Fungal Metabolites Antagonists towards Plant Pests and Human Pathogens: Structure-Activity Relationship Studies
Abstract
1. Introduction
2. Structure–Activity Relationship Studies Performed with Fungal Metabolites
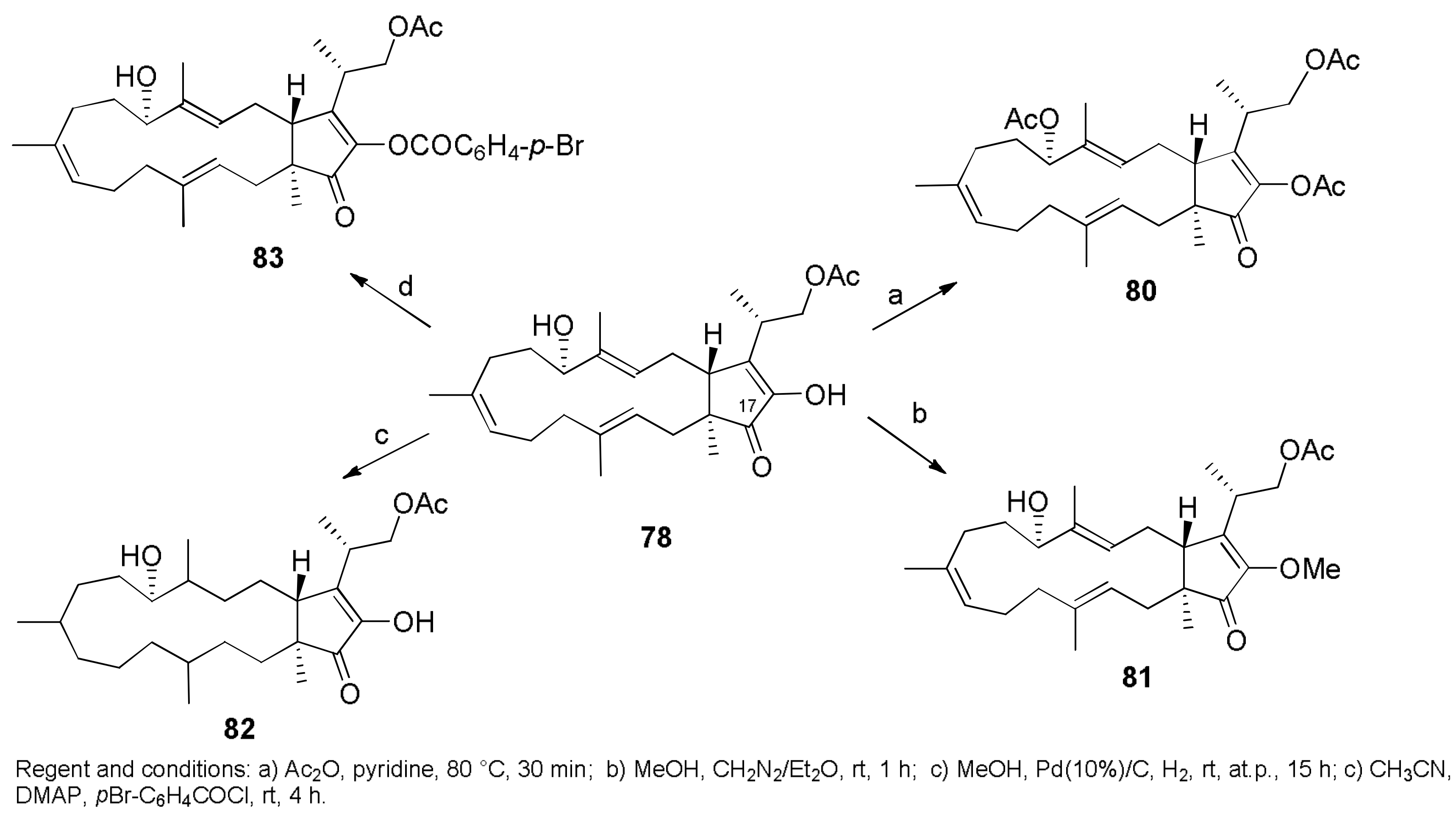
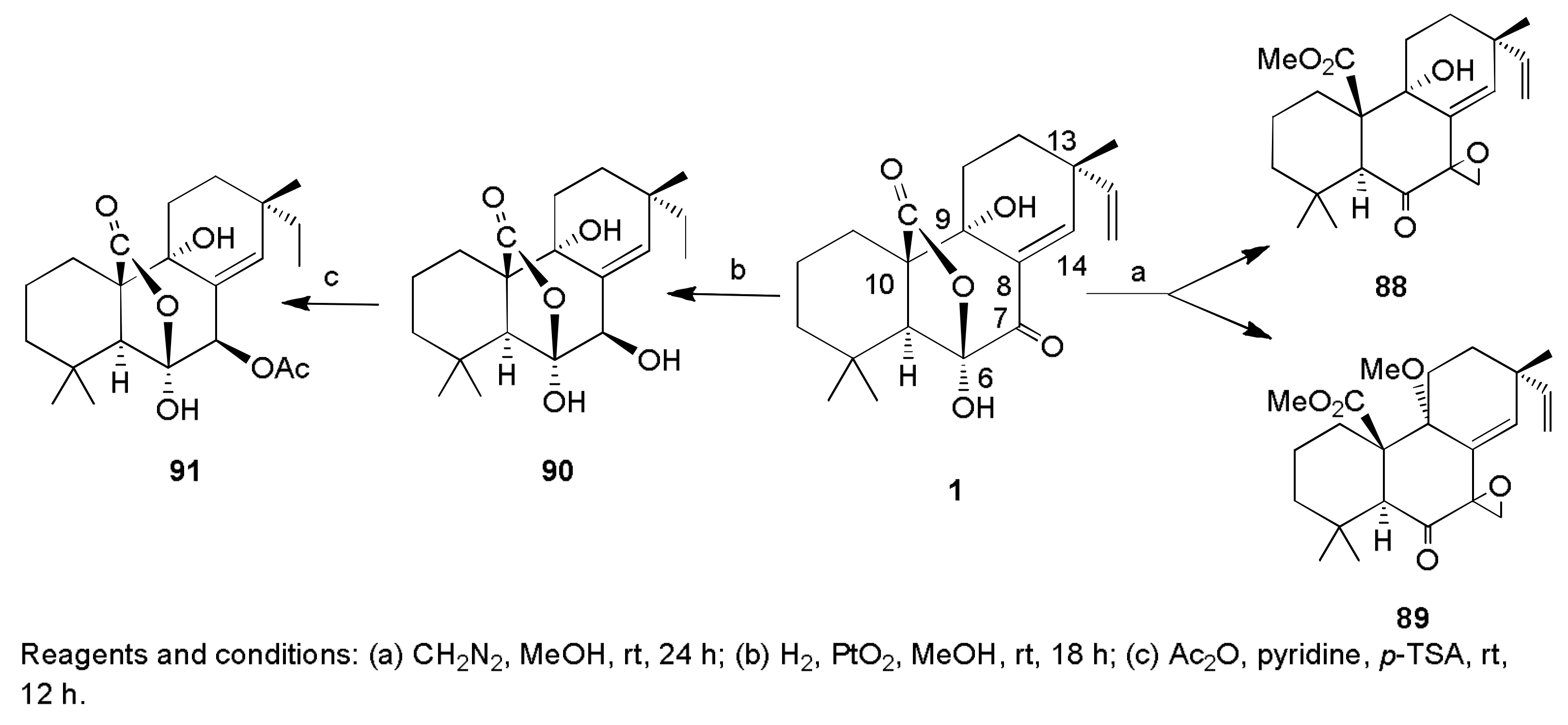
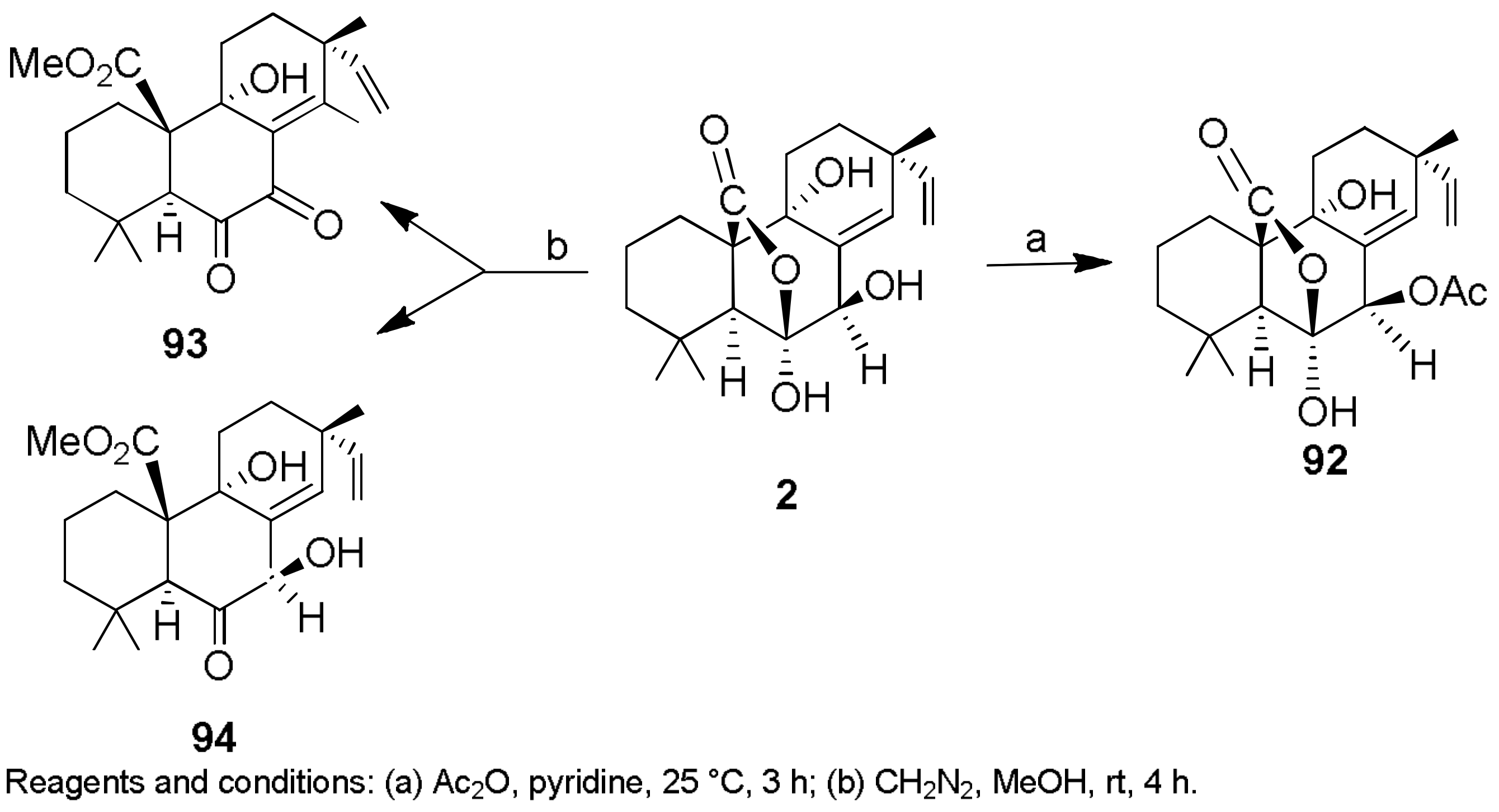
2.1. Fungicides
2.2. Bactericides
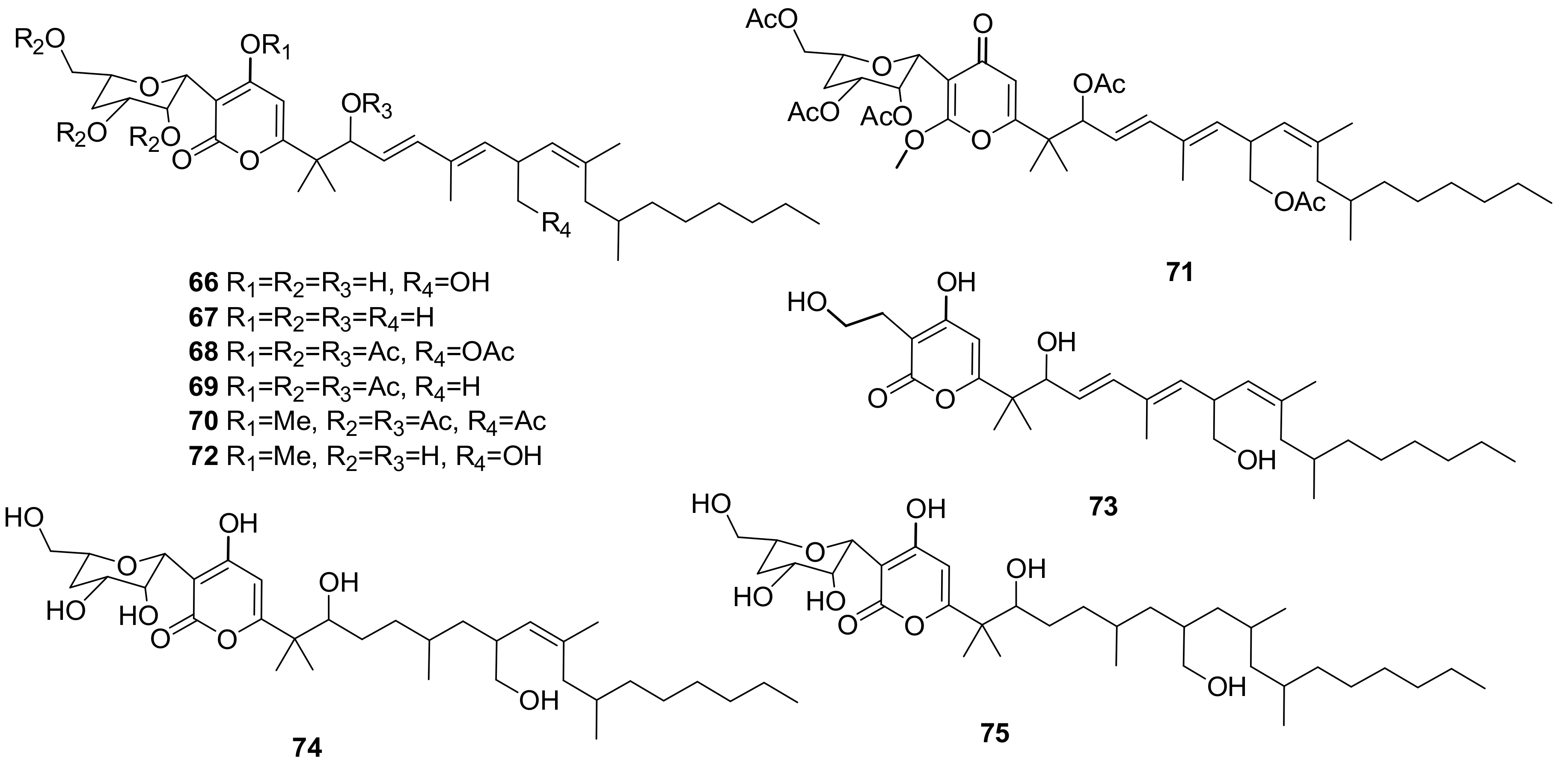
2.3. Insecticides
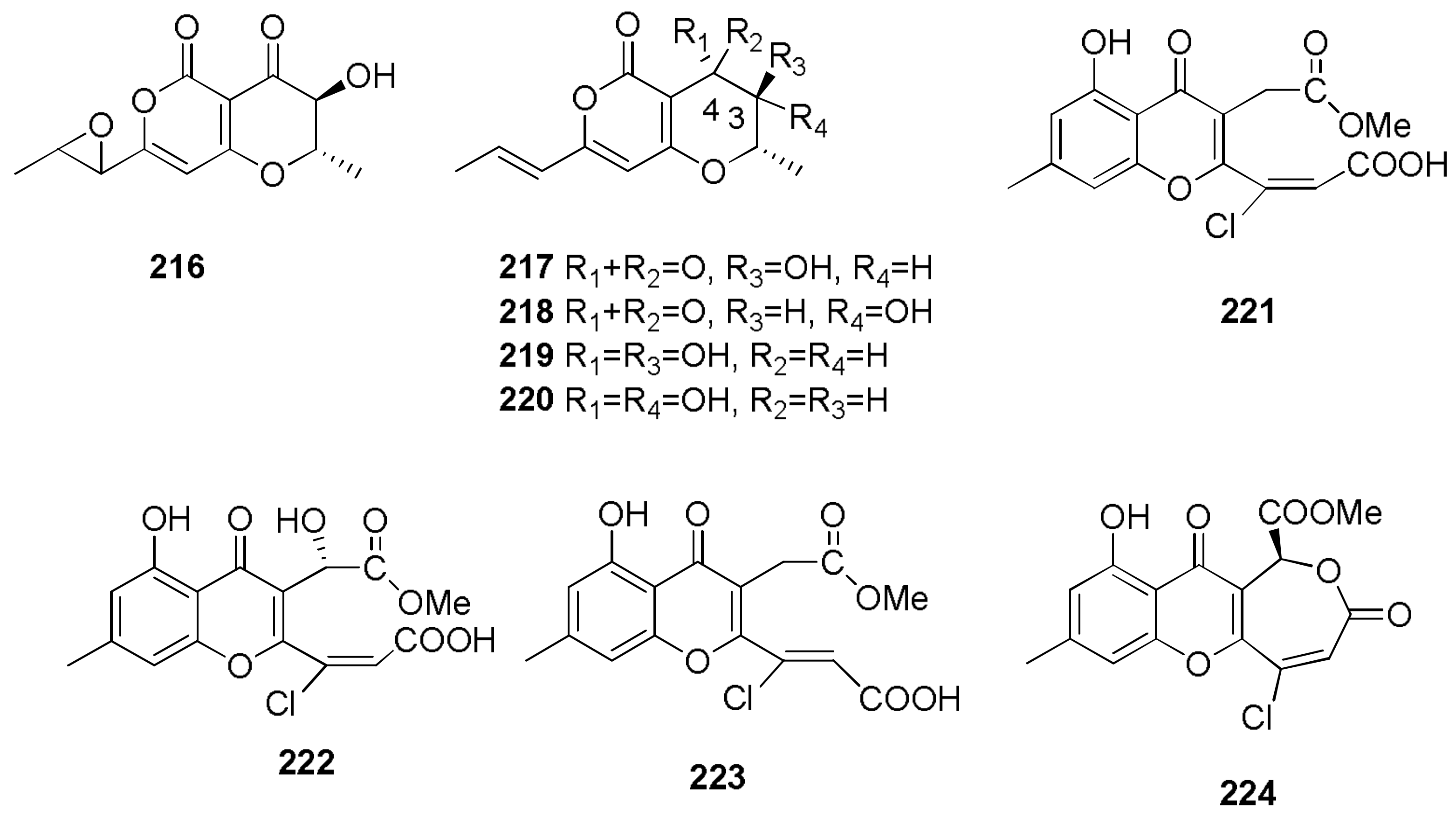
2.4. Herbicides
3. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| Ac2O | Acetic anhydride |
| at.p. | Atmospheric pressure |
| CH2N2 | Diazomethane |
| CH3CN | Acetonitrile |
| DCC | N,N′-Dicyclohexylcarbodiimide |
| DMAP | 4-Dimethylaminopyridine |
| EtOAc | Ethyl acetate |
| Et2O | Diethyl ether |
| MeOH | Methanol |
| Me2CO | Acetone |
| MIC | Minimum inhibitory concentration |
| PDB | Potato dextrose broth |
| p-TSA | p-Toluenesulfonic acid |
| rt | Room temperature |
| THF | Tetrahydrofuran |
References
- Benelli, G.; Jeffries, C.L.; Walker, T. Biological control of mosquito vectors: Past, present, and future. Insects 2016, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, A.; Masi, M.; Evidente, M.; Superchi, S.; Evidente, A. Fungal phytotoxins with potential herbicidal activity: Chemical and biological characterization. Nat. Prod. Rep. 2015, 32, 1629–1653. [Google Scholar] [CrossRef] [PubMed]
- Finizio, A.; Villa, S. Environmental risk assessment for pesticides. Environ. Impact Assess. Rev. 2002, 22, 235–248. [Google Scholar] [CrossRef]
- Aktar, W.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdiscip. Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Vivekanandhan, N.; Duraisamy, A. Ecological impact of pesticides principally organochlorine insecticide endosulfan: A review. Univ. J. Environ. Res. Technol. 2012, 2, 369–376. [Google Scholar]
- Gerwick, B.C.; Sparks, T.C. Natural products for pest control: An analysis of their role, value and future. Pest Manag. Sci. 2014, 70, 1169–1185. [Google Scholar] [CrossRef] [PubMed]
- Gilden, R.C.; Huffling, K.; Sattler, B. Pesticides and health risks. JOGNN J. Obstet. Gynecol. Neonatal. Nurs. 2010, 39, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Eddleston, M.; Bateman, D.N. Pesticides. Medicine 2012, 40, 147–150. [Google Scholar] [CrossRef]
- Pal, K.K.; Mc Spadden Gardener, B. Biological control of plant pathogens. Plant Health Instr. 2006, 2, 1117–1142. [Google Scholar] [CrossRef]
- Bale, J.S.; van Lenteren, J.C.; Bigler, F. Biological control and sustainable food production. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2008, 363, 761–776. [Google Scholar] [CrossRef] [PubMed]
- Balog, A.; Hartel, T.; Loxdale, H.D.; Wilson, K. Differences in the progress of the biopesticide revolution between the EU and other major crop-growing regions. Pest Manag. Sci. 2017, 73, 2203–2208. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Dikshit, A.K. Biopesticides: An ecofriendly approach for pest control. J. Biopestic. 2010, 3, 186–188. [Google Scholar] [CrossRef]
