Dentromers, a Family of Super Dendrimers with Specific Properties and Applications
Abstract
1. Introduction
2. Organoiron Arene Activation
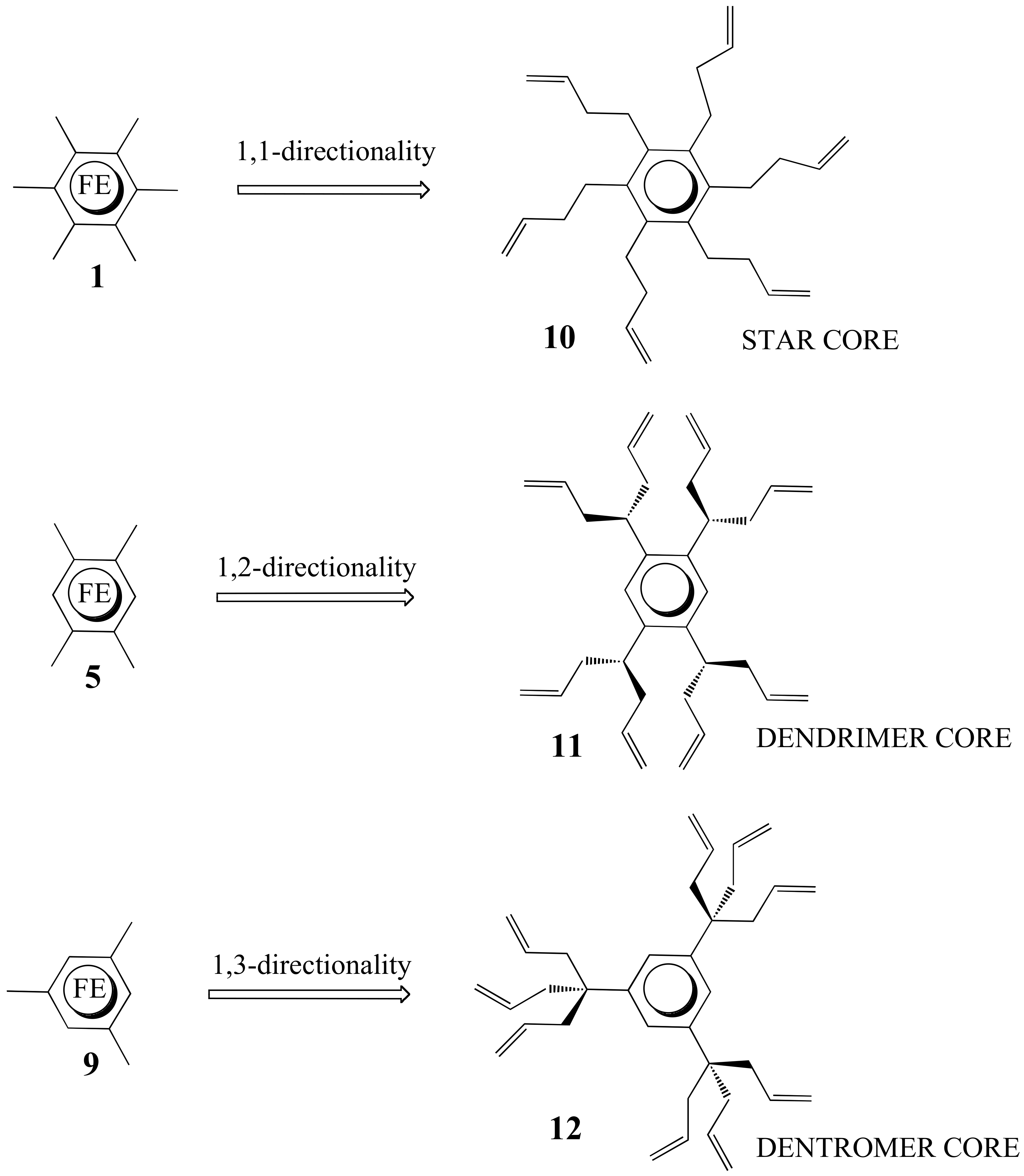
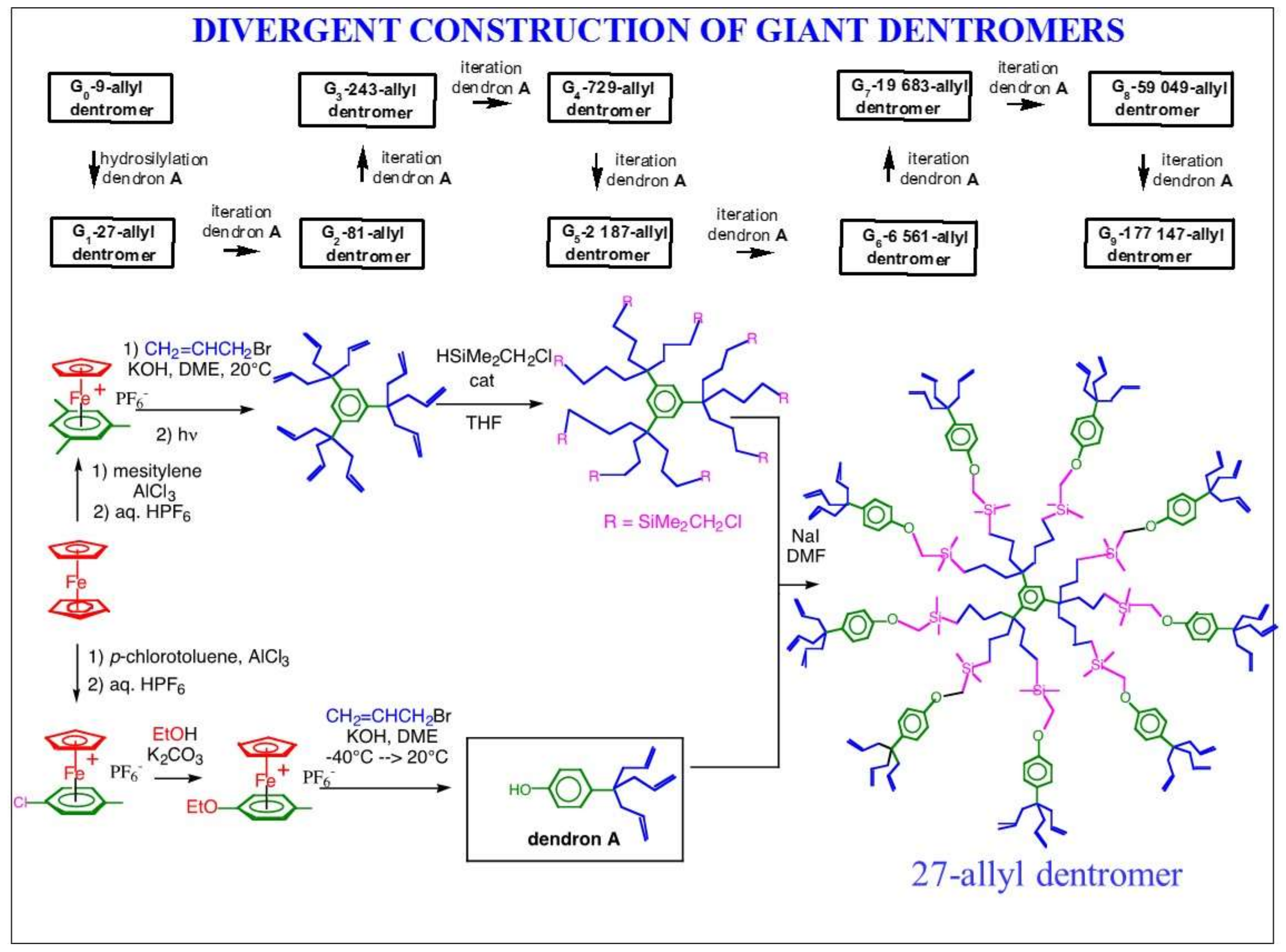
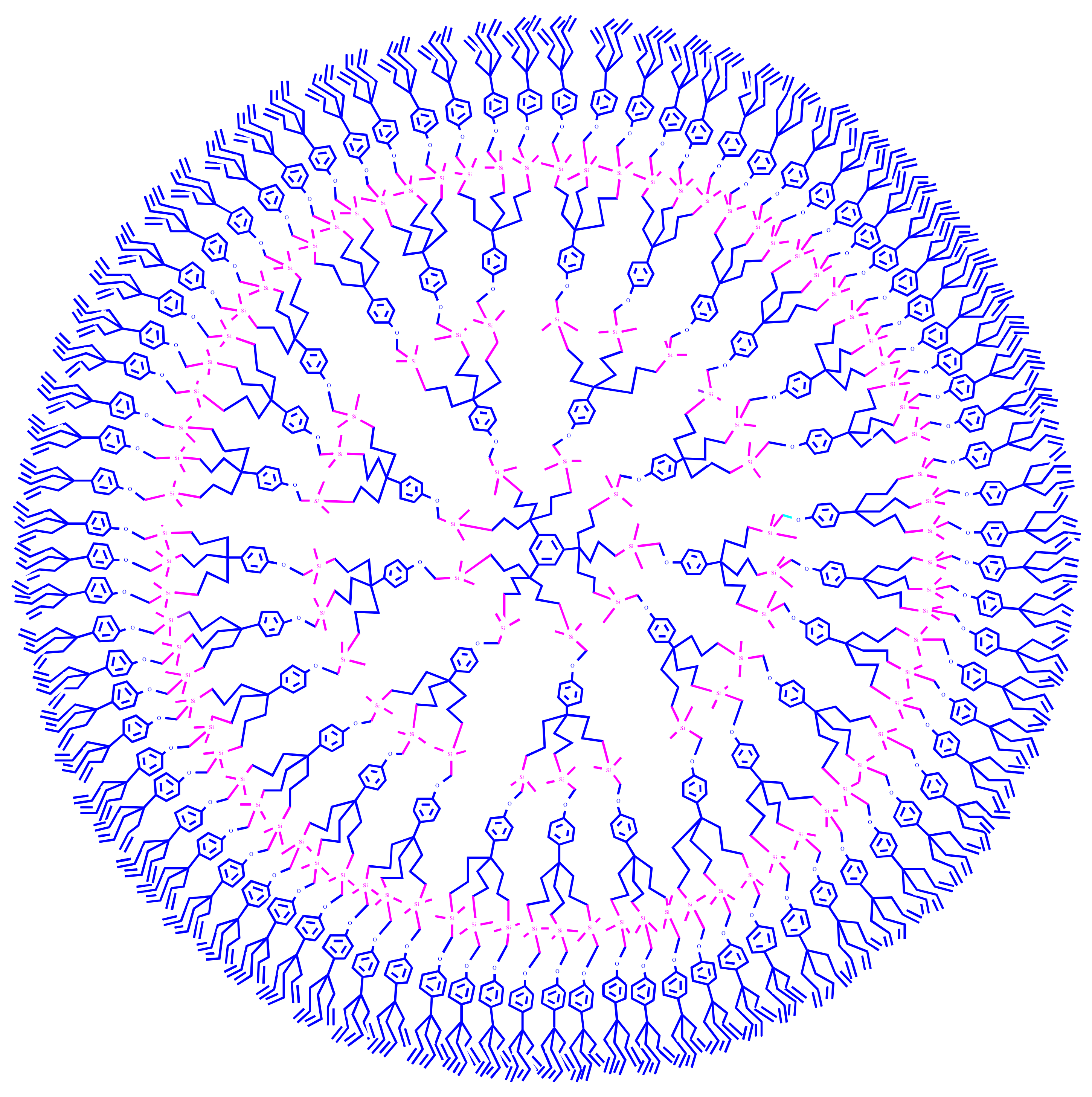
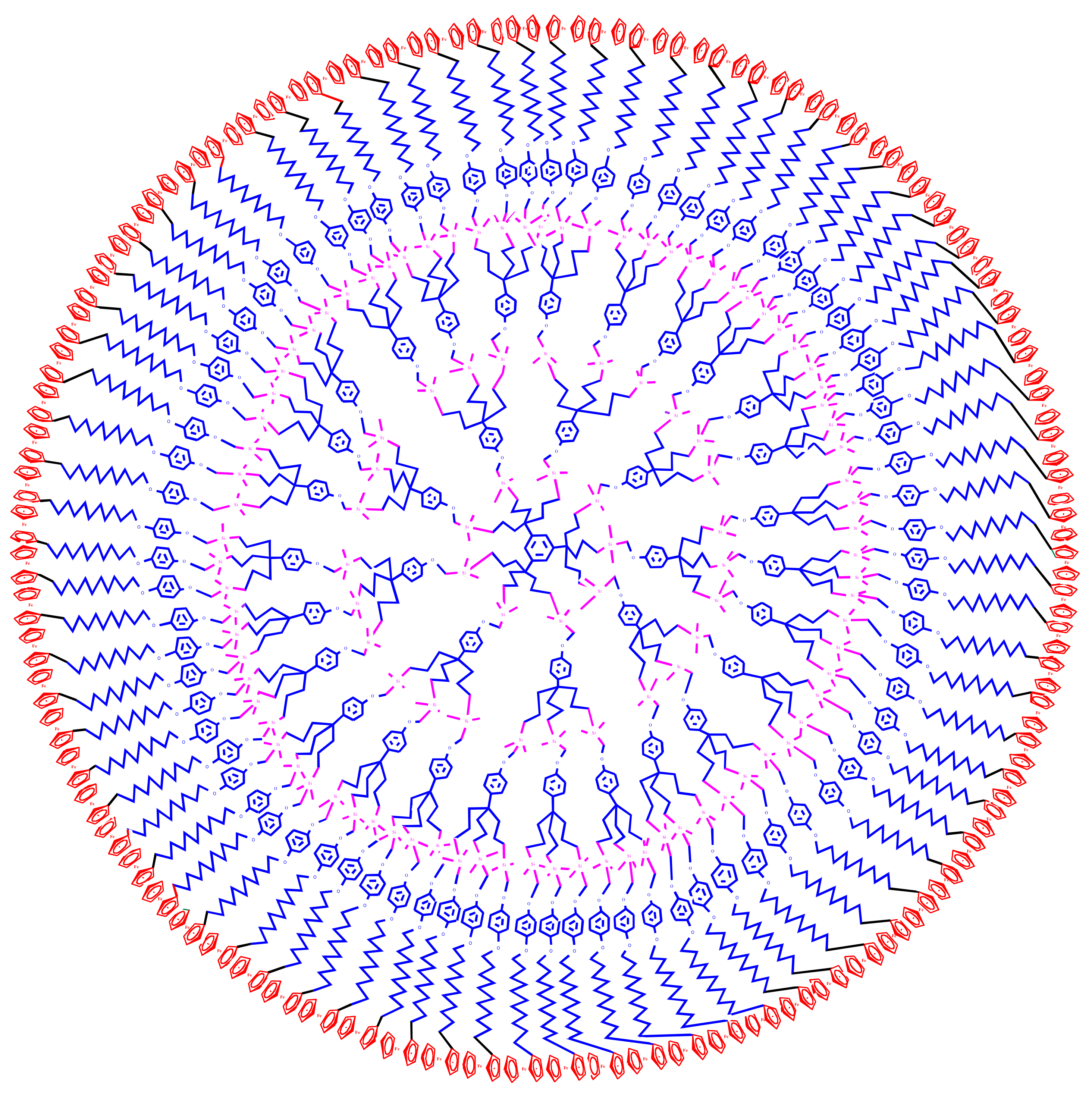
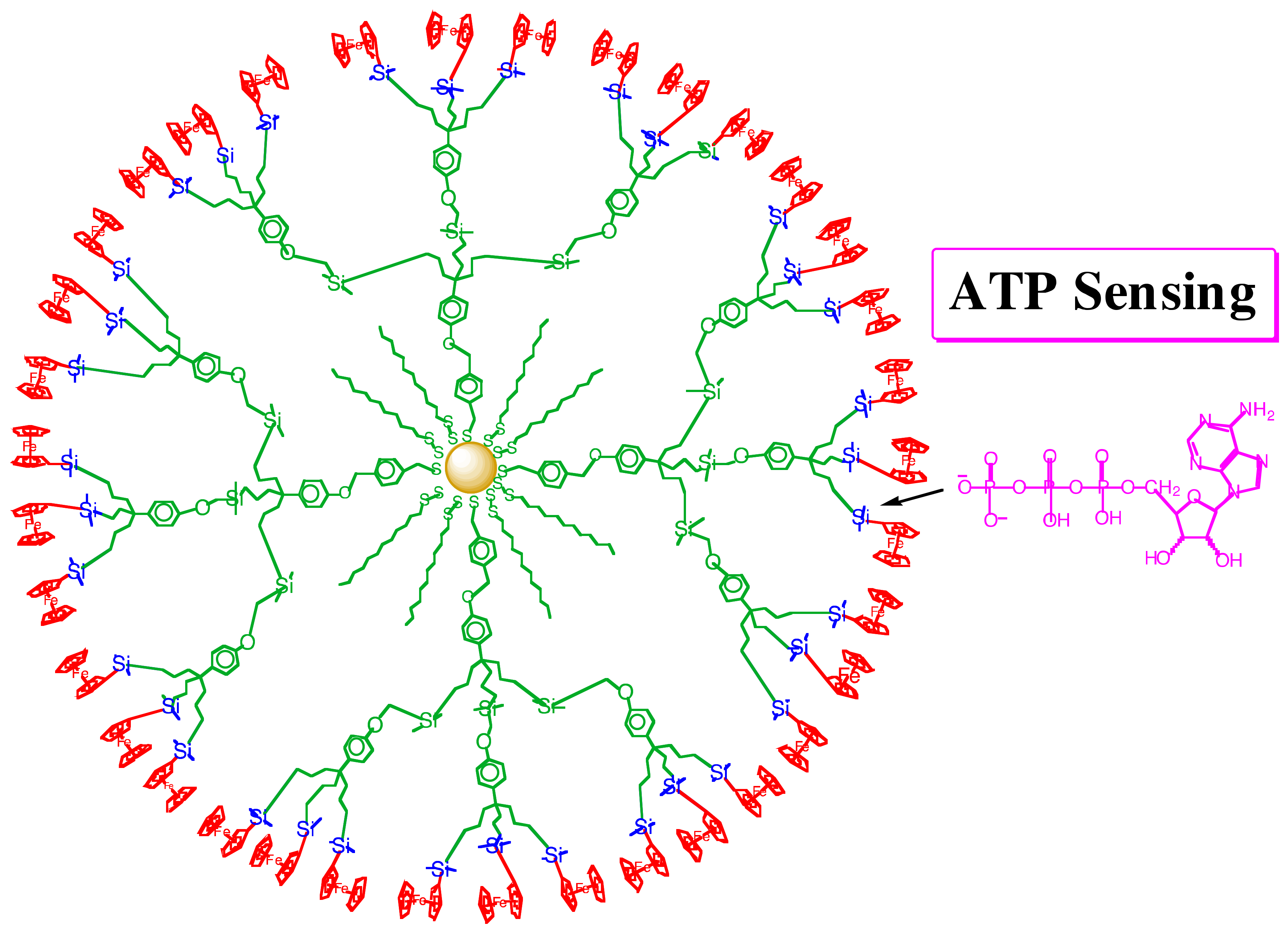
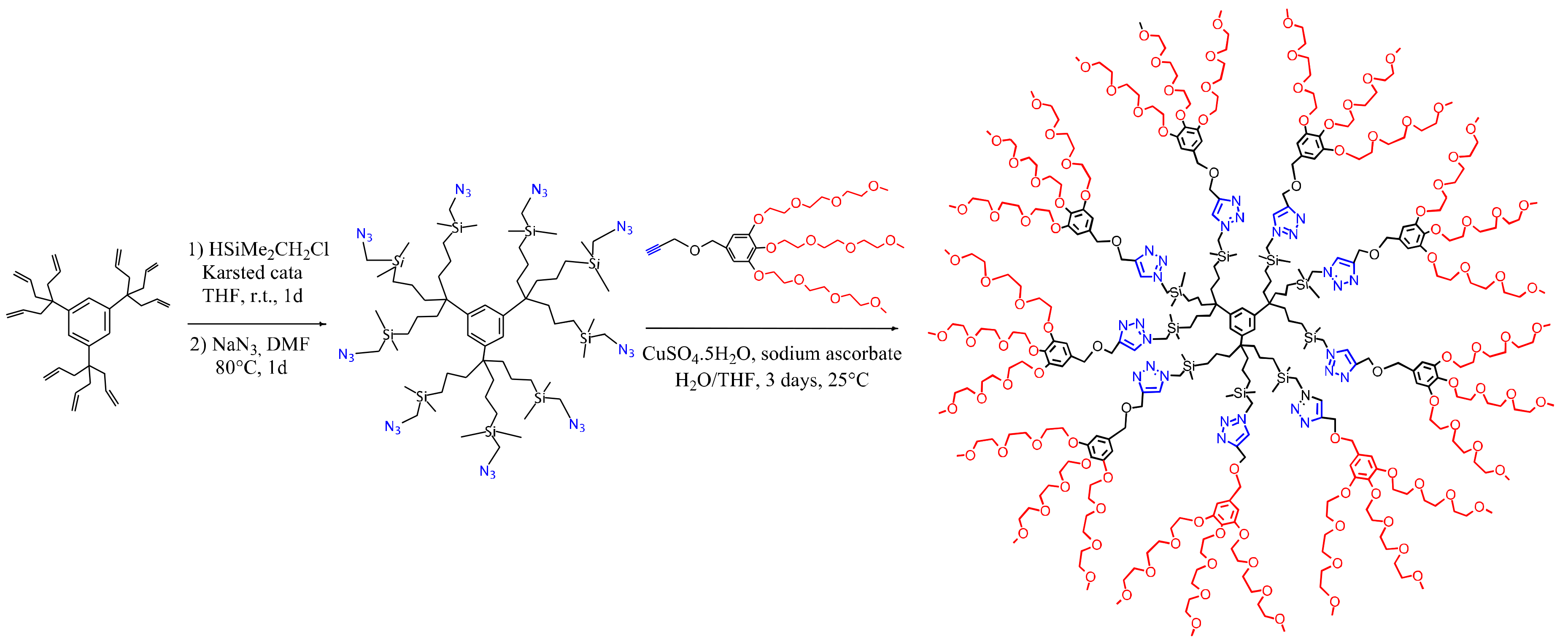
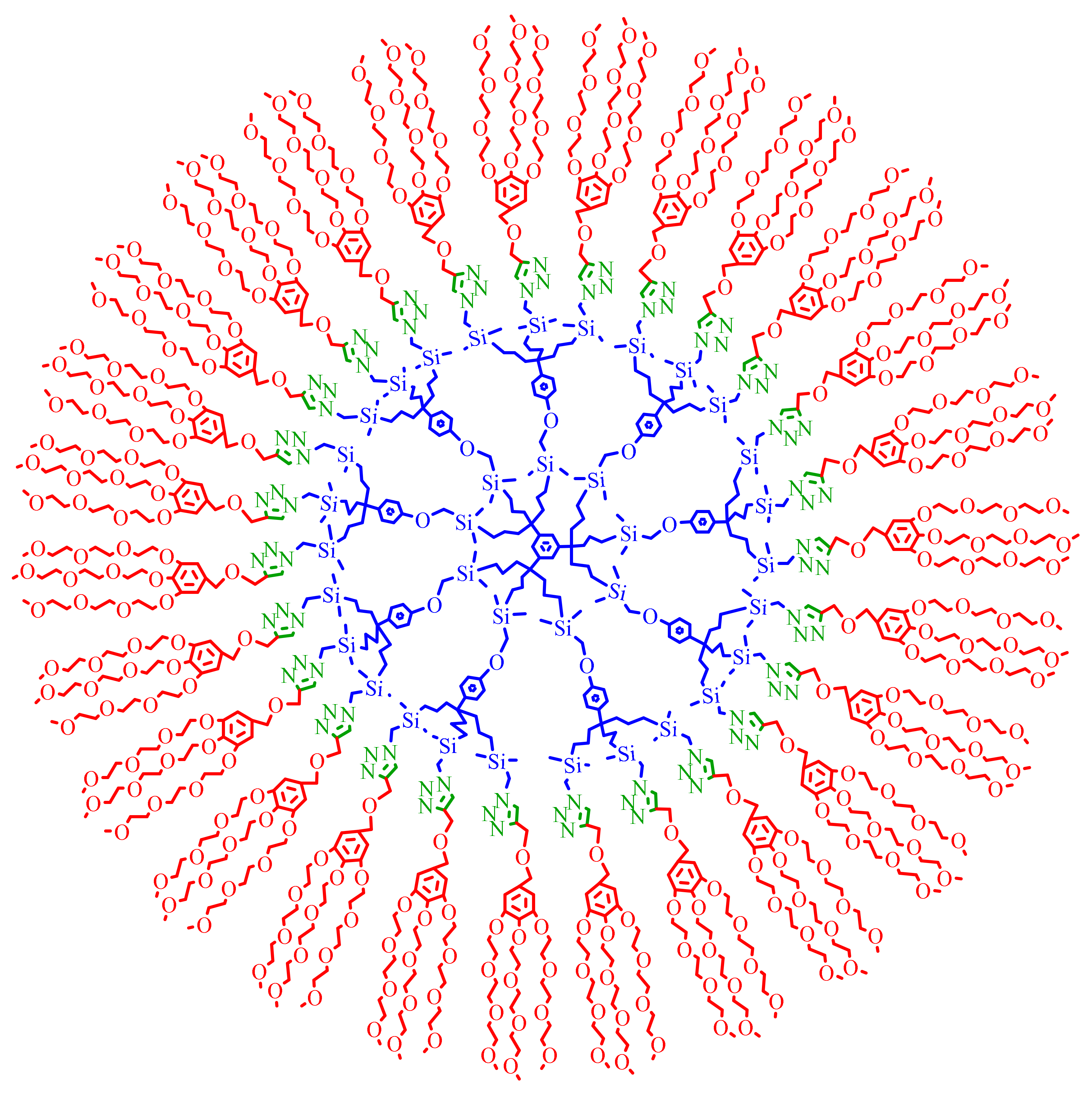
3. Dentromer Construction
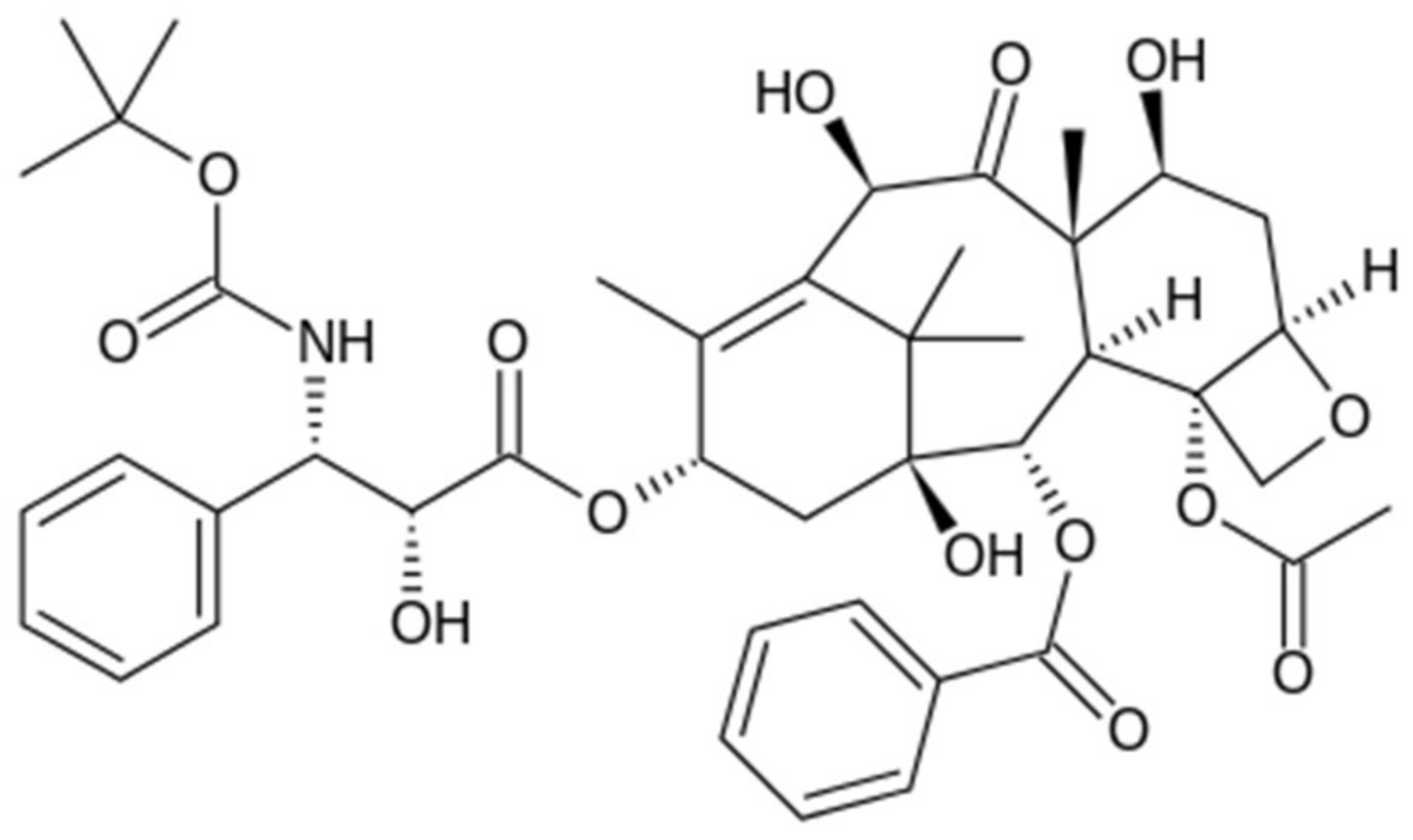
4. The Applications of Dentromers
5. Concluding Remarks
Supplementary Materials
Supplementary File 1Acknowledgments
Conflicts of Interest
References
- Tomalia, D.A.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. A New Class of Polymers: Starburst-Dendritic Macromolecules. Polym. J. 1985, 17, 117–132. [Google Scholar] [CrossRef]
- Newkome, G.R.; Yao, Z.; Baker, G.R.; Gupta, V.K. Micelles. Part 1. Cascade molecules: A new approach to micelles. A [27]-arborol. J. Org. Chem. 1985, 50, 2003–2004. [Google Scholar] [CrossRef]
- Denkewalter, R.G.; Kolc, J.F.; Lukasavage, W.J. Macromolecular Highly Branched Homogeneous Compounds. U.S. Patent 4,410,688, 18 October 1983. [Google Scholar]
- Tomalia, D.A.; Naylor, A.M.; Goddard, W.A. Starburst Dendrimers: Molecular-Level Control of Size, Shape, Surface Chemistry, Topology, and Flexibility from Atoms to Macroscopic Matter. Angew. Chem. Int. Ed. 1990, 29, 138–175. [Google Scholar] [CrossRef]
- Tomalia, D.A. Birth of a New Macromolecular Architecture: Dendrimers as Quantized Building Blocks for Nanoscale Synthetic Polymer Chemistry. Prog. Polym. Sci. 2005, 30, 294–324. [Google Scholar] [CrossRef]
- Wiener, E.C.; Brechbiel, M.W.; Brothers, H.; Magin, R.L.; Gansow, O.A.; Tomalia, D.A.; Lautebur, P.C. Dendrimer-based metal chelates: A new class of magnetic resonance imaging contrast agents. Magn. Reson. Med. 1994, 31, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Esfand, R.; Tomalia, D.A. Poly(amidoamine) (PAMAM) dendrimers: From biomimecry to drug delivery and biomedical applications. Drug. Discov. Today 2001, 427–436. [Google Scholar]
- Lee, C.C.; MacKay, J.A.; Fréchet, J.M.J.; Szoka, F.C. Designing dendrimers for biological applications. Nat. Biotechnol. 2005, 23, 1517–1526. [Google Scholar] [CrossRef] [PubMed]
- Kannan, R.M.; Nance, E.; Kannan, S.; Tomalia, D.A. Emerging concepts in dendrimer-based nanomedicine: From design principles to clinical applications. J. Intern. Med. 2014, 276, 579–617. [Google Scholar] [CrossRef] [PubMed]
- Hawker, C.J.; Fréchet, J.M.J. Preparation of polymers with controlled molecular architecture. A new convergent approach to dendritic macromolecules. J. Am. Chem. Soc. 1990, 112, 7638–7639. [Google Scholar] [CrossRef]
- Miller, T.M.; Neenan, T.X. Convergent synthesis of monodisperse dendrimers based upon 1,3,5-trisubstituted benzenes. Chem. Mater. 1990, 2, 346–349. [Google Scholar] [CrossRef]
- Yamamoto, K.; Higuchi, M.; Shiki, S.; Tsuruta, M.; Chiba, H. Stepwise radial complexation of imine groups in phenylazomethine dendrimers. Nature 2002, 415, 509–511. [Google Scholar] [CrossRef] [PubMed]
