Modified Fusarium Mycotoxins in Cereals and Their Products—Metabolism, Occurrence, and Toxicity: An Updated Review
Abstract
1. Introduction
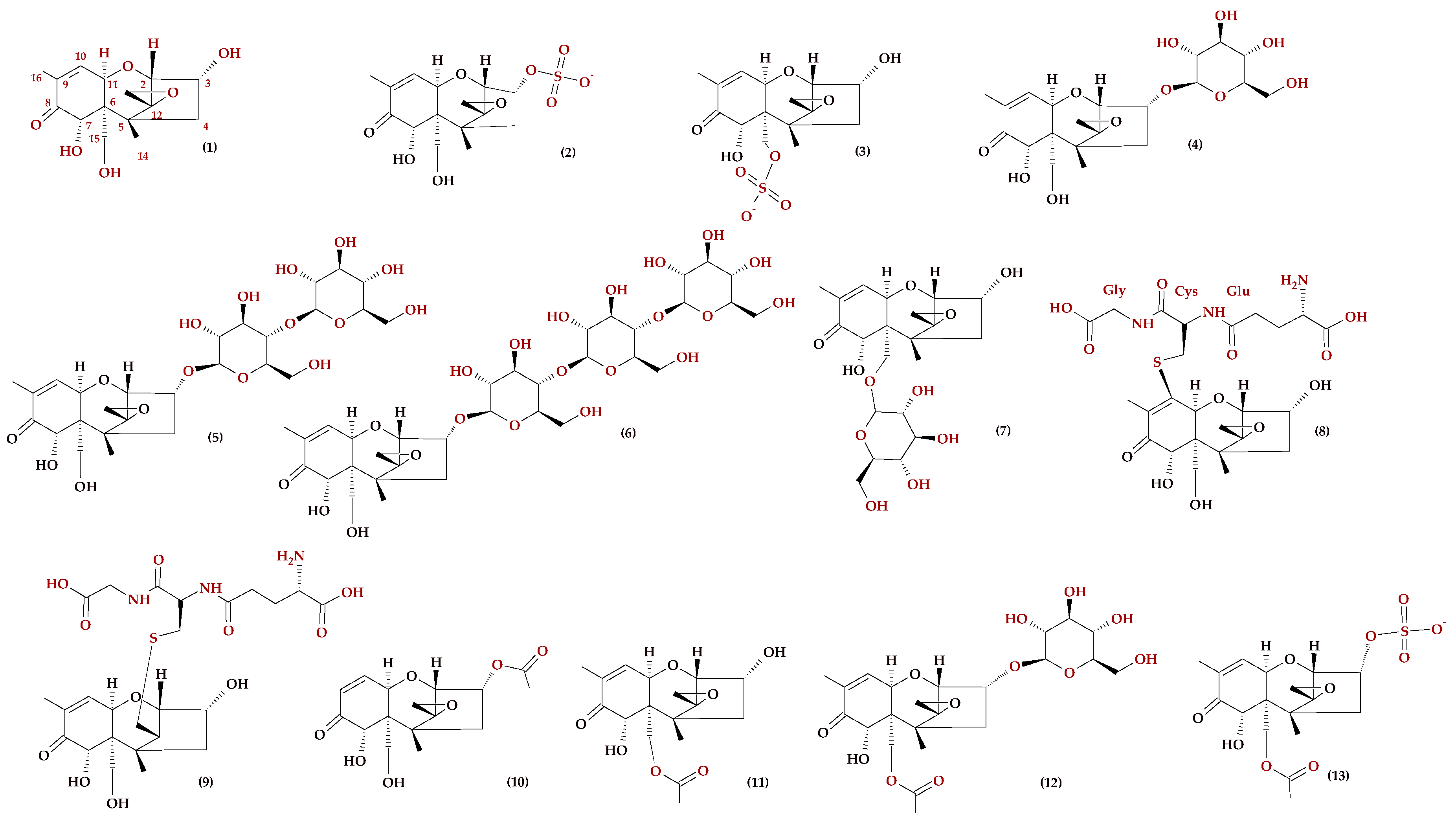
2. Mycotoxin Metabolism
2.1. DON and Its Analogues
2.2. HT-2 and T-2 Toxins and Their Metabolites
2.3. ZEN and Its Metabolites
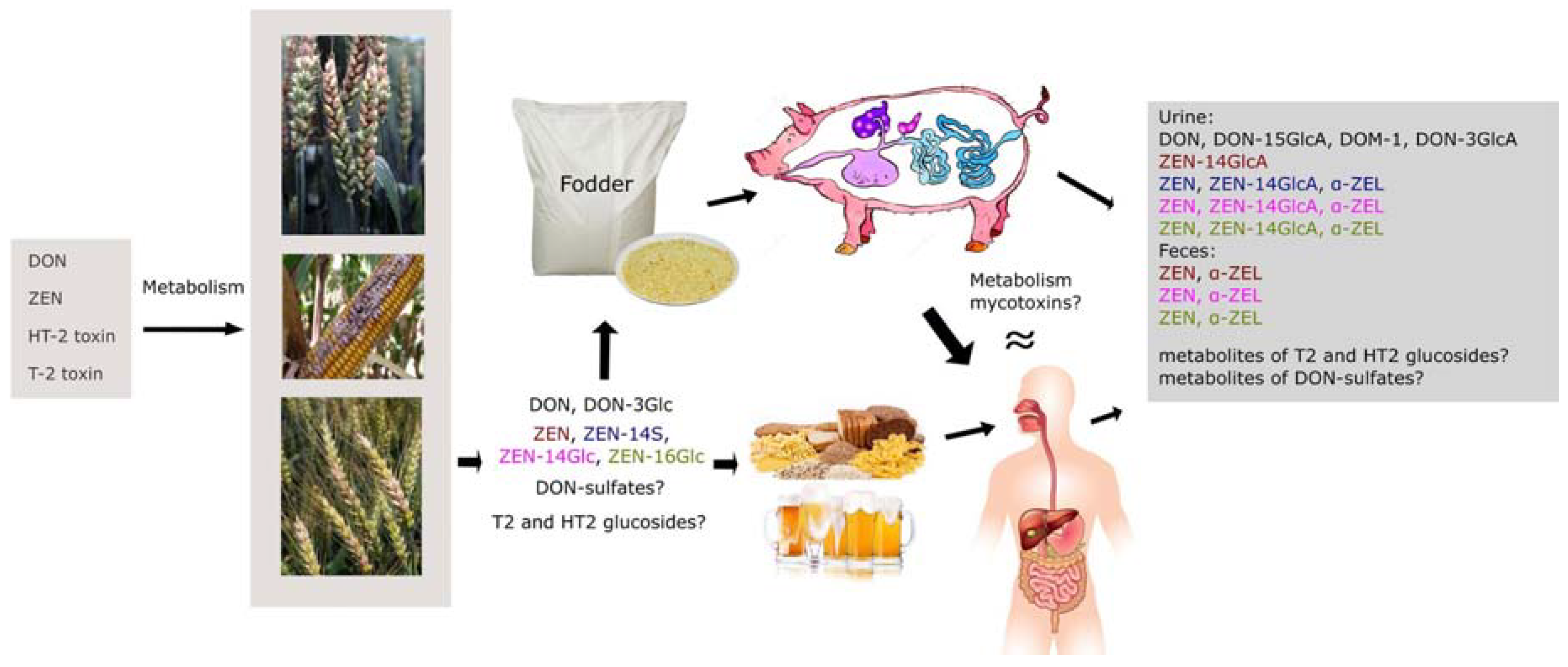
2.4. Metabolism of Mycotoxins in Animals
3. Occurrence
4. Toxicological Properties
4.1. In Vitro Studies on DON and Its Derivatives
4.2. In Vitro Studies on ZEN and Its Derivatives
4.3. In Vivo Studies on DON/ZEN/T-2 Derivatives
Acknowledgments
Conflicts of Interest
References
- Dohlman, E. Mycotoxin hazards and regulations: Impacts on food and animal feed crop trade. In International Trade and Food Safety: Economic Theory and Case Studies; Agricultural Economic Report No. 828; Buzby, J., Ed.; United States Department of Agriculture (USDA): Washington, DC, USA, 2003. [Google Scholar]
- Esper, R.H.; Goncalez, E.; Marques, M.O.; Felicio, R.C.; Felicio, J.D. Potential of essential oils for protection of grains contaminated by aflatoxin produced by Aspergillus flavus. Front. Microbiol. 2014, 5, 269. [Google Scholar] [CrossRef] [PubMed]
- Terzi, V.; Tumino, G.; Stanca, A.M.; Morcia, C. Reducing the incidence of cereal head infection and mycotoxins in small grain cereal species. J. Cereal Sci. 2014, 59, 284–293. [Google Scholar] [CrossRef]
- Tian, Y.; Tan, Y.; Liu, N.; Liao, Y.; Sun, C.; Wang, S.; Wu, A. Functional agents to biologically control deoxynivalenol in cereal grains. Front. Microbiol. 2016, 7, 395. [Google Scholar] [CrossRef] [PubMed]
- Bhat, R.; Rai, R.V.; Karim, A.A. Mycotoxins in food and feed: Present status and future concerns. Compr. Rev. Food Sci. Food Saf. 2010, 9, 57–81. [Google Scholar] [CrossRef]
- Medina, A.; Mohale, S.; Samsudin, N.I.P.; Rodriguez-Sixtos, A.; Rodriguez, A.; Magan, N. Biocontrol of mycotoxins: Dynamics and mechanisms of action. Curr. Opin. Food Sci. 2017, 17, 41–48. [Google Scholar] [CrossRef]
- Stanciu, O.; Banc, R.; Cozma, A.; Filip, L.; Miere, D.; Mañes, J.; Loghin, F. Occurence of Fusarium Mycotoxins in Wheat from Europe—A Review. Acta Univ. Cibiniensis Ser. E Food Technol. 2015, 19, 35–60. [Google Scholar] [CrossRef]
- Medina, A.; Akbar, A.; Baazeem, A.; Rodriguez, A.; Magan, N. Climate change, food security and mycotoxins: Do we know enough? Fungal Biol. Rev. 2017, 31, 143–154. [Google Scholar] [CrossRef]
- Ibáñez-Vea, M.; Lizarraga, E.; González-Peñas, E.; López de Cerain, A. Co-occurrence of type-A and type-B trichothecenes in barley from a northern region of Spain. Food Control 2012, 25, 81–88. [Google Scholar] [CrossRef]
- Commission Regulation (EC) No 1881/2006 of 19 December 2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32006R1881 (accessed on 19 April 2018).
- Commission Recommendation No 2013/165/EU of 27 March 2013 on the Presence of T-2 and HT-2 Toxin in Cereals and Cereal Products. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32013H0165 (accessed on 19 April 2018).
