Polysaccharide of Atractylodes macrocephala Koidz (PAMK) Relieves Immunosuppression in Cyclophosphamide-Treated Geese by Maintaining a Humoral and Cellular Immune Balance
Abstract
:1. Introduction
2. Results
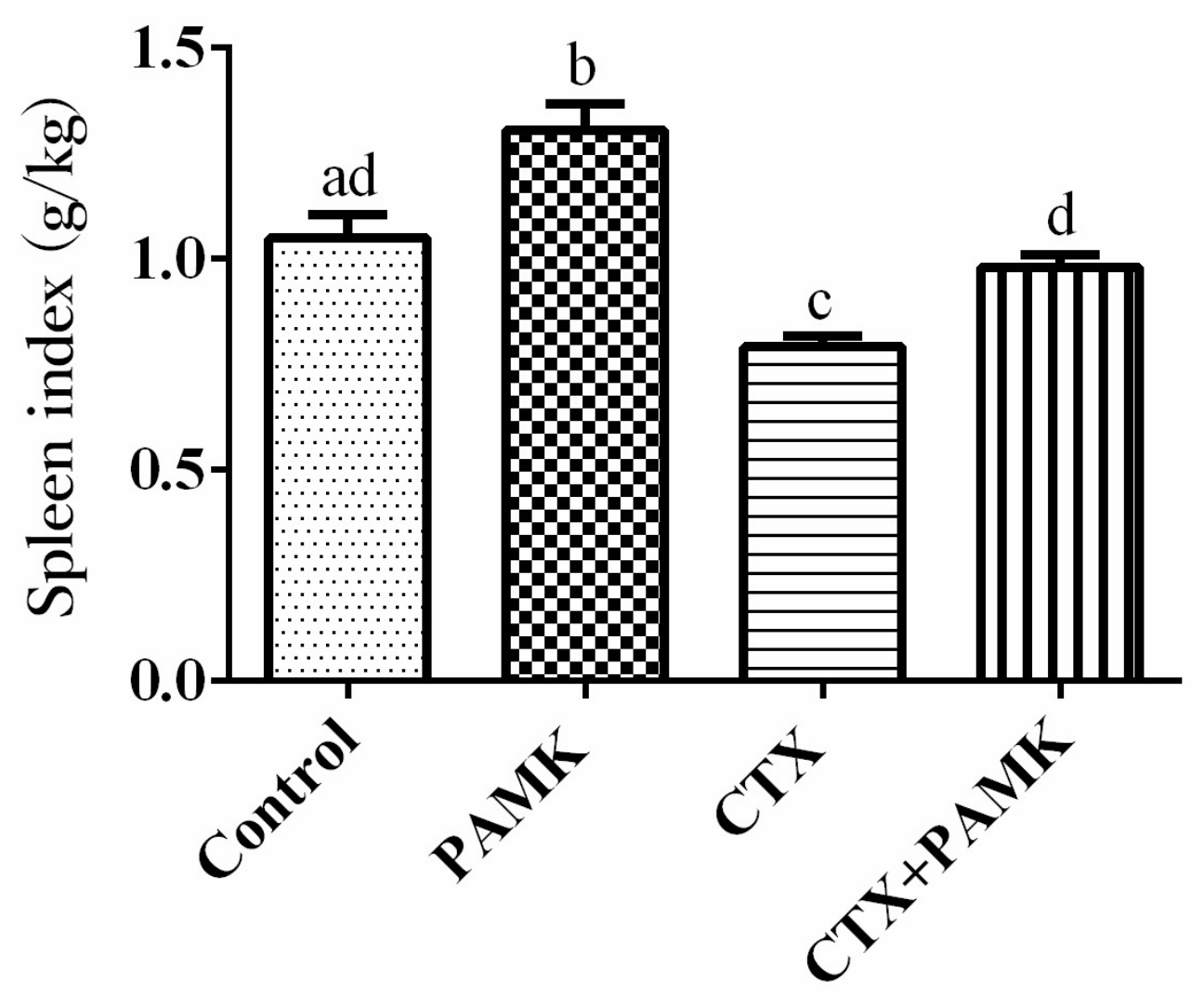
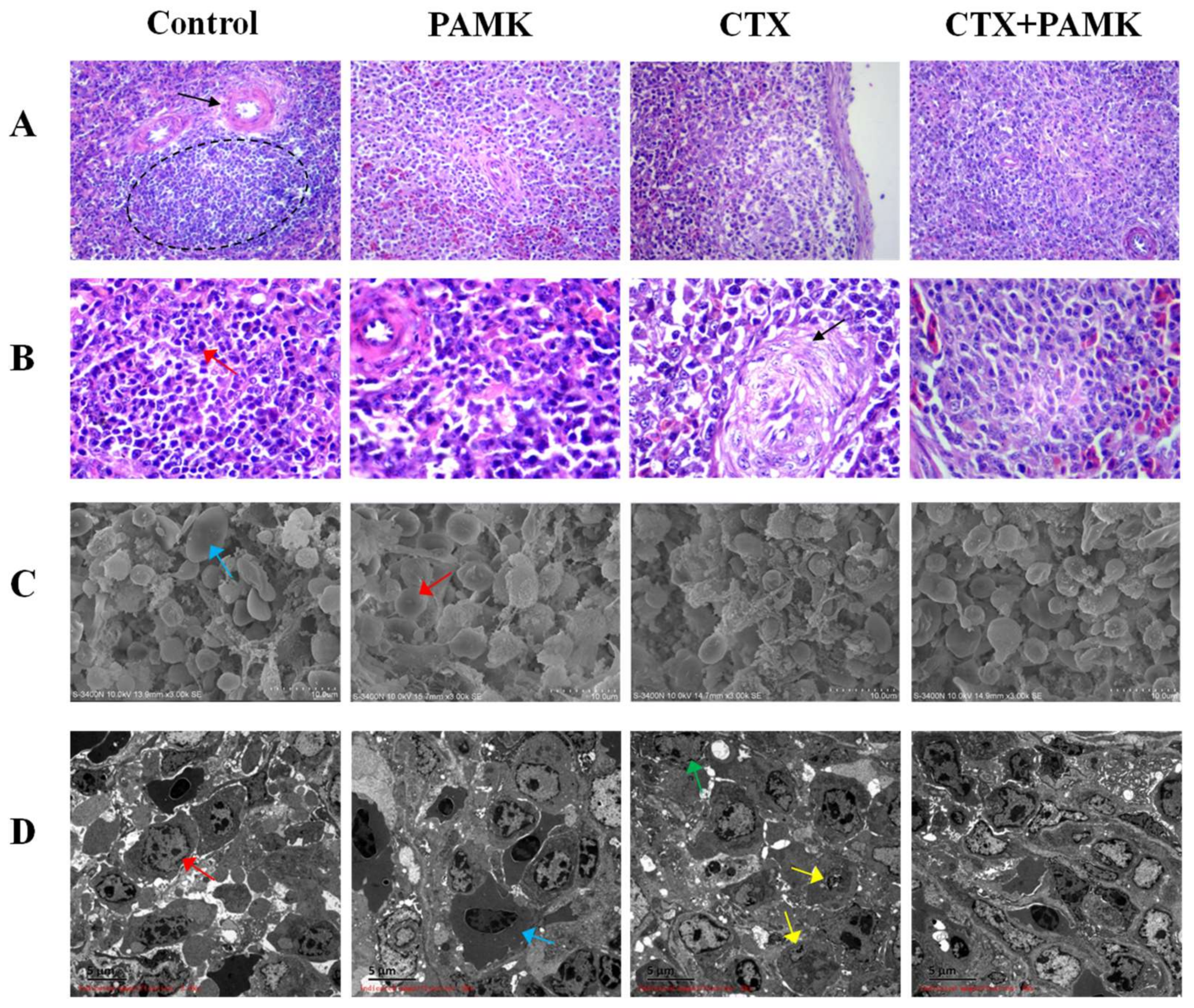
2.1. PAMK Alleviated CTX-Induced Spleen Damage
2.2. PAMK Alleviated the CTX-Induced Leukocyte Ratio Imbalance
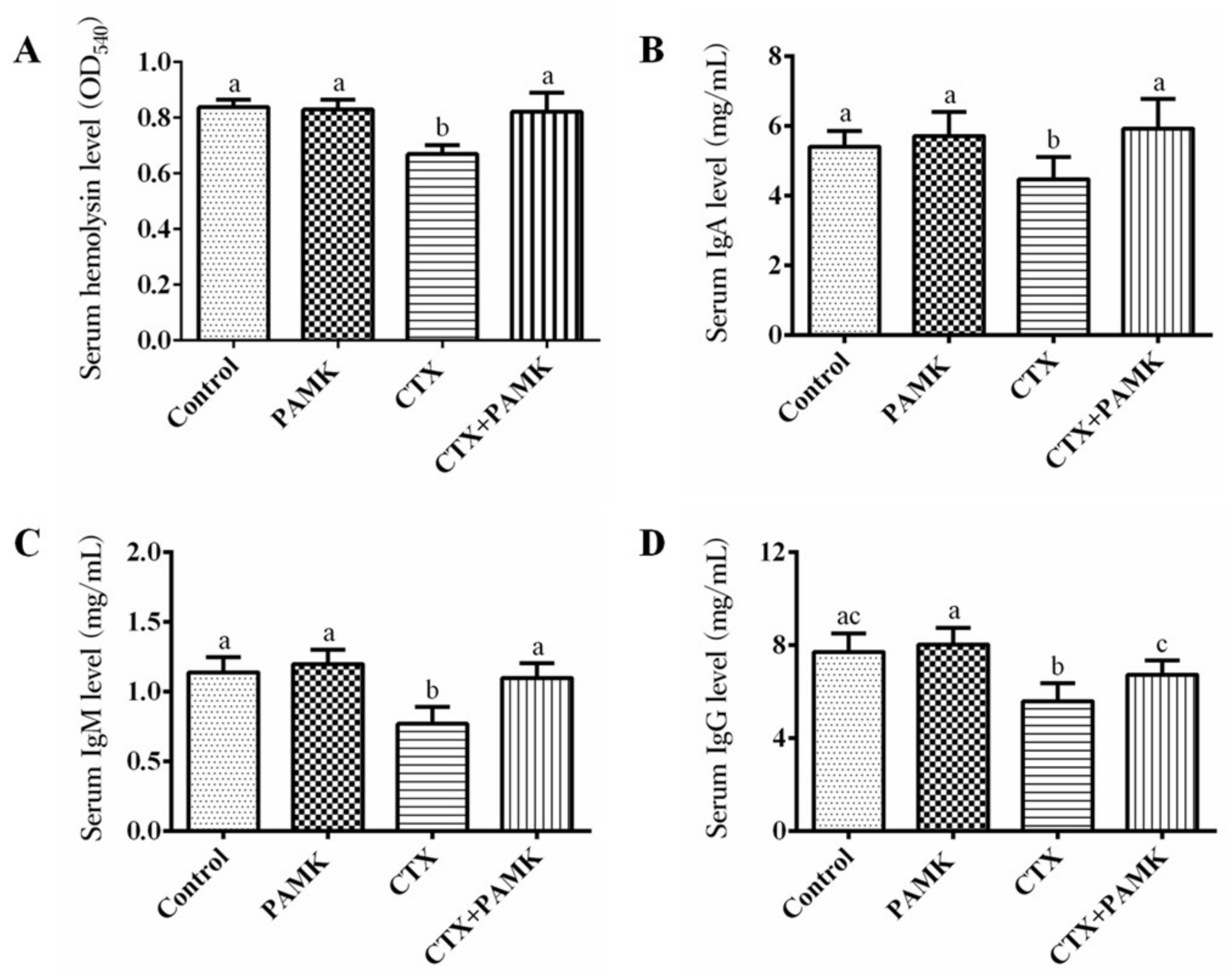
2.3. PAMK Alleviated CTX-Induced Humoral-Immunity Dysfunction
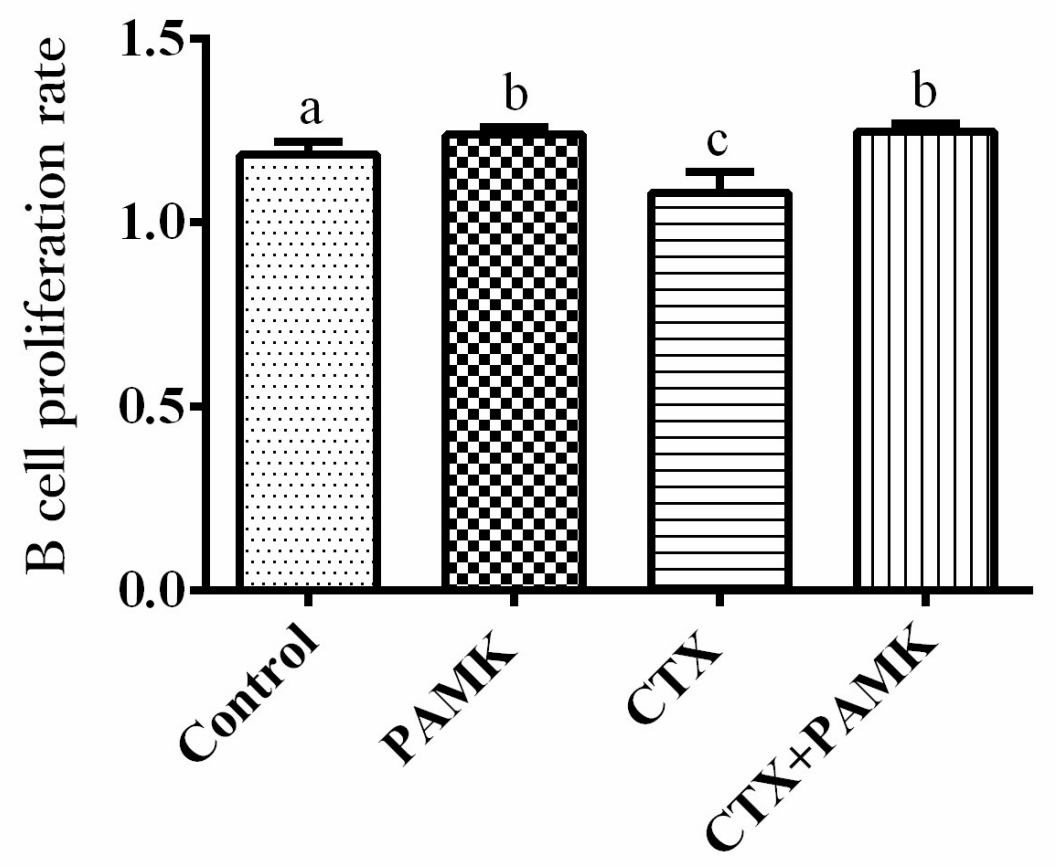
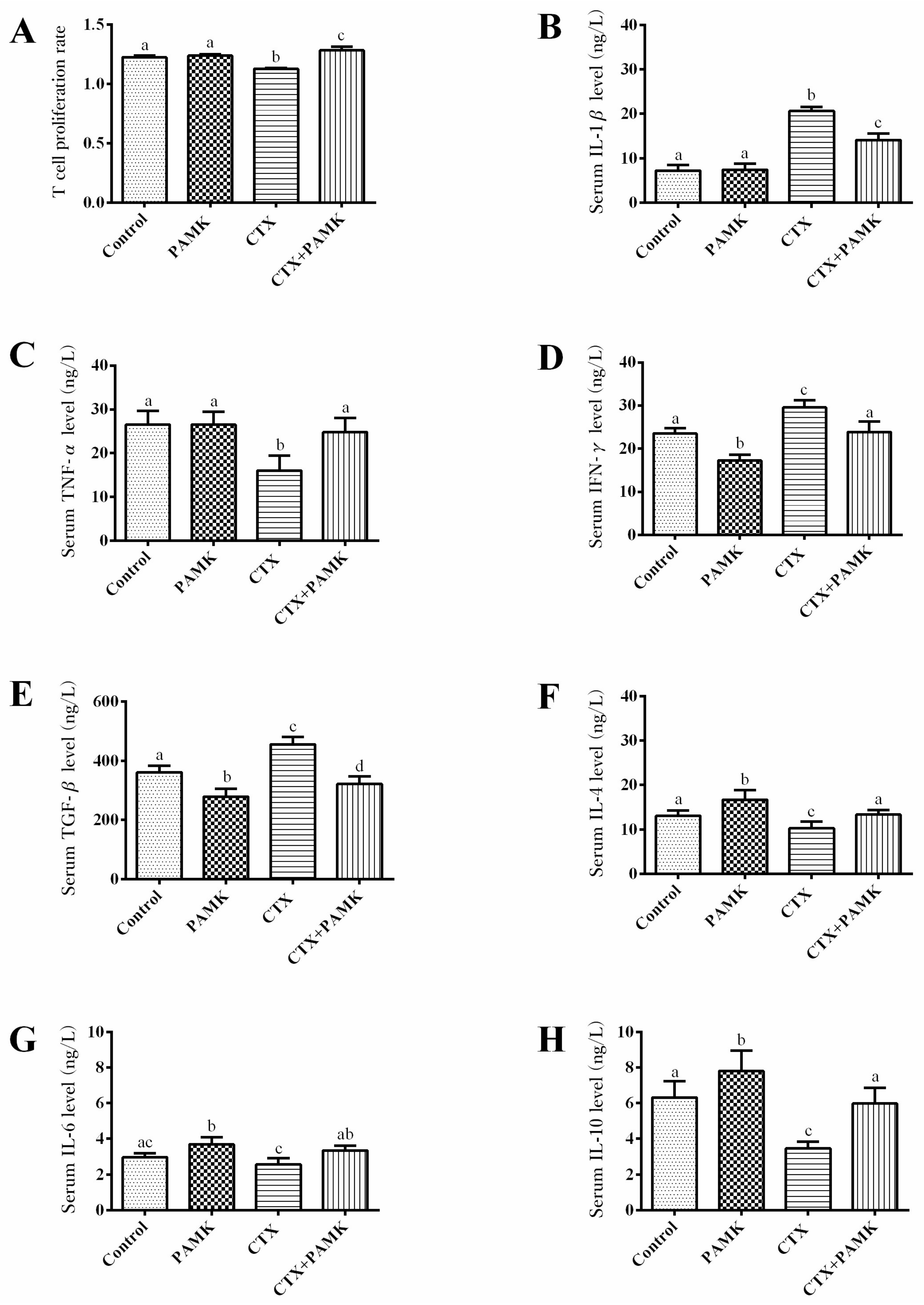
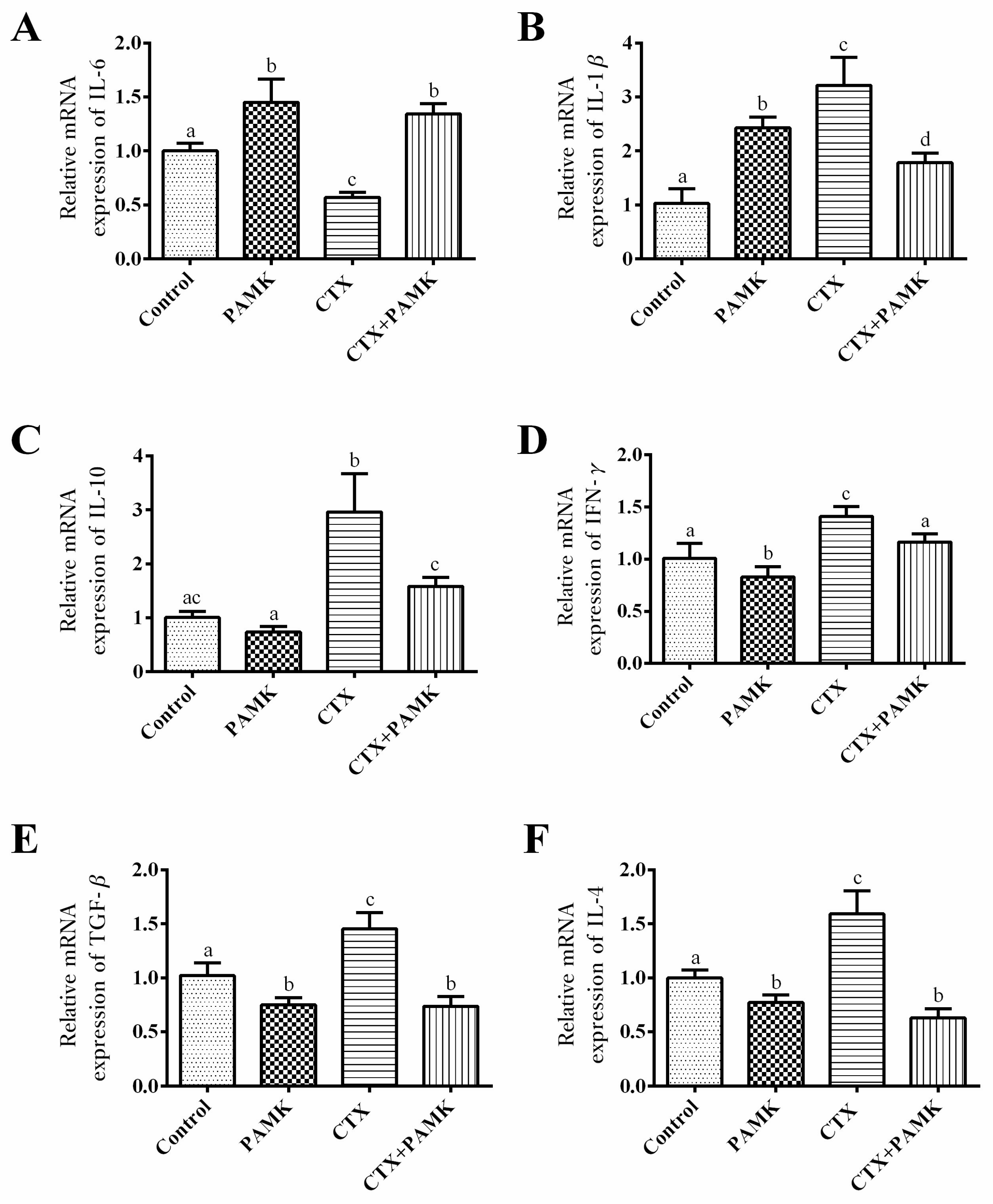
2.4. PAMK Alleviates CTX-Induced Cellular-Immunity Dysfunction
2.5. Principal Component Analysis
3. Discussion
4. Materials and Methods
4.1. Experimental Grouping and Treatments
4.2. The Spleen Index Assay
4.3. Spleen Histology and Ultramicroscopic Morphology Observation
4.4. Leukocyte Counting
4.5. T-Cell and B-Cell Proliferation Assays
4.6. Assays of Serum Cytokines and Immunoglobulins
4.7. The Serum Hemolysin Assay
4.8. Quantitative Reverse-Transcription PCR Analysis
4.9. Principal Component Analysis
4.10. Statistics
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sun, W.; Meng, K.; Qi, C.; Yang, X.; Wang, Y.; Fan, W.; Yan, Z.; Zhao, X.; Liu, J. Immune-enhancing activity of polysaccharides isolated from Atractylodis macrocephalae Koidz. Carbohydr. Polym. 2015, 126, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Li, B.; Cao, N.; Li, W.; Tian, Y.; Huang, Y. The protective effects of polysaccharide of Atractylodes macrocephala Koidz (PAMK) on the chicken spleen under heat stress via antagonizing apoptosis and restoring the immune function. Oncotarget 2017, 8, 70394–70405. