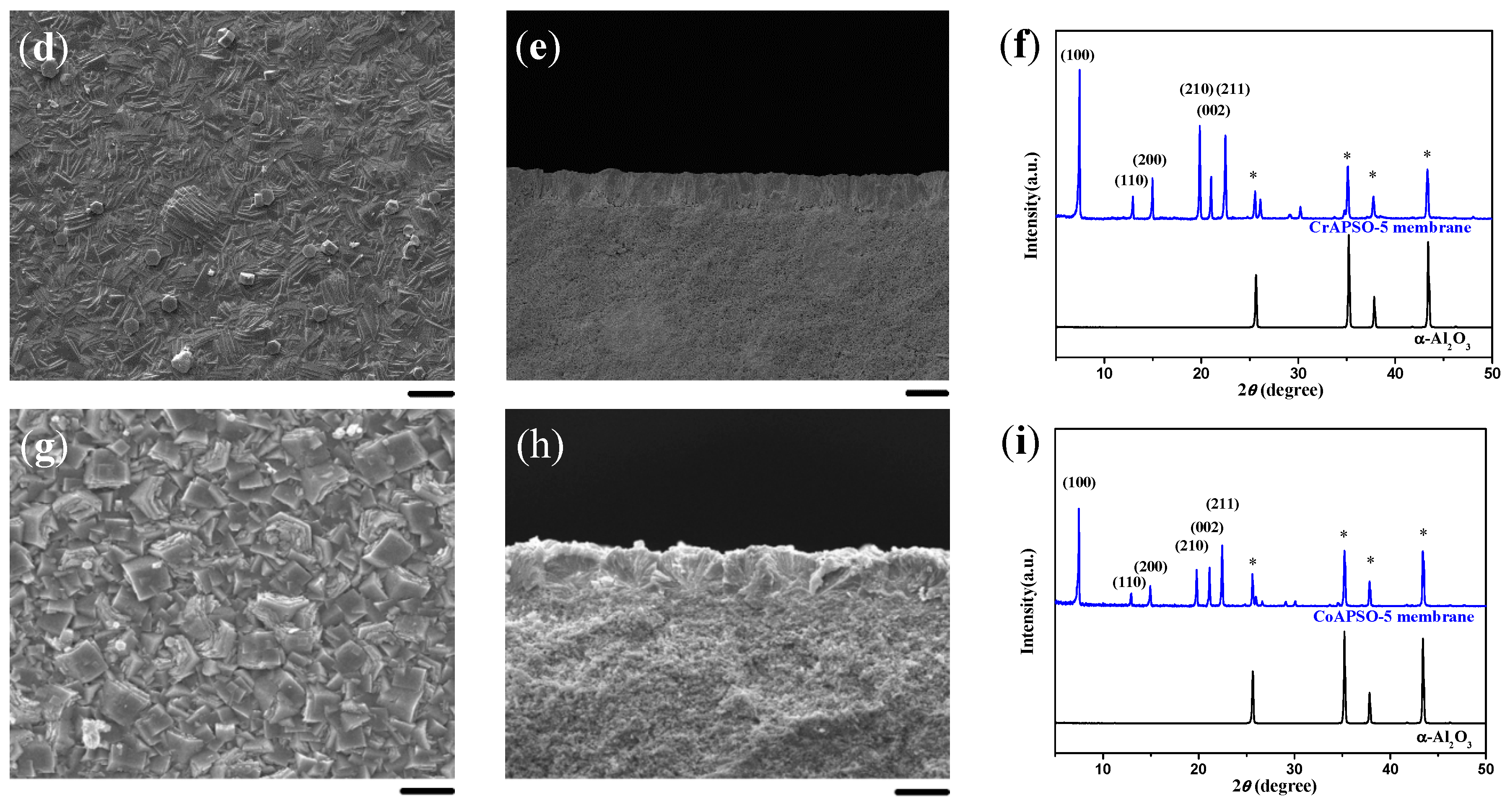
2.3. Single-Layered AFI Membranes of Crystal Agglomerates
2.4. Double-Layered AFI Membranes of Crystal Agglomerates
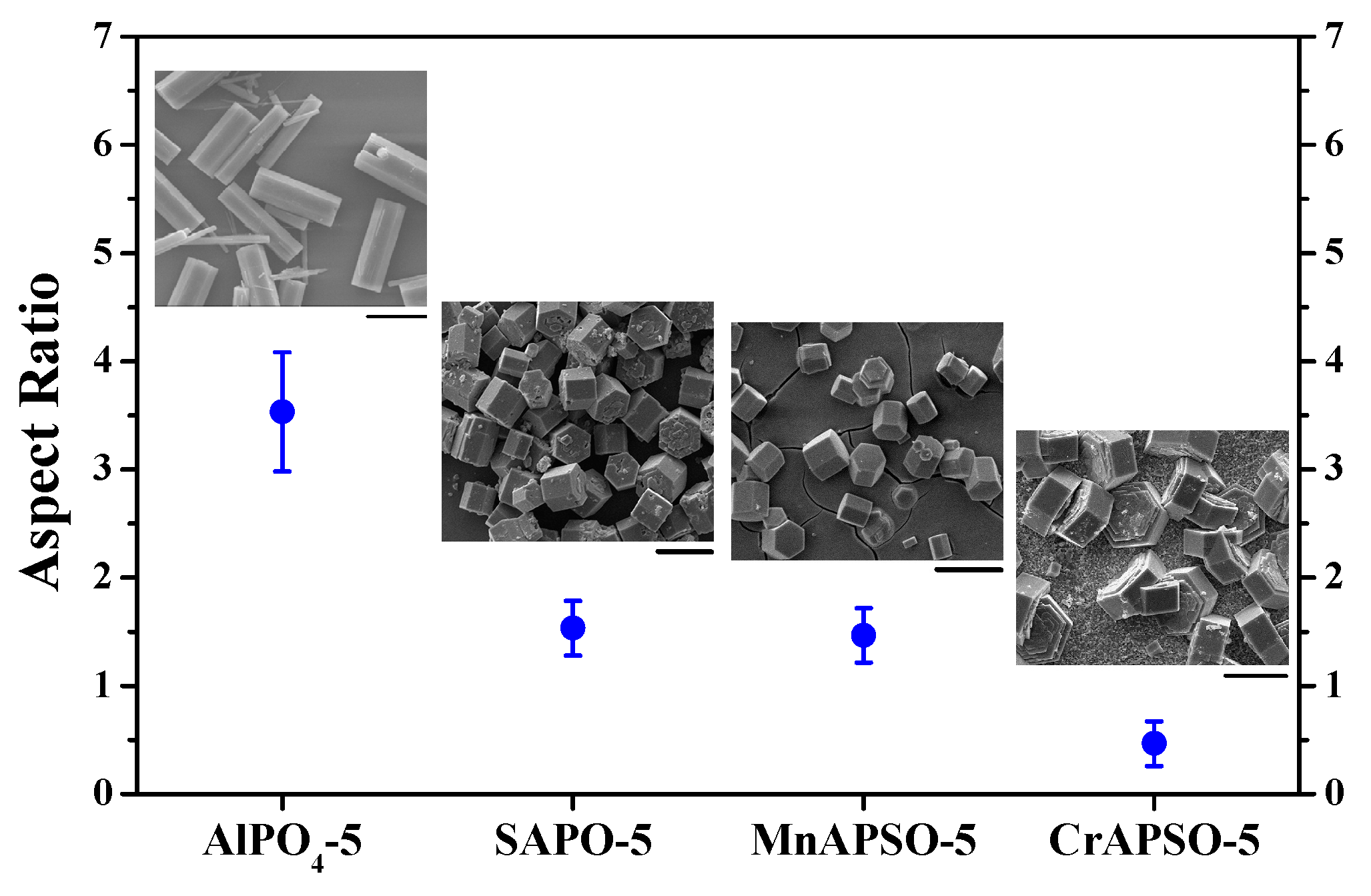
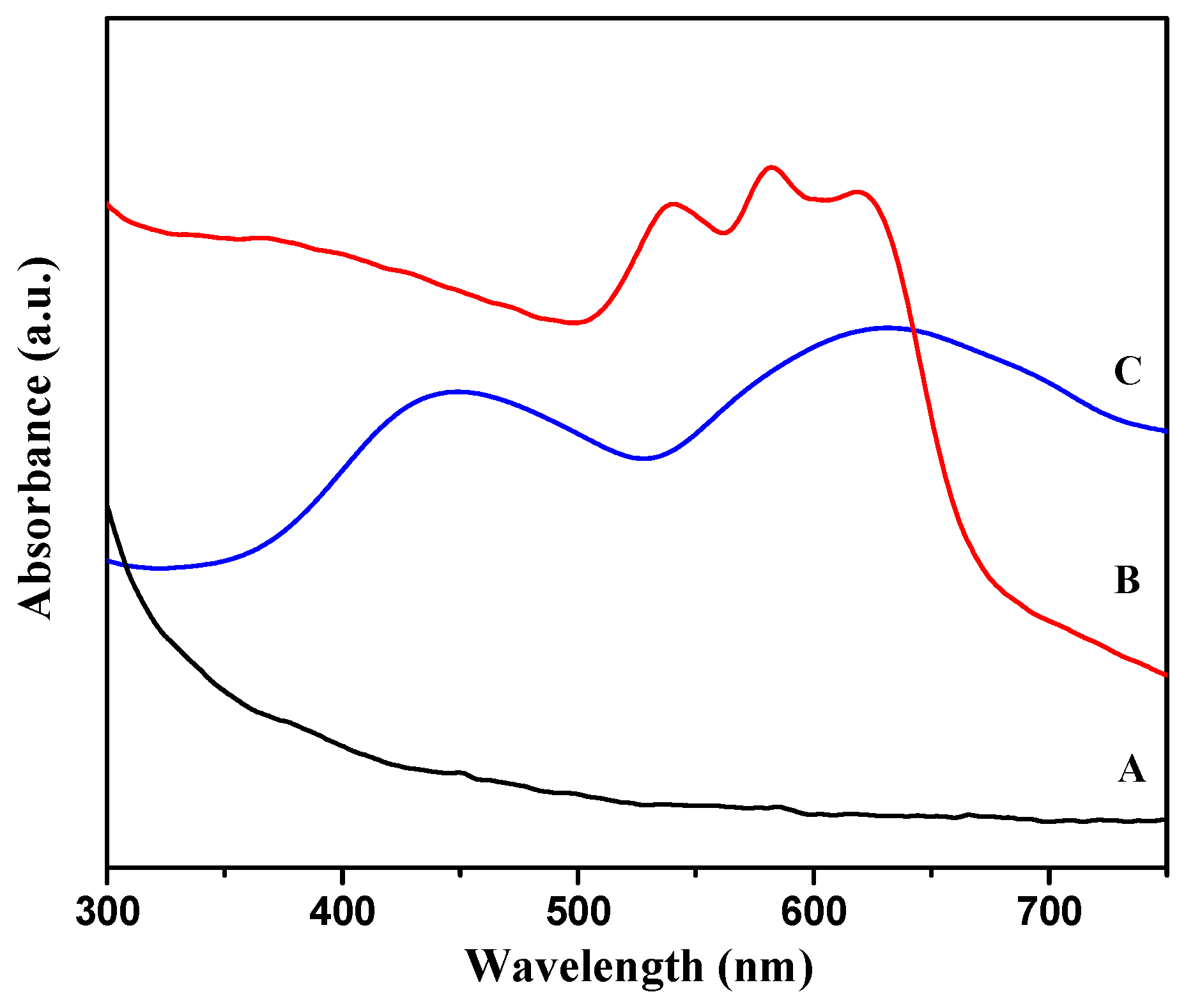
3. Formation Mechanism
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cundy, C.S.; Cox, P.A. The Hydrothermal Synthesis of Zeolites: History and Development from the Earliest Days to the Present Time. Chem. Rev. 2003, 103, 663–702. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.T.; Lok, B.M.; Messina, C.A.; Cannan, T.R.; Flanigen, E.M. Aluminophosphate Molecular Sieves: A New Class of Microporous Crystalline Inorganic Solids. J. Am. Chem. Soc. 1982, 104, 1146–1147. [Google Scholar] [CrossRef]
- Lok, B.M.; Messina, C.A.; Patton, R.L.; Gajek, R.T.; Cannan, T.R.; Flanigen, E.M. Silicoaluminophosphate Molecular Sieves: Another New Class of Microporous Crystalline Inorganic Solids. J. Am. Chem. Soc. 1984, 106, 6092–6093. [Google Scholar] [CrossRef]
- Flanigen, E.M.; Lok, B.M.; Patton, R.L.; Wilson, S.T. Aluminophosphate Molecular Sieves and the Periodic Table. Pure Appl. Chem. 1986, 58, 1351–1358. [Google Scholar] [CrossRef][Green Version]
- Davis, M.E. Ordered Porous Materials for Emerging Applications. Nature 2002, 417, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; Zhang, B.Q.; Liu, X.F. Synthesis and Characterization of SAPO-5 Membranes on Porous α-Al2O3 Substrates. Microporous Mesoporous Mater. 2009, 117, 391–394. [Google Scholar] [CrossRef]
- Jiang, H.Y.; Zhang, B.Q.; Lin, Y.S.; Li, Y.D. Synthesis of Zeolite Membranes. Chin. Sci. Bull. 2004, 49, 2547–2554. [Google Scholar] [CrossRef]
- Caro, J.; Noack, M. Zeolite Membranes-Recent Developments and Progress. Microporous Mesoporous Mater. 2008, 115, 215–233. [Google Scholar] [CrossRef]