- Serroni, S.; Denti, G.; Campagna, S.; Balzani, V. Arborols Based on Luminescent and Redox-Active Transition Metal Complexes. Angew. Chem. Int. Ed. 1992, 31, 1493–1495. [Google Scholar]
- Van der Made, A.W.; van Leeuwen, P.W.N.M. Silane Dendrimers. J. Chem. Soc. Chem. Commun. 1992, 1400–1401. [Google Scholar] [CrossRef]
- Nagasaki, T.; Ukon, M.; Arimori, S.; Shinkai, S. Crowned arborols. J. Chem. Soc. Chem. Commun. 1992, 8, 608–609. [Google Scholar] [CrossRef]
- De Brabander-van den Berg, E.M.M.; Meijer, E.W. Poly(propylene imine) Dendrimers: Large-Scale Synthesis by Hetereogeneously Catalyzed Hydrogenations. Angew. Chem., Int. Ed. Engl. 1993, 105, 1370–1372. [Google Scholar] [CrossRef]
- Moors, R.; Vögtle, F. Dendrimeric Polyamines. Chem. Ber. 1993, 126, 2133–2135. [Google Scholar] [CrossRef]
- Seyferth, D.; Son, D.Y.; Rheingold, A.L.; Ostrander, R.L. Synthesis of an organosilicon dendrimer containing 324 Si-H bonds. Organometallics 1994, 13, 2682–2690. [Google Scholar] [CrossRef]
- Bryce, M.R.; Devonport, W.; Moore, A.J. Dendritic macromolecules incorporating tetrafulvalene units. Angew. Chem. Int. Ed. 1994, 33, 1761–1763. [Google Scholar] [CrossRef]
- Launay, N.; Caminade, A.-M.; Lahana, R.; Majoral, J.-P. A General Synthetic Strategy for Neutral Phosphorus-Containing Dendrimers. Angew. Chem. Int. Ed. 1994, 33, 1589–1592. [Google Scholar] [CrossRef]
- Launay, N.; Caminade, A.-M.; Majoral, J.-P. Synthesis and reactivity of unusual phosphorous dendrimers. A useful divergent growth approach up to the seventh generation. J. Am. Chem. Soc. 1995, 117, 3282–3283. [Google Scholar] [CrossRef]
- Sournies, F.; Crasnier, F.; Graffeuil, M.; Faucher, J.-P.; Labarre, M.-C.; Labarre, J.-F. Spherical Cyclophosphazene Dendrimers to the Fifth Generation. Angew. Chem. Int. Ed. 1995, 34, 578–581. [Google Scholar] [CrossRef]
- Casado, C.M.; Cuadrado, I.; Moran, M.; Alonso, B.; Lobete, F.; Losada, J. Synthesis of the first redox-active organometallic polymers containing cyclosiloxanes as frameworks. Organometallics 1995, 14, 2618–2620. [Google Scholar] [CrossRef]
- Casado, C.M.; Cuadrado, I.; Moran, M.; Alonso, B.; Garcia, B.; Gonzales, B.; Losada, J. Redox-active ferrocenyl dendrimers and polymers in solution and immobilized on electrode surfaces. J. Coord. Chem. Rev. 1998, 185, 53–79. [Google Scholar]
- Balzani, V.; Campagna, S.; Denti, G.; Juris, A.; Serroni, S.; Venturi, M. Designing dendrimers based on transition-metal complexes. Light harvesting properties and predetermined redox patterns. Acc. Chem. Res. 1998, 31, 26–34. [Google Scholar] [CrossRef]
- Armaroli, N.; Boudon, C.; Felder, D.; Gisselbrecht, D.; Gross, J.-P.; Marconi, M.; Nicoud, G.; Nierengarten, J.-F.; Vicinelli, V. A copper (I) bis-phenanthroline complex buried in fullerene-functionalized dendritic black boxes. Angew. Chem. Int. Ed. Engl. 1999, 38, 3730–3733. [Google Scholar] [CrossRef]
- Newkome, G.R.; He, E.; Moorefield, C.N. Suprasupermolecules with novel properties: Metallodendrimers. Chem. Rev. 1999, 99, 1689–1746. [Google Scholar] [CrossRef] [PubMed]