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.O.; Kook, S.H.; Lee, J.C. Glycoproteins and Polysaccharides are the Main Class of Active Constituents Required for Lymphocyte Stimulation and Antigen-Specific Immune Response Induction by Traditional Medicinal Herbal Plants. J. Med. Food 2017, 20, 1011–1021. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, X.; Yue, C.; Hou, R.; Chen, J.; Lu, Y.; Li, X.; Li, R.; Liu, C.; Gao, Z.; et al. Effect of selenylation modification on immune-enhancing activity of Atractylodes macrocephala polysaccharide. Int. J. Biol. Macromol. 2015, 72, 1435–1440. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Tian, Y. Selenium and Polysaccharides of Atractylodes macrocephala Koidz Play Different Roles in Improving the Immune Response Induced by Heat Stress in Chickens. Biol. Trace Elem. Res. 2015, 168, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Liu, Z.; Wang, Z.; Yu, S.; Long, T.; Zhou, X.; Bao, Y. Astragalus polysaccharides exerts immunomodulatory effects via TLR4-mediated MyD88-dependent signaling pathway in vitro and in vivo. Sci. Rep. 2017, 7, 44822. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.L.; Huang, W.M.; Zeng, Q.Y. Atractylenolide I protects mice from lipopolysaccharide-induced acute lung injury. Eur. J. Pharmacol. 2015, 765, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Kim, J.J.; Kang, K.Y.; Hwang, Y.H.; Jeong, G.Y.; Jo, S.K.; Jung, U.; Park, H.R.; Yee, S.T. Herbal preparation (HemoHIM) enhanced functional maturation of bone marrow-derived dendritic cells mediated toll-like receptor 4. BMC Complement. Altern. Med. 2016, 16, 67. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Feng, Y.; Li, J.; Mao, R.; Zou, Y.; Feng, W.; Zheng, D.; Wang, W.; Chen, Y.; Yang, L.; et al. Schisandra polysaccharide evokes immunomodulatory activity through TLR 4-mediated activation of macrophages. Int. J. Biol. Macromol. 