- Yu, M.; Noble, R.D.; Falconer, J.L. Zeolite Membranes: Microstructure Characterization and Permeation Mechanisms. Acc. Chem. Res. 2011, 44, 1196–1206. [Google Scholar] [CrossRef] [PubMed]
- Pera-Titus, M. Porous Inorganic Membranes for CO2 Capture: Present and Prospects. Chem. Rev. 2014, 114, 1413–1492. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Yang, S.W.; Reddy, G.K.; Smirniotis, P.; Dong, J.H. Zeolite Membrane Reactor for High-Temperature Water-Gas Shift Reaction: Effects of Membrane Properties and Operating Conditions. Energy Fuels 2013, 27, 4471–4480. [Google Scholar] [CrossRef]
- Lew, C.M.; Cai, R.; Yan, Y.S. Zeolite Thin Films: From Computer Chips to Space Stations. Acc. Chem. Res. 2010, 43, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Gascon, J.; Kapteijn, F.; Zornoza, B.; Sebastián, V.; Casado, C.; Coronas, J. Practical Approach to Zeolitic Membranes and Coatings: State of the Art, Opportunities, Barriers, and Future Perspectives. Chem. Mater. 2012, 24, 2829–2844. [Google Scholar] [CrossRef]
- Pina, M.P.; Mallada, R.; Arruebo, M.; Urbiztondo, M.; Navascués, N.; de la Iglesia, O.; Santamaria, J. Zeolite Films and Membranes. Emerging Applications. Microporous Mesoporous Mater. 2011, 144, 19–27. [Google Scholar] [CrossRef]
- Caro, J.; Finger, G.; Kornatowski, J.; Richter-Mendau, J.; Werner, L.; Zibrowius, B. Aligned Molecular Sieve Crystals. Adv. Mater. 1992, 4, 273–276. [Google Scholar] [CrossRef]
- Noack, M.; Kölsch, P.; Venzke, D.; Toussaint, P.; Caro, J. New One-dimensional Membrane: Aligned AlPO4-5 Molecular Sieve Crystals in a Nickel Foil. Microporous Mater. 1994, 3, 201–206. [Google Scholar] [CrossRef]
- Girnus, I.; Pohl, M.; Richter-Mendau, J.; Schneider, M.; Noack, M.; Venzke, D.; Caro, J. Synthesis of AlPO4-5 Aluminumphosphate Molecular Sieve Crystals for Membrane Applications by Microwave Heating. Adv. Mater. 1995, 7, 711–714. [Google Scholar] [CrossRef]