- Rychlik, M.; Humpf, H.U.; Marko, D.; Dänicke, S.; Mally, A.; Berthiller, F.; Klaffke, H.; Lorenz, N. Proposal of a comprehensive definition of modified and other forms of mycotoxins including “masked” mycotoxins. Mycotoxin Res. 2014, 30, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Khaneghah, A.M.; Martins, L.M.; von Hertwig, A.M.; Bertoldo, R.; Sant’Ana, A.S. Deoxynivalenol and its masked forms: Characteristics, incidence, control and fate during wheat and wheat based products processing—A review. Trends Food Sci. Technol. 2018, 71, 13–24. [Google Scholar] [CrossRef]
- Gareis, M.; Bauer, J.; Thiem, J.; Plank, G.; Grabley, S.; Gedek, B. Cleavage of zearalenone-glycoside, a “masked” mycotoxin, during digestion in swine. J. Vet. Med. B 1990, 37, 236–240. [Google Scholar] [CrossRef]
- Berthiller, F.; Crews, C.; Dall’Asta, C.; Saeger, S.D.; Haesaert, G.; Karlovsky, P.; Oswald, I.P.; Seefelder, W.; Speijers, G.; Stroka, J. Masked mycotoxins: A review. Mol. Nutr. Food Res. 2013, 57, 165–186. [Google Scholar] [CrossRef] [PubMed]
- Broekaert, N.; Devreese, M.; de Baere, S.; de Backer, P.; Croubels, S. Modified Fusarium mycotoxins unmasked: From occurrence in cereals to animal and human excretion. Food Chem. Toxicol. 2015, 80, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.O.D.; Blake-Kalff, M.M.A.; Davies, T.G.E. Detoxification of xenobiotics by plants: Chemical modification and vacuolar compartmentation. Trends Plant Sci. 1997, 2, 144–151. [Google Scholar] [CrossRef]
- Audenaert, K.; Troch, V.; Landschoot, S.; Haesaert, G. Biotic stresses in the anthropogenic hybrid triticale (×Triticosecale Wittmack): Current knowledge and breeding challenges. Eur. J. Plant. Pathol. 2014, 140, 615–630. [Google Scholar] [CrossRef]
- Gorst-Allman, C.P.; Steyn, P.S.; Vleggaar, R.; Rabie, C. Structure elucidation of a novel trichothecene glycoside using 1H and 13C nuclear magnetic resonance spectroscopy. J. Chem. Soc. Perkin Trans. 1985, 1, 1553–1555. [Google Scholar] [CrossRef]
- Shin, S.; Torres-Acosta, J.A.; Heinen, S.J.; McCormick, S.; Lemmens, M.; Paris, M.P.; Berthiller, F.; Adam, G.; Muehlbauer, G.J. Transgenic Arabidopsis thaliana expressing a barley UDP-glucosyltransferase exhibit resistance to the mycotoxin deoxynivalenol. J. Exp. Bot. 2012, 63, 4731–4740. [Google Scholar] [CrossRef] [PubMed]
- Schweiger, W.; Boddu, J.; Shin, S.; Poppenberger, B.; Berthiller, F.; Lemmens, M.; Muehlbauer, G.J.; Adam, G. Validation of a candidate deoxynivalenol-inactivating UDP-glucosyltransferase from barley by heterologous expression in yeast. Mol. Plant Microbe Interact. 2010, 23, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Ovando-Martinez, M.; Ozsisli, B.; Anderson, J.; Whitney, K.; Ohm, J.-B.; Simsek, S. Analysis of deoxynivalenol and deoxynivalenol-3-glucoside in hard red spring wheat inoculated with Fusarium graminearum. Toxins 2013, 5, 2522–2532. [Google Scholar] [CrossRef] [PubMed]
- Gratz, S.W. Do Plant-Bound Masked Mycotoxins Contribute to Toxicity? Toxins 2017, 9, 85. [Google Scholar] [CrossRef] [PubMed]
- Freire, L.; Sant’Ana, A.S. Modified mycotoxins: An uptake review on their formation, detection, occurrence and toxic effects. Food Chem. Toxicol. 2018, 111, 189–205. [Google Scholar] [CrossRef] [PubMed]
- Berthiller, F.; Dall’Asta, C.; Schuhmacher, R.; Lemmens, M.; Adam, G.; Krska, R. Masked mycotoxins: Determination of a deoxynivalenol glucoside in artificially and naturally contaminated wheat by liquid chromatography-tandem mass spectrometry. J. Agric. Food Chem. 2005, 53, 3421–3425. [Google Scholar] [CrossRef] [PubMed]
- Cirlini, M.; Generotti, S.; Dall’Erta, A.; Lancioni, P.; Ferrazzano, G.; Massi, A.; Galaverna, G.; Dall’Asta, C. Durum wheat (Triticum Durum Desf.) lines show different abilities to form masked mycotoxins under greenhouse conditions. Toxins 2014, 6, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Kostelanska, F.; Hajslova, J.; Zachariasova, M.; Malachova, A.; Kalachova, K.; Poustka, J.; Fiala, J.; Scott, P.M.; Berthiller, F.; Krska, R. Occurence of Deoxynivalenol and its major conjugate, deoxynivalenol-3-glucoside, in beer and some brewing intermediate. J. Agric. Food Chem. 2009, 57, 3187–3194. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, B. Fate of deoxynivalenol and deoxynivalenol-3-glucoside during wheat milling and Chinese steamed bread processing. Food Control 2014, 44, 86–91. [Google Scholar] [CrossRef]
- Nagl, V.; Wöchtl, B.; Schwartz-Zimmermann, H.E.; Hennig-Pauka, I.; Moll, W.D.; Adam, G.; Berthiller, F. Metabolism of the masked mycotoxin deoxynivalenol-3-glucoside in pigs. Toxicol. Lett. 2014, 229, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Berthiller, F.; Krska, R.; Domig, K.J.; Kneifel, W.; Juge, N.; Schuhmacher, R.; Adam, G. Hydrolytic fate of deoxynivalenol-3-glucoside during digestion. Toxicol. Lett. 2011, 206, 264–267. [Google Scholar] [CrossRef] [PubMed]
- Dall’Erta, A.; Cirlini, M.; Dall’Asta, M.; Del Rio, D.; Galaverna, G.; Dall’Asta, C. Masked mycotoxins are efficiently hydrolyzed by human colonic microbiota releasing their aglycones. Chem. Res. Toxicol. 2013, 26, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, S.A.; Boddu, J.; Berthiller, F.; Hametner, C.; Stupar, R.M.; Adam, G.; Muehlbauer, G.J. Transcriptome analysis of the barley-deoxynivalenol interaction: Evidence for a role of glutathione in deoxynivalenol detoxification. Mol. Plant Microbe Interact. 2010, 23, 962–976. [Google Scholar] [CrossRef] [PubMed]
- Kluger, B.; Bueschl, C.; Lemmens, M.; Berthiller, F.; Häubl, G.; Jaunecker, G.; Adam, G.; Krska, R.; Schuhmacher, R. Stable isotopic labelling-assisted untargeted metabolic profiling reveals novel conjugates of the mycotoxin deoxynivalenol in wheat. Anal. Bioanal. Chem. 2013, 405, 5031–5036. [Google Scholar] [CrossRef] [PubMed]
- Schroder, P.; Scheer, C.E.; Diekmann, F.; Stampfl, A. How plants cope with foreign compounds—Translocation of xenobiotic glutathione conjugates in roots of barley (Hordeum vulgare). Environ. Sci. Pollut. Res. 2007, 14, 114–122. [Google Scholar]
- De Zutter, N.; Audenaert, K.; Arroyo-Manzanares, N.; De Boevre, M.; Van Poucke, C.; De Saeger, S.; Haesaert, G.; Smagghe, G. Aphids transform and detoxify the mycotoxin deoxynivalenol via a type II biotransformation mechanism yet unknown in animals. Sci. Rep. 2016, 6, 38640. [Google Scholar] [CrossRef] [PubMed]
- Berthiller, F.; Brera, C.; Crews, C.; Iha, M.H.; Krsha, R.; Lattanzio, V.M.T.; MacDonald, S.; Malone, R.J.; Maragos, C.; Solfrizzo, M.; et al. Developments in mycotoxin analysis: An update for 2013–2014. World Mycotoxin J. 2015, 8, 5–35. [Google Scholar] [CrossRef]
- Warth, B.; Fruhmann, P.; Wiesenberger, G.; Kluger, B.; Sarkanj, B.; Lemmens, M.; Hametner, C.; Frohlich, J.; Adam, G.; Krska, R.; et al. Deoxynivalenol-sulfates: Identification and quantification of novel conjugated (masked) mycotoxins in wheat. Anal. Bioanal. Chem. 2015, 407, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Kluger, B.; Bueschl, C.; Lemmens, M.; Michlmayr, H.; Malachova, A.; Koutnik, A.; Maloku, I.; Berthiller, F.; Adam, G.; Krska, R.; et al. Biotransformation of the mycotoxin deoxynivalenol in Fusarium resistant and susceptible near isogenic wheat lines. PLoS ONE 2015, 10, 119656. [Google Scholar] [CrossRef] [PubMed]
- Uhlig, S.; Stanic, A.; Hofgaard, I.S.; Kluger, B.; Schuhmacher, R.; Miles, C.O. Glutathione-conjugates of deoxynivalenol in naturally contaminated grain are primarily linked via the epoxide group. Toxins 2016, 11, e329. [Google Scholar] [CrossRef] [PubMed]
- Schmeitzl, C.; Warth, B.; Fruhmann, P.; Michlmayr, H.; Malachová, A.; Berthiller, F.; Schuhmacher, R.; Krska, R.; Adam, G. The metabolic fate of deoxynivalenol and its acetylated derivatives in a wheat suspension culture: Identification and detection of DON-15-O-glucoside, 15-acetyl-DON-3-O-glucoside and 15-acetyl-DON-3-sulfate. Toxins 2015, 7, 3112–3126. [Google Scholar] [CrossRef] [PubMed]
- Busman, M.; Poling, S.M.; Maragos, C.M. Observation of T-2 toxin and HT-2 toxin glucosides from Fusarium sporotrichioides by liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS). Toxins 2011, 3, 1554–1568. [Google Scholar] [CrossRef] [PubMed]
- Veprikova, Z.; Vaclavikova, M.; Lacina, O.; Dzuman, Z.; Zachariasova, M.; Hajslova, J. Occurrence of mono- and di-glycosylated conjugates of T-2 and HT-2 toxins in naturally contaminated cereals. World Mycotoxin J. 2012, 5, 231–240. [Google Scholar] [CrossRef]
- Lattanzio, V.M.T.; Visconti, A.; Haidukowski, M.; Pascale, M. Identification and characterization of new Fusarium masked mycotoxins, T2 and HT2 glycosyl derivatives, in naturally contaminated wheat and oats by liquid chromatography–high-resolution mass spectrometry. J. Mass Spectrom. 2012, 47, 466–475. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, E.; Monaci, L.; Pascale, M.; Visconti, A. Fate of deoxynivalenol, T-2 and HT-2 toxins and their glucoside conjugates from flour to bread: An investigation by high-performance liquid chromatography high-resolution mass spectrometry. Food Addit. Contam. 2013, 30, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, H.; Sakamoto, S.; Sago, Y.; Nagashima, H. Detection of type A trichothecene di-glucosides produced in corn by high-resolution liquid chromatography-orbitrap mass spectrometry. Toxins 2013, 5, 590–604. [Google Scholar] [CrossRef] [PubMed]
