Poly(vinyl Chloride) Photostabilization in the Presence of Schiff Bases Containing a Thiadiazole Moiety
Abstract
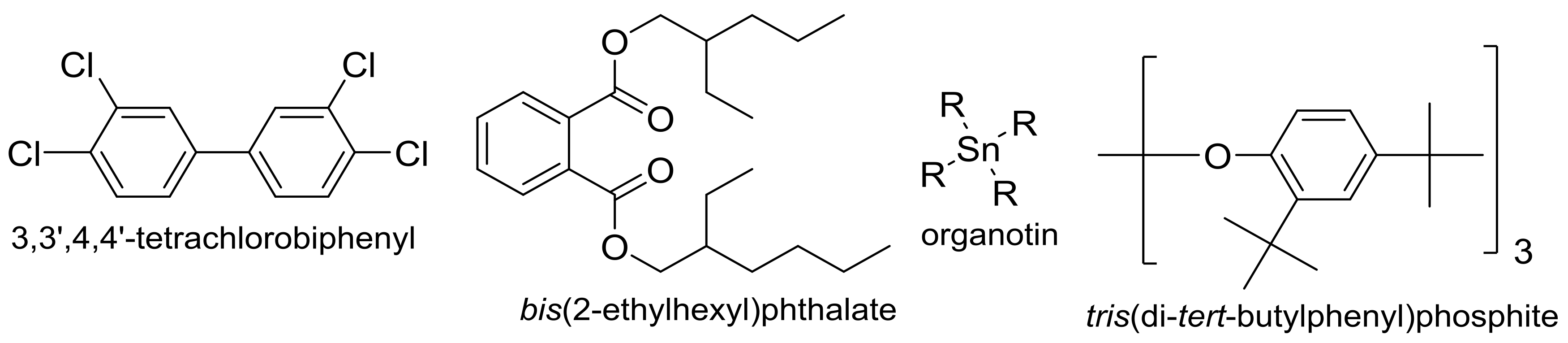
1. Introduction
2. Results and Discussions
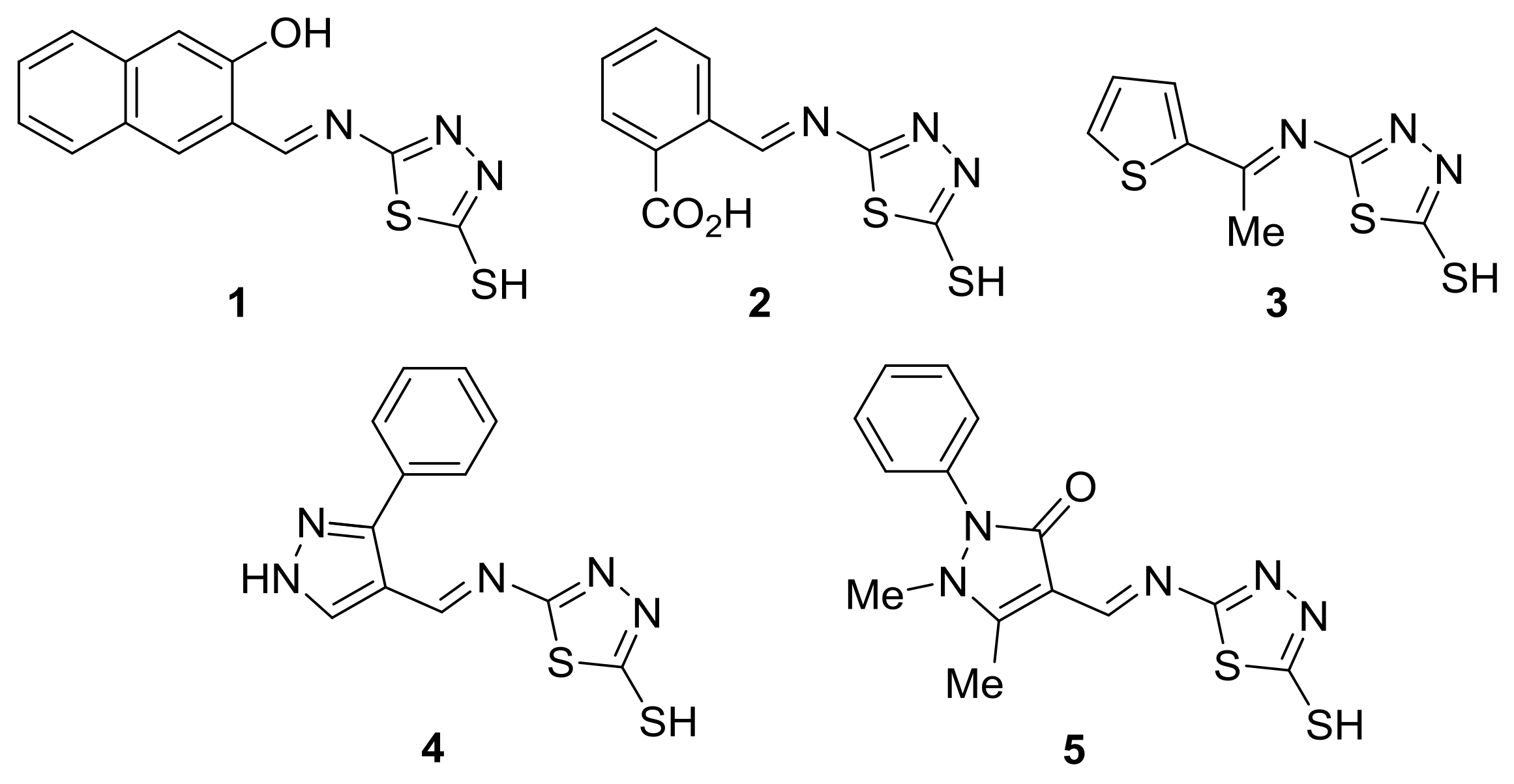
2.1. Schiff Bases 1–5
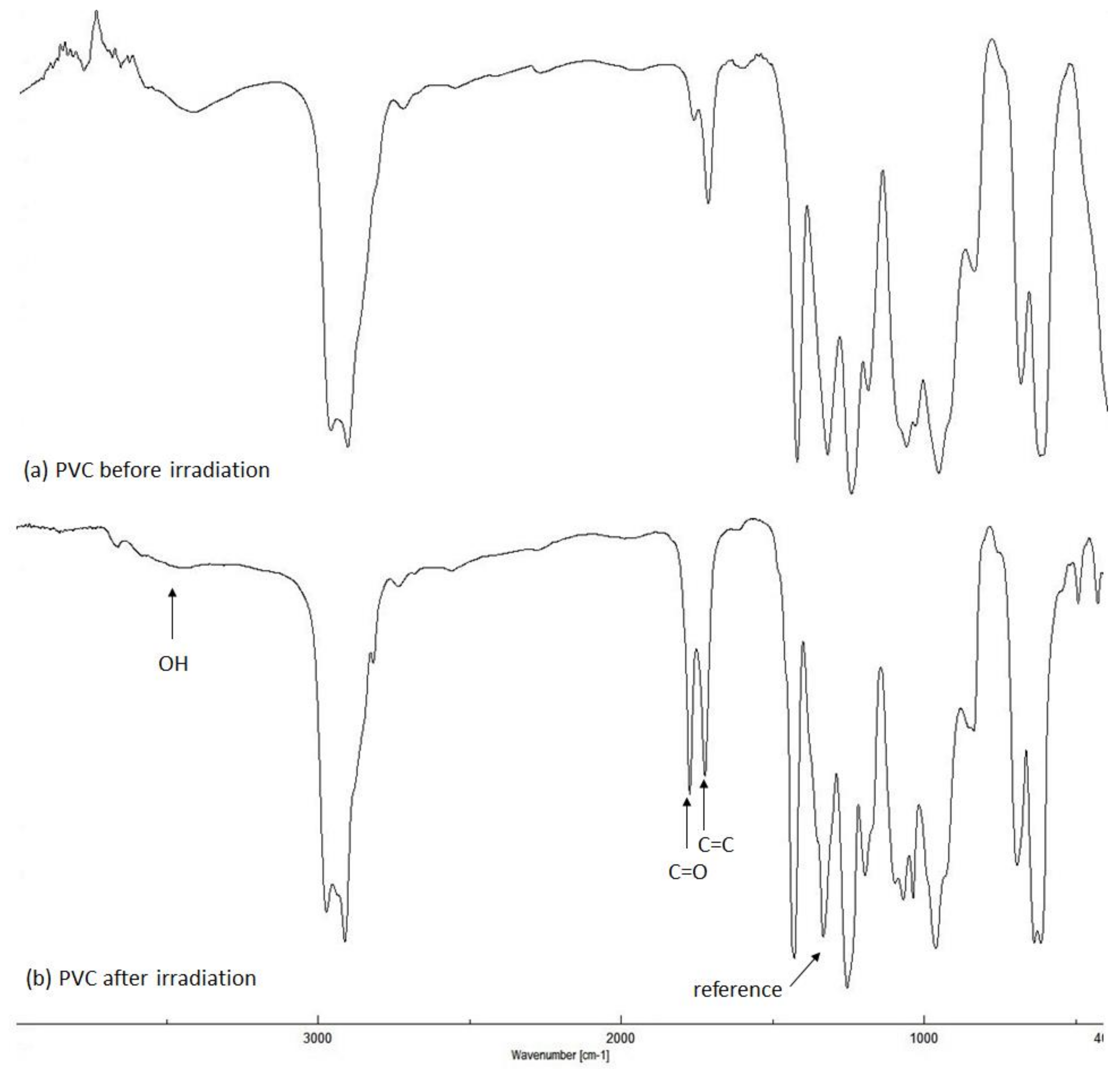
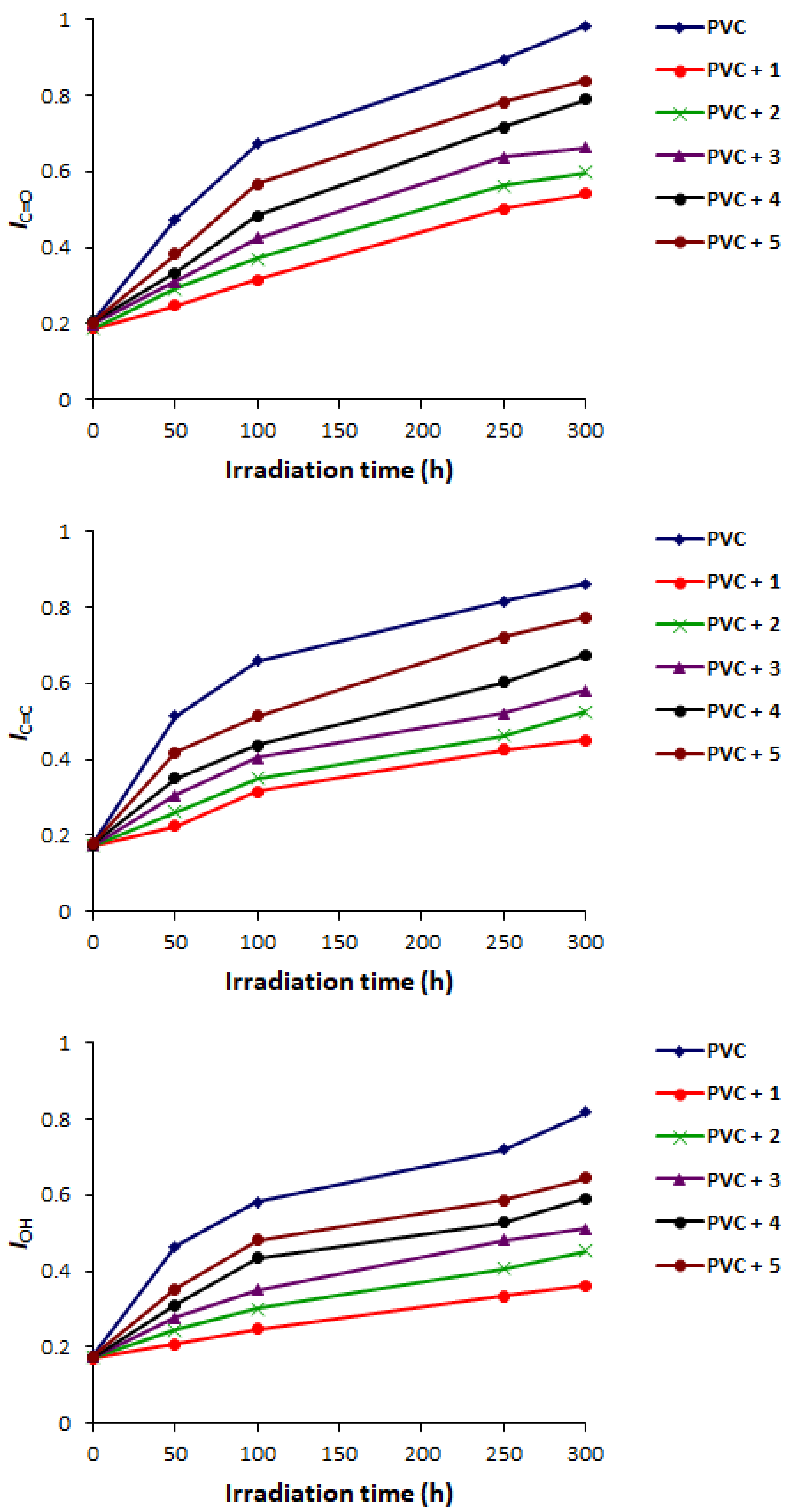
2.2. PVC Photodegradation by Fourier Transform Infrared (FTIR) Spectroscopy
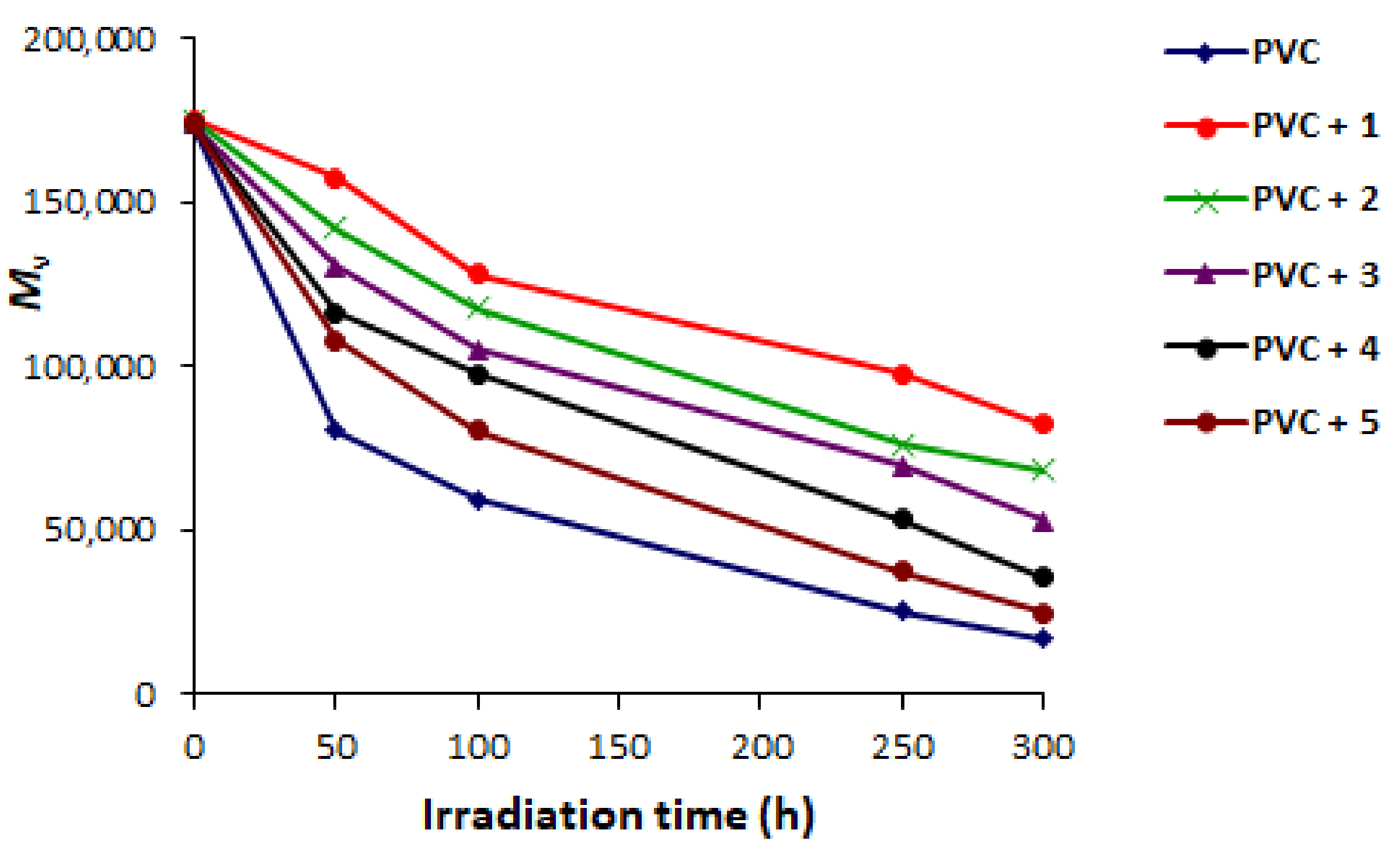
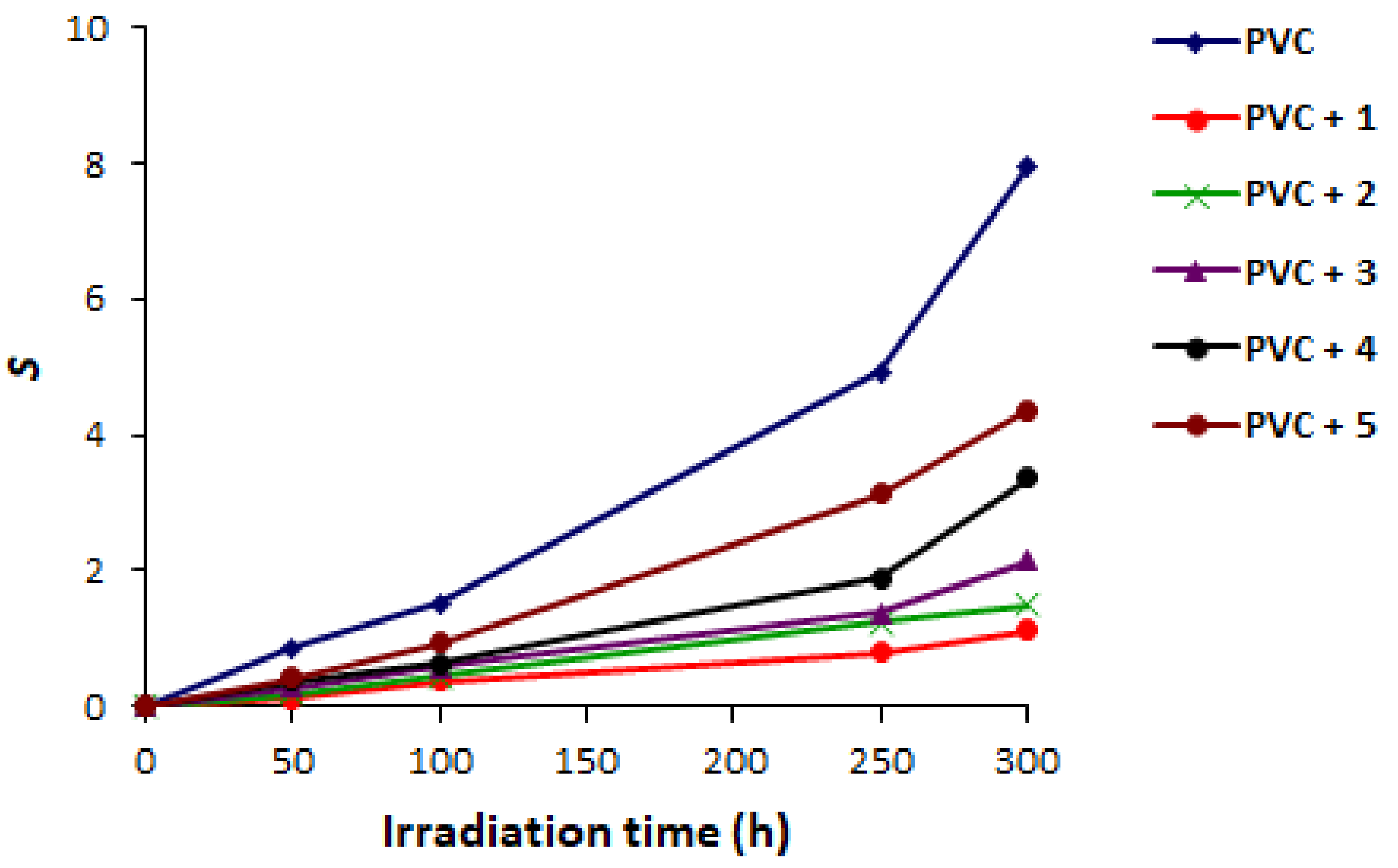
2.3. PVC Photodegradation by Viscosity
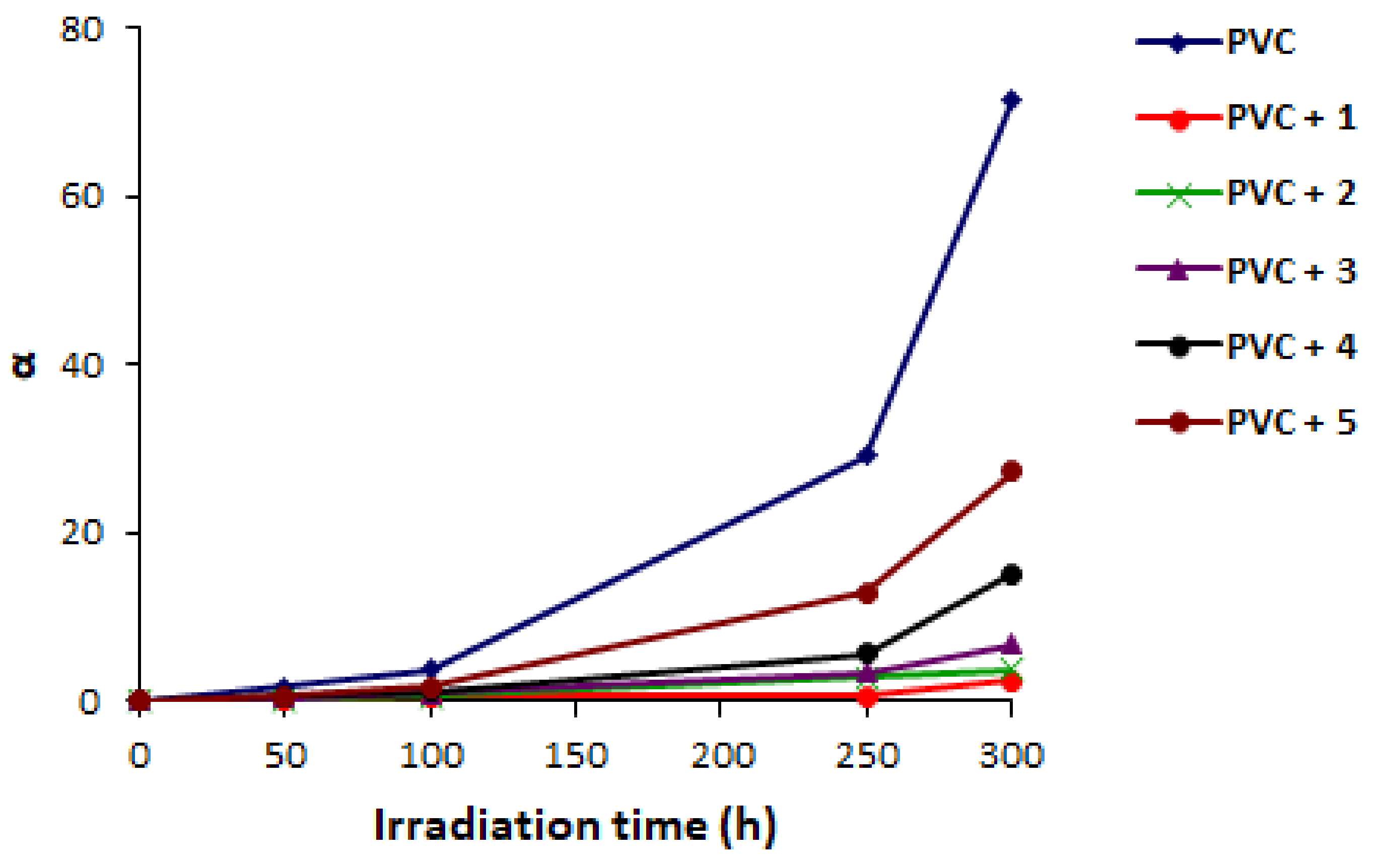
2.4. PVC Photodegradation by Surface Morphology
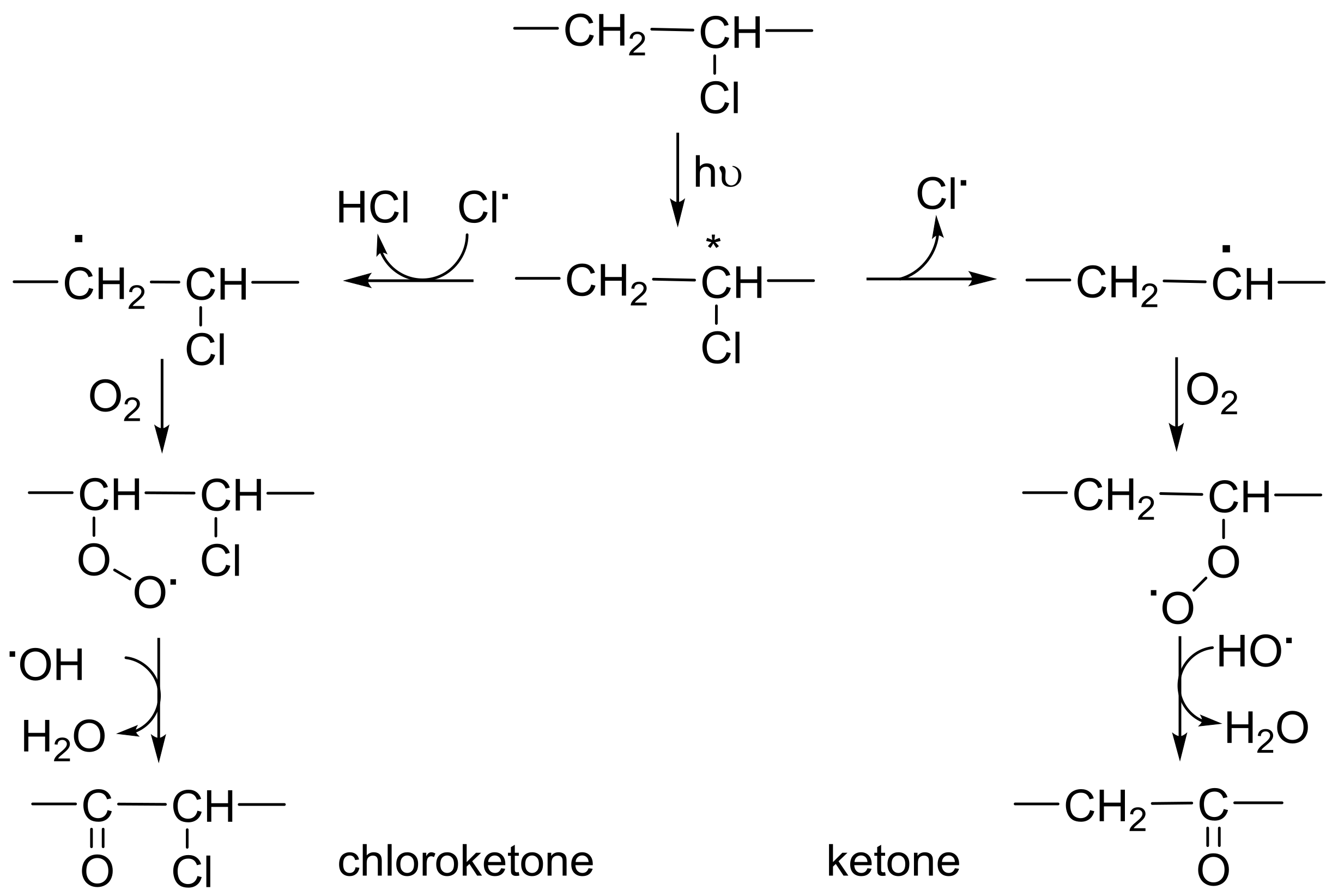
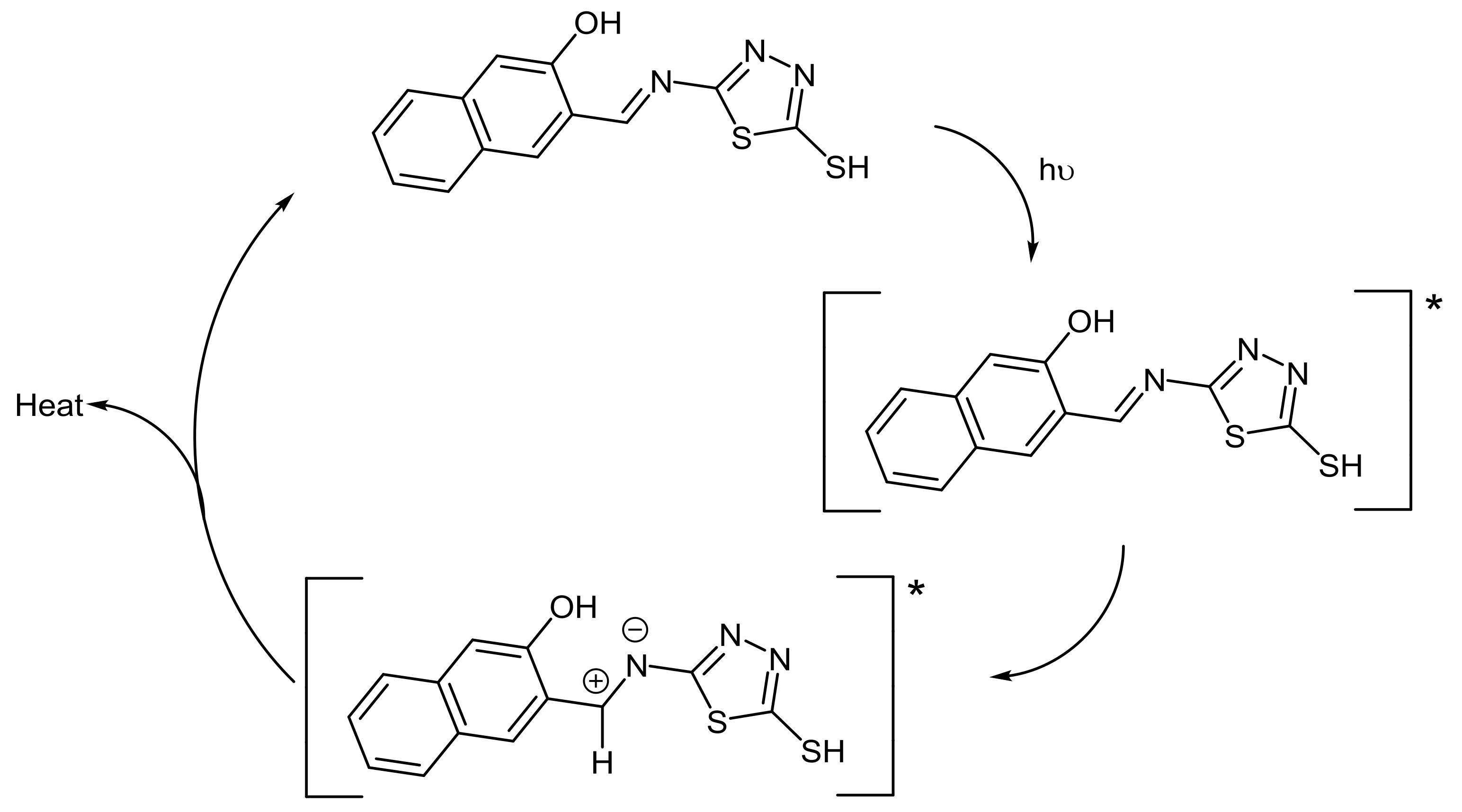
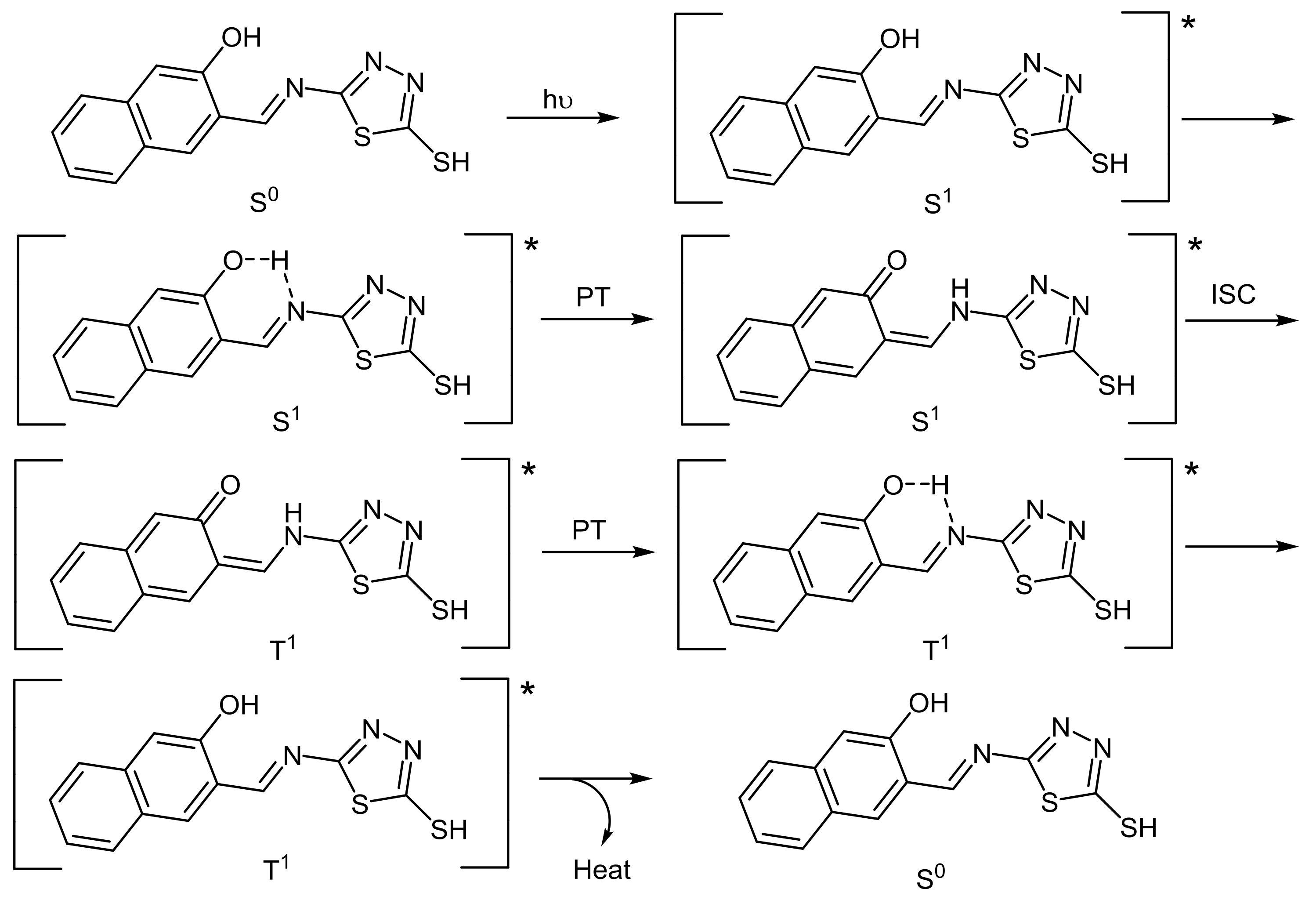
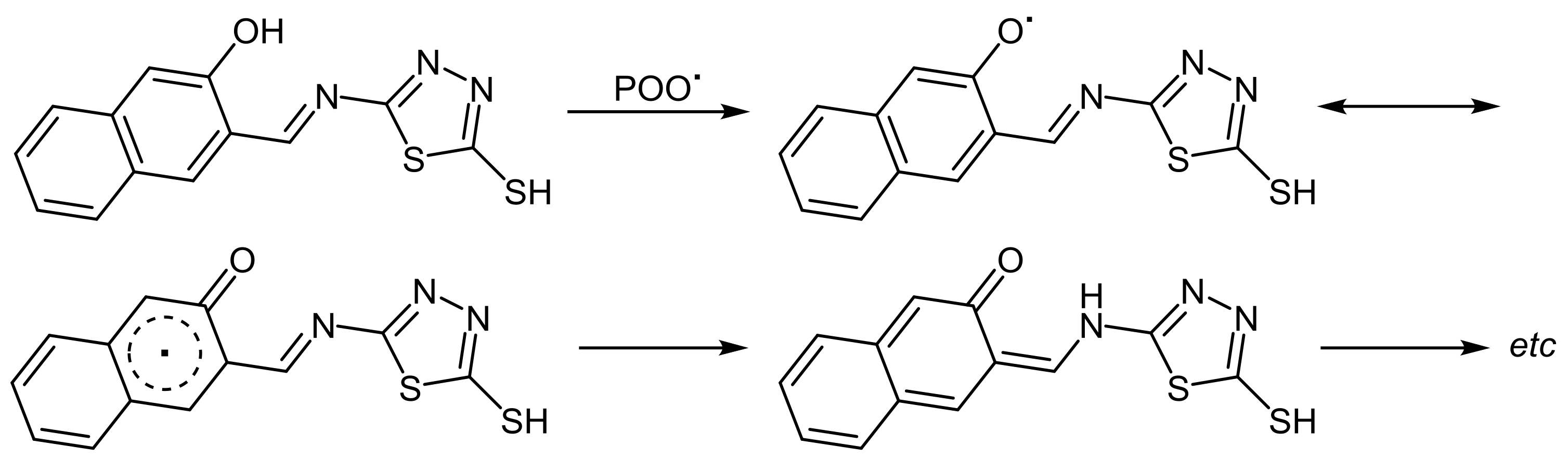
2.5. PVC Photostabilization Mechanisms
3. Experimental
3.1. General
3.2. Synthesis of Schiff Bases
3.3. PVC Films Preparation
3.4. PVC Photodegradation by FTIR Spectrophotometry
3.5. PVC Photodegradation by Viscometry
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Allsopp, M.W.; Vianello, G. Poly(Vinyl Chloride). In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 1992; Volume A21. [Google Scholar]
- Burgess, R.H. Manufacture and Processing of PVC; Applied Science Publishers: London, UK, 1982. [Google Scholar]
- Wickson, E.J. Handbook of Polyvinyl Chloride Formulating; John Wiley and Sons: New York, NY, USA, 1993. [Google Scholar]
- Patrick, S.G. Practical Guide to Polyvinyl Chloride; Rapra Technology Limited: Shrewsbury, UK, 2005. [Google Scholar]
- Chanda, M.; Roy, S.K. Plastics Technology Handbook, 4th ed.; Plastics Engendering Series; CRC Press: Boca Raton, FL, USA, 2006; pp. 1–6. [Google Scholar]
- Harper, C.A. Handbook of Plastics, Elastomers, and Composites, 4th ed.; McGraw Hill: New York, NY, USA, 2002. [Google Scholar]
- Cooray, B.; Scott, G. The effect of thermal processing on PVC–VI. The role of hydrogen chloride. Eur. Polym. J. 1980, 16, 169–177. [Google Scholar] [CrossRef]
- Rabek, J.F. Comprehensive Chemical Kinetic. Degradation of Polymers; Bamford, C.H., Tipper, C.H.F., Eds.; Elsevier: Amsterdam, The Netherlands, 1975; Volume 1. [Google Scholar]
- Jellinek, H.H.G. Aspects of Degradation and Stabilization of Polymers; Elsevier: Amsterdam, The Netherlands, 1978. [Google Scholar]