- Dayan, F.E.; Duke, S.O. Natural compounds as next generation herbicides. Plant Physiol. 2014, 166, 1090–1105. [Google Scholar] [CrossRef] [PubMed]
- Seiber, J.N.; Coats, J.; Duke, S.O.; Gross, A.D. Biopesticides: State of the art and future opportunities. J. Agric. Food Chem. 2014, 62, 11613–11619. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.B.; Tiwari, V.K. Natural products: An evolving role in future drug discovery. Eur. J. Med. Chem. 2011, 46, 4769–4807. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.L. Natural products in drug discovery. Drug Discov. Today 2008, 13, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.L.; Edrada-Ebel, R.; Quinn, R.J. The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug Discov. 2015, 14, 111–129. [Google Scholar] [CrossRef] [PubMed]
- Lahlou, M. The success of natural products in drug discovery. Pharmacol. Pharm. 2013, 4, 17–31. [Google Scholar] [CrossRef]
- Rossolini, G.M.; Arena, F.; Pecile, P.; Pollini, S. Update on the antibiotic resistance crisis. Curr. Opin. Pharmacol. 2014, 18, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. The antibiotic resistance crisis. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Neu, H.C. The crisis in antibiotic resistance. Science 1992, 257, 1064–1073. [Google Scholar] [CrossRef] [PubMed]
- Giedraitienė, A.; Vitkauskienė, A.; Naginienė, R.; Pavilonis, A. Antibiotic resistance mechanisms of clinically important bacteria. Medicina 2011, 47, 137–146. [Google Scholar] [PubMed]
- Taiwo, S.S. Antibiotic-resistant bugs in the 21st century: A public health challenge. World J. Clin. Infect. Dis. 2011, 1, 11–16. [Google Scholar] [CrossRef]
- Smith, K.F.; Guégan, J.F. Changing geographic distributions of human pathogens. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 231–250. [Google Scholar] [CrossRef][Green Version]
- Katz, L.; Baltz, R.H. Natural product discovery: Past, present, and future. J. Ind. Microbiol. Biotechnol. 2016, 43, 155–176. [Google Scholar] [CrossRef] [PubMed]
- Di Santo, R. Natural products as antifungal agents against clinically relevant pathogens. Nat. Prod. Rep. 2010, 27, 1084–1098. [Google Scholar] [CrossRef] [PubMed]
- Gyawali, R.; Ibrahim, S.A. Natural products as antimicrobial agents. Food Control 2014, 46, 412–429. [Google Scholar] [CrossRef]
- Narsing Rao, M.P.; Xiao, M.; Li, W.J. Fungal and bacterial pigments: Secondary metabolites with wide applications. Front. Microbiol. 2017, 8, 1113. [Google Scholar] [CrossRef] [PubMed]
- Kornienko, A.; Evidente, A.; Vurro, M.; Mathieu, V.; Cimmino, A.; Evidente, M.; van Otterlo, W.A.L.; Dasari, R.; Lefranc, F.; Kiss, R. Toward a cancer drug of fungal origin. Med. Res. Rev. 2015, 35, 937–967. [Google Scholar] [CrossRef] [PubMed]