- Lattanzio, V.M.; Ciasca, B.; Haidukowski, M.; Infantino, A.; Visconti, A.; Pascale, M. Mycotoxin profile of Fusarium langsethiae isolated from wheat in Italy: Production of type-A trichothecenes and relevant glucosyl derivatives. J. Mass Spectrom. 2013, 48, 1291–1298. [Google Scholar] [CrossRef] [PubMed]
- McCormick, S.P.; Kato, T.; Maragos, C.M.; Busman, M.; Lattanzio, V.M.T.; Galaverna, G.; Dall-Asta, C.; Crich, D.; Price, N.P.J.; Kurtzman, C.P. Anomericity of T-2 toxin-glucoside: Masked mycotoxin in cereal crops. J. Agric. Food Chem. 2015, 63, 731–738. [Google Scholar] [CrossRef] [PubMed]
- De Boevre, M.; Vanheule, A.; Audenaert, K.; Bekaert, B.; Di Mavungu, J.D.; Werbrouck, S.; Haesaert, G.; De Saeger, S. Detached leaf in vitro model for masked mycotoxin biosynthesis and subsequent analysis of unknown conjugates. World Mycotoxin J. 2014, 7, 305–312. [Google Scholar] [CrossRef]
- McCormick, S.P.; Price, N.P.J.; Kurtzman, C.P. Glucosylation and other biotransformations of T-2 toxin by yeasts of the Trichomonascus Clade. Appl. Environ. Microbiol. 2012, 78, 8694–8702. [Google Scholar] [CrossRef] [PubMed]
- Nathanail, A.V.; Varga, E.; Meng-Reiterer, J.; Bueschl, C.; Michlmayr, H.; Malachova, A.; Fruhmann, P.; Jestoi, M.; Peltonen, K.; Adam, G.; et al. Metabolism of the Fusarium mycotoxins T-2 toxin and HT-2 toxin in wheat. J. Agric. Food Chem. 2015, 63, 7862–7872. [Google Scholar] [CrossRef] [PubMed]
- Meng-Reiterer, J.; Varga, E.; Nathanail, A.V.; Bueschl, C.; Rechthaler, J.; McCormick, S.P.; Michlmayr, H.; Malachová, A.; Fruhmann, F.; Adam, G.; et al. Tracing the metabolism of HT-2 toxin and T-2 toxin in barley by isotope-assisted untargeted screening and quantitative LC-HRMS analysis. Anal. Bioanal. Chem. 2015, 407, 8019–8033. [Google Scholar] [CrossRef] [PubMed]
- Meng-Reiterer, J.; Bueschl, C.; Rechthaler, J.; Berthiller, F.; Lemmens, M.; Schuhmacher, R. Metabolism of HT-2 toxin and T-2 toxin in oats. Toxins 2016, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Tiemann, U.; Viergutz, T.; Jonas, L.; Schneider, F. Influence of the mycotoxins a- and b- zearalenol and deoxynivalenol on the cell cycle of cultured porcine endometrial cells. Reprod. Toxicol. 2003, 17, 209–218. [Google Scholar] [CrossRef]
- D’Mello, J.P.F.; Placinta, C.M.; Macdonald, A.M.C. Fusarium mycotoxins: A review of global implications for animal health, welfare and productivity. Anim. Feed Sci. Technol. 1999, 80, 183–205. [Google Scholar] [CrossRef]
- Engelhardt, G.; Zill, G.; Wohner, B.; Wallnöfer, P.R. Transformation of the Fusarium mycotoxin zearalenone in maize cell suspension cultures. Naturwissenschaften 1988, 75, 309–310. [Google Scholar] [CrossRef] [PubMed]
- Dellafiora, L.; Galaverna, G.; Righi, F.; Cozzini, P.; Dall’Asta, C. Assessing the hydrolytic fate of the masked mycotoxin zearalenone-14-glucoside—A warning light for the need to look at the “maskedome”. Food Chem Toxicol. 2017, 99, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Schneeweis, I.; Meyer, K.; Engelhardt, G.; Bauer, J. Occurrence of zearalenone-4-β-d-glucopyranoside in wheat. J. Agric. Food Chem. 2002, 50, 1736–1738. [Google Scholar] [CrossRef]
- Poppenberger, B.; Berthiller, F.; Bachmann, H.; Lucyshyn, D.; Peterbauer, C.; Mitterbauer, R.; Schuhmacher, R.; Krska, R.; Glössl, J.; Adam, G. Heterologous expression of Arabidopsis UDP-glucosyltransferases in Saccharomyces cerevisiae for production of zearalenone-4-O-glucoside. Appl. Environ. Microbiol. 2006, 72, 4404–4410. [Google Scholar] [CrossRef] [PubMed]
- Krenn, P.; Berthiller, F.; Schweiger, W.; Hametner, C.; Ludwig, R.; Adam, G.; Krska, R.; Schuhmacher, R. Production of zearalenone-4-glucoside, a-zearalenol-4-glucoside and ß-zearalenol-4-glucoside. Mycotoxin Res. 2007, 23, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Berthiller, F.; Hametner, C.; Krenn, P.; Schweiger, W.; Ludwig, R.; Adam, G.; Krska, R.; Schuhmacher, R. Preparation and characterization of the conjugated Fusarium mycotoxins zearalenone-4-O-β-d-glucopyranoside, α-zearalenol-4-O-β-d-glucopyranoside and β-zearalenol-4-O-β-d-glucopyranoside by MS/MS and two-dimensional NMR. Food Addit. Contam. 2009, 26, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Berthiller, F.; Werner, U.; Sulyok, M.; Krska, R.; Hauser, M.-T.; Schuhmacher, R. Liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) determination of phase II metabolites of the mycotoxin zearalenone in the model plant Arabidopsis thaliana. Food Addit. Contam. 2006, 23, 1194–1200. [Google Scholar] [CrossRef] [PubMed]
- Kovalsky, M.P.; Schweiger, W.; Hametner, C.; Stuckler, R.; Muehlbauer, G.J.; Varga, E.; Krska, R.; Berthiller, F.; Adam, G. Zearalenone-16-O-glucoside: A new masked mycotoxin. J. Agric. Food Chem. 2014, 62, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Michlmayr, H.; Varga, E.; Lupi, F.; Malachová, A.; Hametner, C.; Berthiller, F.; Adam, G. Synthesis of mono- and di-glucosides of zearalenone and α-/β-zearalenol by recombinant barley glucosyltransferase HvUGT14077. Toxins 2017, 9, E58. [Google Scholar] [CrossRef] [PubMed]
- Plasencia, J.; Mirocha, C.J. Isolation and characterization of zearalenone sulfate produced by Fusarium spp. Appl. Environ. Microbiol. 1991, 57, 146–150. [Google Scholar] [PubMed]
- De Boevre, M.; Di Mavungu, J.D.; Landschoot, S.; Audenaert, K.; Eeckhout, M.; Maene, P.; Haesaert, G.; de Saeger, S. Natural occurrence of mycotoxins and their masked forms in food and feed products. World Mycotoxin J. 2012, 5, 207–219. [Google Scholar] [CrossRef]
- Vendl, O.; Crews, C.; MacDonald, S.; Krska, R.; Berthiller, F. Occurrence of free and conjugated Fusarium mycotoxins in cereal-based food. Food Addit. Contam. 2010, 27, 1148–1152. [Google Scholar] [CrossRef] [PubMed]
- González Pereyra, M.L.; Sulyok, M.; Baralla, V.; Dalcero, A.M.; Krska, R.; Chulze, S.; Cavaglieri, L.R. Evaluation of zearalenone, α-zearalenol, β-zearalenol, zearalenone 4-sulfate and β-zearalenol 4-glucoside levels during the ensiling process. World Mycotoxin J. 2014, 7, 291–295. [Google Scholar] [CrossRef]
- El-Sharkaway, S.H.; Selim, M.I.; Afifi, M.S.; Halaweish, F.T. Microbial transformation of zearalenone to a zearalenone sulfate. Appl. Environ. Microbiol. 1991, 57, 549–552. [Google Scholar] [PubMed]
- Jard, G.; Liboz, T.; Mathieu, F.; Guyonvarc'h, A.; André, F.; Delaforge, M.; Lebrihi, A.G. Transformation of zearalenone to zearalenone-sulfate by Aspergillus spp. World Mycotoxin J. 2010, 3, 183–191. [Google Scholar] [CrossRef]
- Brodehl, A.; Möller, A.; Kunte, H.J.; Koch, M.; Maul, R. Biotransformation of the mycotoxin zearalenone by fungi of the genera Rhizopus and Aspergillus. Microbiol. Lett. 2014, 359, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Berthiller, F.; Lemmens, M.; Werner, U.; Krska, R.; Hauser, M.T.; Adam, G.; Schuhmacher, R. Short review: Metabolism of the Fusarium mycotoxins deoxynivalenol and zearalenone in plants. Mycotoxin Res. 2007, 23, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Pierron, A.; Mimoun, S.; Murate, L.S.; Loiseau, N.; Lippi, Y.; Bracarense, A.P.F.L.; Liaubet, L.; Schatzmayr, G.; He, J.W.; Zhou, T.; et al. Microbial biotransformation of DON: Molecular basis for reduced toxicity. Sci. Rep. 2016, 6, 29105. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Dohnal, V.; Huang, L.; Kuca, K.; Yuan, Z. Metabolic pathways of trichothecenes. Drug Metab. Rev. 2010, 42, 250–267. [Google Scholar] [CrossRef] [PubMed]
- Dhanasekaran, D.; Shanmugapriya, S.; Thajuddin, N.; Panneerselvam, A. Panneerselvam, A. Panneerselvam, aflatoxins and aflatoxicosis in human and animals. In Aflatoxins—Biochemistry and Molecular Biology; Guevara-Gonzalez, R.G., Ed.; InTech: London, UK, 2011; pp. 221–254. [Google Scholar]
- Wen, J.K.; Mu, P.Q.; Deng, Y.Q. Mycotoxins: Cytotoxicity and biotransformation in animal cells. Toxicol. Res. 2016, 5, 377–387. [Google Scholar] [CrossRef]
- Turner, P.C.; Hopton, R.P.; Lecluse, Y.; White, K.L.; Fisher, J.; Lebailly, P. Determinants of urinary deoxynivalenol and de-epoxy deoxynivalenol in male farmers from Normandy, France. J. Agric. Food Chem. 2010, 58, 5206–5212. [Google Scholar] [CrossRef] [PubMed]
- Uhlig, S.; Ivanova, L.; Faeste, C.K. Enzyme-assisted synthesis and structural characterization of the 3-, 8-, and 15-glucuronides of deoxynivalenol. J. Agric. Food Chem. 2012, 61, 2006–2012. [Google Scholar] [CrossRef] [PubMed]
- Welsch, T.; Humpf, H.U. HT-2 toxin 4-glucuronide as new T-2 toxin metabolite: Enzymatic synthesis, analysis, and species specific formation of T-2 and HT-2 toxin glucuronides by rat, mouse, pig, and human liver microsomes. J. Agric. Food Chem. 2012, 60, 10170–10178. [Google Scholar] [CrossRef] [PubMed]