- Grassie, N.; Scott, G. Polymer Degradation and Stabilisation; Cambridge University Press: Cambridge, UK, 1988. [Google Scholar]
- Chaochanchaikul, K.; Rosarpitak, V.; Sombatsompop, N. Photodegradation profiles of PVC compound and wood/PVC composites under UV weathering. Express Polym. Lett. 2013, 7, 146–160. [Google Scholar] [CrossRef]
- Ye, X.; Pi, H.; Guo, S. A novel route for preparation of PVC sheets with high UV irradiation resistance. J. Appl. Polym. Sci. 2010, 117, 2899–2906. [Google Scholar] [CrossRef]
- Decker, C.; Balandier, M. Photo-oxidation of poly(vinyl chloride). Polym. Photochem. 1981, 1, 221–232. [Google Scholar] [CrossRef]
- Real, L.P.; Rocha, A.P.; Grandette, J.-L. Artificial accelerated weathering of poly(vinyl chloride) for outdoor applications: the evolution of the mechanical and molecular properties. Polym. Degrad. Stab. 2003, 82, 235–243. [Google Scholar] [CrossRef]
- Cadogan, D.F.; Howick, C.J. Plasticizers. In Ullmann's Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2000. [Google Scholar]
- Porta, M.; Zumeta, E. Implementing the Stockholm treaty on persistent organic pollutants. Occup. Environ. Med. 2002, 59, 651–652. [Google Scholar] [CrossRef] [PubMed]
- Grossman, R.F. Mixed metal vinyl stabilizer synergism. II: Reactions with zinc replacing cadmium. J. Vinyl Addit. Technol. 1990, 12, 142–145. [Google Scholar] [CrossRef]
- Li, D.; Xie, L.; Fu, M.; Zhang, J.; Indrawirawan, S.; Zhang, Y.; Tang, S. Synergistic effects of lanthanum-pentaerythritol alkoxide with zinc stearates and with beta-diketone on the thermal stability of poly(vinyl chloride). Polym. Degd. Stab. 2015, 114, 52–59. [Google Scholar] [CrossRef]
- Fu, M.; Li, D.; Liu, H.; Ai, H.; Zhang, Y.; Zhang, L. Synergistic effects of zinc-mannitol alkoxide with calcium/zinc stearates and with β-diketone on thermal stability of rigid poly(vinyl chloride). J. Polym. Res. 2016, 23, 13. [Google Scholar] [CrossRef]
- Yousif, E.; Salimon, J.; Salih, N.; Jawad, A.; Win, Y.-F. New stabilizers for PVC based on some diorganotin(IV) complexes with bezamidoleucine. Arab. J. Chem. 2016, 9, S1394–S1401. [Google Scholar] [CrossRef]
- Martins, L.M.D.R.S.; Hazra, S.; Guedes de Silva, M.F.C.; Pombeiro, A.J.L. A sulfonated Schiff base dimethyltin(IV) coordination polymer: Synthesis, characterization and application as a catalyst for ultrasound- or microwave-assisted Baeyer-Villiger oxidation under solvent-free conditions. RSC Adv. 2016, 6, 78225–78233. [Google Scholar] [CrossRef]