- Dewick, P.M. Medicinal Natural Products: A Biosynthetic Approach, 3rd ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2009; ISBN 9780470741689. [Google Scholar]
- Cole, R.J.; Cox, R.H. Handbook of Toxic Fungal Metabolites; Elsevier Inc.: Amsterdam, The Netherlands, 1981; ISBN 9780121797607. [Google Scholar]
- Masi, M.; Maddau, L.; Linaldeddu, B.T.; Scanu, B.; Evidente, A.; Cimmino, A. Bioactive metabolites from pathogenic and endophytic fungi of forest trees. Curr. Med. Chem. 2018, 25, 208–252. [Google Scholar] [CrossRef] [PubMed]
- Evidente, A.; Masi, M.; Linaldeddu, B.T.; Franceschini, A.; Scanu, B.; Cimmino, A.; Andolfi, A.; Motta, A.; Maddau, L. Afritoxinones A and B, dihydrofuropyran-2-ones produced by Diplodia africana the causal agent of branch dieback on Juniperus phoenicea. Phytochemistry 2012, 77, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Andolfi, A.; Maddau, L.; Basso, S.; Linaldeddu, B.T.; Cimmino, A.; Scanu, B.; Deidda, A.; Tuzi, A.; Evidente, A. Diplopimarane, a 20-nor-ent-pimarane produced by the oak pathogen Diplodia quercivora. J. Nat. Prod. 2014, 77, 2352–2360. [Google Scholar] [CrossRef] [PubMed]
- Masi, M.; Maddau, L.; Linaldeddu, B.T.; Cimmino, A.; D’Amico, W.; Scanu, B.; Evidente, M.; Tuzi, A.; Evidente, A. Bioactive secondary metabolites produced by the oak pathogen Diplodia corticola. J. Agric. Food Chem. 2016, 64, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, A.; Maddau, L.; Masi, M.; Evidente, M.; Linaldeddu, B.T.; Evidente, A. Further secondary metabolites produced by Diplodia corticola, a fungal pathogen involved in cork oak decline. Tetrahedron 2016, 72, 6788–6793. [Google Scholar] [CrossRef]
- Lallemand, B.; Masi, M.; Maddau, L.; De Lorenzi, M.; Dam, R.; Cimmino, A.; Moreno, Y.; Banuls, L.; Andolfi, A.; Kiss, R.; et al. Evaluation of in vitro anticancer activity of sphaeropsidins A–C, fungal rearranged pimarane diterpenes, and semisynthetic derivatives. Phytochem. Lett. 2012, 5, 770–775. [Google Scholar] [CrossRef]
- Mathieu, V.; Chantôme, A.; Lefranc, F.; Cimmino, A.; Miklos, W.; Paulitschke, V.; Mohr, T.; Maddau, L.; Kornienko, A.; Berger, W.; et al. Sphaeropsidin A shows promising activity against drug-resistant cancer cells by targeting regulatory volume increase. Cell. Mol. Life Sci. 2015, 72, 3731–3746. [Google Scholar] [CrossRef] [PubMed]
- Masi, M.; Cimmino, A.; Maddau, L.; Kornienko, A.; Tuzi, A.; Evidente, A. Crystal structure and absolute configuration of sphaeropsidin A and its 6-O-p-bromobenzoate. Tetrahedron Lett. 2016, 57, 4592–4594. [Google Scholar] [CrossRef]
- Ingels, A.; Dinhof, C.; Garg, A.D.; Maddau, L.; Masi, M.; Evidente, A.; Berger, W.; Dejaegher, B.; Mathieu, V. Computed determination of the in vitro optimal chemocombinations of sphaeropsidin A with chemotherapeutic agents to combat melanomas. Cancer Chemother. Pharmacol. 2017, 79, 971–983. [Google Scholar] [CrossRef] [PubMed]