2014, 65, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Angel-Morales, G.; Noratto, G.; Mertens-Talcott, S.U. Standardized curcuminoid extract (Curcuma longa L.) decreases gene expression related to inflammation and interacts with associated microRNAs in human umbilical vein endothelial cells (HUVEC). Food Funct. 2012, 3, 1286–1293. [Google Scholar] [CrossRef] [PubMed]
- Ji, G.-Q.; Chen, R.-Q.; Zheng, J.-X. Macrophage activation by polysaccharides from Atractylodes macrocephala Koidz through the nuclear factor-κB pathway. Pharm. Biol. 2014, 53, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Liu, J.; Hu, Y.; Wang, D.; Li, Z.; Zhang, J.; Qin, T.; Liu, X.; Liu, C.; Zhao, X.; et al. Astragalus polysaccharide and sulfated epimedium polysaccharide synergistically resist the immunosuppression. Carbohydr. Polym. 2012, 90, 1055–1060. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Meng, X.Y.; Yang, R.L.; Qin, T.; Wang, X.Y.; Zhang, K.Y.; Fei, C.Z.; Li, Y.; Hu, Y.; Xue, F.Q. Cordyceps militaris polysaccharides can enhance the immunity and antioxidation activity in immunosuppressed mice. Carbohydr. Polym. 2012, 89, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.T.; Li, J.; Wang, H.L.; Cheng, W.M.; Zhang, L.; Ge, J.F. Immunomodulating effects of fractioned polysaccharides isolated from Yu-Ping-Feng-Powder in cyclophosphamide-treated mice. Am. J. Chin. Med. 2006, 34, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Xu, P.; Wang, X.; Huang, Y.; Wu, L.; Chen, Y.; Teng, L.; Wang, D. Investigation on the immunomodulatory activities of Sarcodon imbricatus extracts in a cyclophosphamide (CTX)-induced immunosuppressanted mouse model. Saudi Pharm. J. 2017, 25, 460–463. [Google Scholar] [CrossRef] [PubMed]
