Liposomes as Gene Delivery Vectors for Human Placental Cells
Abstract
:1. Introduction
2. Results
2.1. Formulation Characterization
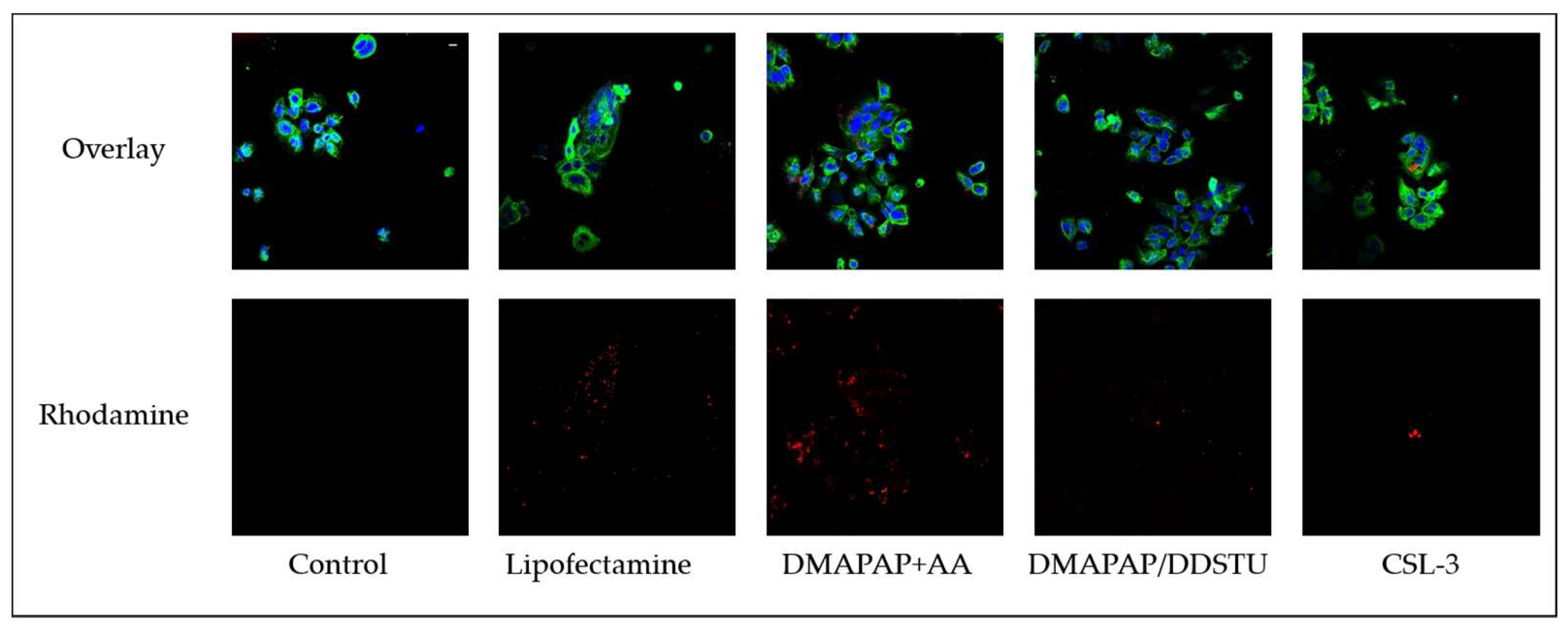
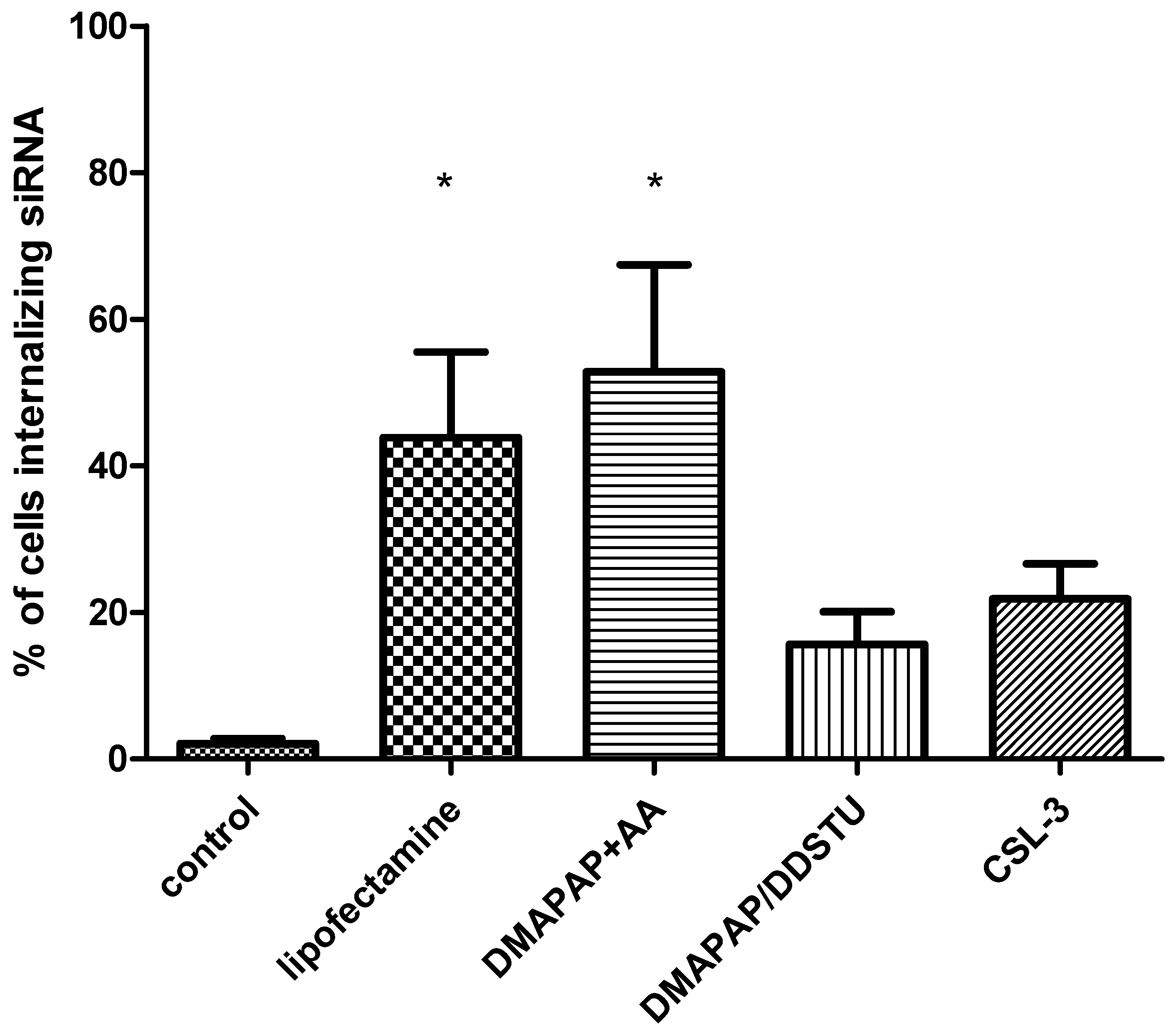
2.2. siRNA Delivery to Primary Cytotrophoblasts
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Liposome and Lipoplex Preparation
4.3. Liposome and Lipoplex Characterization
4.4. Placentae Collection
4.5. Primary Villous Cytotrophoblasts Isolation and Purification
4.6. In Vitro siRNA Internalization
4.7. Confocal Microscopy
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations:
| DMAPAP | dimyristoylaminopropylaminopropyl |
| CSL-3 | cationic switchable lipid 3 |
| DDSTU | didecylserinethiourea |
| VCT | primary villous cytotrophoblasts |
References
- Shields, K.E.; Lyerly, A.D. Exclusion of pregnant women from industry-sponsored clinical trials. Obstet. Gynecol. 2013, 122, 1077–1081. [Google Scholar] [CrossRef] [PubMed]
- Scaffidi, J.; Mol, B.; Keelan, J. The pregnant women as a drug orphan: A global survey of registered clinical trials of pharmacological interventions in pregnancy. BJOG Int. J. Obstet. Gynaecol. 2017, 124, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Baylis, F.; MacQuarrie, R. Why Physicians and Women Should Want Pregnant Women Included in Clinical Trials; Springer: Cham, Switzerland, 2016; pp. 17–31. [Google Scholar]
- Keelan, J.A.; Leong, J.W.; Ho, D.; Iyer, K.S. Therapeutic and safety considerations of nanoparticle-mediated drug delivery in pregnancy. Nanomedicine 2015, 10, 2229–2247. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, N.; Udayakumar, T.; D’Souza, W.; Simone, C., II; Raghavan, S.; Polf, J.; Mahmood, J. Liposomes: Clinical Applications and Potential for Image-Guided Drug Delivery. Molecules 2018, 23, 288. [Google Scholar] [CrossRef] [PubMed]
- Alhareth, K.; Sancey, L.; Tsapis, N.; Mignet, N. How should we plan the future of nanomedicine for cancer diagnosis and therapy? Int. J. Pharm. 2017, 532, 657–659. [Google Scholar] [CrossRef] [PubMed]