- Ali, M.M.; El-Hiti, G.A.; Yousif, E. Photostabilizng efficiency of poly(vinyl chloride) in the presence of organotin(IV) complexes as photostabilizers. Molecules 2016, 21, 1151. [Google Scholar] [CrossRef] [PubMed]
- Ghazi, D.; El-Hiti, G.A.; Yousif, E.; Ahmed, D.S.; Alotaibi, M.H. The effect of ultraviolet irradiation on the physicochemical properties of poly(vinyl chloride) films containing organotin (IV) complexes as photostabilizers. Molecules 2018, 23, 254. [Google Scholar] [CrossRef] [PubMed]
- Yousif, E.; El-Hiti, G.A.; Hussain, Z.; Altaie, A. Viscoelastic, spectroscopic and microscopic study of the photo irradiation effect on the stability of PVC in the presence of sulfamethoxazole Schiff’s bases. Polymers 2015, 7, 2190–2204. [Google Scholar] [CrossRef]
- Ali, G.Q.; El-Hiti, G.A.; Tomi, I.H.R.; Haddad, R.; Al-Qaisi, A.J.; Yousif, E. Photostability and performance of polystyrene films containing 1,2,4-triazole-3-thiol ring system Schiff bases. Molecules 2016, 21, 1699. [Google Scholar] [CrossRef] [PubMed]
- Yousif, E.; Hasan, A.; El-Hiti, G.A. Spectroscopic, physical and topography of photochemical process of PVC films in the presence of Schiff base metal complexes. Polymers 2016, 8, 204. [Google Scholar] [CrossRef]
- Ahmed, D.S.; El-Hiti, G.A.; Hameed, A.S.; Yousif, E.; Ahmed, A. New tetra-Schiff bases as efficient photostabilizers for poly(vinyl chloride). Molecules 2017, 22, 1506. [Google Scholar] [CrossRef] [PubMed]
- Balakit, A.A.; Ahmed, A.; El-Hiti, G.A.; Smith, K.; Yousif, E. Synthesis of new thiophene derivatives and their use as photostabilizers for rigid poly(vinyl chloride). Int. J. Polym. Sci. 2015, 2015, 510390. [Google Scholar] [CrossRef]
- Sabaa, M.W.; Oraby, E.H.; Abdel Naby, A.S.; Mohammed, R.R. Anthraquinone derivatives as organic stabilizers for rigid poly(vinyl chloride) against photo-degradation. Eur. Polym. J. 2005, 41, 2530–2543. [Google Scholar] [CrossRef]
- Sabaa, M.W.; Mikhael, M.G.; Mohamed, N.A.; Yassin, A.A. N-substituted maleimides as thermal stabilizers for rigid poly(vinyl chloride). Angew. Makromol. Chem. 1989, 168, 23–25. [Google Scholar] [CrossRef]