- Yunus, A.W.; Valenta, H.; Abdel-Raheem, S.M.; Döll, S.; Dänicke, S.; Böhm, J. Blood plasma levels of deoxynivalenol and its de-epoxy metabolite in broilers after a single oral dose of the toxin. Mycotoxin Res. 2010, 26, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Wan, D.; Huang, L.; Pan, Y.; Wu, Q.; Chen, D.; Tao, Y.; Wang, X.; Liu, Z.; Li, J.; Wang, L.; et al. Metabolism, distribution, and excretion of deoxynivalenol with combined techniques of radiotracing, high-performance liquid chromatography ion trap time-of-flight mass spectrometry, and online radiometric detection. J. Agric. Food Chem. 2014, 62, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Devreese, M.; Antonissen, G.; Broekaert, N.; De Mil, T.; De Baere, S.; Vanhaecke, L.; De Backer, P.; Croubels, S. Toxicokinetic study and oral bioavailability of DON in turkey poults, and comparative biotransformation between broilers and turkeys. World Mycotoxin J. 2015, 8, 533–539. [Google Scholar] [CrossRef]
- Maul, R.; Warth, B.; Schebb, N.H.; Krska, R.; Koch, M.; Sulyok, M. In vitro glucuronidation kinetics of deoxynivalenol by human and animal microsomes and recombinant human UGT enzymes. Arch. Toxicol. 2015, 89, 949–960. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, H. Research on mycotoxin glucosides (masked mycotoxins). JSM Mycotoxins 2016, 66, 21–25. [Google Scholar] [CrossRef]
- Wegulo, S.W.; Baenzige, P.S.; Nopsa, J.H.; Bocku, W.W.; Hallen-Adams, H. Management of Fusarium head blight of wheat and barley. Crop Prot. 2015, 73, 100–107. [Google Scholar] [CrossRef]
- Wu, L.; Qiu, L.; Zhang, H.; Sun, J.; Hu, X.; Wang, B. Optimization for the production of deoxynivalenol and zearalenone by Fusarium graminearum using response surface methodology. Toxins 2017, 9, 57. [Google Scholar] [CrossRef] [PubMed]
- Simsek, S.; Burgess, K.; Whitney, K.L.; Gu, Y.; Qian, S.Y. Analysis of deoxynivalenol and deoxynivalenol-3-glucoside in wheat. Food Control 2012, 26, 287–292. [Google Scholar] [CrossRef]
- Lenc, L.; Czecholiński, G.; Wyczling, D.; Turów, T.; Kaźmierczak, A. Fusarium head blight (FHB) and Fusarium spp. on grain of spring wheat cultivars grown in Poland. J. Plant Prot. Res. 2015, 55, 266–267. [Google Scholar] [CrossRef]
- Wegulo, S. Factors influencing deoxinivalenol accumulation in small grain cereals. Toxins 2012, 4, 1157–1180. [Google Scholar] [CrossRef] [PubMed]
- Simsek, S.; Ovando-Martínez, M.; Ozsisli, B.; Whitney, K.; Ohm, J.B. Occurrence of deoxynivalenol and deoxynivalenol-3-glucoside in hard red spring wheat grown in the USA. Toxins 2013, 5, 2656–2670. [Google Scholar] [CrossRef] [PubMed]
- Muhovski, Y.; Batoko, H.; Jacquemin, J.M. Identification, characterization and mapping of differentially expressed genes in a winter wheat cultivar (Centenaire) resistant to Fusarium graminearum infection. Mol. Biol. Rep. 2012, 39, 9583–9600. [Google Scholar] [CrossRef] [PubMed]
- Nazari, L.; Pattori, E.; Terzi, V.; Morcia, C.; Rossi, V. Influence of temperature on infection, growth, and mycotoxin production by Fusarium langsethiae and F. sporotrichioides in durum wheat. Food Microbiol. 2014, 39, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Lindblad, M.; Gidlund, A.; Sulyok, M.; Börjesson, T.; Krska, R.; Olsen, M.; Fredlund, E. Deoxynivalenol and other selected Fusarium toxins in Swedish wheat—Occurrence and correlation to specific Fusarium species. Int. J. Food Microbiol. 2013, 167, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Edwards, S.G.; Imathiu, S.M.; Ray, R.V.; Back, M.; Hare, M.C. Molecular studies to identify the Fusarium species responsible for HT-2 and T-2 mycotoxins in UK oats. Int. J. Food Microbiol. 2012, 156, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Medina, A.; Magan, N. Comparisons of water activity and temperature impacts on growth of Fusarium langsethiae strains from northern Europe on oat-based media. Int. J. Food Microbiol. 2010, 142, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Nordkvist, E.; Häggblom, P. Fusarium mycotoxin contamination of cereals and bedding straw at Swedish pig farms. Anim. Feed Sci. Technol. 2014, 198, 231–237. [Google Scholar] [CrossRef]
- Van Der Fels-Klerx, H.J.; Klemsdal, S.; Hietaniemi, V.; Lindblad, M.; Ioannou-Kakouri, E.; Van Asselt, E.D. Mycotoxin contamination of cereal grain commodities in relation to climate in North West Europe. Food Addit. Contam. A 2012, 29, 1581–1592. [Google Scholar] [CrossRef] [PubMed]
- Medina, A.; Magan, N. Temperature and water activity effects on production of T-2 and HT-2 by Fusarium langsethiae strains from north European countries. Food Microbiol. 2011, 28, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Strub, C.; Pocaznoi, D.; Lebrihi, A.; Fournier, R.; Mathieu, F. Influence of barley malting operating parameters on T-2 and HT-2 toxinogenesis of Fusarium langsethiae, a worrying contaminant of malting barley in Europe. Food Addit. Contam. 2010, 27, 1247–1252. [Google Scholar] [CrossRef] [PubMed]
- Kokkonen, M.; Ojala, L.; Parikka, P.; Jestoi, M. Mycotoxin production of selected Fusarium species at different culture conditions. Int. J. Food Microbiol. 2010, 143, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Imathiu, S.M.; Edwards, S.G.; Ray, R.V.; Back, M.A. Fusarium langsethiae—A HT-2 and T-2 toxins producer that needs more attention. J. Phytopathol. 2013, 161, 1–10. [Google Scholar] [CrossRef]
- Peraica, M.; Rašić, D. The impact of mycotoxicoses on human history. Arh. Hig. Rada Toksikol. 2012, 63, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Brown-Guedira, G.; Van Sanford, D.; Ohm, H.; Dong, Y.; McKendry, A.L. Novel QTL associated with the Fusarium head blight resistance in Truman soft red winter wheat. Euphytica 2016, 207, 571–592. [Google Scholar] [CrossRef]
- Patriarca, A.; Pinto, V.F. Prevalence of mycotoxins in food and decontamination. Curr. Opin. Food Sci. 2017, 14, 50–60. [Google Scholar] [CrossRef]
- Atanasova-Penichon, V.; Barreau, C.; Richard-Forget, F. Antioxidant secondary metabolites in cereals: Potential involvement in resistance to Fusarium and mycotoxin accumulation. Front Microbiol. 2016, 22, 566. [Google Scholar] [CrossRef] [PubMed]
- Generotti, S.; Cirlini, M.; Šarkanj, B.; Sulyok, M.; Berthiller, F.; Dall’Asta, C.; Suman, M. Formulation and processing factors affecting trichothecene mycotoxins within industrial biscuit-making. Food Chem. 2017, 229, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Kostelanska, M.; Zachariasova, M.; Lacina, O.; Fenclova, M.; Kollos, A.; Jana Hajslova, J. The study of deoxynivalenol and its masked metabolites fate during the brewing process realised by UPLC–TOFMS method. Food Chem. 2011, 126, 1870–1876. [Google Scholar] [CrossRef] [PubMed]
- Zachariasova, M.; Vaclavikova, M.; Lacina, O.; Vaclavik, L.; Hajslova, J. Deoxynivalenol oligoglycosides: New “masked” Fusarium toxins occurring in malt, beer, and breadstuff. J. Agric. Food Chem. 2012, 60, 9280–9291. [Google Scholar] [CrossRef] [PubMed]
- Lancova, K.; Hajslova, J.; Poustka, J.; Krplova, A.; Zachariasova, M.; Dostalek, P.; Sachambula, L. Transfer of Fusarium mycotoxins and ‘masked’ deoxynivalenol (deoxynivalenol-3-glucoside) from field barley through malt to beer. Food Addit. Contam. 2008, 25, 732–744. [Google Scholar] [CrossRef] [PubMed]
- Generotti, S.; Cirlini, M.; Malachova, A.; Sulyok, M.; Berthiller, F.; Dall’Asta, C.; Suman, M. Deoxynivalenol and deoxynivalenol-3-glucoside mitigation through bakery production strategies: Effective experimental dising within industrial rusk-making technology. Toxins 2015, 7, 2773–2790. [Google Scholar] [CrossRef] [PubMed]
- Suman, M.; Generotti, S. Transformation of mycotoxins upon food processing: Masking, binding and degradation phenomena. In Masked Mycotoxins in Food: Formation, Occurrence and Toxicological Relevance; Dall’Asta, C., Berthiller, F., Eds.; RSC Publishing: London, UK, 2015. [Google Scholar]
- Karlovsky, P.; Suman, M.; Berthiller, F.; De Meester, J.; Eisenbrand, G.; Perrin, I.; Dussort, P. Impact of food processing and detoxification treatments on mycotoxin contamination. Mycotoxin Res. 2016, 32, 179–205. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Kuča, K.; Humpf, H.; Klímová, B.; Cramer, B. Fate of deoxynivalenol and deoxynivalenol-3-glucoside during cereal-based thermal food processing: A review study. Mycotoxin Res. 2017, 33, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Kostelanska, M.; Dzuman, Z.; Malachova, A.; Capouchova, I.; Prokinova, E.; Skerikova, A.; Hajslova, J. Effects of milling and baking technologies on levels of deoxynivalenol and its masked form deoxynivalenol-3-glucoside. J. Agric. Food Chem. 2011, 59, 9303–9312. [Google Scholar] [CrossRef] [PubMed]
- Vidal, A.; Ambrosio, A.; Sanchis, V.; Ramos, A.J.; Marín, S. Enzyme bread improvers affect the stability of deoxynivalenol and deoxynivalenol-3-glucoside during breadmaking. Food Chem. 2016, 208, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Commission Recommendation (EU) No 2016/1319 of 29 July 2016 Amending Recommendation 2006/576/EC as Regards Deoxynivalenol, Zearalenone and Ochratoxin A in Pet Food. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv:OJ.L_.2016.208.01.0058.01.ENG&toc=OJ:L:2016:208:FULL (accessed on 19 April 2018).