- Evidente, A.; Sparapano, L.; Motta, A.; Giordano, F.; Fierro, O.; Frisullo, S. A phytotoxic pimarane diterpene of Sphaeropsis sapinea f. sp. cupressi, the pathogen of a canker disease of cypress. Phytochemistry 1996, 42, 1541–1546. [Google Scholar] [CrossRef]
- Evidente, A.; Sparapano, L.; Fierro, O.; Bruno, G.; Giordano, F.; Motta, A. Sphaeropsidins B and C, phytotoxic pimarane diterpenes from Sphaeropsis sapinea f. sp. cupressi and Diplodia mutila. Phytochemistry 1997, 45, 705–713. [Google Scholar] [CrossRef]
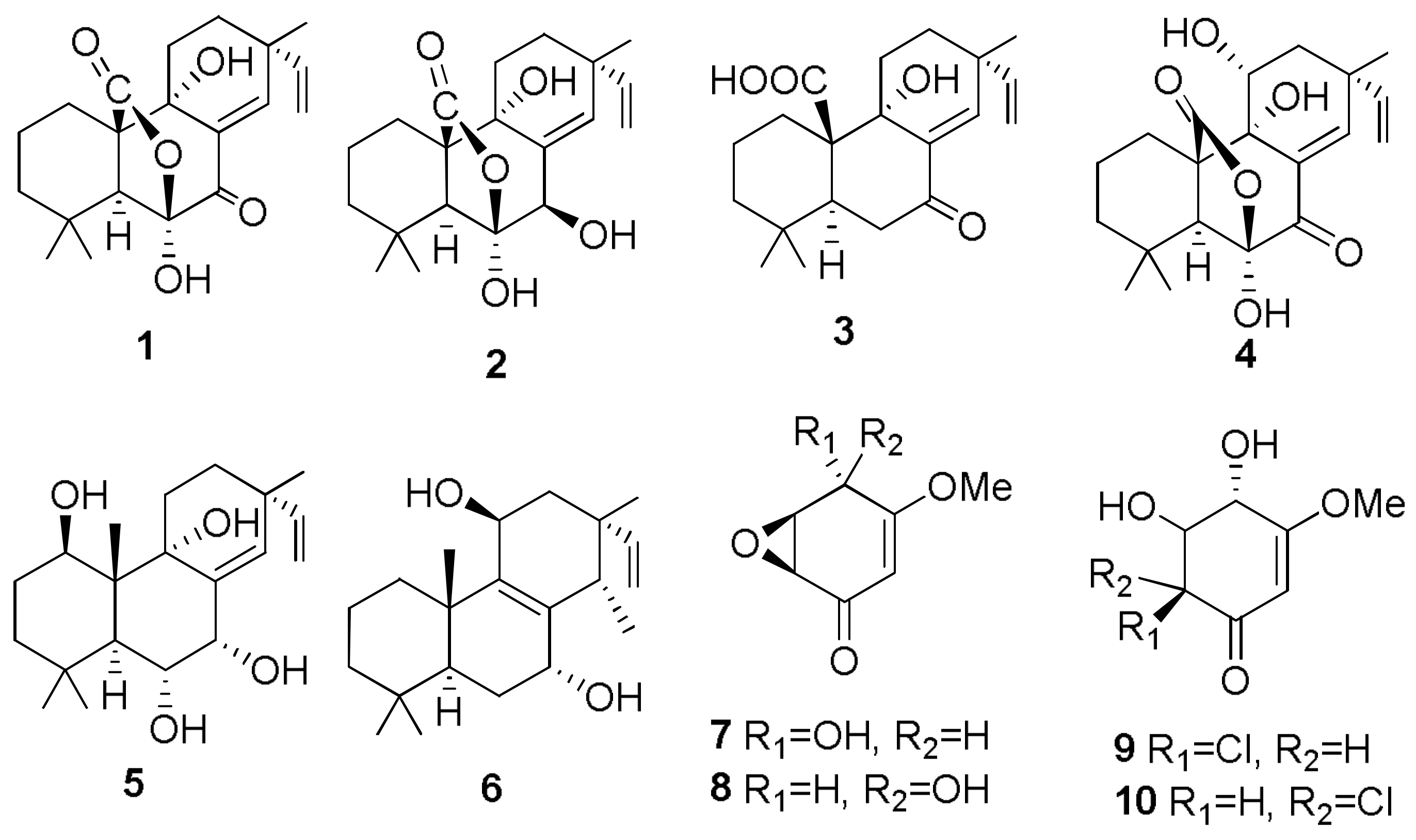
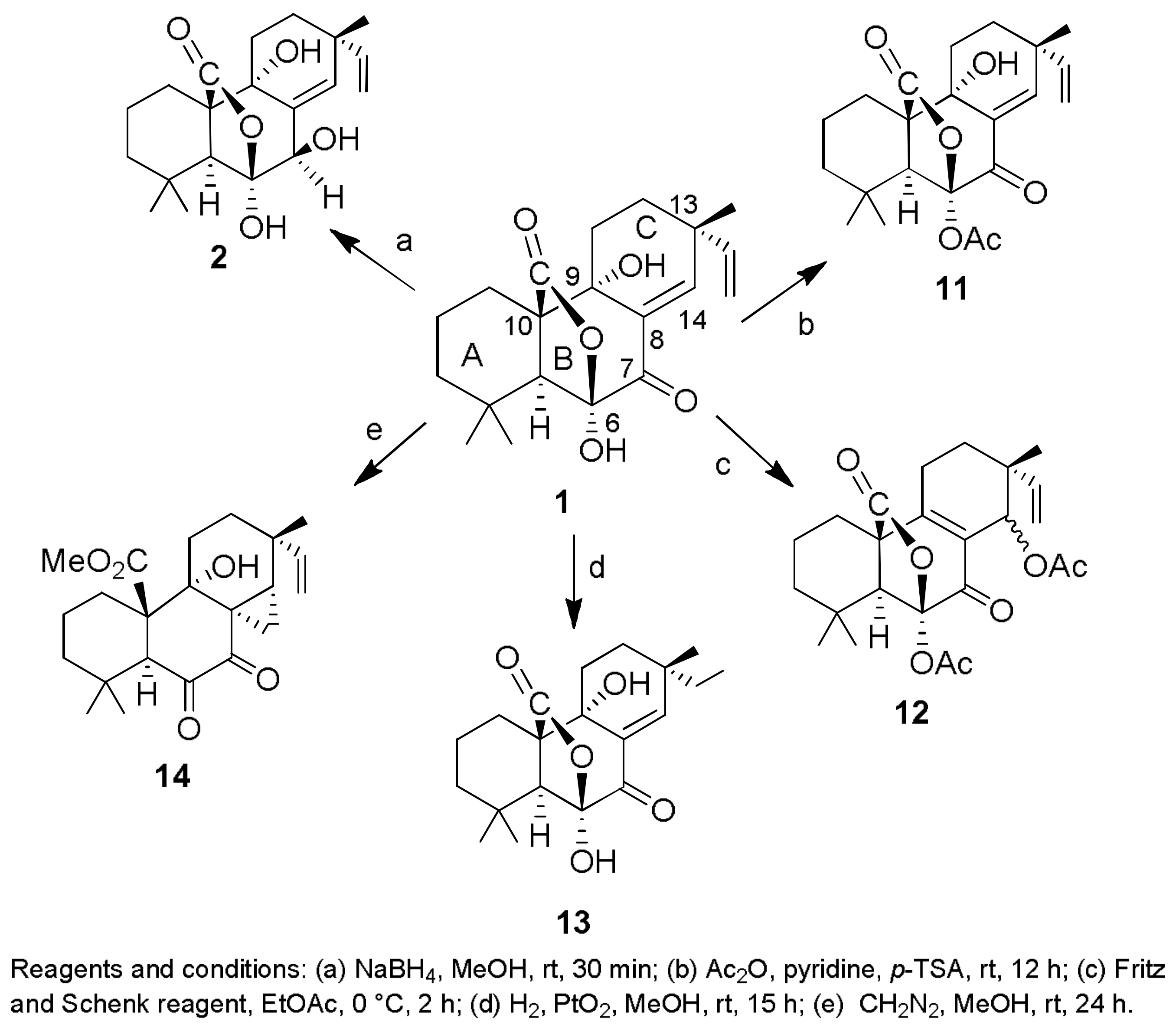
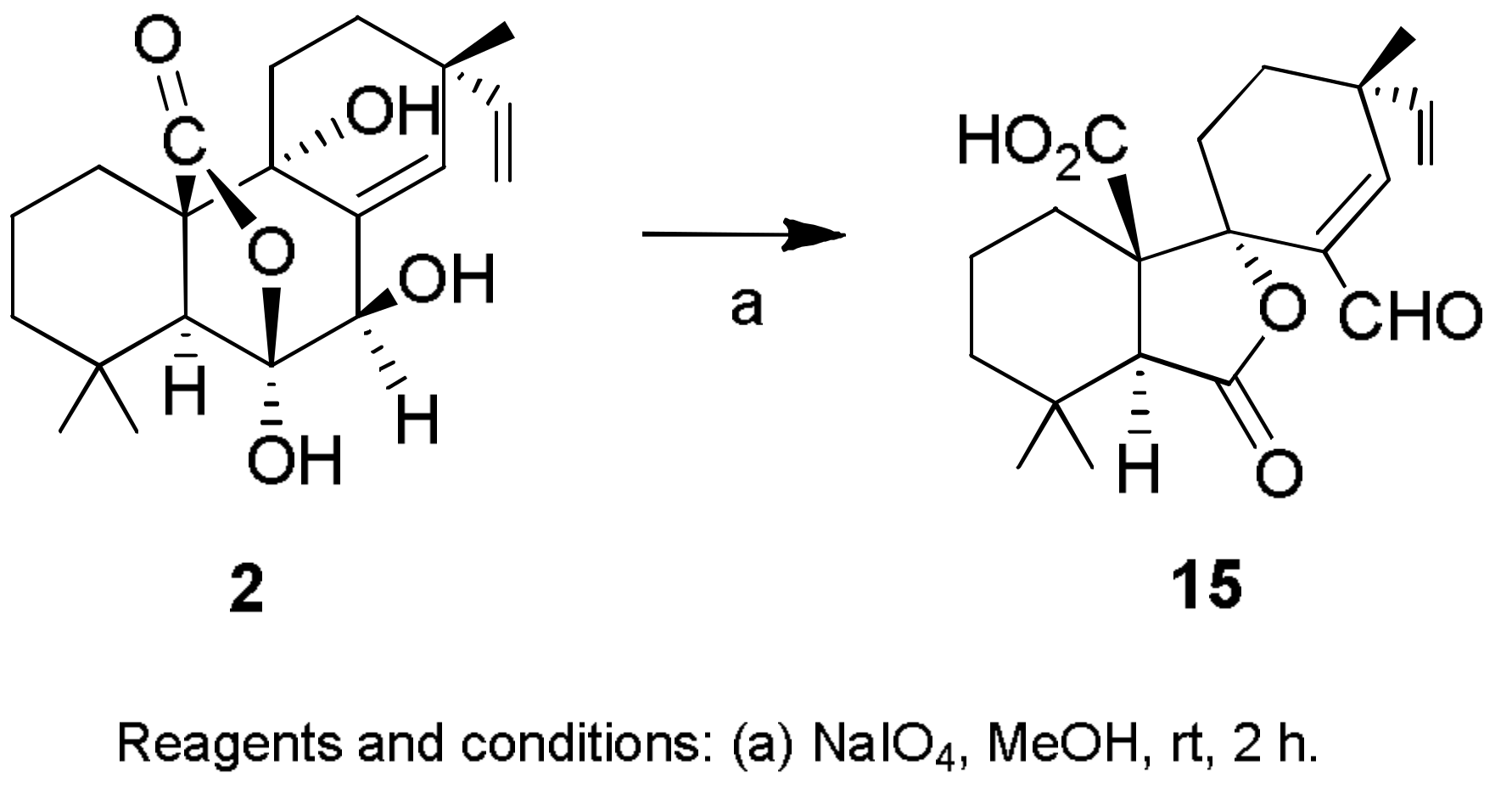
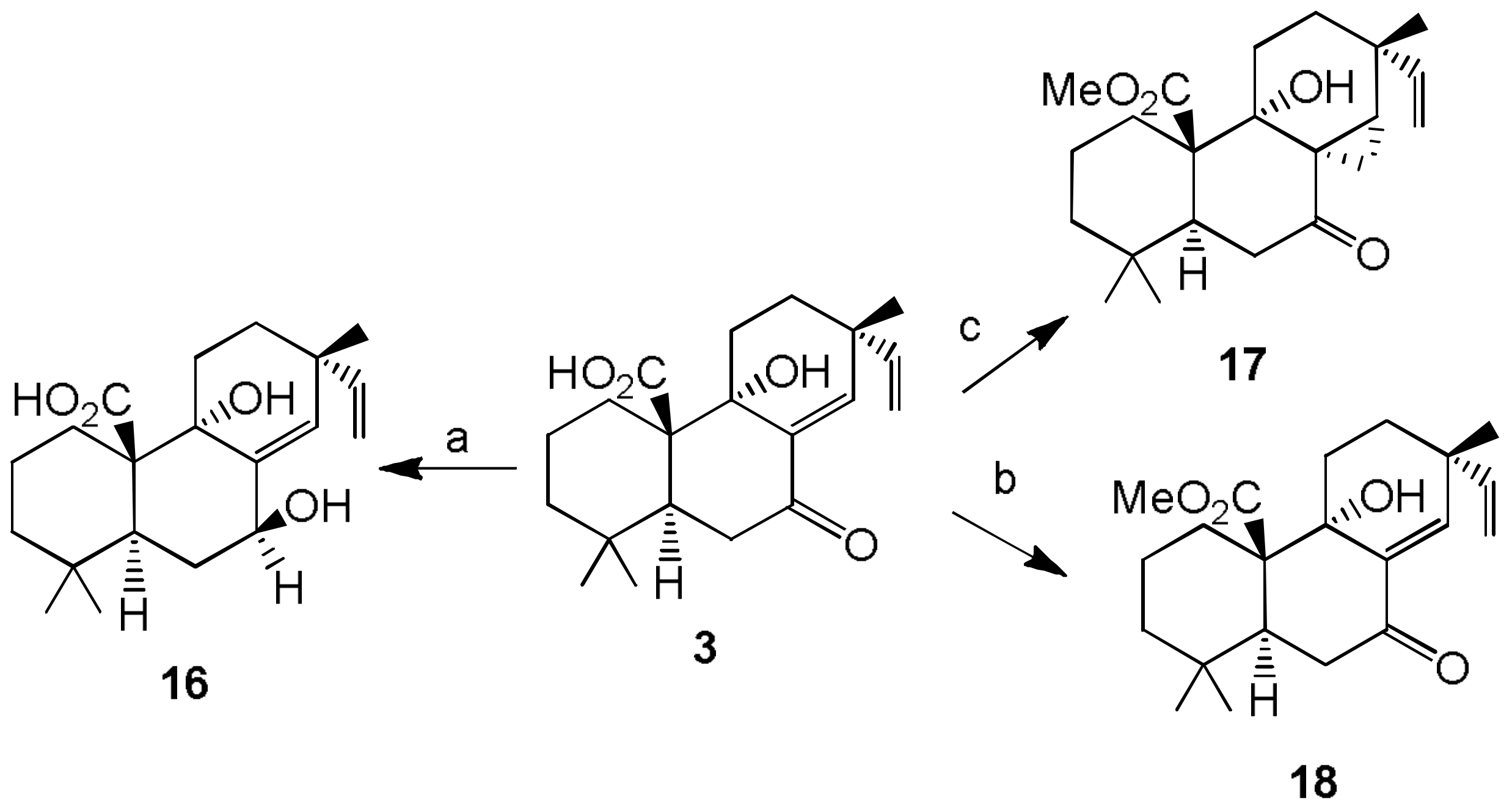
- Sparapano, L.; Bruno, G.; Fierro, O.; Evidente, A. Studies on structure-activity relationship of sphaeropsidins A–F, phytotoxins produced by Sphaeropsis sapinea f. sp. cupressi. Phytochemistry 2004, 65, 189–198. [Google Scholar] [CrossRef] [PubMed]
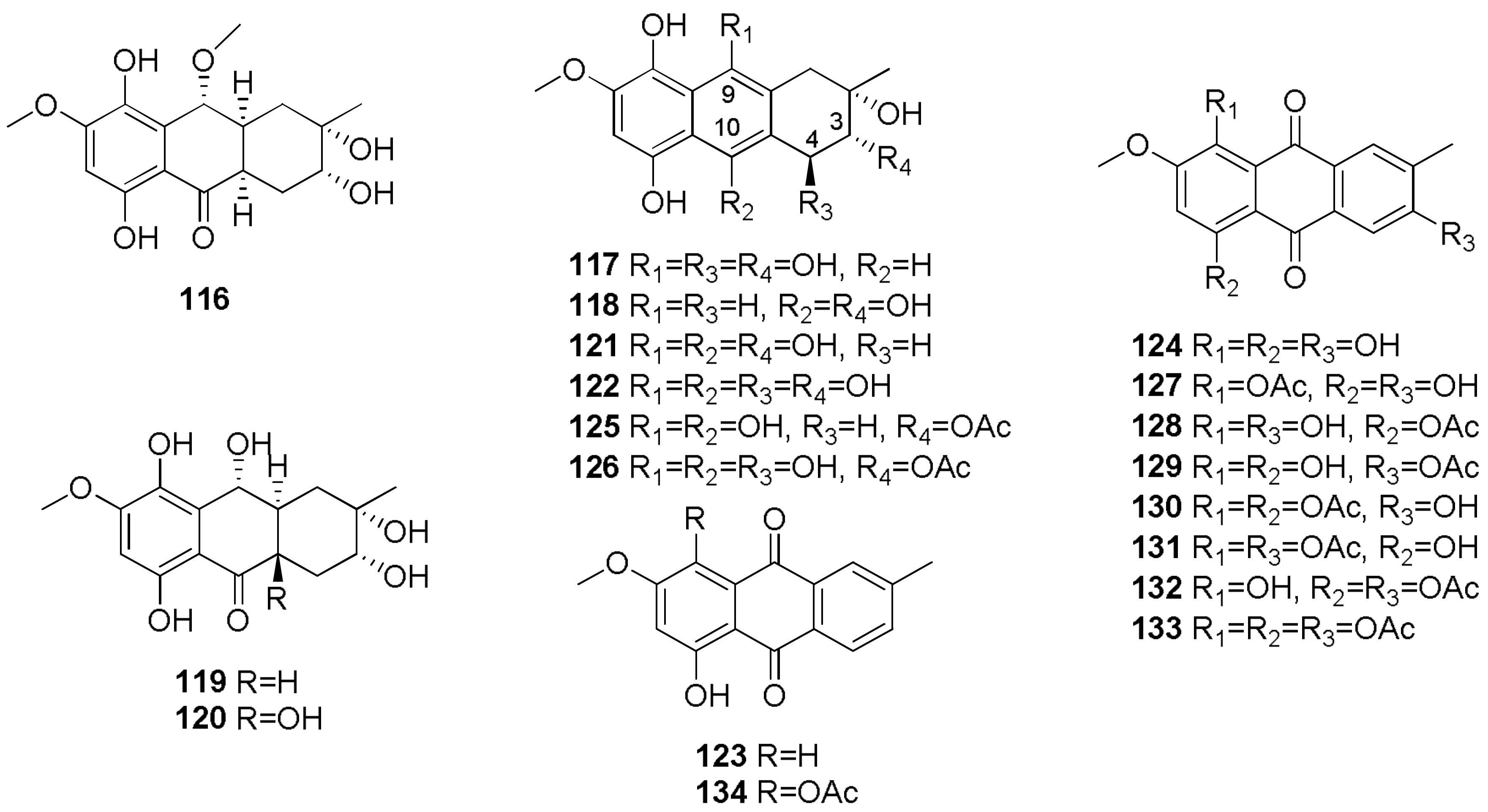
- Evidente, A.; Maddau, L.; Scanu, B.; Andolfi, A.; Masi, M.; Motta, A.; Tuzi, A. Sphaeropsidones, phytotoxic dimedone methyl ethers produced by Diplodia cupressi: A structure—Activity relationship study. J. Nat. Prod. 2011, 74, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Andolfi, A.; Maddau, L.; Linaldeddu, B.T.; Scanu, B.; Cimmino, A.; Basso, S.; Evidente, A. Bioactivity studies of oxysporone and several derivatives. Phytochem. Lett. 2014, 10, 40–45. [Google Scholar] [CrossRef]
- Scherlach, K.; Boettger, D.; Remme, N.; Hertweck, C. The chemistry and biology of cytochalasans. Nat. Prod. Rep. 2010, 27, 869–886. [Google Scholar] [CrossRef] [PubMed]
- Masi, M.; Evidente, A.; Meyer, S.; Nicholson, J.; Munoz, A. Effect of strain and cultural conditions on the production of cytochalasin B by the potential mycoherbicide Pyrenophora semeniperda (Pleosporaceae, Pleosporales). Biocontrol. Sci. Technol. 2014, 24, 53–64. [Google Scholar] [CrossRef]
- Masi, M.; Meyer, S.; Cimmino, A.; Clement, S.; Black, B.; Evidente, A. Pyrenophoric acids B and C, two new phytotoxic sesquiterpenoids produced by Pyrenophora semeniperda. J. Agric. Food Chem. 2014, 62, 10304–10311. [Google Scholar] [CrossRef] [PubMed]
- Skellam, E. The biosynthesis of cytochalasans. Nat. Prod. Rep. 2017, 34, 1252–1263. [Google Scholar] [CrossRef] [PubMed]
- Bottalico, A.; Capasso, R.; Evidente, A.; Randazzo, G.; Vurro, M. Cytochalasins: Structure-activity relationships. Phytochemistry 1990, 29, 93–96. [Google Scholar] [CrossRef]
- Li, X.J.; Zhang, Q.; Zhang, A.L.; Gao, J.M. Metabolites from Aspergillus fumigatus, an endophytic fungus associated with Melia azedarach, and their antifungal, antifeedant, and toxic activities. J. Agric. Food Chem. 2012, 60, 3424–3431. [Google Scholar] [CrossRef] [PubMed]