- Mohammed, R.; El-Hiti, G.A.; Ahmed, A.; Yousif, E. Poly (vinyl chloride) doped by 2-(4-isobutylphenyl) propanoate metal complexes: Enhanced resistance to UV irradiation. Arab. J. Sci. Eng. 2017, 42, 4307–4315. [Google Scholar] [CrossRef]
- Folarin, O.M.; Sadiku, E.R. Thermal stabilizers for poly(vinyl chloride): A review. Int. J. Phys. Sci. 2011, 6, 4323–4330. [Google Scholar]
- Cheng, Q.; Li, C.; Pavlinek, V.; Saha, P.; Wang, H. Surface-modified antibacterial TiO2/Ag+ nanoparticles: Preparation and properties. Appl. Surf. Sci. 2006, 252, 4154–4160. [Google Scholar] [CrossRef]
- Chen, X.; Li, C.; Zhang, L.; Xu, S.; Zhou, Q.; Zhu, Y.; Qu, X. Main factors in preparation of antibacterial particles / PVC composite. China Particuol. 2004, 2, 226–229. [Google Scholar] [CrossRef]
- Birmingham, J.N. The effect of surface oxidation and titanium dioxide on exterior PVC color retention. J. Vinyl Addit. Technol. 1995, 1, 84–87. [Google Scholar] [CrossRef]
- Deanin, R.D.; Reynolds, H.H.; Ozcayir, Y. Thermal stabilization of polyvinyl chloride by group II metal laurates. J. Appl. Polym. Sci. 1969, 13, 1247–1252. [Google Scholar] [CrossRef]
- Chen, T.-P.; Lin, C.-W.; Li, S.-S.; Tsai, Y.-H.; Wen, C.-Y.; Lin, W.J.; Hsiao, F.-M.; Chiu, Y.-P.; Tsukagoshi, K.; Osada, M.; et al. Self-assembly atomic stacking transport layer of 2D layered titania for perovskite solar cells with extended UV stability. Adv. Energy Mater. 2018, 8, 1701722. [Google Scholar] [CrossRef]
- Abate, A.; Correa-Baena, J.-P.; Saliba, M.; Su'ait, M.S.; Bella, F. Frontispiece: Perovskite solar cells: From the laboratory to the assembly line. Chem. Eur. J. 2018, 24, 3083–3100. [Google Scholar] [CrossRef] [PubMed]
- Pintossi, D.; Iannaccone, G.; Colombo, A.; Bella, F.; Välimäki, M.; Väisänen, K.-L.; Hast, J.; Levi, M.; Gerbaldi, C.; Dragonetti, C.; et al. Luminescent downshifting by photo-induced sol-gel hybrid coatings: Accessing multifunctionality on flexible organic photovoltaics via ambient temperature material processing. Adv. Energy Mater. 2016, 2, 1600288. [Google Scholar] [CrossRef]
- Bella, F.; Lamberti, A.; Bianco, S.; Tresso, E.; Gerbaldi, C.; Pirri, C.F. Floating photovoltaics: Floating, flexible polymeric dye-sensitized solar-cell architecture: The way of near-future photovoltaics. Adv. Mater. Technol. 2016, 1, 1600002. [Google Scholar] [CrossRef]