- Lin, J.C.; Yates, M.Z.; Petkoska, A.T.; Jacobs, S. Electric-Field-Driven Assembly of Oriented Molecular-Sieve Films. Adv. Mater. 2004, 16, 1944–1948. [Google Scholar] [CrossRef]
- Lin, J.C.; Yates, M.Z. Oriented Molecular Sieve Thin Films from Aligned Seed Layers. Chem. Mater. 2006, 18, 4137–4141. [Google Scholar] [CrossRef]
- Wu, C.N.; Chao, K.J.; Tsai, T.G.; Chiou, Y.H.; Shih, H.C. Oriented Growth of Molecular Sieves on Inorganic Membranes. Adv. Mater. 1996, 8, 1008–1012. [Google Scholar] [CrossRef]
- Tsai, T.G.; Chao, K.J.; Guo, X.J.; Sung, S.L.; Wu, C.N.; Wang, Y.L.; Shih, H.C. Aligned Aluminophosphate Molecular Sieves Crystallized on Floating Anodized Alumina by Hydrothermal Microwave Heating. Adv. Mater. 1997, 9, 1154–1157. [Google Scholar] [CrossRef]
- Tsai, T.G.; Shih, H.C.; Liao, S.J.; Chao, K.J. Well-aligned SAPO-5 Membrane: Preparation and Characterization. Microporous Mesoporous Mater. 1998, 22, 333–341. [Google Scholar] [CrossRef]
- Xu, R.; Zhu, G.S.; Yin, X.J.; Wan, X.; Qiu, S.L. Epitaxial Growth of Highly-Oriented AlPO4-5 Molecular Sieve Films for Microlaser Systems. J. Mater. Chem. 2006, 16, 2200–2204. [Google Scholar] [CrossRef]
- Hu, E.P.; Huang, Y.; Yan, Q.; Liu, D.P.; Lai, Z.P. Synthesis of Highly c-Oriented AFI Membranes by Epitaxial Growth. Microporous Mesoporous Mater. 2009, 126, 81–86. [Google Scholar] [CrossRef]
- Xue, M.; Fan, L.L.; Kang, Z.X.; Zhang, W.T.; Li, H.; Qiu, S.L. Dye-Modified Nanochannels of c-Oriented AFI Film for Heavy Metal Ion Sensing. J. Mater. Chem. 2012, 22, 17644–17648. [Google Scholar] [CrossRef]
- Ji, C.; Tian, Y.; Li, Y.; Lin, Y.S. Thin Oriented AFI Zeolite Membranes for Molecular Sieving Separation. Microporous Mesoporous Mater. 2014, 186, 80–83. [Google Scholar] [CrossRef]