- Evidente, A.; Conti, L.; Altomare, C.; Bottalico, A.; Sindona, G.; Segre, A.L.; Logrieco, A. Fusapyrone and deoxyfusapyrone, two antifungal α-pyrones from Fusarium semitectum. Nat. Toxins 1994, 2, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Altomare, C.; Pengue, R.; Favilla, M.; Evidente, A.; Visconti, A. Structure-activity relationships of derivatives of fusapyrone, an antifungal metabolite of Fusarium semitectum. J. Agric. Food Chem. 2004, 52, 2997–3001. [Google Scholar] [CrossRef] [PubMed]
- Cooney, J.M.; Lauren, D.R. Trichoderma/pathogen interactions: Measurement of antagonistic chemicals produced at the antagonist/pathogen interface using a tubular bioassay. Lett. Appl. Microbiol. 1998, 27, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Jeleń, H.; Błaszczyk, L.; Chełkowski, J.; Rogowicz, K.; Strakowska, J. Formation of 6-n-pentyl-2H-pyran-2-one (6-PAP) and other volatiles by different Trichoderma species. Mycol. Prog. 2014, 13, 589–600. [Google Scholar] [CrossRef]
- Evidente, A.; Cabras, A.; Maddau, L.; Serra, S.; Andolfi, A.; Motta, A. Viridepyronone, a New antifungal 6-substituted 2H-Pyran-2-one produced by Trichoderma viride. J. Agric. Food Chem. 2003, 51, 6957–6960. [Google Scholar] [CrossRef] [PubMed]
- Sarrocco, S. Dung-inhabiting fungi: A potential reservoir of novel secondary metabolites for the control of plant pathogens. Pest Manag. Sci. 2016, 72, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, A.; Sarrocco, S.; Masi, M.; Diquattro, S.; Evidente, M.; Vannacci, G.; Evidente, A. Fusaproliferin, terpestacin and their derivatives display variable allelopathic activity against some Ascomycetous fungi. Chem. Biodivers. 2016, 13, 1593–1600. [Google Scholar] [CrossRef] [PubMed]
- Oerke, E.C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, X.F.; Zhou, M.G.; Kuang, J.; Zhang, Y.; Shang, Y.; Wang, J.X. Status of streptomycin resistance development in Xanthomonas oryzae pv. oryzae and Xanthomonas oryzae pv. oryzicola in China and their resistance characters. J. Phytopathol. 2010, 158, 601–608. [Google Scholar] [CrossRef]
- Mansfield, J.; Genin, S.; Magori, S.; Citovsky, V.; Sriariyanum, M.; Ronald, P.; Dow, M.; Verdier, V.; Beer, S.V.; Machado, M.A.; et al. Top 10 plant pathogenic bacteria in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 614–629. [Google Scholar] [CrossRef] [PubMed]
- Evidente, A.; Venturi, V.; Masi, M.; Degrassi, G.; Cimmino, A.; Maddau, L.; Andolfi, A. In vitro antibacterial activity of sphaeropsidins and chemical derivatives toward Xanthomonas oryzae pv. oryzae, the causal agent of rice bacterial blight. J. Nat. Prod. 2011, 74, 2520–2525. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Yang, Y.; Zhang, J.-R. Molecular basis of host specificity in human pathogenic bacteria. Emerg. Microbes Infect. 2014, 3, e23. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.L.; Le, X.; Li, H.J.; Yang, X.L.; Chen, J.X.; Xu, J.; Liu, H.L.; Wang, L.Y.; Wang, K.T.; Hu, K.C.; et al. Exploring the chemodiversity and biological activities of the secondary metabolites from the marine fungus Neosartorya pseudofischeri. Mar. Drugs 2014, 12, 5657–5676. [Google Scholar] [CrossRef] [PubMed]