- EFSA. Scientific Opinion on the risk for human and animal health related to the presence of modified forms of certain mycotoxins in food and feed. EFSA J. 2014, 12, 3916. [Google Scholar]
- De Boevre, M.; Di Mavungu, J.D.; Maene, P.; Audenaert, K.; Deforce, D.; Haesaert, G.; Eeckhout, M.; Callebaut, A.; Berthiller, F.; Van Peteghem, C.; et al. Development and validation of an LC-MS/MS method for the simultaneous determination of deoxynivalenol, zearalenone, T-2-toxin and some masked metabolites in different cereals and cereal-derived food. Food Addit. Contam. 2012, 29, 819–835. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, P.H.; Nielsen, K.F.; Ghorbani, F.; Spliid, N.H.; Nielsen, G.C.; Jørgensen, L.N. Occurrence of different trichothecenes and deoxynivalenol-3-β-d-glucoside in naturally and artificially contaminated Danish cereal grains and whole maize plants. Mycotoxin Res. 2012, 28, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Rao, Q.; Song, S.; Liu, N.; Han, Z.; Hou, J.; Wu, A. Simultaneous determination of major type B trichothecenes and deoxynivalenol-3-glucoside in animal feed and raw materials using improved DSPE combined with LC-MS/MS. J. Chromatogr. B 2014, 963, 75–82. [Google Scholar] [CrossRef] [PubMed]
- De Boevre, M.; Landschoot, S.; Audenaert, K.; Maene, P.; Di Mavungu, J.D.; Eeckhout, M.; Haesaert, G.; De Saeger, S. Occurrence and within field variability of Fusarium mycotoxins and their masked forms in maize crops in Belgium. World Mycotoxin J. 2014, 7, 91–102. [Google Scholar] [CrossRef]
- Wei, W.; Jiao-Jie, M.; Chuan-Chuan, Y.; Xiao-Hui, L.; Hong-Ru, J.; Bing, S.; Feng-Qin, L. Simultaneous determination of masked deoxynivalenol and some important type B trichothecenes in Chinese corn kernels and corn-based products by ultra-performance liquid chromatography-tandem mass spectrometry. J. Agric. Food Chem. 2012, 60, 11638–11646. [Google Scholar] [CrossRef] [PubMed]
- Kovalsky, P.; Kos, G.; Nährer, K.; Schwab, C.; Jenkins, T.; Schatzmayr, G.; Sulyok, M.; Krska, R. Co-occurrence of regulated, masked and emerging mycotoxins and secondary metabolites in finished feed and maize-an extensive survey. Toxins 2016, 8, 363. [Google Scholar] [CrossRef] [PubMed]
- Nathanail, A.V.; Syvähuoko, J.; Malachová, A.; Jestoi, M.; Varga, E.; Michlmayr, H.; Adam, G.; Sieviläinen, E.; Berthiller, F.; Peltonen, K. Simultaneous determination of major type A and B trichothecenes, zearalenone and certain modified metabolites in Finnish cereal grains with a novel liquid chromatography-tandem mass spectrometric method. Anal. Bioanal. Chem. 2015, 407, 4745–4755. [Google Scholar] [CrossRef] [PubMed]
- Bryła, M.; Waśkiewicz, A.; Podolska, G.; Szymczyk, K.; Jędrzejczak, R.; Damaziak, K.; Sułek, A. Occurrence of 26 mycotoxins in the grain of cereals cultivated in Poland. Toxins 2016, 8, 160. [Google Scholar] [CrossRef] [PubMed]
- Bryła, M.; Ksieniewicz-Woźniak, E.; Waśkiewicz, A.; Szymczyk, K.; Jędrzejczak, R. Natural occurrence of nivalenol, deoxynivalenol, and deoxynivalenol-3-glucoside in Polish winter wheat. Toxins 2018, 10, 81. [Google Scholar] [CrossRef] [PubMed]
- Trombete, F.; Barros, A.; Vieira, M.; Saldanha, T.; Venancio, A.; Fraga, M. Simultaneous determination of deoxynivalenol, deoxynivalenol-3-glucoside and nivalenol in wheat grains by HPLC-PDA with immunoaffinity column cleanup. Food Anal. Methods 2016, 9, 2579–2586. [Google Scholar] [CrossRef]
- Jin, Z.; Zhou, B.; Gillespie, J.; Gross, T.; Barr, J.; Simsek, S.; Brueggeman, R.; Schwatz, P. Production of deoxynivalenol (DON) and DON-3Glucoside during the malting of Fusarium infected hard red spring wheat. Food Control 2018, 85, 6–10. [Google Scholar] [CrossRef]
- Palacios, S.A.; Erazo, J.G.; Ciasca, B.; Lattanzio, V.M.T.; Reynoso, M.M.; Farnochi, M.C. Occurrence of deoxynivalenol and deoxynivalenol-3-glucoside in durum wheat from Argentina. Food Chem. 2017, 230, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Malachova, A.; Dzuman, Z.; Veprikova, Z.; Vaclavikova, M.; Zachariasova, M.; Hajslova, J. Deoxynivalenol, deoxynivalenol-3-glucoside, and enniatins: The major mycotoxins found in cereal-based products on the Czech market. J. Agric. Food Chem. 2011, 59, 12990–12997. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Jiang, D.; Zhou, J.; Chen, J.; Li, W.; Zheng, F. Mycotoxins in wheat flour and intake assessment in Shandong province of China. Food Addit. Contam. B 2016, 9, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Zachariasova, M.; Hajslova, J.; Kostelanska, M.; Poustka, J.; Krplova, A.; Cuhra, P.; Hocher, I. Deoxynivalenol and its conjugates in beer: A critical assessment of data obtained by enzyme-linked immunosorbent assay and liquid chromatography coupled to tandem mass spectrometry. Anal. Chim. Acta 2008, 625, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Varga, E.; Malachova, A.; Schwartz, H.; Krska, R.; Berthiller, F. Survey of deoxynivalenol and its conjugates deoxynivalenol-3-glucoside and 3-acetyl-deoxynivalenol in 374 beer samples. Food Addit. Contam. A 2013, 30, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Lattanzio, V.M.; Ciasca, B.; Terzi, V.; Ghizzoni, R.; McCormick, S.P.; Pascale, M. Study of the natural occurrence of T-2 and HT-2 toxins and their glucosyl derivatives from field barley to malt by high-resolution Orbitrap mass spectrometry. Food Addit. Contam. A 2015, 32, 1647–1655. [Google Scholar] [CrossRef] [PubMed]
- Dellafiora, L.; Ruotolo, R.; Perotti, A.; Cirlini, M.; Galaverna, G.; Cozzini, P.; Buschini, A.; Dall’Asta, C. Molecular insights on xenoestrogenic potential of zearalenone-14-glucoside through a mixed in vitro/in silico approach. Food Chem Toxicol. 2017, 108, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Fruhmann, P.; Warth, B.; Hametner, C.; Berthiller, F.; Horkel, E.; Adam, G.; Sulyok, M.; Krska, R.; Fröhlich, J.; Berthiller, F.; et al. Synthesis of deoxynivalenol-3-ß-D-O-glucuronide for its use as biomarker for dietary deoxynivalenol exposure. World Mycotoxin J. 2012, 5, 127–132. [Google Scholar] [CrossRef]
- Mikula, H.; Weber, J.; Svatunek, D.; Skrinjar, P.; Adam, G.; Krska, R.; Hametner, C.; Fröhlich, J. Synthesis of zearalenone-16-β,d-glucoside and zearalenone-16-sulfate: A tale of protecting resorcylic acid lactones for regiocontrolled conjugation. Beilstein J. Org. Chem. 2014, 10, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Habler, K.; Frank, O.; Rychlik, M. Chemical Synthesis of Deoxynivalenol-3-β-d-[13C6]-glucoside and Application in Stable Isotope Dilution Assays. Molecules 2016, 21, 838. [Google Scholar] [CrossRef] [PubMed]
- Amarasinghe, C.C.; Dilantha Fernando, W.G. Comparative analysis of deoxynivalenol biosynthesis related gene expression among different chemotypes of Fusarium graminearum in spring wheat. Front. Microbiol. 2016, 7, 1229. [Google Scholar] [CrossRef] [PubMed]
- Schmeitzl, C.; Varga, E.; Warth, B.; Kugler, K.G.; Malachová, A.; Michlmayr, H.; Wiesenberger, G.; Mayer, K.F.X.; Mewes, H.W.; Krska, R.; et al. Identification and characterization of carboxylesterases from brachypodium distachyon deacetylating trichothecene mycotoxins. Toxins 2016, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, Z.; van der Lee, T.; Chen, W.Q.; Xu, J.; Xu, J.S.; Yang, L.; Yu, D.; Waalwijk, C.; Feng, J. Population genetic analyses of Fusarium asiaticum populations from barley suggest a recent shift favoring 3AcDON producers in southern China. Phytopathology 2010, 100, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Evaluations of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). Available online: http://apps.who.int/food-additives-contaminants-jecfa-database/chemical.aspx?chemID=2947 (accessed on 19 April 2018).