- Caminade, A.-M.; Majoral, J.-P. Dendrimers containin heteroatoms (Si, P, B, Ge or Bi). Chem. Rev. 1999, 99, 845–880. [Google Scholar]
- Bosman, A.W.; Janssen, H.M.; Meijer, E.W. About dendrimers: Structure, physical properties and applications. Chem. Rev. 1999, 99, 1665–1688. [Google Scholar] [CrossRef] [PubMed]
- Nierengarten, J.F. Fullerodendrimers: A new class of compounds for supramolecular chemistry and material science applications. Chem. Eur. J. 2000, 6, 3667–3670. [Google Scholar] [CrossRef]
- Grayson, S.M.; Fréchet, J.M.J. Convergent dendrons and dendrimers: From synthesis to applications. Chem. Rev. 2001, 101, 3819–3867. [Google Scholar] [CrossRef] [PubMed]
- Newkome, G.R.; Moorefield, C.N.; Vögtle, F. Dendrimers and Dendrons: Concepts, Syntheses, Applications; Wiley-vch: Weinheim, Germany, 2001. [Google Scholar]
- Tomalia, D.A.; Fréchet, J.M.J. (Eds.) Dendrimers and Other Dendritic Polymers; Wiley: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Newkome, G.R.; Shreiner, C.D. Poly(amidoamine), polypropylenimine, and related dendrimers and dendrons possessing different 1 → 2 branching motifs: An overview of the divergent procedures. Polymer 2008, 49, 1–173. [Google Scholar] [CrossRef]
- Astruc, D.; Boisselier, E.; Ornelas, C. Dendrimers Designed for Functions: From Physical, Photophysical and Supramolecular Properties to Applications in Sensing, Catalysis, Molecular Electronics, Photonics and Nanomedicine. Chem. Rev. 2010, 110, 1857–1959. [Google Scholar] [CrossRef] [PubMed]
- Caminade, A.-M.; Turrin, C.-O.; Laurent, R.; Ouali, A.; Delavaux-Nicot, B. Dendrimers Towards Catalytic, Material and Biomedical Uses; John Wiley & Sons: Chichester, UK, 2011. [Google Scholar]
- Tomalia, D.A.; Christensen, J.B.; Boas, U. Dendrimers, Dendrons and Dendritic Polymers; Cambridge University Press: Cambridge, UK, 2012. [Google Scholar]
- Campagna, S.; Ceroni, P.; Puntoriero, F. (Eds.) Designing Dendrimers; Wiley: Hoboken, NJ, USA, 2012. [Google Scholar]
- Newkome, G.R.; Shreiner, C. Dendrimers derived from 1 → 3 branching motifs. Chem. Rev. 2010, 110, 6338–6442. [Google Scholar] [CrossRef] [PubMed]
- Rengan, K.; Engel, R. Ammonium cascade molecules. J. Chem. Soc. Chem. Commun. 1992, 757–758. [Google Scholar] [CrossRef]
- Denliker, P.J.; Diederich, F.; Gross, M.; Knobler, C.B.; Louati, A.; Sanford, E.M. Dendritic Porphyrins: Modulating Redox Potentials of Electroactive Chromophores with Pendant Multifunctionality. Angew. Chem. Int. Ed. 1994, 33, 1739. [Google Scholar]
- Percec, V.; Mitchell, C.M.; Cho, W.D.; Uchida, S.; Glodde, M.; Goran, U.; Zeng, X.; Liu, Y.; Balagurusamy, V.S.K.; Heiney, P.A. Designing Libraries of First Generation AB3 and AB2 Self-Assembling Dendrons via the Primary Structure Generated from Combinations of (AB)y-AB3 and (AB)-AB2 Bilding Blocks. J. Am. Chem. Soc. 2004, 126, 6078–6094. [Google Scholar] [CrossRef] [PubMed]
- Rosen, B.M.; Wilson, C.J.; Wilson, D.A.; Percec, V. Dendron-Mediated Self-Assembly, Diassembly, and Self-Organization of Complex Systems. Chem. Rev. 2009, 109, 6275–6540. [Google Scholar] [CrossRef] [PubMed]