- Masi, M.; Andolfi, A.; Mathieu, V.; Boari, A.; Cimmino, A.; Moreno, Y.; Banuls, L.; Vurro, M.; Kornienko, A.; Kiss, R.; et al. Fischerindoline, a pyrroloindole sesquiterpenoid isolated from Neosartorya pseudofischeri, with in vitro growth inhibitory activity in human cancer cell lines. Tetrahedron 2013, 69, 7466–7470. [Google Scholar] [CrossRef]
- Schnekenburger, M.; Mathieu, V.; Lefranc, F.; Jang, J.Y.; Masi, M.; Kijjoa, A.; Evidente, A.; Kim, H.-J.; Kiss, R.; Dicato, M.; et al. The fungal metabolite eurochevalierine, a sequiterpene alkaloid, displays anti-cancer properties through selective sirtuin 1/2 inhibition. Molecules 2018, 23, 333. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Jiang, N.; Han, B.; Mei, Y.; Ge, H.; Guo, Z.; Weng, N.; Tan, R. Antibacterial epipolythiodioxopiperazine and unprecedented sesquiterpene from Pseudallescheria boydii, a beetle (coleoptera)-associated fungus. Org. Biomol. Chem. 2014, 12, 9405–9412. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.L.; Wei, M.Y.; Shao, C.L.; Fu, X.M.; Guo, Z.Y.; Xu, R.F.; Zheng, C.J.; She, Z.G.; Lin, Y.C.; Wang, C.Y. Antibacterial anthraquinone derivatives from a sea anemone-derived fungus Nigrospora sp. J. Nat. Prod. 2012, 75, 935–941. [Google Scholar] [CrossRef] [PubMed]
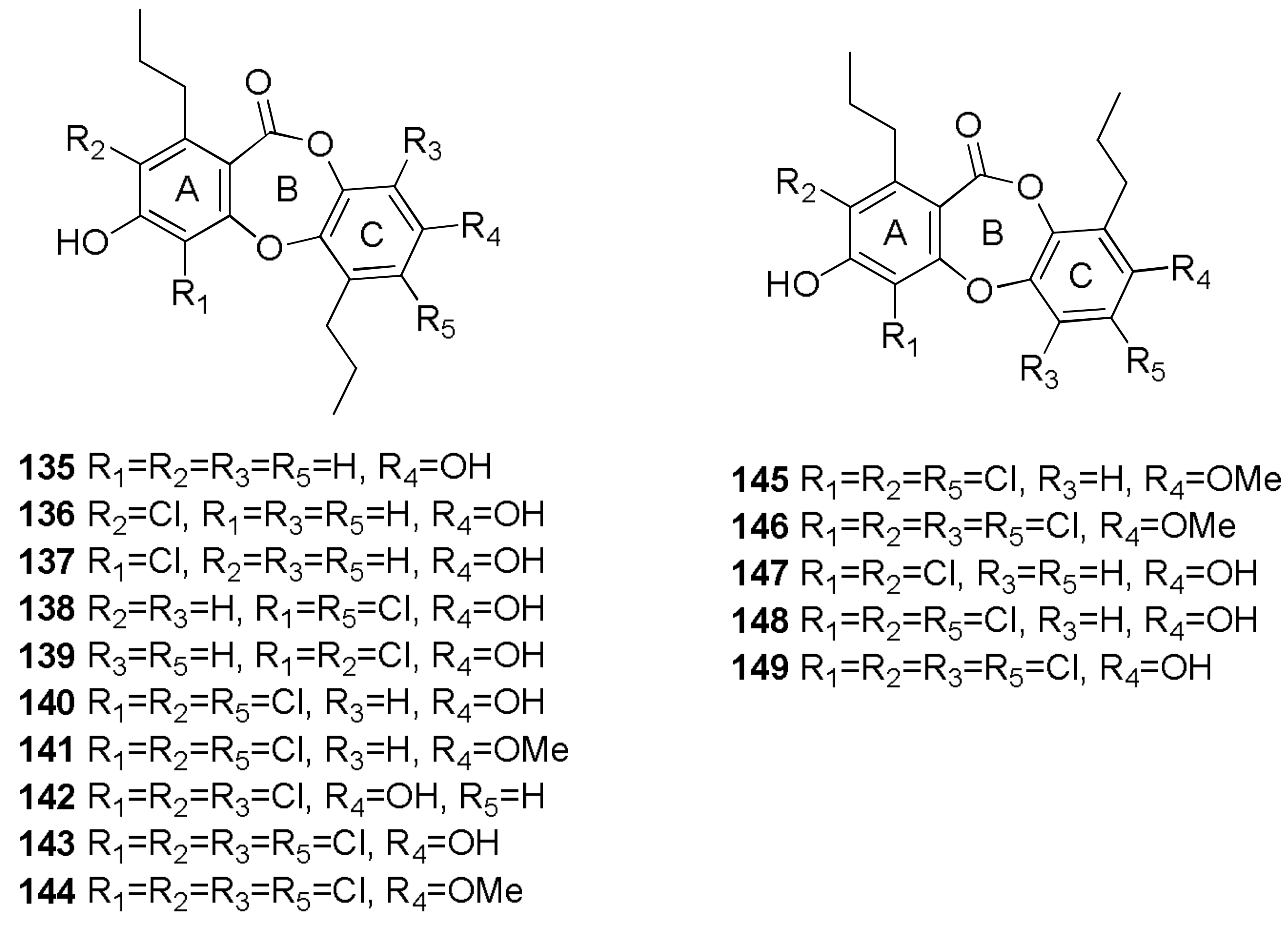
- Niu, S.; Liu, D.; Hu, X.; Proksch, P.; Shao, Z.; Lin, W. Spiromastixones A–O, antibacterial chlorodepsidones from a deep-sea-derived Spiromastix sp. fungus. J. Nat. Prod. 2014, 77, 1021–1030. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chen, R.; Liu, M.; Liu, D.; Li, X.; Wang, S.; Niu, S.; Guo, P.; Lin, W.; Jacobson, P.B. Spiromastixones inhibit foam cell formation via regulation of cholesterol efflux and uptake in RAW264.7 macrophages. Mar. Drugs 2015, 13, 6352–6365. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.C.; Milatovic, D. Insecticides. In Biomarkers in Toxicology; Academic Press: Cambridge, MA, USA, 2014; pp. 389–407. ISBN 9780124046306. [Google Scholar]
- Anke, H.; Sterner, O. Insecticidal and nematicidal metabolites from fungi. In The Mycota X. Industrial Applications; Osiewacz, H.D., Ed.; Springer: Berlin, Germany, 2002; pp. 109–127. [Google Scholar]
- Geris, R.; Ribeiro, P.R.; Da Silva Brandão, M.; Da Silva, H.H.G.; Da Silva, I.G. Chapter 10—Bioactive Natural Products as Potential Candidates to Control Aedes aegypti, the Vector of Dengue; Elsevier: New York, NY, USA, 2012; Volume 37. [Google Scholar]
- Masi, M.; Cala, A.; Tabanca, N.; Cimmino, A.; Green, I.R.; Bloomquist, J.R.; Van Otterlo, W.A.L.; Macias, F.A.; Evidente, A. Alkaloids with activity against the zika virus vector Aedes aegypti (L.)-crinsarnine and sarniensinol, two new crinine and mesembrine type alkaloids isolated from the South African plant Nerine sarniensis. Molecules 2016, 21, 1432. [Google Scholar] [CrossRef] [PubMed]