- Patil, M.S.; Kim, S.C.; Seo, J.-H.; Lee, M.-Y. Review of the thermo-physical properties and performance characteristics of a refrigeration system using refrigerant-based nanofluids. Energies 2016, 9, 22. [Google Scholar] [CrossRef]
- Smith, K.; Balakit, A.A.; El-Hiti, G.A. Synthesis and characterization of a new photochromic alkylene sulfide derivative. J. Sulfur. Chem. 2018, 39, 182–192. [Google Scholar]
- Ahmed, D.S.; El-Hiti, G.A.; Yousif, E.; Hameed, A.S.; Abdalla, M. New eco-friendly phosphorus organic polymers as gas storage media. Polymers 2017, 9, 336. [Google Scholar] [CrossRef]
- Altaee, N.; El-Hiti, G.A.; Fahdil, A.; Sudesh, K.; Yousif, E. Screening and evaluation of poly(3-hydroxybutyrate) with Rhodococcus. equi using different carbon sources. Arab. J. Sci. Eng. 2017, 42, 2371–2379. [Google Scholar] [CrossRef]
- Altaee, N.; El-Hiti, G.A.; Fahdil, A.; Sudesh, K.; Yousif, E. Biodegradation of different formulations of polyhydroxybutyrate films in soil. SpringerPlus 2016, 5, 762. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.; Al-Zuhairi, A.J.; El-Hiti, G.A.; Alshammari, M.B. Comparison of cyclic and polymeric disulfides as catalysts for the regioselective chlorination of phenols. J. Sulfur Chem. 2015, 36, 74–85. [Google Scholar] [CrossRef]
- Yousif, E.; El-Hiti, G.A.; Haddad, R.; Balakit, A.A. Photochemical stability and photostabilizing efficiency of poly(methyl methacrylate) based on 2-(6-methoxynaphthalen-2-yl) propanoate metal ion complexes. Polymers 2015, 7, 1005–1019. [Google Scholar] [CrossRef]
- Smith, K.; Balakit, A.A.; El-Hiti, G.A. Poly (propylene sulfide)-borane: Convenient and versatile reagent for organic synthesis. Tetrahedron 2012, 68, 7834–7839. [Google Scholar] [CrossRef]
- Smith, K.; Balakit, A.A.; Pardasani, R.T.; El-Hiti, G.A. New polymeric sulfide-borane complexes: Convenient hydroborating and reducing reagents. J. Sulfur Chem. 2011, 32, 287–295. [Google Scholar] [CrossRef]
- Shaalan, N.; Laftah, N.; Muslih, R.; Yousif, E. Photostability study of some modified poly(vinyl chloride) containing pendant Schiff’s bases. Baghdad Sci. J. 2016, 13, 188–195. [Google Scholar] [CrossRef]
- Sodhi, R.K.; Paul, S.; Clark, J.H. A comparative study of different metal acetylacetonates covalently anchored onto amine functionalized silica: A study of the oxidation of aldehydes and alcohols to corresponding acids in water. Green Chem. 2012, 14, 1649–1656. [Google Scholar] [CrossRef]