- Newkome, G.R. Suprasupermolecular chemistry: The chemistry within the dendrimer. Pure Appl. Chem. 1998, 70, 2337–2343. [Google Scholar] [CrossRef][Green Version]
- Newkome, G.R.; Yao, Z.-Q.; Baker, G.R.; Gupta, V.K.; Russo, P.S.; Saunders, M.J. Chemistry of micelles series. Part 2. Cascade molecules. Synthesis and characterization of a benzene[9]3-arborol. J. Am. Chem. Soc. 1986, 108, 849–850. [Google Scholar] [CrossRef]
- Newkome, G.R.; Moorefield, C.N.; Baker, G.R. Building Blocks for Dendritic Macromolecules. Aldrichim. Acta 1992, 25, 31–38. [Google Scholar]
- Newkome, G.R.; Moorefield, C.N.; Theriot, K.J. A convenient synthesis of bis-homotris: 4-amino-4-[1-(3-hydroxypropyl)]-1,7-heptanediol and 1-azoniaprpellane. J. Org. Chem. 1988, 53, 5552–5554. [Google Scholar] [CrossRef]
- Moorefield, C.N.; Newkome, G.R. A Review of Dendritic Macromolecules. In Advances in Dendritic Molecules; Newkome, G.R., Ed.; JAI: Greenwich, UK, 1994; Volume 1, pp. 1–67. [Google Scholar]
- Twyman, L.J.; Beezer, A.E.; Esfand, R.; Hardy, M.J.; Mitchell, J.C. The Synthesis of Water-Soluble Dendrimers and their Application as Possible Drug Delivery Systems. Tetrahedron Lett. 1999, 1743–1746. [Google Scholar] [CrossRef]
- Newkome, G.R.; Hu, Y.; Saunders, M.J.; Fronzsek, S.R. Silvanols: Water-Soluble Calixarenes. Tetrahedron Lett. 1991, 32, 1133. [Google Scholar] [CrossRef]
- Rudick, J.G.; Percec, V. Induced Helical Backbone Conformations of Self-Organizable Dendronized Polymers. Acc. Chem. Res. 2008, 41, 1641–1652. [Google Scholar] [CrossRef] [PubMed]
- Abd-El-Aziz, A.S.; Bernardin, S. Synthesis and reactivity of arenes coordinated to cyclopentadienyliron cations. Coord. Chem. Rev. 2000, 203, 219–267. [Google Scholar] [CrossRef]
- Catheline, D.; Astruc, D. The Use of Ferrocene in Organometallic Synthesis: A two Step Preparation of Cyclopentadienyliron Acetonitrile and Phosphine Cations via Photolysis of Cyclopentadienyliron Tricarbonyl or Arene Cations. J. Organomet. Chem. 1984, 272, 417–426. [Google Scholar] [CrossRef]
- Astruc, D.; Hamon, J.-R.; Althoff, G.; Román, E.; Batail, P.; Michaud, P.; Mariot, J.-P.; Varret, F.; Cozak, D. Design, Stabilization and Efficiency of Organometallic “Electron Reservoirs”. 19-Electron Sandwichs η5-C5R5Fe(I) η6-C6R6, a Key Class Active in Redox Catalysis. J. Am. Chem. Soc. 1979, 101, 5445–5447. [Google Scholar] [CrossRef]
- Astruc, D.; Roman, E.; Hamon, J.-R.; Batail, P. Novel Reactions of Dioxygen in Organometallic Chemistry. Hydrogen Atom Abstraction versus Dimerization of the 19-Electron Complexes η6‑Cyclopentadienyl Iron(I) η6-Arene. J. Am. Chem. Soc. 1979, 101, 2240–2242. [Google Scholar] [CrossRef]
- Moinet, C.; Roman, E.; Astruc, D. Electrochemical Reduction of Cations η5-Cyclopentadienyl Fe+ η6‑Arene in Basic Media. Study of the Behavior of the Radicals Formed “in Situ”. J. Electroanal. Chem. 1981, 121, 241–253. [Google Scholar] [CrossRef]
- Astruc, D. Why is Ferrocene so Exceptional. Eur. J. Inorg. Chem. 2017, 6–29. [Google Scholar] [CrossRef]