- EFSA. Risks to human and animal health related to the presence of deoxynivalenol and its acetylated and modified forms in food and feed. EFSA J. 2017, 15, 1–345. [Google Scholar]
- Pinton, P.; Tsybulskyy, D.; Lucioli, J.; Laffitte, J.; Callu, P.; Lyazhri, F.; Grosjean, F.; Bracarense, A.P.; Kolf-Clauw, M.; Oswald, I.P. Toxicity of deoxynivalenol and its acetylated derivatives on the intestine: Differential effects on morphology, barrier function, tight junction proteins, and mitogen-activated protein kinases. Toxicol. Sci. 2012, 130, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Iwahashi, Y. Acetylated deoxynivalenol generates differences of gene expression that discriminate trichothecene toxicity. Toxins 2016, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Broekaert, N.; Devreese, M.; Demeyere, K.; Berthiller, F.; Michlmayr, H.; Varga, E.; Adam, G.; Meyera, E.; Croubels, S. Comparative in vitro cytotoxicity of modified deoxynivalenol on porcine intestinal epithelial cells. Food Chem. Toxicol. 2016, 95, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, A.; Braber, S.; Akbari, P.; Kraneveld, A.; Garssen, J.; Fink-Gremmels, J. Deoxynivalenol and its modified forms: Are there major differences? Toxins 2016, 8, 334. [Google Scholar] [CrossRef] [PubMed]
- De Loubresse, N.G.; Prokhorova, I.; Holtkamp, W.; Rodnina, M.V.; Yusupova, G.; Yusupov, M. Structural basis for the inhibition of the eukaryotic ribosome. Nature 2014, 513, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.H.; Dohnal, V.; Kuca, K.; Yuan, Z.H. Trichothecenes: Structure-toxic activity relationships. Curr. Drug Metab. 2013, 14, 641–660. [Google Scholar] [CrossRef] [PubMed]
- Gratz, S.W.; Duncan, G.; Richardson, A.J. The human fecal microbiota metabolizes deoxynivalenol and deoxynivalenol-3-glucoside and may be responsible for urinary deepoxy-deoxynivalenol. Appl. Environ. Microbiol. 2013, 79, 1821–1825. [Google Scholar] [CrossRef] [PubMed]
- Gratz, S.W.; Currie, V.; Richardson, A.J.; Duncan, G.; Holtrop, G.; Farquharson, F.; Louis, P.; Pinton, P.; Oswald, I.P. Porcine small and large intestinal microbiota rapidly hydrolyze the masked mycotoxin deoxynivalenol-3-glucoside and release deoxynivalenol in spiked batch cultures in vitro. Appl. Environ. Microbiol. 2018, 84, 2106–2117. [Google Scholar] [CrossRef] [PubMed]
- De Nijs, M.; Van den Top, H.J.; Portier, L.; Oegema, G.; Kramer, E.; Van Egmond, H.P.; Hoogenboom, L.A.P. Digestibility and absorption of deoxynivalenol-3-β-glucoside in in vitro models. World Mycotoxin J. 2012, 5, 319–324. [Google Scholar] [CrossRef]
- De Angelis, E.; Monaci, L.; Visconti, A. Investigation on the stability of deoxynivalenol and DON-3 glucoside during gastro-duodenal in vitro digestion of a naturally contaminated bread model food. Food Control 2014, 43, 270–275. [Google Scholar] [CrossRef]
- Suzuki, T.; Iwahashi, Y. Low toxicity of deoxynivalenol-3-glucoside in microbial cells. Toxins 2015, 7, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Pierron, A.; Mimoun, S.; Murate, L.S.; Loiseau, N.; Lippi, Y.; Bracarense, A.P.F.L.; Liaubet, L.; Schatzmayr, G.; Berthiller, F.; Moll, W.D.; et al. Intestinal toxicity of the masked mycotoxin deoxynivalenol-3-β-d-glucoside. Arch. Toxicol. 2016, 90, 2037–2046. [Google Scholar] [CrossRef] [PubMed]
- Nagl, V.; Schatzmayr, G. Deoxynivalenol and its masked forms in food and feed. Curr. Opin. Food Sci. 2015, 5, 43–49. [Google Scholar] [CrossRef]
- Tian, Y.; Tan, Y.; Liu, N.; Yan, Z.; Liao, Y.; Chen, J.; de Saeger, S.; Yang, H.; Zhang, Q.; Wu, A. Detoxification of deoxynivalenol via glycosylation represents novel insights on antagonistic activities of Trichoderma when confronted with Fusarium graminearum. Toxins 2016, 8, 335. [Google Scholar] [CrossRef] [PubMed]
- Malekinejad, H.; Colenbrander, B.; Fink-Gremmels, J. Hydroxysteroid dehydrogenases in bovine and porcine granulosa cells convert zearalenone into its hydroxylated metabolites alpha-zearalenol and beta-zearalenol. Vet. Res. Commun. 2006, 30, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Videmann, B.; Mazallon, M.; Tep, J.; Lecoeur, S. Metabolism and transfer of the mycotoxin zearalenone in human intestinal Caco-2 cells. Food Chem. Toxicol. 2008, 46, 3279–3286. [Google Scholar] [CrossRef] [PubMed]
- Belhassen, H.; Jiménez-Díaz, I.; Ghali, R.; Ghorbel, H.; Molina-Molina, J.M.; Olea, N.; Hedili, A. Validation of a UHPLC-MS/MS method for quantification of zearalenone, α-zearalenol, β-zearalenol, α-zearalanol, β-zearalanol and zearalanone in human urine. J. Chromatogr. B 2014, 962, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Othmen, Z.O.B.; El Golli, E.; Abid-Essefi, S.; Bacha, H. Cytotoxicity effects induced by zearalenone metabolites, α zearalenol and β zearalenol, on cultured Vero cells. Toxicology 2008, 252, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Murata, H.; Sultana, P.; Shimada, N.; Yoshioka, M. Structure–activity relationships among zearalenone and its derivatives based on bovine neutrophil chemiluminescence. Vet. Hum. Toxicol. 2003, 45, 18–20. [Google Scholar] [PubMed]
- Luongo, D.; Severino, L.; Bergamo, P.; De Luna, R.; Lucisano, A.; Rossi, M. Interactive effects of fumonisin B1 and alpha-zearalenol on proliferation and cytokine expression in Jurkat T cells. Toxicol. In Vitro 2006, 20, 1403–1410. [Google Scholar] [CrossRef] [PubMed]
- Abid-Essefi, S.; Bouaziz, C.; Golli-Bennour, E.E.; Ouanes, Z.; Bacha, H. Comparative study of toxic effects of zearalenone and its two major metabolites alpha-zearalenol and beta-zearalenol on cultured human Caco-2 cells. J. Biochem. Mol. Toxicol. 2009, 23, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Minervini, F.; Lacalandra, G.M.; Filannino, A.; Nicassio, M.; Visconti, A.; Dell’Aquila, M.E. Effects of in vitro exposure to natural levels of zearalenone and its derivatives on chromatin structure stability in equine spermatozoa. Theriogenology 2010, 73, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Ayed, Y.; Ayed-Boussema, I.; Ouanes, Z.; Bacha, H. In vitro and in vivo induction of chromosome aberrations by alpha- and beta-zearalenols: Comparison with zearalenone. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2011, 276, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Tatay, E.; Meca, G.; Font, G.; Ruiz, M.J. Interactive effects of zearalenone and its metabolites on cytotoxicity and metabolization in ovarian CHO-K1 cells. Toxicol. In Vitro 2014, 28, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Marin, D.E.; Motiu, M.; Taranu, I. Food contaminant zearalenone and its metabolites affect cytokine synthesis and intestinal epithelial integrity of porcine cells. Toxins 2015, 7, 1979–1988. [Google Scholar] [CrossRef] [PubMed]
- Ben Salem, I.; Prola, A.; Boussabbeh, M.; Guilbert, A.; Bacha, H.; Lemaire, C.; Abid-Essefi, S. Activation of ER stress and apoptosis by α- and β-zearalenol in HCT116 cells, protective role of Quercetin. NeuroToxicology 2016, 53, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Drzymala, S.S.; Herrmann, A.J.; Maul, R.; Pfeifer, D.; Garbe, L.A.; Koch, M. In vitro phase I metabolism of cis-zearalenone. Chem. Res. Toxicol. 2014, 27, 1972–1978. [Google Scholar] [CrossRef] [PubMed]