- Gardette, J.L.; Gaumet, S.; Lemaire, J. Photooxidation of poly(viny1 chloride). 1. A reexamination of the mechanism. Macromolecules 1989, 22, 2576–2581. [Google Scholar] [CrossRef]
- Gaumet, S.; Gardette, J.-L. Photo-oxidation of poly(vinyl chloride): Part 2—A comparative study of the carbonylated products in photo-chemical and thermal oxidations. Polym. Degrad. Stab. 1991, 33, 17–34. [Google Scholar] [CrossRef]
- Sabaa, M.W.; Oraby, E.H.; Abdul Naby, A.S.; Mohamed, R.R. N-Phenyl-3-substituted-5-pyrazolone derivatives as organic stabilizer for rigid PVC against photodegradation. J. Appl. Polym. Sci. 2005, 101, 1543–1555. [Google Scholar] [CrossRef]
- Yousif, E.; Salih, N.; Salimon, J. Improvement of the photostabilization of PVC films in the presence of 2N-salicylidene-5-(substituted)-1,3,4-thiadiazole. J. Appl. Polym. Sci. 2011, 120, 2207–2214. [Google Scholar] [CrossRef]
- Mori, F.; Koyama, M.; Oki, Y. Studies on photodegradation of poly(vinyl chloride (part I). Macromol. Mater. Eng. 1977, 64, 89–99. [Google Scholar]
- Braun, D.; Rabie, S.T.; Khaireldin, N.Y.; Abd El-Ghaffar, M.A. Preparation and evaluation of some benzophenone terpolymers as photostabilizers for rigid PVC. J. Vinyl Addit. Technol. 2011, 17, 147–155. [Google Scholar] [CrossRef]
- Scott, G. Polymers and Ecological Problems; Plennm Press: New York, NY, USA, 1973; Volume 3, pp. 27–35. [Google Scholar]
- Gugumus, F. Mechanism of Polymer Degradation and Stabilization; Elsevier: Amsterdam, The Netherlands, 1990. [Google Scholar]
- Shyichuk, A.V.; White, J.R. Analysis of chain-scission and crosslinking rates on the photooxidation of polystyrene. J. Appl. Polym. Sci. 2000, 77, 3015–3023. [Google Scholar] [CrossRef]
- Awaja, F.; Zhang, S.; Tripathi, M.; Nikiforov, A.; Pugno, N. Cracks, microcracks and fracture in polymer structures: Formation, detection, autonomic repair. Prog. Mater. Sci. 2016, 83, 536–573. [Google Scholar] [CrossRef]
- Valko, L.; Klein, E.; Kovařík, P.; Bleha, T.; Šimon, P. Kinetic study of thermal dehydrochlorination of poly(vinyl chloride) in the presence of oxygen: III. Statistical thermodynamic interpretation of the oxygen catalytic activity. Eur. Polym. J. 2001, 37, 1123–1133. [Google Scholar] [CrossRef]