- Ikezawa, Y.; Nakazawa, M.; Tamura, C.; Takahashi, K.; Minami, M.; Ikezawa, Z. Cyclophosphamide decreases the number, percentage and the function of CD25+ CD4+ regulatory T cells, which suppress induction of contact hypersensitivity. J. Dermatol. Sci. 2005, 39, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, F.; Alp, Ö.S.; Maschmeyer, P.; McGrath, M.; Mashreghi, M.-F.; Hojyo, S.; Chang, H.-D.; Tokoyoda, K.; Radbruch, A. Maintenance of CD8+ memory T lymphocytes in the spleen but not in the bone marrow is dependent on proliferation. Euro. J. Immunol. 2017, 47, 1900–1905. [Google Scholar] [CrossRef] [PubMed]
- Tanahashi, T.; Sekiguchi, N.; Matsuda, K.; Matsumoto, A.; Ito, T.; Nakazawa, H.; Ishida, F. A screening method with lymphocyte percentage and proportion of granular lymphocytes in the peripheral blood for large granular lymphocyte (LGL) leukemia. Int. J. Hematol. 2016, 105, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, J.; Gao, K.; Ye, X.; Lei, W.; Jin, T.; Dong, L.; Xuan, Q.; Zhang, Z.; Feng, M.; et al. Lymphocyte-to-monocyte ratio is associated with prognosis of diffuse large B-cell lymphoma_correlation with CD163 positive M2 type tumor-associated macrophages, not PD-1 positive tumor-infiltrating lymphocytes. Oncotarget 2017, 8, 5414–5425. [Google Scholar] [PubMed]
- Sun, C.; Yang, J.; Pan, L.; Guo, N.; Li, B.; Yao, J.; Wang, M.; Qi, C.; Zhang, G.; Liu, Z. Improvement of icaritin on hematopoietic function in cyclophosphamide-induced myelosuppression mice. Immunopharmacol. Immunotoxicol. 2017, 40, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Zhang, J.; Liu, Y.; Hao, Y.; Yang, C.; Wu, K.; Cao, S.; Wu, C. Immunomodulatory effects of polysaccharopeptide in immunosuppressed mice induced by cyclophosphamide. Mol. Med. Rep. 2013, 8, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.P.; Gao, L.X.; Hou, L.F.; Yang, X.Q.; He, P.L.; Yang, Y.F.; Tang, W.; Yue, J.M.; Li, J.; Zuo, J.P. Astragaloside II triggers T cell activation