- Hamon, J.-R.; Saillard, J.-Y.; Le Beuze, A.; McGlinchey, M.; Astruc, D. Multiple Formation and Cleavage of C-C Bonds in CpFe+(Arene) Sandwichs and the Unusual C6Et6 Geometry in the X-Ray Crystal Structure of CpFe+(η6-C6Et6) PF6-. J. Am. Chem. Soc. 1982, 104, 7549–7555. [Google Scholar] [CrossRef]
- Moulines, F.; Astruc, D. Tentacled Iron Sandwichs. Angew. Chem. Int. Ed. Engl. 1988, 27, 1347–1349. [Google Scholar] [CrossRef]
- Moulines, F.; Gloaguen, B.; Astruc, D. One-Pot Multifunctionalization of Polymethyl Hydrocarbon π-Ligands. Maximum Space Occupancy by Double Branching and Formation of Arborols. Angew. Chem. Int. Ed. Eng. 1992, 28, 458–460. [Google Scholar] [CrossRef]
- Gloaguen, B.; Astruc, D. Chiral Pentaisopropyl- and Pentaisopentyl Complexes: One-Pot Synthesis by Formation of Ten Carbon-Carbon Bonds from Pentamethylcobaticinium. J. Am. Chem. Soc. 1990, 112, 4607–4609. [Google Scholar] [CrossRef]
- Moulines, F.; Djakovitch, L.; Boese, R.; Gloaguen, B.; Thiel, W.; Fillaut, J.-L.; Delville, M.-H.; Astruc, D. Organometallic Molecular Trees as Multi-Electron and Proton Reservoirs: CpFe+ Induced Nona-Allylation of Mesitylene and Phase-Transfer Catalyzed Synthesis of a Redox Active Nona-Iron Complex. Angew. Chem., Int. Ed. Engl. 1993, 32, 1075–1077. [Google Scholar] [CrossRef]
- Sartor, V.; Djakovitch, L.; Fillaut, J.-L.; Moulines, F.; Neveu, F.; Marvaud, V.; Guittard, J.; Blais, F.; Neveu, J.-C.; Astruc, D. Organoiron Routes to a New Dendron for Fast Dendritic Syntheses Using Divergent and Convergent Methods. J. Am. Chem. Soc. 1999, 121, 2929–2930. [Google Scholar] [CrossRef]
- Ruiz, J.; Lacoste, M.; Astruc, D. Arene Exchange by P Donors in the 19-Electron Complexes FeICp(Arene): Kinetics, Mechanism and Salt Effects; Characterization and Reactivity of the New 17-Electron and 19-Electron Radicals FeICp(PR3)n (n = 2 and 3). J. Am. Chem. Soc. 1990, 112, 5471–5483. [Google Scholar] [CrossRef]
- Nlate, S.; Ruiz, J.; Blais, J.-C.; Astruc, D. Ferrocenylsilylation of Dendrons: A Fast Convergent Route to Redox-Stable Ferrocene Dendrimers. Chem. Commun. 2000, 5, 417–418. [Google Scholar] [CrossRef]
- Ruiz, J.; Lafuente, G.; Marcen, S.; Ornelas, C.; Lazare, S.; Cloutet, E.; Blais, J.-C.; Astruc, D. Construction of Giant Dendrimers Using a Tripodal Buiding Block. J. Am. Chem. Soc. 2003, 125, 7250–7257. [Google Scholar] [CrossRef] [PubMed]
- Kob, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Functions from a Few Good Reactions. Angew. Chem., Int. Ed. 2001, 40, 2004–2021. [Google Scholar]
- Binder, W.H.; Sachsenhofer, R. Click Chemistry in Polymer and Materials Science. Macromol. Commun. 2007, 28, 15–54. [Google Scholar] [CrossRef]
- Franc, G.; Kakkar, A.K. Click methodologies: Efficient, simple and greener routes to design dendrimers. Chem. Soc. Rev. 2010, 39, 1536–1544. [Google Scholar] [CrossRef] [PubMed]