- Yousif, E.; Bakir, E.; Salimon, J.; Salih, N. Evaluation of Schiff bases of 2,5-dimercapto-1,3,4-thiadiazole as photostabilizer for poly(methyl methacrylate). J. Saudi Chem. Soc. 2012, 16, 279–285. [Google Scholar] [CrossRef]
- Starnes, W.H.; Du, B.; Kim, S.; Zaikov, V.G.; Ge, X.; Culyba, E.K. Thermal stabilization and plasticization of poly(vinyl chloride) by ester thiols: Update and current status. Thermochim. Acta 2006, 442, 78–80. [Google Scholar] [CrossRef]
- Kasha, M. Characterization of electronic transitions in complex molecules. Discuss. Faraday Soc. 1950, 9, 14–19. [Google Scholar] [CrossRef]
- Pospíšil, J.; Klemchuk, P.P. Oxidation Inhibition in Organic Materials; CRC Press: Boca Raton, FL, USA, 1989; pp. 48–49. [Google Scholar]
- Li, D.; Zhou, M.; Xie, L.; Yu, X.; Yu, Y.; Ai, H.; Tang, S. Synergism of pentaerythritol-zinc with β-diketone and calcium stearate in poly(vinyl chloride) thermal stability. Polym. J. 2013, 45, 775–782. [Google Scholar] [CrossRef]
- Witwit, N.A. Study of the optical properties of poly(vinyl chloride)-4-[(5-mercapto-1,3,4-thiadiazol-2-yl) diazenyl] phenol complexes. Eur. J. Chem. 2014, 5, 652–656. [Google Scholar] [CrossRef][Green Version]
- Mori, F.; Koyama, M.; Oki, Y. Studies on photodegradation of poly(vinyl chloride), part 3. Macromol. Mater. Eng. 1979, 75, 113–122. [Google Scholar]
- Kwei, K.P.S. Photo-oxidation of poly(vinyl chloride). J. Polym. Sci. A 1969, 7, 1075–1088. [Google Scholar] [CrossRef]
- Mark, J.E. Physical Properties of Polymers Handbook; Springer: New York, NY, USA, 2007. [Google Scholar]
Sample Availability: Samples of the Schiff bases are available from the authors. |
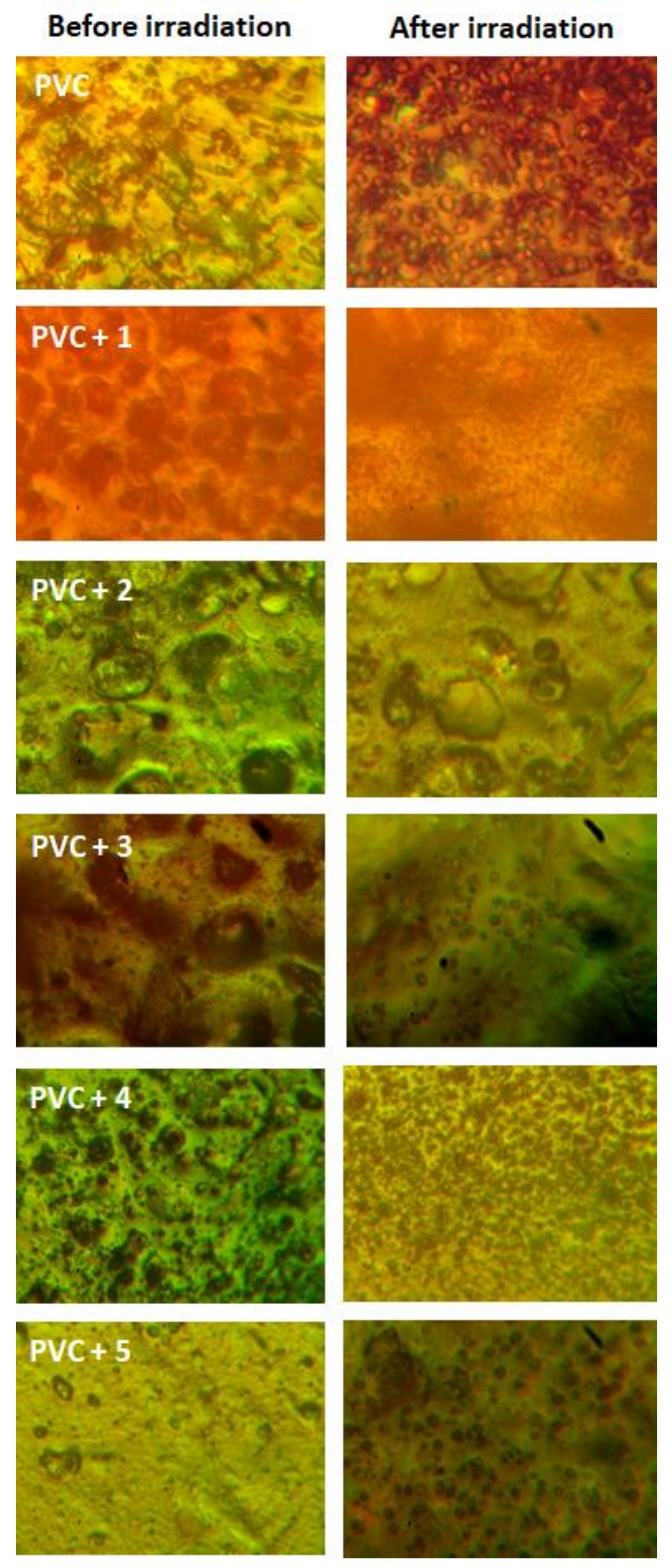
| Additive | FR-IR (υ, cm−1) | Absorption (nm) | |||||
| OH/NH | SH | C=O | CH=N | C-S | π–π* | n–π* | |
| 1 | 3318 | 2588 | — | 1618 | 621 | 274, 331 | 383, 484 |
| 2 | 3124 | 2604 | 1786 | 1610 | 638 | 304 | 459 |
| 3 | — | 2584 | — | 1608 | 644 | 304 | 347 |
| 4 | 3178 | 2592 | — | 1610 | 630 | 279 | 434 |
| 5 | — | 2564 | 1685 | 1597 | 634 | 307 | 416 |
| Additive | 1H-NMR (400 MHz: DMSO-d6, δ, ppm, J in Hz) |
|---|---|
| 1 | 12.97 (s, exch, 1 H), 9.41 (s, 1 H), 8.80 (d, J = 8.1 Hz, 1 H), 8.10 (d, J = 8.1 Hz, 1 H), 7.89 (d, J = 8.1 Hz, 1 H), 7.63 (t, J = 8.1 Hz, 1 H), 7.44 (t, J = 8.1 Hz, 1 H), 7.25 (d, J = 8.1 Hz, 1 H) |