- Frizzell, C.; Ndossi, D.; Verhaegen, S.; Dahl, E.; Eriksen, G.; Sørlie, M.; Ropstad, E.; Muller, M.; Elliotta, C.T.; Connolly, L. Endocrine disrupting effects of zearalenone, alpha- and beta-zearalenol at the level of nuclear receptor binding and steroidogenesis. Toxicol. Lett. 2011, 206, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Molina-Molina, J.M.; Real, M.; Jimenez-Diaz, I.; Belhassen, H.; Hedhili, A.; Torné, P.; Fernández, M.F.; Olea, N. Assessment of estrogenic and anti-androgenic activities of the mycotoxin zearalenone and its metabolites using in vitro receptor-specific bioassays. Food Chem. Toxicol. 2014, 74, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Frizzell, C.; Uhlig, S.; Miles, C.O.; Verhaegen, S.; Elliott, C.T.; Eriksen, G.S.; Sørlie, M.; Ropstad, E.; Connolly, L. Biotransformation of zearalenone and zearalenols to their major glucuronide metabolites reduces estrogenic activity. Toxicol. In Vitro 2015, 29, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Metzler, M.; Pfeiffer, E.; Hildebrand, A. Zearalenone and its metabolites as endocrine disrupting chemicals. World Mycotoxin J. 2010, 3, 385–401. [Google Scholar] [CrossRef]
- Drzymala, S.S.; Binder, J.; Brodehl, A.; Penkert, M.; Rosowski, M.; Garbe, L.A.; Koch, M. Estrogenicity of novel phase i and phase ii metabolites of zearalenone and cis-zearalenone. Toxicon 2015, 105, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Dellaflora, L.; Perotti, A.; Galaverna, G.; Buschini, A.; Dall’Asta, C. On the masked mycotoxin zearalenone-14-glucoside. Does the mask truly hide? Toxicon 2016, 111, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Cirlini, M.; Barilli, A.; Galaverna, G.; Michlmayr, H.; Adam, G.; Barthiller, F.; Dall’Asta, C. Study on the uptake and deglycosylation of the masked forms of zearalenone in human intestinal Caco-2 cell. Food Chem. Toxicol. 2016, 98, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Turner, P.C.; Flannery, B.; Isitt, C.; Ali, M.; Pestka, J. The role of biomarkers in evaluating human health concerns from fungal contaminants in food. Nutr. Res. Rev. 2012, 25, 162–179. [Google Scholar] [CrossRef] [PubMed]
- Broekaert, N.; Devreese, M.; van Bergen, T.; Schauvliege, S.; De Boevre, M.; De Saeger, S.; Vanhaecke, L.; Berthiller, F.; Michlmayr, H.; Malachová, A.; et al. In vivo contribution of deoxynivalenol-3-β-d-glucoside to deoxynivalenol exposure in broiler chickens and pigs: Oral bioavailability, hydrolysis and toxicokinetics. Arch. Toxicol. 2016, 111, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Schwartz-Zimmermann, H.E.; Fruhmann, P.; Dänicke, S.; Wiesenberger, G.; Caha, S.; Weber, J.; Berthiller, F. Metabolism of deoxynivalenol and deepoxy-deoxynivalenol in broiler chickens, pullets, roosters and turkeys. Toxins 2015, 7, 4706–4729. [Google Scholar] [CrossRef] [PubMed]
- Versilovskis, A.; Geys, J.; Huybrechts, B.; Goossens, E.; De Saeger, S.; Callebaut, A. Simultaneous determination of masked forms of deoxynivalenol and zearalenone after oral dosing in rats by LC-MS/MS. World Mycotoxin J. 2012, 5, 303–318. [Google Scholar] [CrossRef]
- Nagl, V.; Schwartz, H.; Krska, R.; Moll, W.D.; Knasmüller, S.; Ritzmann, M.; Adam, G.; Berthiller, F. Metabolism of the masked mycotoxin deoxynivalenol-3-glucoside in rats Toxicol. Lett. 2013, 213, 367–373. [Google Scholar]
- EFSA. Risks for animal health related to the presence of zearalenone and its modified forms in feed. EFSA J. 2017, 15, 4851. [Google Scholar]
- Binder, S.B.; Schwartz-Zimmermann, H.E.; Varga, E.; Bichl, G.; Michlmayr, H.; Adam, G.; Berthiller, F. Metabolism of zearalenone and its major modified forms in pigs. Toxins 2017, 9, 56. [Google Scholar] [CrossRef] [PubMed]
- Broekaert, N.; Devreese, M.; De Mil, T.; Fraeyman, S.; De Boevre, M.; De Saeger, S.; De Backer, P.; Croubels, S. In vivo hydrolysis and toxicokinetics of T-2 toxin and T2-glucoside in broiler chickens. In Proceedings of the 37th Mycotoxin Workshop, Bratislava, Slovakia, 1–3 June 2015; Society of Mycotoxin Research: Munich, Germany, 2015. [Google Scholar]



| Mycotoxin | Cereals/Cereals Based Food | Positive Samples | Min–Max [µg/kg] | References |
|---|---|---|---|---|
| DON | maize | 6/6 (100%) | 255–5245 | [117] |
| 3/3 (100%) | 90–680 | [25] | ||
| 10/10 (100%) | 32–2246 | [118] | ||
| 9/10 (90%) | 74–1382 | [119] | ||
| - | LOQ *–5660 | [120] | ||
| 51–100% | 0.3–4374 | [121] | ||
| maize silage for feed and ready feed | 799/1113 (71.8%) | 1.5–13,488 | [122] | |
| maize products | 91–97% | 0.3–2803 | [121] | |
| barley | 28/34 (82.4%) | LOQ–1180 | [123] | |
| 15/15 (100%) | <60 | [118] | ||
| 20/24 (83%) | 54–1602 | [124] | ||
| wheat | 76/92 (83%) | 11–1265 | [125] | |
| 29/30 (97.6%) | LOQ–5510 | [123] | ||
| 4/6 (66.7%) | 16–150 | [117] | ||
| 5/5 (100%) | 540–5080 | [25] | ||
| 6/6 (100%) | 46–2683 | [118] | ||
| 46/99 (46.5%) | 25–2975 | [124] | ||
| 5/5 (100%) | 4680–36,720 ** | [28] | ||
| 6/13 (46.1%) | LOQ–297 | [126] | ||
| 15/20 (75%) | LOQ–10,130 | [127] | ||
| durum wheat | 84/84 (100%) | 1750 (average) | [128] | |
| triticale | 4/5 (80.0%) | 43–737 | [118] | |
| 20/20 (100%) | 196–1326 | [124] | ||
| rye | 12/12 (100.0%) | <50 | [118] | |
| oat | 31/31 (100%) | 2690 (average) | [123] | |
| 1/6 (16.7%) | 46 | [117] | ||
| 9/11 (81.8%) | 62–2216 | [118] | ||
| 4/4 (100%) | 67–149 | [124] | ||
| bread | 4/6 (66.7%) | 20–102 | [117] | |
| corn flakes | 1/6 (16.7%) | 207 | ||
| white flour products | 16/17 (94.1%) | 5–30 | [129] | |
| mixed flour and whole-corn products | 32/36 (88.9%) | 13–431 | ||
| breakfast cereals | 2/7 (28.6%) | 31–347 | ||
| snacks | 21/34 (61.8%) | 13–320 | ||
| flours | 16/22 (72.3%) | 28–594 | ||
| DON | wheat flour | 349/359 (97.2%) | 1.3–825.9 | [130] |
| 4/4 (100%) | 78.9–325.8 | [126] | ||
| 5/5 (100%) | 940–5890 ** | [28] | ||
| feed for swine | 15/16 (93.8%) | 50–931 | [119] | |
| feed for poultry | 14/15 (93.3%) | 157–1231 | ||
| beer | 18/20 (90%) | LOQ–35.9 | [131] | |
| light beer | 118/217 (54.4%) | <LOQ–89.3 | [132] | |
| wheat beer | 36/46 (78.3%) | <LOQ–49.6 | ||
| dark beer | 14/47 (29.8%) | <LOQ–45.0 | ||
| bock beer | 18/20 (90.0%) | LOQ–27.1 | ||
| alcohol free beer | 5/19 (26.3%) | LOQ–26.1 | ||
| shandy beer | 13/25 (52.0%) | LOQ–12.7 | ||
| 3Ac-DON | maize | 6/6 (100%) | 63–643 | [117] |
| 1/3 (33%) | 10 | [25] | ||
| 0/10 (0%) | - | [119] | ||
| 33–57% | 0.3–368 | [121] | ||
| maize silage for feed and ready feed | 71/1113 (6.4%) | LOD ***–527 | [122] | |
| maize products | 22–73% | 0.3–105 | [121] | |
| barley | 14/34 (41.2%) | LOQ–23.6 | [123] | |
| wheat | 14/30 (46.7%) | LOQ–71.0 | [123] | |
| 1/6 (16.7%) | 17 | [117] | ||
| 3/5 (80.0%) | LOQ–50 | [25] | ||
| oat | 24/31 (77.4%) | LOQ–2720 | [123] | |
| 6/6 (100%) | 34–116 | [117] | ||
| bread | 6/6 (100%) | 29–51 | [117] | |
| corn flakes | 5/6 (83.3%) | 29–52 | [117] | |
| wheat flour | 40/359 (11.1%) | 0.6–3.6 | [130] | |
| feed for swine | 1/16 (6.3%) | 14 | [119] | |
| feed for poultry | 2/15 (13.3%) | 19–21 | ||
| light beer | 0/217 (0%) | - | [132] | |
| wheat beer | 0/46 (0%) | - | ||
| dark beer | 0/47 (0%) | - | ||
| bock beer | 0/20 (0%) | - | ||
| alcohol free beer | 0/19 (0%) | - | ||
| shandy beer | 0/25 (0%) | - | ||
| 15Ac-DON | maize | 6/6 (100%) | 61–792 | [117] |
| 0/3 (0%) | - | [25] | ||
| 6/10 (60%) | 15–79 | [119] | ||
| 48–90% | 0.3–1734 | [121] | ||
| maize silage for feed and ready feed | 128/1113 (11.5%) | LOD–2177 | [122] | |
| maize products | 52–92% | 0.3–1519 | [121] | |
| wheat | 0/6 (0%) | - | [117] | |
| 1/5 (20.0%) | 20 | [25] | ||
| oat | 2/6 (33.3%) | <LOQ–27 | [117] | |
| bread | 1/6 (16.7%) | 18 | ||
| corn flakes | 1/6 (16.7%) | 17 | ||
| wheat flour | 51/359 (14.2%) | 2.0–11.1 | [130] | |
| feed for swine | 10/16 (62.5%) | 10–34 | [119] | |
| feed for poultry | 7/15 (46.7%) | 17–45 | ||
| 3Ac-DON & 15Ac-DON | maize | - | <LOQ–11,900 | [120] |
| barley | 1/24 (4.2%) | 62 | [124] | |
| wheat | 34/99 (34.3%) | 25–98 | ||
| durum wheat | 41/84 (49%) | LOQ–190 | [128] | |
| triticale | 20/20 (100%) | 36–374 | [124] | |
| DON-3Glc | maize | 6/6 (100%) | 36–1003 | [117] |
| 2/3 (67%) | <LOQ–70 | [25] | ||
| 10/10 (100%) | <35 | [118] | ||
| 8/10 (80%) | 14–121 | [119] | ||
| - | <LOQ–1100 | [120] | ||