- Karanikolos, G.N.; Wydra, J.W.; Stoeger, J.A.; Garcia, H.; Corma, A.; Tsapatsis, M. Continuous c-Oriented AlPO4-5 Films by Tertiary Growth. Chem. Mater. 2007, 19, 792–797. [Google Scholar] [CrossRef]
- Karanikolos, G.N.; Garcia, H.; Corma, A.; Tsapatsis, M. Growth of AlPO4-5 and CoAPO-5 Films from Amorphous Seeds. Microporous Mesoporous Mater. 2008, 115, 11–22. [Google Scholar] [CrossRef]
- Veziri, C.M.; Palomino, M.; Karanikolos, G.N.; Corma, A.; Kanellopoulos, N.K.; Tsapatsis, M. Toward Submicrometer c-Oriented Nanoporous Films with Unidimensional Pore Network: AFI Film Morphology Control by Precursor Mixture Manipulation. Chem. Mater. 2010, 22, 1492–1502. [Google Scholar] [CrossRef]
- Stoeger, J.A.; Veziri, C.M.; Palomino, M.; Corma, A.; Kanellopoulos, N.K.; Tsapatsis, M.; Karanikolos, G.N. On Stability and Performance of Highly c-Oriented Columnar AlPO4-5 and CoAPO-5 Membranes. Microporous Mesoporous Mater. 2012, 147, 286–294. [Google Scholar] [CrossRef]
- Stoeger, J.A.; Palomino, M.; Agrawal, K.V.; Zhang, X.; Karanikolos, G.N.; Valencia, S.; Corma, A.; Tsapatsis, M. Oriented CoSAPO-5 Membranes by Microwave-Enhanced Growth on TiO2-Coated Porous Alumina. Angew. Chem. Int. Ed. 2012, 51, 2470–2473. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Bein, T. Vertical Aluminophosphate Molecular Sieve Crystals Grown at Inorganic-Organic Interfaces. Science 1994, 265, 1839–1841. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; Liu, X.F.; Zhang, L.; Zhang, B.Q. In Situ Synthesis and Microstructure Manipulation of SAPO-5 Films over Porous α-Al2O3 Substrates. Langmuir 2009, 25, 2271–2277. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Z.; Liu, X.F.; Li, J.; Zhang, B.Q. Microwave-Assisted Seeded Growth of the Submicrometer-Thick and Pure b-Oriented MFI zeolite Films Using an Ultra-Dilute Synthesis Solution. CrystEngComm 2013, 15, 6301–6304. [Google Scholar] [CrossRef]