- Ornelas, C.; Ruiz, J.; Cloutet, E.; Astruc, D. Click Assembly of 1,2,3-Triazole-Linked Dendrimers Including Ferrocenyl Dendrimers that Sense Both Oxo-anions and Metal Cations. Angew. Chem. Int. Ed. 2007, 46, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Diallo, A.K.; Ornelas, C.; Salmon, L.; Ruiz, J.; Astruc, D. Homeopathic Catalytic Activity and Atom-Leaching Mechanism in the Miyaura-Suzuki Reactions under Ambient Conditions Using Precise “Click” Dendrimer-Stabilized Pd Nanoparticles. Angew. Chem. Int. Ed. Engl. 2007, 46, 8644–8648. [Google Scholar] [CrossRef] [PubMed]
- Ornelas, C.; Ruiz, J.; Belin, C.; Astruc, D. Giant Dendritic Molecular Electrochrome Batteries with Ferrocenyl and Pentamethylferrocenyl Termini. J. Am. Chem. Soc. 2009, 131, 590–601. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Salmon, L.; Ruiz, J.; Astruc, D. Metallodendrimers in Three Oxidation States with Electronically Interacting Metals and Stabilization of Size-Selected Gold Nanoparticles. Nat. Commun. 2014, 5, 3489. [Google Scholar] [CrossRef] [PubMed]
- Djeda, R.; Rapakousiou, A.; Liang, L.; Guidolin, N.; Ruiz, J.; Astruc, D. Click Syntheses of Large 1,2,3-Triazolyl Biferrocenyl Dendrimers and Selective Roles of the Inner and Outer Ferrocenyl Groups in the Redox Recognition of the ATP2- Anion and PdII Cation. Angew. Chem. Int. Ed. 2010, 49, 8152–8156. [Google Scholar] [CrossRef] [PubMed]
- Boisselier, E.; Diallo, A.K.; Salmon, L.; Ornelas, C.; Ruiz, J.; Astruc, D. Encapsulation and Stabilization of Gold Nanoparticles with “Click” Polyethyleneglycol Dendrimers. J. Am. Chem. Soc. 2010, 132, 2729–2742. [Google Scholar] [CrossRef] [PubMed]
- Astruc, D.; Liang, L.; Rapakousiou, A.; Ruiz, J. Click Dendrimers and Triazole-Related Aspects: Catalysts, Mechanism, Synthesis, and Functions. A Bridge between Dendritic Architectures and Nanomaterials. Acc. Chem. Res. 2012, 45, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Valério, C.; Fillaut, J.-L.; Ruiz, J.; Guittard, J.; Blais, J.-C.; Astruc, D. The Dendritic Effect in Molecular Recognition: Ferrocene Dendrimers and their Use as Supramolecular Redox Sensors for the Recognition of Small Inorganic Anions. J. Am. Chem. Soc. 1997, 119, 2588–2589. [Google Scholar] [CrossRef]
- Valério, C.; Alonso, E.; Ruiz, J.; Blais, J.-C.; Astruc, D. A Polycationic Metallodendrimer with 24 Organoiron Termini which Senses Chloride and Bromide Anions. Angew. Chem. Int. Ed. Engl. 1999, 38, 1747–1751. [Google Scholar] [CrossRef]
- Astruc, D.; Ornelas, C.; Ruiz, J. Metallocenyl Dendrimers and their Applications in Molecular Electronics, Sensing and Catalysis. Acc. Chem. Res. 2008, 41, 841–856. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.; Belin, C.; Astruc, D. Assembly of Dendrimers with Redox-Active Clusters [CpFe(μ3-CO)]4 at the Periphery and Application to Oxo-anion and Adenosyne-5’-Triphosphate (ATP) Sensing. Angew. Chem. Int. Ed. 2006, 45, 132–136. [Google Scholar]
- Daniel, M.-C.; Ruiz, J.; Nlate, S.; Blais, J.-C.; Astruc, D. Nanoscopic Assemblies Between Supramolecular Redox Active Metallodendrons and Gold Nanoparticles: Syntheses, Characterization and Selective Recognition of H2PO4-, HSO4- and Adenosine-5’-Triphosphate (ATP2-) Anions. J. Am. Chem. Soc. 2003, 125, 2617–2628. [Google Scholar] [CrossRef] [PubMed]