- Valero, L.; Alhareth, K.; Gil, S.; Lecarpentier, E.; Tsatsaris, V.; Mignet, N.; Fournier, T.; Andrieux, K. Nanomedicine as a potential approach to empower the new strategies for the treatment of preeclampsia. Drug Discov. Today 2018. [Google Scholar] [CrossRef] [PubMed]
- Valero, L.; Alhareth, K.; Gil, S.; Simasotchi, C.; Roques, C.; Scherman, D.; Mignet, N.; Fournier, T.; Andrieux, K. Assessment of dually labelled PEGylated liposomes transplacental passage and placental penetration using a combination of two ex-vivo human models: The dually perfused placenta and the suspended villous explants. Int. J. Pharm. 2017, 532, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Jia, J.; Guo, X.; Chen, R.; Feng, L. Modulating circulating sFlt1 in an animal model of preeclampsia using PAMAM nanoparticles for siRNA delivery. Placenta 2017, 58, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, A.; Largeau, C.; Bigey, P.; Bessodes, M.; Lebozec, K.; Scherman, D.; Escriou, V. Anionic polymers for decreased toxicity and enhanced in vivo delivery of siRNA complexed with cationic liposomes. J. Controll. Release 2011, 152, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Seguin, J.; Dhotel, H.; Kai-Luen, R.; Bessodes, M.; Mignet, N. Fine tuning of mixed ionic and hydrogen bond interactions for plasmid delivery using lipoplexes. Eur. J. Pharm. Biopharm. 2015, 90, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Viricel, W.; Poirier, S.; Mbarek, A.; Derbali, R.M.; Mayer, G.; Leblond, J. Cationic switchable lipids: PH-triggered molecular switch for siRNA delivery. Nanoscale 2017, 9, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Byk, G.; Dubertret, C.; Escriou, V.; Frederic, M.; Jaslin, G.; Rangara, R.; Pitard, B.; Crouzet, J.; Wils, P.; Schwartz, B.; et al. Synthesis, Activity, and Structure−Activity Relationship Studies of Novel Cationic Lipids for DNA Transfer. J. Med. Chem. 1998, 41, 224–235. [Google Scholar] [CrossRef]
- Breton, M.; Leblond, J.; Seguin, J.; Midoux, P.; Scherman, D.; Herscovici, J.; Pichon, C.; Mignet, N. Comparative gene transfer between cationic and thiourea lipoplexes. J. Gene Med. 2010, 12, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Viricel, W.; Mbarek, A.; Leblond, J. Switchable Lipids: Conformational Change for Fast pH-Triggered Cytoplasmic Delivery. Angew. Chem. Int. Ed. 2015, 54, 12743–12747. [Google Scholar] [CrossRef] [PubMed]