through regulation of CD45 protein tyrosine phosphatase activity. Acta Pharmacol. Sin. 2013, 34, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Hughes, F.M.; Vivar, N.P.; Kennis, J.G.; Pratt-Thomas, J.D.; Lowe, D.W.; Shaner, B.E.; Nietert, P.J.; Spruill, L.S.; Purves, J.T. Inflammasomes are important mediators of cyclophosphamide-induced bladder inflammation. Am. J. Physiol. Renal Physiol. 2014, 306, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Lu, J.; Peng, X.; Wang, J.; Liu, X.; Chen, X.; Jiang, Y.; Li, X.; Zhang, B. Integrated analysis of microRNA regulatory network in nasopharyngeal carcinoma with deep sequencing. J. Exp. Clin. Cancer Res. 2016, 35, 17. [Google Scholar] [CrossRef] [PubMed]
- Khong, A.; Matheny, T.; Jain, S.; Mitchell, S.F.; Wheeler, J.R.; Parker, R. The Stress Granule Transcriptome Reveals Principles of mRNA Accumulation in Stress Granules. Mol. Cell 2017, 68, 808–820. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Li, W.; Huang, Y.; He, J.; Tian, Y. The effect of selenium and polysaccharide of Atractylodes macrocephala Koidz. (PAMK) on immune response in chicken spleen under heat stress. Biol. Trace Elem. Res. 2014, 160, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Zhang, P.J.; Bao, C.Q.; Gu, Y.L.; Xu, B.H.; Li, C.W.; Li, J.P.; Bo, P.; Liu, X.N. Protective effects of Atractylodes macrocephala polysaccharide on liver ischemia-reperfusion injury and its possible mechanism in rats. Am. J. Chin. Med. 2011, 39, 489–502. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, L.; Ran, X.; Dou, D.; Li, B.; Yang, B.; Li, W.; Koike, K.; Kuang, H. What caused the changes in the usage of Atractylodis Macrocephalae Rhizoma from ancient to current times? J. Nat. Med. 2016, 70, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Cao, N.; Li, W.; Li, B.; Tian, Y.; Xu, D. Transcriptome profiling reveals the immune response of goose T cells under selenium stimuli. Anim. Sci. J. 2017, 88, 2001–2009. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.D.; Wu, Q.; Zhang, Z.W.; Li, S.; Wang, X.L.; Lei, X.G.; Xu, S.W. Selenoprotein W serves as an antioxidant in chicken myoblasts. Biochim. Biophys. Acta 2013, 1830, 3112–3120. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |

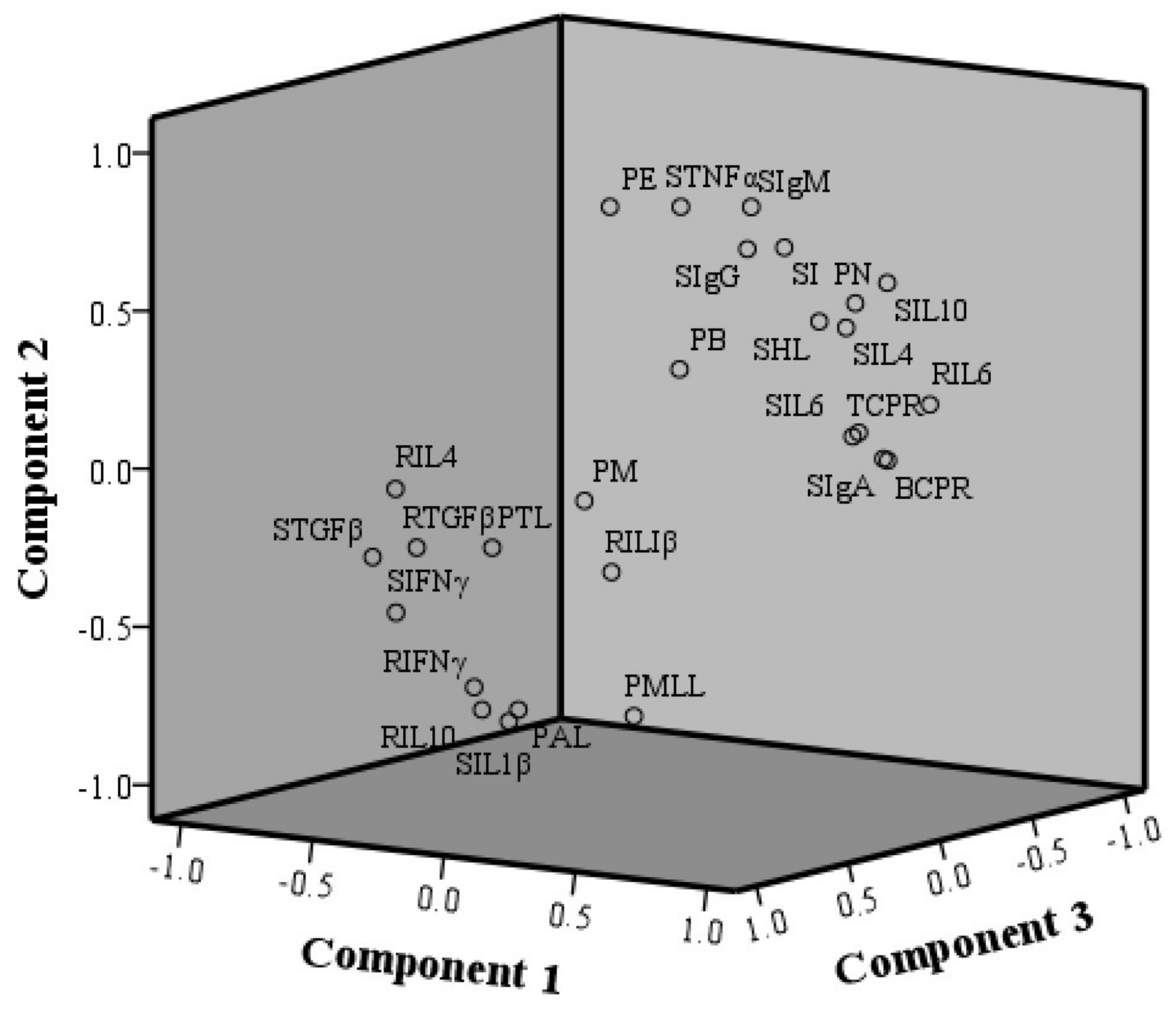
| Component | Eigenvalue | % of Variance | Cumulative % |
|---|---|---|---|
| 1 | 13.119 | 48.589 | 48.589 |
| 2 | 3.888 | 14.398 | 62.988 |
| 3 | 3.222 | 11.934 | 74.922 |
| Indicators | Abbreviation | Component 1 | Component 2 | Component 3 |
|---|---|---|---|---|
| Spleen index | SI | 0.002 | 0.071 | 0.034 |
| Percentage of neutrophil | PN | 0.072 | 0.020 | −0.020 |
| Percentage of eosinophils | PE | −0.009 | 0.222 | 0.205 |
| Percentage of basophils | PB | 0.159 | −0.079 | −0.037 |
| Percentage of monocytes | PM | −0.024 | 0.081 | −0.044 |
| Percentage of medium and large lymphocytes | PMLL | 0.168 | 0.008 | 0.149 |
| Percentage of total lymphocytes | PTL | 0.149 | −0.081 | 0.024 |
| Percentage of atypical lymphocytes | PAL | −0.032 | 0.236 | 0.251 |
| Serum hemolysin level | SHL | −0.103 | 0.032 | 0.066 |
| Serum IgM level | SIgM | 0.089 | −0.067 | −0.021 |
| Serum IgA level | SIgA | −0.008 | 0.019 | −0.160 |
| Serum IgG level | SIgG | 0.036 | 0.004 | −0.142 |
| B-cell proliferation rate | BCPR | −0.039 | 0.035 | 0.161 |
| T-cell proliferation rate | TCPR | 0.028 | −0.107 | 0.046 |
| Serum TNF-α level | STNFα | −0.031 | −0.035 | −0.250 |
| Serum TGF-β level | STGFβ | −0.109 | 0.116 | −0.057 |
| Serum IL-6 level | SIL6 | 0.005 | −0.058 | 0.041 |
| Serum IL-4 level | SIL4 | 0.021 | −0.086 | −0.093 |
| Serum IL-10 level | SIL10 | 0.105 | −0.176 | −0.037 |