| 33–83% | 3–978 | [121] | ||
| maize silage for feed and ready feed | 701/1113 (63.0%) | 1.0–3159 | [122] | |
| maize products | 54–86% | 3–844 | [121] | |
| barley | 25/34 (73.5%) | <LOQ–1300 | [123] | |
| 0/15 (0%) | - | [118] | ||
| 7/24 (29%) | 43–277 | [124] | ||
| wheat | 25/30 (83.3%) | <LOQ–922 | [123] | |
| 1/6 (16.7%) | 18 | [117] | ||
| 5/5 (100%) | 50–200 | [25] | ||
| 5/6 (83.3%) | 43–737 | [118] | ||
| 27/99 (27.3%) | 40–356 | [124] | ||
| 25/92 (27%) | 16–138 | [125] | ||
| 4/13 (31%) | <LOQ | [126] | ||
| 15/20 (75%) | 100–1230 | [127] | ||
| 5/5 (100%) | 170–1040 ** | [28] | ||
| durum wheat | 80/84 (94%) | LOQ–850 | [128] | |
| triticale | 1/5 (20.0%) | 109 | [118] | |
| 15/20 (75.0%) | 40–434 | [125] | ||
| DON-3Glc | rye | 0/12 (0%) | - | [118] |
| oat | 27/31 (87.1%) | <LOQ–6600 | [123] | |
| 6/6 (100%) | 28–97 | [117] | ||
| 5/11 (45.5%) | 162–287 | [118] | ||
| 0/4 (0%) | - | [124] | ||
| bread | 5/6 (83.3%) | 26–29 | [117] | |
| corn flakes | 3/6 (50.0%) | 24–28 | ||
| white flour products | 14/17 (82.4%) | 5–30 | [129] | |
| mixed flour and whole-corn products | 28/36 (77.8%) | 7–41 | [129] | |
| breakfast cereals | 6/7 (85.7%) | 19–66 | [129] | |
| snacks | 28/34 (82.4%) | 11–94 | ||
| flours | 15/22 (68.2%) | 5–72 | ||
| wheat flour | 5/5 (100%) | 110–770 ** | [28] | |
| 120/359 (33.4%) | 0.2–15.7 | [130] | ||
| feed for swine | 15/16 (93.8%) | 6–80 | [119] | |
| feed for poultry | 14/15 (93.3%) | 30–107 | ||
| beer | 19/20 (95%) | LOQ–27.5 | [131] | |
| light beer | 142/217 (65.4%) | <LOQ–81.3 | [132] | |
| wheat beer | 32/46 (69.6%) | <LOQ–28.4 | ||
| dark beer | 28/47 (59.6%) | <LOQ–26.2 | ||
| bock beer | 20/20 (100%) | 2.4–33.3 | ||
| alcohol free beer | 9/19 (47.4%) | <LOQ–6.6 | ||
| shandy beer | 20/25 (80.0%) | <LOQ–7.9 | ||
| NIV | barley | 25/34 (73.5%) | <LOQ–302 | [123] |
| wheat | 13/30 (43.3%) | <LOQ–74.0 | ||
| oat | 22/31 (71.0%) | <LOQ–4940 | ||
| wheat flour | 4/4 (100%) | LOQ–140.6 | [126] | |
| NIV-3Glc | barley | 21/34 (61.8%) | <LOQ–65.3 | [123] |
| wheat | 13/30 (43.3%) | <LOQ–33.6 | ||
| oats | 6/31 (16.1%) | <LOQ–58.3 | ||
| HT-2 | barley | 12/34 (35.3%) | <LOQ–39.5 | [123] |
| 8/8 (100%) | 23–233 | [42] | ||
| 18/18 (100%) | 3–213 | [133] | ||
| wheat | 4/4 (100%) | 19–96 | [42] | |
| 19/30 (63.3%) | <LOQ–39.5 | [123] | ||
| 7/9 (77.8%) | 26–85 | [43] | ||
| HT-2 | oat | 8/8 (100%) | 11–187 | [42] |
| 23/31 (74.2%) | <LOQ–1830 | [123] | ||
| 8/9 (88.9%) | 21–851 | [43] | ||
| HT-2-Glc | barley | 18/34 (52.9%) | <LOQ–38.5 | [123] |
| 6/8 (75%) | - **** | [43] | ||
| 17/18 (94.4%) | 0.6–162.8 | [133] | ||
| wheat | 3/4 (75%) | - **** | [42] | |
| 16/30 (53.3%) | <LOQ–15.9 | [123] | ||
| oat | 6/8 (75%) | - **** | [42] | |
| 5/31 (16.1%) | <LOQ–300 | [123] | ||
| HT-2-di-Glc | barley | 2/8 (25%) | - **** | [42] |
| wheat | 0/4 (0%) | - **** | ||
| oats | 0/8 (0%) | - **** | ||
| T-2 | barley | 8/8 (100%) | 41–160 | [42] |
| 18/18 (100%) | 1–154 | [133] | ||
| wheat | 4/4 (100%) | 17–76 | [42] | |
| 5/9 (55.6%) | 11–23 | [43] | ||
| oat | 8/8 (100%) | 32–165 | [42] | |
| 8/9 (88.9%) | 10–377 | [43] | ||
| T-2-Glc | barley | 7/8 (87.5%) | - **** | [42] |
| 9/18 (50.0%) | 0.1–14.5 | [133] | ||
| wheat | 3/4 (75%) | - **** | [42] | |
| oat | 6/8 (75%) | - **** | [42] | |
| T-2-di-Glc | barley | 0/8 (0%) | - **** | [42] |
| wheat | 0/4 (0%) | - **** | ||
| oat | 0/8 (0%) | - **** | ||
| T-2 & HT-2 | maize | - | <LOQ–103 | [120] |
| ZEN | maize | 5/6 (83%) | 59–1071 | [117] |
| - | <LOQ–15,700 | [120] | ||
| maize silage for feed and ready feed | 884/1113 (79.4%) | 20–11,192 | [122] | |
| barley | 2/34 (5.9%) | <LOQ–17 | [123] | |
| 16/24 (67%) | 2–31 | [124] | ||
| wheat | 14/30 (46.7%) | <LOQ–234 | [123] | |
| 5/6 (83.3%) | 12–109 | [117] | ||
| ZEN | wheat | 47/99 (47.5%) | 1–100 | [124] |
| triticale | 20/20 (100%) | 4–86 | [124] | |
| oat | 13/31 (41.9%) | <LOQ–675 | [112] | |
| 4/6 (66.7%) | 13–85 | [117] | ||
| 4/4 (100%) | 5–15 | [124] | ||
| bread | 5/6 (83.3%) | 19–53 | [117] | |
| corn flakes | 5/6 (83.3%) | 34–90 | ||
| ZEN-14Glc | maize | 1/6 (17%) | 274 | [117] |
| barley | 6/34 (17.6%) | <LOQ–9.6 | [123] | |
| wheat | 2/30 (6.7%) | <LOQ–0.6 | [123] | |
| 0/6 (0%) | - | [117] | ||
| oats | 1/31 (3.2%) | <LOQ | [123] | |
| 0/6 (0%) | - | [117] | ||
| bread | 2/6 (33.3%) | 20–20 | [117] | |
| corn flakes | 0/6 (0%) | - | [117] | |
| ZEN-16Glc | barley | 8/34 (23.5%) | <LOQ | [123] |
| wheat | 2/30 (6.7%) | <LOQ–2.8 | ||
| oat | 18/31 (58.1%) | <LOQ–7.9 | ||
| ZEN-14S | maize | 1/6 (17%) | 51 | [117] |
| maize silage for feed and ready feed | 471/1113 (42.3%) | 2–4318 | [122] | |
| barley | 3/34 (8.8%) | <LOQ–26.1 | [123] | |
| wheat | 2/6 (33.3%) | 11 (average) | [117] | |
| 12/30 (40.0%) | <LOQ–22.5 | [123] | ||
| oats | 1/6 (16.7%) | 12 | [117] | |
| 9/31 (29.0%) | <LOQ–220 | [123] | ||
| bread | 1/6 (16.7%) | 24 | [117] | |
| corn flakes | 0/6 (0%) | - | [117] | |
| α-ZEL | maize | 6/6 (100%) | 22–262 | [117] |
| barley | 1/34 (2.9%) | <LOQ–0.6 | [123] | |
| 0/24 (0%) | - | [124] | ||
| wheat | 4/30 (13.3%) | <LOQ–0.7 | [123] | |
| 2/6 (33.3%) | 16 | [117] | ||
| 1/99 (1.0%) | 8 | [124] | ||
| triticale | 0/20 (0%) | - | [124] | |
| α-ZEL-14Glc | oat | 3/31 (9.7%) | <LOQ–2.2 | [123] |
| 2/6 (33.3%) | <LOQ–68 | [117] | ||
| 0/4 (100%) | - | [124] | ||
| bread | 2/6 (33.3%) | 18–110 | [117] | |
| corn flakes | 2/6 (33.3%) | 26–34 | ||
| maize | 1/6 (17%) | 283 | [117] | |
| barley | 8/34 (23.5%) | <LOQ–5.1 | [123] | |
| wheat | 5/30 (16.7%) | <LOQ–4.4 | [123] | |
| 0/6 (0%) | - | [117] | ||
| oat | 0/6 (0%) | - | [117] | |
| 0/31 (0%) | - | [123] | ||
| bread | 0/6 (0%) | - | [117] | |
| corn flakes | 0/6 (0%) | - | ||
| β-ZEL | maize | 4/6 (67%) | 12–103 | [117] |
| barley | 1/34 (2.9%) | <LOQ–2.0 | [123] | |
| wheat | 2/30 (6.7%) | <LOQ–10 | [123] | |
| 1/6 (16.7%) | 49 | [117] | ||
| 30/99 (30.3%) | 2–24 | [124] | ||
| triticale | 0/20 (0%) | - | [124] | |
| oat | 10/31 (32.3%) | <LOQ–6.0 | [123] | |
| 1/6 (16.7%) | 46 | [117] | ||
| 2/4 (50.0%) | 6–9 | [124] | ||
| bread | 3/6 (50.0%) | 55–96 | [117] | |
| corn flakes | 4/6 (50.0%) | 44–63 | ||
| β-ZEL-14Glc | maize | 3/6 (50%) | 92–193 | [117] |
| barley | 1/34 (2.9%) | <LOQ–0.7 | [123] | |
| wheat | 0/6 (0%) | - | [117] | |
| 0/30 (0%) | - | [123] | ||
| oat | 1/6 (16.7%) | 20 | [117] | |
| 0/31 (0%) | - | [123] | ||
| bread | 0/6 (0%) | - | [117] | |
| corn flakes | 0/6 (0%) | - | ||
| α-ZEL & β-ZEL | maize | - | <LOQ–7970 | [120] |
| ZEN-14Glc ZEN-14S α-ZEL-14Glc β-ZEL-14Glc | maize | - | <LOQ–9750 (total) | [120] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bryła, M.; Waśkiewicz, A.; Ksieniewicz-Woźniak, E.; Szymczyk, K.; Jędrzejczak, R. Modified Fusarium Mycotoxins in Cereals and Their Products—Metabolism, Occurrence, and Toxicity: An Updated Review. Molecules 2018, 23, 963. https://doi.org/10.3390/molecules23040963
Bryła M, Waśkiewicz A, Ksieniewicz-Woźniak E, Szymczyk K, Jędrzejczak R. Modified Fusarium Mycotoxins in Cereals and Their Products—Metabolism, Occurrence, and Toxicity: An Updated Review. Molecules. 2018; 23(4):963. https://doi.org/10.3390/molecules23040963
Chicago/Turabian StyleBryła, Marcin, Agnieszka Waśkiewicz, Edyta Ksieniewicz-Woźniak, Krystyna Szymczyk, and Renata Jędrzejczak. 2018. "Modified Fusarium Mycotoxins in Cereals and Their Products—Metabolism, Occurrence, and Toxicity: An Updated Review" Molecules 23, no. 4: 963. https://doi.org/10.3390/molecules23040963
APA StyleBryła, M., Waśkiewicz, A., Ksieniewicz-Woźniak, E., Szymczyk, K., & Jędrzejczak, R. (2018). Modified Fusarium Mycotoxins in Cereals and Their Products—Metabolism, Occurrence, and Toxicity: An Updated Review. Molecules, 23(4), 963. https://doi.org/10.3390/molecules23040963