- Forbes, K.; Desforges, M.; Garside, R.; Aplin, J.D.; Westwood, M. Methods for siRNA-mediated Reduction of mRNA and Protein Expression in Human Placental Explants, Isolated Primary Cells and Cell Lines. Placenta 2009, 30, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Bedarida, T.; Domingues, A.; Baron, S.; Ferreira, C.; Vibert, F.; Cottart, C.-H.; Paul, J.-L.; Escriou, V.; Bigey, P.; Gaussem, P.; et al. Reduced endothelial thioredoxin-interacting protein protects arteries from damage induced by metabolic stress in vivo. FASEB J. 2018. [Google Scholar] [CrossRef] [PubMed]
- Orendi, K.; Kivity, V.; Sammar, M.; Grimpel, Y.; Gonen, R.; Meiri, H.; Lubzens, E.; Huppertz, B. Placental and trophoblastic in vitro models to study preventive and therapeutic agents for preeclampsia. Placenta 2011, 32, S49–S54. [Google Scholar] [CrossRef] [PubMed]
- Bajoria, R.; Sooranna, S.R.; Contractor, S.F. Endocytotic uptake of small unilamellar liposomes by human trophoblast cells in culture. Hum. Reprod. 1997, 12, 1343–1348. [Google Scholar] [CrossRef] [PubMed]
- Pinnapireddy, S.R.; Duse, L.; Strehlow, B.; Schäfer, J.; Bakowsky, U. Composite liposome-PEI/nucleic acid lipopolyplexes for safe and efficient gene delivery and gene knockdown. Colloids Surf. B Biointerfaces 2017, 158, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Mignet, N.; Richard, C.; Seguin, J.; Largeau, C.; Bessodes, M.; Scherman, D. Anionic pH-sensitive pegylated lipoplexes to deliver DNA to tumors. Int. J. Pharm. 2008, 361, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Mignet, N.; Seguin, J.; Ramos Romano, M.; Brullé, L.; Touil, Y.S.; Scherman, D.; Bessodes, M.; Chabot, G.G. Development of a liposomal formulation of the natural flavonoid fisetin. Int. J. Pharm. 