- Daniel, M.-C.; Ruiz, J.; Astruc, D. Supramolecular H-bonded Assemblies of Redox-Active Metallodendrimers and Positive and Unusual Dendritic Effects on the Recognition of H2PO4−. J. Am. Chem. Soc. 2003, 125, 1150–1151. [Google Scholar] [CrossRef] [PubMed]
- Stryer, L. Biochemistry, 2nd ed.; Freeman: New York, NY, USA, 1981; p. 240. [Google Scholar]
- Crooks, R.M.; Zhao, M.; Sun, L.; Chechik, V.; Yeung, L.K. Dendrimer-Encapsulated Metal Nanoparticles: Synthesis, Characterization, and Application to Catalysis. Acc. Chem. Res. 2001, 34, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Scott, R.W.J.; Wilson, O.M.; Crooks, R.M. Synthesis, Characterization, and Applications of Dendrimer-Encapsulated Nanoparticles. J. Phys. Chem. B 2005, 109, 692–704. [Google Scholar] [CrossRef] [PubMed]
- Toshima, N.; Yonezawa, T. Bimetallic nanoparticles—novel materials for chemical and physical applications. New J. Chem. 1998, 22, 1179–1201. [Google Scholar] [CrossRef]
- Deraedt, C.; Astruc, D. “Homeopathic” Palladium Nanoparticle Catalysis of Cross Carbon–Carbon Coupling Reactions. Acc. Chem. Res. 2014, 47, 494–503. [Google Scholar] [CrossRef] [PubMed]
- De Vries, J.G. A unifying mechanism for all high-temperature Heck reactions. The role of palladium colloids and anionic species. Dalton Trans. 2006, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Diallo, A.K.; Boisselier, E.; Liang, L.; Ruiz, J.; Astruc, D. Dendrimer-induced Molecular Catalysis in Water: The Example of Olefin Metathesis. Chem. Eur. J. 2010, 16, 11832–11835. [Google Scholar] [CrossRef] [PubMed]
- Deraedt, C.; Pinaud, N.; Astruc, D. A Recyclable Catalytic Dendrimer Nanoreactor for Part-Per-Million Cu(I) Catalysis of “click” Reactions in Water. J. Am. Chem. Soc. 2014, 136, 12092–12098. [Google Scholar] [CrossRef] [PubMed]
- Rapakousiou, A.; Deraedt, C.; Gu, H.; Salmon, L.; Belin, C.; Ruiz, J.; Astruc, D. Mixed-Valent Intertwinned Polymer Units Containing Biferrocenium Chloride Side Chains Form Nanosnakes that Encapsulate Gold Nanoparticles. J. Am. Chem. Soc. 2014, 136, 13995–13998. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Gregurec, D.; Irigoyen, J.; Martinez, A.; Moya, S.; Ciganda, R.; Hermange, P.; Ruiz, J.; Astruc, D. Precise Localization of Metal Nanoparticles in Dendrimer Nanosnakes or Inner Periphery and Consequences in Catalysis. Nat. Commun. 2016, 7, 13152. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ciganda, R.; Salmon, L.; Gregurec, D.; Irigoyen, J.; Moya, S.; Ruiz, J.; Astruc, D. Highly efficient transition metal nanoparticle catalysts in aqueous solutions. Angew. Chem. Int. Ed. 2016, 55, 3091–3095. [Google Scholar] [CrossRef] [PubMed]
- Astruc, D.; Wang, Q.; Fu, F.; Escobar, A.; Moya, S.; Ruiz, J. “Click” dendrimer-stabilized nanocatalysts for hydrogen release upon ammonia-borane hydrolysis. ChemCatChem 2018, 0. [Google Scholar] [CrossRef]
- Ornelas, C. Brief Timelapse on dendrimer Chemistry: Advances, limitations and Expectations. Macromol. Chem. Phys. 2016, 217, 149–174. [Google Scholar] [CrossRef]