- Masi, M.; van der Westhuyzen, A.E.; Tabanca, N.; Evidente, M.; Cimmino, A.; Green, I.R.; Bernier, U.R.; Becnel, J.J.; Bloomquist, J.R.; van Otterlo, W.A.L.; et al. Sarniensine, a mesembrine-type alkaloid isolated from Nerine sarniensis, an indigenous South African Amaryllidaceae, with larvicidal and adulticidal activities against Aedes aegypti. Fitoterapia 2017, 116, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Masi, M.; Cimmino, A.; Tabanca, N.; Becnel, J.J.; Bloomquist, J.R.; Evidente, A. A survey of bacterial, fungal and plant metabolites against Aedes aegypti (Diptera: Culicidae), the vector of yellow and dengue fevers and Zika virus. Open Chem. 2017, 15, 156–166. [Google Scholar] [CrossRef]
- Cimmino, A.; Andolfi, A.; Avolio, F.; Ali, A.; Tabanca, N.; Khan, I.A.; Evidente, A. Cyclopaldic acid, seiridin, and sphaeropsidin A as fungal phytotoxins, and larvicidal and biting deterrents against Aedes aegypti (Diptera: Culicidae): Structure-activity relationships. Chem. Biodivers. 2013, 10, 1239–1251. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, A.; Evidente, M.; Masi, M.; Ali, A.; Tabanca, N.; Khan, I.A.; Evidente, A. Papyracillic acid and its derivatives as biting deterrents against Aedes aegypti (Diptera: Culicidae): Structure-activity relationships. Med. Chem. Res. 2015, 24, 3981–3989. [Google Scholar] [CrossRef]
- Graniti, A.; Sparapano, L.; Evidente, A. Cyclopaldic acid, a major phytotoxic metabolite of Seiridium cupressi, the pathogen of a canker disease of cypress. Plant Pathol. 2007, 41, 563–568. [Google Scholar] [CrossRef]
- Sparapano, L.; Evidente, A. Biological activity of cyclopaldic acid, a major toxin of Seiridium cupressi, its six derivatives, andiso-cyclopaldic acid. Nat. Toxins 1995, 3, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Evidente, A.; Randazzo, G.; Ballio, A. Structure determination of seiridin and isoseiridin, phytotoxic butenolides from culture filtrate of Seiridium cardinale. J. Nat. Prod. 1986, 49, 593–601. [Google Scholar] [CrossRef]
- Shan, R.; Anke, H.; Stadler, M.; Sterner, O. Papyracillic acid, a new penicillic acid analogue from the ascomycete Lachnum papyraceum. Tetrahedron 1996, 52, 10249–10254. [Google Scholar] [CrossRef]
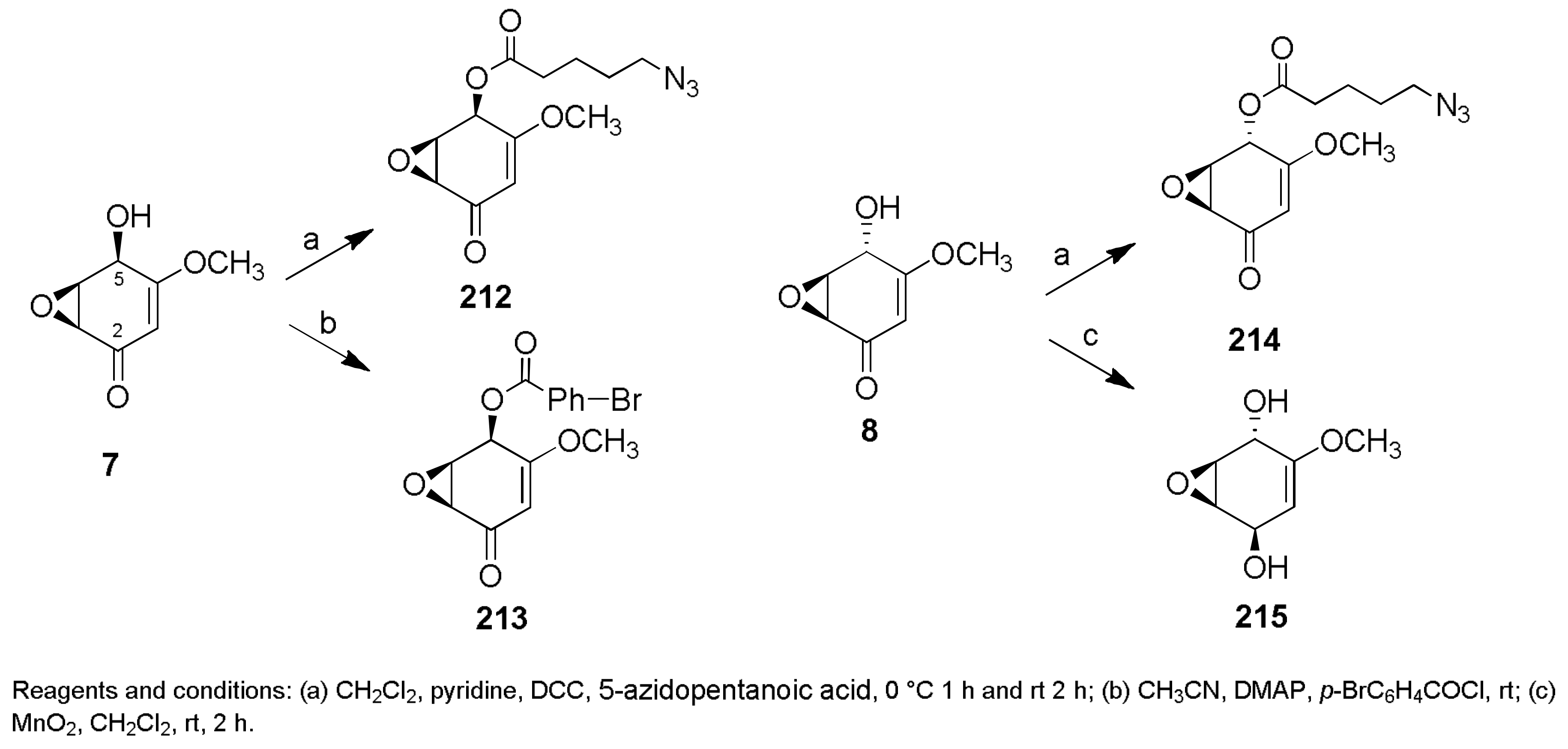
- Evidente, A.; Berestetskiy, A.; Cimmino, A.; Tuzi, A.; Superchi, S.; Melck, D.; Andolfi, A. Papyracillic acid, a phytotoxic 1,6-dioxaspiro[4,4]nonene produced by Ascochyta agropyrina var. nana, a potential mycoherbicide for Elytrigia repens biocontrol. J. Agric. Food Chem. 2009, 57, 11168–11173. [Google Scholar] [CrossRef] [PubMed]
- Shan, R.; Stadler, M.; Anke, H.; Sterner, O. The reactivity of the fungal toxin papyracillic acid. Tetrahedron 1997, 53, 6209–6214. [Google Scholar] [CrossRef]