- Zhou, M.; Zhang, B.Q.; Liu, X.F. Oriented Growth and Assembly of Zeolite Crystals on Substrates. Chin. Sci. Bull. 2008, 53, 801–816. [Google Scholar] [CrossRef][Green Version]
- Huanbutta, K.; Cheewatanakornkool, K.; Terada, K.; Nunthanid, J.; Sriamornsak, P. Impact of Salt Form and Molecular Weight of Chitosan on Swelling and Drug Release from Chitosan Matrix Tablets. Carbohydr. Polym. 2013, 97, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Finger, G.; Richter-Mendau, J.; Bülow, M.; Kornatowski, J. On Synthesis Conditions for Tailoring AlPO4-5 Crystal Dimensions. Zeolites 1991, 11, 443–448. [Google Scholar] [CrossRef]
- Demuth, D.; Stucky, G.D.; Unger, K.K.; Schüth, F. Synthesis of Large Optically Clear Silicoaluminophosphate Crystals with AFI Structure. Microporous Mater. 1995, 3, 473–487. [Google Scholar] [CrossRef]
- Schunk, S.A.; Demuth, D.G.; Schulz-Dobrick, B.; Unger, K.K.; Schüth, F. Element Distribution and Growth Mechanism of Large SAPO-5 Crystals. Microporous Mater. 1996, 6, 273–285. [Google Scholar] [CrossRef]
- Iwasaki, A.; Sano, T.; Kodaira, T.; Kiyozumi, Y. Growth Behaviors of AFI Type Crystals. Microporous Mesoporous Mater. 2003, 64, 145–153. [Google Scholar] [CrossRef]
- Jhung, S.H.; Chang, J.S.; Hwang, Y.K.; Park, S.E. Crystal Morphology Control of AFI Type Molecular Sieves with Microwave Irradiation. J. Mater. Chem. 2004, 14, 280–285. [Google Scholar] [CrossRef]
- Jhung, S.H.; Chang, J.S.; Kim, D.S.; Park, S.E. Effects of Silica on the Synthesis of AFI Molecular Sieve in Acid and Base Conditions under Microwave Irradiation. Microporous Mesoporous Mater. 2004, 71, 135–142. [Google Scholar] [CrossRef]