| 2 | 13.68 (s, exch, 1 H), 9.03 (d, J = 8.9 Hz, 1 H), 7.91 (s, 1 H), 7.86 (t, J = 8.9 Hz, 1 H), 7.75 (d, J = 8.9 Hz, 1 H), 6.97 (d, J = 8.9 Hz, 1 H) |
| 3 | 13.01 (s, exch, 1 H), 7.62 (m, 1 H), 7.56 (m, 1 H), 7.17 (m, 1 H), 2.51 (s, 3 H) |
| 4 | 14.30 (s, exch, 1 H), 9.90 (s, 1 H), 8.49 (s, exch, 1 H), 7.79–7.73 (m, 3 H), 7.52 (d, J = 7.9 Hz, 2 H) |
| 5 | 12.61 (exch, s, 1 H), 8.22 (s, 1 H), 7.58–7.51 (m, 3 H), 7.45 (d, J = 8.0 Hz, 2 H), 3.38 (s, 3 H), 2.86 (s, 3 H) |
| Additive | MS (m/z; %) |
|---|---|
| 1 | 288 ([M + 1]+, 17), 287 (M+, 100), 270 (30), 254 (32), 211 (64), 196 (33), 182 (35), 169 (44), 154 (23), 127 (96), 115 (35), 77 (30) |
| 2 | 266 ([M + 1]+, 12), 265 (M+, 90), 220 (5), 133 (100), 105 (20), 77 (22) |
| 3 | 242 ([M + 1]+, 22), 241 (M+, 61), 227 (38), 133 (100), 74 (22), 57 (50) |
| 4 | 288 ([M + 1]+, 18), 287 (M+, 100), 255 (12), 240 (10), 182 (36), 155 (39), 128 (33), 77 (73), 57 (70) |
| 5 | 332 ([M + 1]+, 16), 331 (M+, 94), 313 (10), 273 (15), 258 (20), 226 (21), 214 (35), 183 (15), 133 (47), 77 (61), 56 (100) |
| PVC Film | Φcs |
|---|---|
| PVC | 3.09 × 10–8 |
| PVC + 1 | 8.82 × 10–10 |
| PVC + 2 | 1.42 × 10–9 |
| PVC + 3 | 2.65 × 10–9 |
| PVC + 4 | 6.29 × 10–9 |
| PVC + 5 | 1.35 × 10–8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaalan, N.; Laftah, N.; El-Hiti, G.A.; Alotaibi, M.H.; Muslih, R.; Ahmed, D.S.; Yousif, E. Poly(vinyl Chloride) Photostabilization in the Presence of Schiff Bases Containing a Thiadiazole Moiety. Molecules 2018, 23, 913. https://doi.org/10.3390/molecules23040913
Shaalan N, Laftah N, El-Hiti GA, Alotaibi MH, Muslih R, Ahmed DS, Yousif E. Poly(vinyl Chloride) Photostabilization in the Presence of Schiff Bases Containing a Thiadiazole Moiety. Molecules. 2018; 23(4):913. https://doi.org/10.3390/molecules23040913
Chicago/Turabian StyleShaalan, Naser, Nawres Laftah, Gamal A. El-Hiti, Mohammad Hayal Alotaibi, Raad Muslih, Dina S. Ahmed, and Emad Yousif. 2018. "Poly(vinyl Chloride) Photostabilization in the Presence of Schiff Bases Containing a Thiadiazole Moiety" Molecules 23, no. 4: 913. https://doi.org/10.3390/molecules23040913
APA StyleShaalan, N., Laftah, N., El-Hiti, G. A., Alotaibi, M. H., Muslih, R., Ahmed, D. S., & Yousif, E. (2018). Poly(vinyl Chloride) Photostabilization in the Presence of Schiff Bases Containing a Thiadiazole Moiety. Molecules, 23(4), 913. https://doi.org/10.3390/molecules23040913