- Boisselier, E.; Ruiz, J.; Astruc, D. Encapsulation de la vitamine C dans des dendrimères solubles dans l’eau. French Patent 101305FR, 22 January 2008. [Google Scholar]
- Zhao, P.; Ruiz, J.; Astruc, D. Encapsulation of water-soluble vitamins in hydrophobic media. Chem. Lett. 2012, 41, 1107–1109. [Google Scholar] [CrossRef]
- Ornelas, C.; Boisselier, E.; Martinez, V.; Pianet, I.; Ruiz, J.; Astruc, D. New Water-soluble Polyanionic Dendrimers and Binding of Acetylcholin in Water by Mean of Supramolecular Interactions. Chem. Commun. 2007, 5093–5095. [Google Scholar] [CrossRef] [PubMed]
- Boisselier, E.; Ornelas, C.; Pianet, I.; Ruiz, J.; Astruc, D. Four Generations of Water Soluble Dendrimers with 9 to 243 Benzoate Tethers: Synthesis and Dendritic Effects on Their Ion Paring with Acetylcholine, Benzyltriethylammonium and Dopamine in Water. Chem. Eur. J. 2008, 14, 5577–5587. [Google Scholar] [CrossRef] [PubMed]
- Diallo, A.K.; Boisselier, E.; Ruiz, J.; Astruc, D. Nouveau Procédé de métathèse d’oléfines ou d’énynes. French Patent 08/05548FR, 8 October 2008. [Google Scholar]
- François, A.; Laroche, A.; Pinaud, N.; Salmon, L.; Ruiz, J.; Robert, J.; Astruc, D. Encapsulation of docetaxel into PEGylated gold nanoparticles for vectorization to cancer cells and in vitro results. ChemMedChem 2011, 6, 2003–2008. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Astruc, D. Docetaxel nanotechnology in anti-cancer therapy. ChemMedChem 2012, 7, 952–972. [Google Scholar] [CrossRef] [PubMed]
- Bossard, C.; Rigaut, S.; Astruc, D.; Delville, M.-H.; Félix, G.; Février-Bouvier, A.; Amiell, J.; Flandrois, S.; Delhaès, P. One-, Two- and Three-Electron Reduction of C60 Using the Electron-Reservoir Complex [FeICp(C6Me6)]. J. Chem. Soc. Chem. Commun. 1993, 3, 333–334. [Google Scholar] [CrossRef]
- Zhou, F.M.; Jehoulet, C.; Bard, A.J. Reduction and electrochemistry of fullerene C60 in liquid ammonia. J. Am. Chem. Soc. 1992, 114, 11004. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Astruc, D.; Deraedt, C.; Djeda, R.; Ornelas, C.; Liu, X.; Rapakousiou, A.; Ruiz, J.; Wang, Y.; Wang, Q. Dentromers, a Family of Super Dendrimers with Specific Properties and Applications. Molecules 2018, 23, 966. https://doi.org/10.3390/molecules23040966
Astruc D, Deraedt C, Djeda R, Ornelas C, Liu X, Rapakousiou A, Ruiz J, Wang Y, Wang Q. Dentromers, a Family of Super Dendrimers with Specific Properties and Applications. Molecules. 2018; 23(4):966. https://doi.org/10.3390/molecules23040966
Chicago/Turabian StyleAstruc, Didier, Christophe Deraedt, Rodrigue Djeda, Catia Ornelas, Xiang Liu, Amalia Rapakousiou, Jaime Ruiz, Yanlan Wang, and Qi Wang. 2018. "Dentromers, a Family of Super Dendrimers with Specific Properties and Applications" Molecules 23, no. 4: 966. https://doi.org/10.3390/molecules23040966
APA StyleAstruc, D., Deraedt, C., Djeda, R., Ornelas, C., Liu, X., Rapakousiou, A., Ruiz, J., Wang, Y., & Wang, Q. (2018). Dentromers, a Family of Super Dendrimers with Specific Properties and Applications. Molecules, 23(4), 966. https://doi.org/10.3390/molecules23040966