- Liu, D.C.; Zhang, B.Q.; Liu, X.F.; Li, J. Cyclohexane Oxidation over AFI Molecular Sieves: Effects of Cr, Co Incorporation and Crystal Size. Catal. Sci. Technol. 2015, 5, 3394–3402. [Google Scholar] [CrossRef]
- Lang, L.; Liu, X.F.; Zhang, B.Q. Synthesis and Characterization of (h0h)-Oriented Silicalite-1 Films on α-Al2O3 Substrates. Appl. Surf. Sci. 2008, 254, 2353–2358. [Google Scholar] [CrossRef]
- Yan, J.; Li, J.; Zhang, B.Q.; Liu, X.F. Synthesis and Characterization of Low Aspect Ratio CrCoAPO-5 Molecular Sieves. Chin. J. Inorg. Chem. 2015, 31, 749–754. [Google Scholar] [CrossRef]
- Fan, W.B.; Schoonheydt, R.A.; Weckhuysen, B.M. Hydrothermal Synthesis of Co-Rich CoAPO-5 Molecular Sieves. Phys. Chem. Chem. Phys. 2001, 3, 3240–3246. [Google Scholar] [CrossRef]
- Grandjean, D.; Beale, A.M.; Petukhov, A.V.; Weckhuysen, B.M. Unraveling the Crystallization Mechanism of CoAPO-5 Molecular Sieves under Hydrothermal Conditions. J. Am. Chem. Soc. 2005, 127, 14454–14465. [Google Scholar] [CrossRef] [PubMed]
- Kornatowski, J.; Zadrozna, G.; Rozwadowski, M.; Zibrowius, B.; Marlow, F.; Lercher, J.A. New Strategy for Chromium Substitution and Crystal Morphology Control-Synthesis and Characteristics of CrAPO-5. Chem. Mater. 2001, 13, 4447–4456. [Google Scholar] [CrossRef]
- Laha, S.C.; Kamalakar, G.; Gläser, R. Microwave-Assisted Synthesis of [Cr]APO-5. Microporous Mesoporous Mater. 2006, 90, 45–52. [Google Scholar] [CrossRef]
- Kim, J.; Biswas, K.; Jhon, K.W.; Jeong, S.Y.; Ahn, W.S. Synthesis of AlPO4-5 and CrAPO-5 Using Aluminum Dross. J. Hazard. Mater. 2009, 169, 919–925. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, Z.J.; Bozhilov, K.N.; Chen, Z.W.; Yan, Y.S. TEM Investigation of Formation Mechanism of Monocrystal-Thick b-Oriented Pure Silica Zeolite MFI Film. J. Am. Chem. Soc. 2004, 126, 10732–10737. [Google Scholar] [CrossRef] [PubMed]