| Serum IFN-γ level | SIFNγ | 0.058 | 0.134 | 0.391 |
| Serum IL-1β level | SIL1β | 0.010 | −0.158 | −0.050 |
| Relative mRNA expression of TGF-β | RTGFβ | −0.110 | −0.051 | −0.071 |
| Relative mRNA expression of IL-6 | RIL6 | 0.140 | −0.088 | −0.038 |
| Relative mRNA expression of IL-4 | RIL4 | −0.158 | 0.068 | −0.015 |
| Relative mRNA expression of IL-10 | RIL10 | 0.003 | −0.134 | −0.007 |
| Relative mRNA expression of IFN-γ | RIFNγ | 0.023 | −0.053 | 0.116 |
| Relative mRNA expression of IL-1β | RILIβ | −0.060 | −0.042 | −0.100 |
| Groups | Component 1 | Component 2 | Component 3 | Total | Ranking |
|---|---|---|---|---|---|
| Control | −0.177 | 0.942 | 0.461 | 1.226 | 2 |
| PAMK | 0.513 | 0.374 | −1.348 | −0.462 | 3 |
| CTX | −1.385 | −1.249 | 0.117 | −2.516 | 4 |
| CTX+PAMK | 0.965 | −0.116 | 0.746 | 1.594 | 1 |
| Indicators | Component 1 (48.59%) | Component 2 (14.40%) | Component 3 (11.93%) |
|---|---|---|---|
| SI | 0.319 | 0.644 | −0.284 |
| SHL | 0.563 | 0.457 | −0.125 |
| SIgM | 0.37 | 0.814 | −0.03 |
| SIgA | 0.856 | 0.062 | −0.055 |
| SIgG | 0.184 | 0.627 | −0.275 |
| TCPR | 0.916 | 0.175 | 0.198 |
| BCPR | 0.82 | 0.042 | −0.131 |
| STNFα | 0.191 | 0.815 | 0.097 |
| STGFβ | −0.781 | −0.351 | 0.383 |
| SIL6 | 0.581 | 0.08 | −0.314 |
| SIL4 | 0.323 | 0.342 | −0.614 |
| SIL10 | 0.538 | 0.518 | −0.531 |
| SIFNγ | −0.529 | −0.468 | 0.616 |
| SIL1β | −0.265 | −0.819 | 0.379 |
| PN | 0.214 | 0.379 | −0.819 |
| PE | −0.291 | 0.722 | −0.207 |
| PB | 0.022 | 0.249 | −0.138 |
| PM | −0.109 | −0.133 | 0.192 |
| PMLL | 0.138 | −0.777 | 0.275 |
| PTL | 0.057 | −0.158 | 0.929 |
| PAL | −0.36 | −0.819 | 0.192 |
| RTGFβ | −0.809 | −0.365 | 0.103 |
| RIL6 | 0.857 | 0.198 | −0.306 |
| RIL4 | −0.893 | −0.188 | 0.099 |
| RIL10 | −0.418 | −0.809 | 0.307 |
| RIFNγ | −0.285 | −0.69 | 0.54 |
| RILIβ | −0.325 | −0.447 | −0.265 |