2012, 423, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Handschuh, K.; Guibourdenche, J.; Tsatsaris, V.; Guesnon, M.; Laurendeau, I.; Evain-Brion, D.; Fournier, T. Human Chorionic Gonadotropin Produced by the Invasive Trophoblast But Not the Villous Trophoblast Promotes Cell Invasion and Is Down-Regulated by Peroxisome Proliferator-Activated Receptor-γ. Endocrinology 2007, 148, 5011–5019. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds DMAPAP, CSL-3; DDSTU and Dodagly-PEG are available from the authors. |
| DMAPAP+AA | DMAPAP/DDSTU | CSL-3 | ||||
|---|---|---|---|---|---|---|
| Liposomes | Lipoplexes | Liposomes | Lipoplexes | Liposomes | Lipoplexes | |
| Mean hydrodynamic diameter (nm) | 104 ± 4 | 121 ± 2 | 72 ± 4 | 86 ± 7 | 170 ± 9 | 317 ± 7 |
| Polydispersity Index | 0.25 ± 0.02 | 0.18 ± 0.01 | 0.22 ± 0.01 | 0.24 ± 0.02 | 0.25 ± 0.01 | 0.25 ± 0.01 |
| Zeta potential (mV) | 28 ± 4 | −5 ± 2 | 12 ± 5 | −2 ± 0.4 | 31 ± 2 | −2 ± 1 |
| siRNA encapsulation (%) | 100 | 100 | 98 | |||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valero, L.; Alhareth, K.; Espinoza Romero, J.; Viricel, W.; Leblond, J.; Chissey, A.; Dhotel, H.; Roques, C.; Campiol Arruda, D.; Escriou, V.; et al. Liposomes as Gene Delivery Vectors for Human Placental Cells. Molecules 2018, 23, 1085. https://doi.org/10.3390/molecules23051085
Valero L, Alhareth K, Espinoza Romero J, Viricel W, Leblond J, Chissey A, Dhotel H, Roques C, Campiol Arruda D, Escriou V, et al. Liposomes as Gene Delivery Vectors for Human Placental Cells. Molecules. 2018; 23(5):1085. https://doi.org/10.3390/molecules23051085
Chicago/Turabian StyleValero, Lucie, Khair Alhareth, Jenifer Espinoza Romero, Warren Viricel, Jeanne Leblond, Audrey Chissey, Hélène Dhotel, Caroline Roques, Danielle Campiol Arruda, Virginie Escriou, and et al. 2018. "Liposomes as Gene Delivery Vectors for Human Placental Cells" Molecules 23, no. 5: 1085. https://doi.org/10.3390/molecules23051085
APA StyleValero, L., Alhareth, K., Espinoza Romero, J., Viricel, W., Leblond, J., Chissey, A., Dhotel, H., Roques, C., Campiol Arruda, D., Escriou, V., Mignet, N., Fournier, T., & Andrieux, K. (2018). Liposomes as Gene Delivery Vectors for Human Placental Cells. Molecules, 23(5), 1085. https://doi.org/10.3390/molecules23051085