- Lang, L.; Liu, X.F.; Jiang, H.Y.; Lin, J.Y.S.; Zhang, B.Q. Direct Evidence of the Evolutionary mechanism of Zeolite Monolayers on the Substrate Surface in a Hydrothermal Reaction. Langmuir 2010, 26, 5895–5900. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.F.; Wang, X.D.; Na, P.; Jiang, H.Y.; Lang, L.; Zhao, H.S.; Zhang, B.Q. Evolutionary Mechanism of b-Oriented TS-1 Film on Porous α-Al2O3 Supported Chitosan Surface in Hydrothermal Reactions. Chin. Sci. Bull. 2010, 55, 3131–3137. [Google Scholar] [CrossRef]
- Krajewska, B. Membrane-Based Processes Performed with Use of Chitin/Chitosan Materials. Sep. Purif. Technol. 2005, 41, 305–312. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available on request from the authors. |


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geng, L.; Dong, H.; Liu, X.; Zhang, B. Efficient Manipulation of Continuous AFI-Type Aluminophosphate Membranes with Distinctive Microstructures on Macroporous α-Al2O3 Substrates. Molecules 2018, 23, 1127. https://doi.org/10.3390/molecules23051127
Geng L, Dong H, Liu X, Zhang B. Efficient Manipulation of Continuous AFI-Type Aluminophosphate Membranes with Distinctive Microstructures on Macroporous α-Al2O3 Substrates. Molecules. 2018; 23(5):1127. https://doi.org/10.3390/molecules23051127
Chicago/Turabian StyleGeng, Luwei, Hongfeng Dong, Xiufeng Liu, and Baoquan Zhang. 2018. "Efficient Manipulation of Continuous AFI-Type Aluminophosphate Membranes with Distinctive Microstructures on Macroporous α-Al2O3 Substrates" Molecules 23, no. 5: 1127. https://doi.org/10.3390/molecules23051127
APA StyleGeng, L., Dong, H., Liu, X., & Zhang, B. (2018). Efficient Manipulation of Continuous AFI-Type Aluminophosphate Membranes with Distinctive Microstructures on Macroporous α-Al2O3 Substrates. Molecules, 23(5), 1127. https://doi.org/10.3390/molecules23051127