| cDNA | Primer Sequence (5′→3′) |
|---|---|
| IFNG-F | CCAGATTGTTTCCCTGTACTTG |
| IFNG-R | CATCAGAAAGGGTGTCTCTCA |
| IL1β-F | ACGGTGTGGGGACATTCATC |
| IL1β-R | AGGCGAAGCTTCTTCTGTGG |
| IL4-F | GGCATCTACCTCAACTTGCT |
| IL4-R | CTCTTTCGCTACTCGTTGGA |
| IL6-F | ACGATAAGGCAGATGGTGAT |
| IL6-R | TCCAGGTCTTATCCGACTTC |
| IL10-F | ATCATGACATGGACCCGGTA |
| IL10-R | ATTGCTCCATGACAGTTGCT |
| TGF-β-F | CATCACAGAGACAGGAACCTT |
| TGF-β-R | CTTTCACATCACCACTGGAA |
| β-actin-F | GCACCCAGCACGATGAAAAT |
| β-actin-R | GACAATGGAGGGTCCGGATT |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Guo, S.; Xu, D.; Li, B.; Cao, N.; Tian, Y.; Jiang, Q. Polysaccharide of Atractylodes macrocephala Koidz (PAMK) Relieves Immunosuppression in Cyclophosphamide-Treated Geese by Maintaining a Humoral and Cellular Immune Balance. Molecules 2018, 23, 932. https://doi.org/10.3390/molecules23040932
Li W, Guo S, Xu D, Li B, Cao N, Tian Y, Jiang Q. Polysaccharide of Atractylodes macrocephala Koidz (PAMK) Relieves Immunosuppression in Cyclophosphamide-Treated Geese by Maintaining a Humoral and Cellular Immune Balance. Molecules. 2018; 23(4):932. https://doi.org/10.3390/molecules23040932
Chicago/Turabian StyleLi, Wanyan, Sixuan Guo, Danning Xu, Bingxin Li, Nan Cao, Yunbo Tian, and Qingyan Jiang. 2018. "Polysaccharide of Atractylodes macrocephala Koidz (PAMK) Relieves Immunosuppression in Cyclophosphamide-Treated Geese by Maintaining a Humoral and Cellular Immune Balance" Molecules 23, no. 4: 932. https://doi.org/10.3390/molecules23040932
APA StyleLi, W., Guo, S., Xu, D., Li, B., Cao, N., Tian, Y., & Jiang, Q. (2018). Polysaccharide of Atractylodes macrocephala Koidz (PAMK) Relieves Immunosuppression in Cyclophosphamide-Treated Geese by Maintaining a Humoral and Cellular Immune Balance. Molecules, 23(4), 932. https://doi.org/10.3390/molecules23040932