- Geris dos Santos, R.M.; Rodrigues-Fo, E. Meroterpenes from Penicillium sp. found in association with Melia azedarach. Phytochemistry 2002, 61, 907–912. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Zhang, X.; Lai, D.; Zhou, L. Structural diversity and biological activities of the cyclodipeptides from fungi. Molecules 2017, 22, 2026. [Google Scholar] [CrossRef] [PubMed]
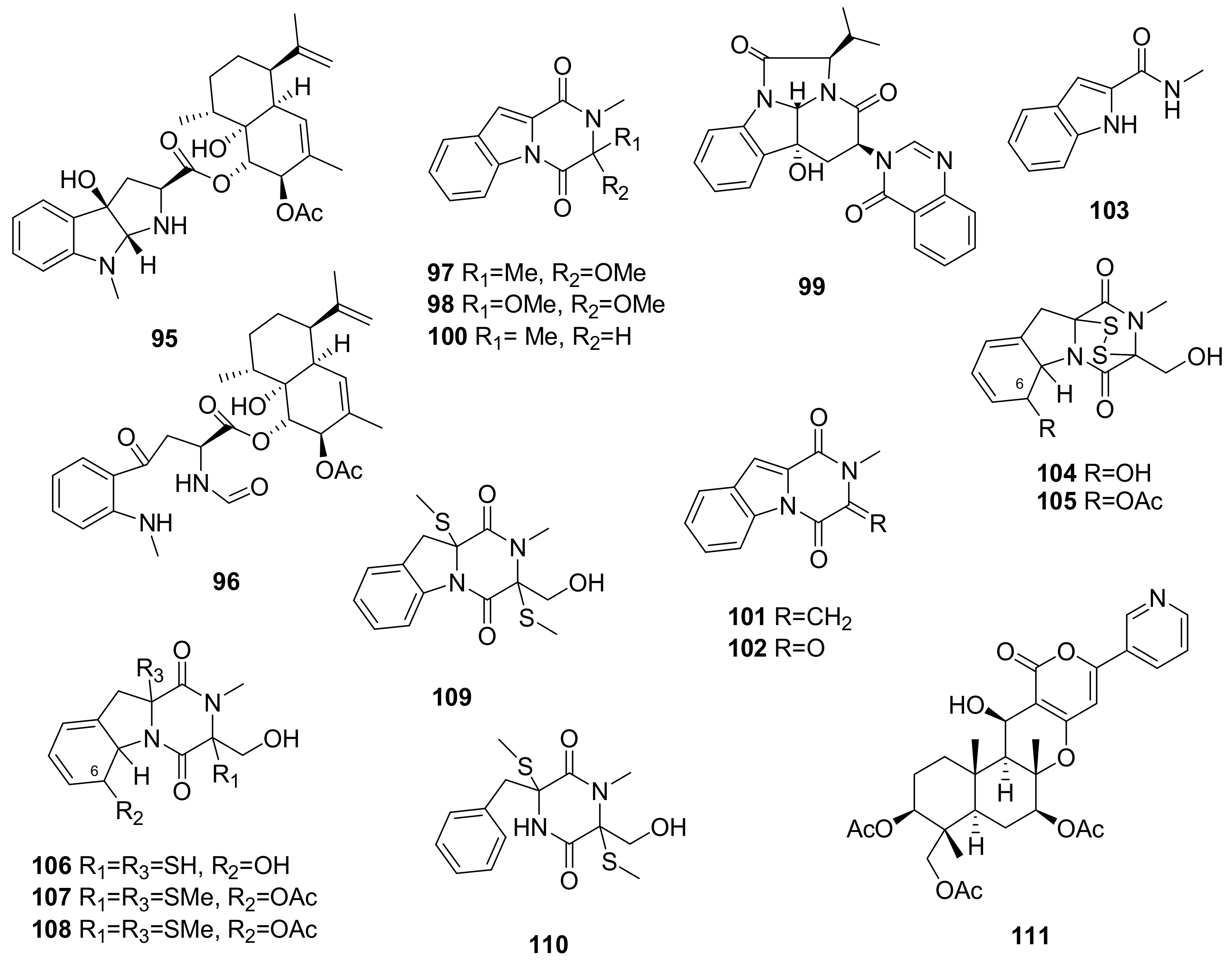
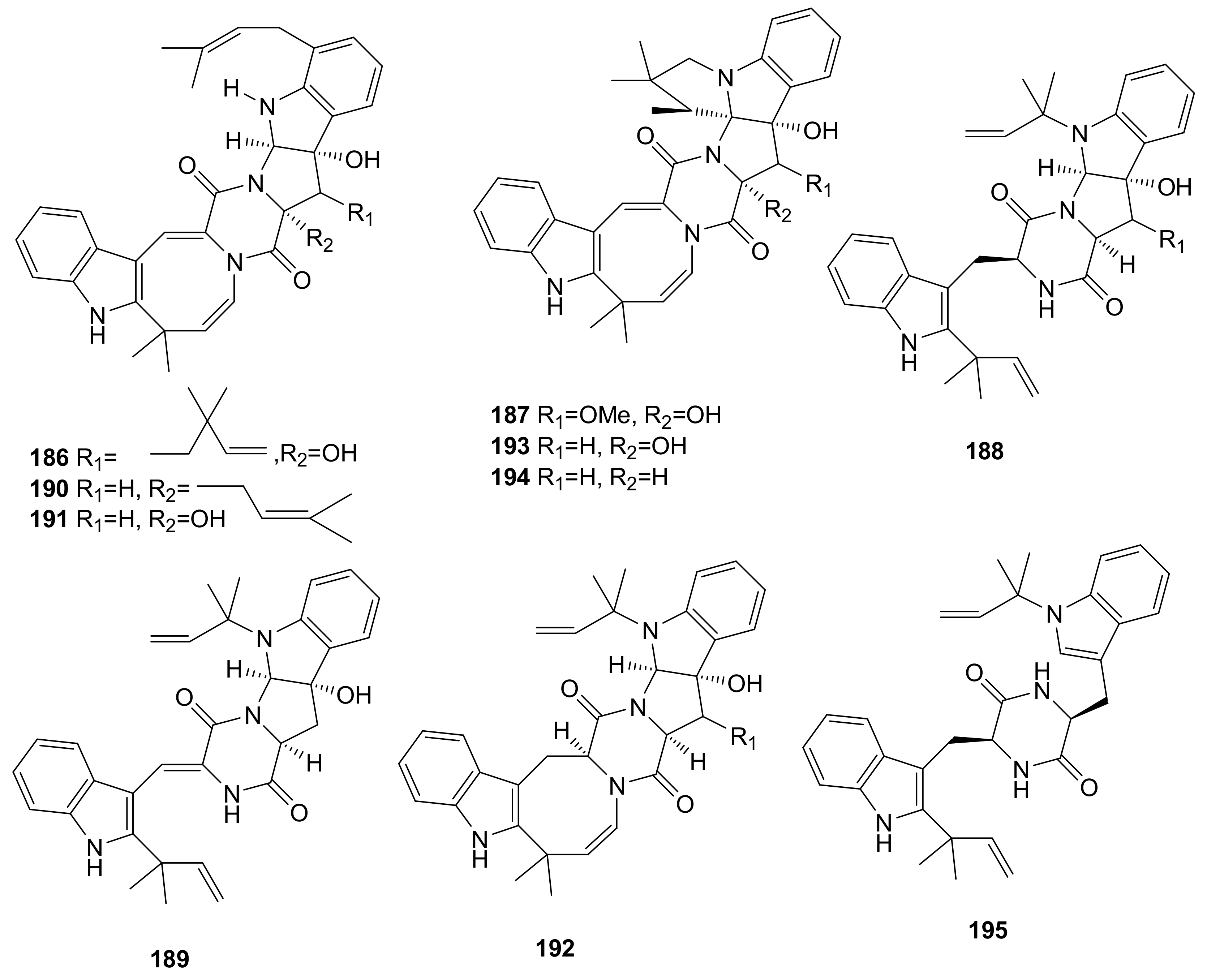
- Kato, N.; Furutani, S.; Otaka, J.; Noguchi, A.; Kinugasa, K.; Kai, K.; Hayashi, H.; Ihara, M.; Takahashi, S.; Matsuda, K.; et al. Biosynthesis and structure–activity relationship studies of okaramines that target insect glutamate-gated chloride channels. ACS Chem. Biol. 2018, 13, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, H.; Takiuchi, K.; Murao, S.; Arai, M. Structure and insecticidal activity of new indole alkaloids, okaramines A and B, from Penicillium simplicissimum ak-40. Agric. Biol. Chem. 1989, 53, 461–469. [Google Scholar] [CrossRef]
- Harvey, R.J.; Vreugdenhill, E.; Zaman, S.H.; Bhandal, N.S.; Usherwood, P.N.R.; Barnard, E.A.; Darlison, M.G.; Harvey, R.J.; ffrench-Constant, R.H.; Rocheleau, T.A.; et al. GluCl a target of indole alkaloid okaramines: A 25 year enigma solved. Br. J. Pharmacol. 1996, 119, 62–67. [Google Scholar] [CrossRef]
- Cimmino, A.; Andolfi, A.; Zonno, M.C.; Avolio, F.; Santini, A.; Tuzi, A.; Berestetskyi, A.; Vurro, M.; Evidente, A. Chenopodolin: A phytotoxic unrearranged ent-pimaradiene diterpene produced by phoma chenopodicola, a fungal pathogen for Chenopodium album biocontrol. J. Nat. Prod. 2013, 76, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Evidente, M.; Cimmino, A.; Zonno, M.C.; Masi, M.; Berestetskyi, A.; Santoro, E.; Superchi, S.; Vurro, M.; Evidente, A. Phytotoxins produced by Phoma chenopodiicola, a fungal pathogen of Chenopodium album. Phytochemistry 2015, 117, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Berestetskiy, A.; Dmitriev, A.; Mitina, G.; Lisker, I.; Andolfi, A.; Evidente, A. Nonenolides and cytochalasins with phytotoxic activity against Cirsium arvense and Sonchus arvensis: A structure-activity relationships study. Phytochemistry 2008, 69, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Andolfi, A.; Cimmino, A.; Vurro, M.; Berestetskiy, A.; Troise, C.; Zonno, M.C.; Motta, A.; Evidente, A. Agropyrenol and agropyrenal, phytotoxins from Ascochyta agropyrina var. nana, a fungal pathogen of Elitrigia repens. Phytochemistry 2012, 79, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, A.; Zonno, M.C.; Andolfi, A.; Troise, C.; Motta, A.; Vurro, M.; Evidente, A. Agropyrenol, a phytotoxic fungal metabolite, and its derivatives: A structure-activity relationship study. J. Agric. Food Chem. 2013, 61, 1779–1783. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, A.; Andolfi, A.; Zonno, M.C.; Troise, C.; Santini, A.; Tuzi, A.; Vurro, M.; Ash, G.; Evidente, A. Phomentrioloxin: A phytotoxic pentasubstituted geranylcyclohexentriol produced by Phomopsis sp., a potential mycoherbicide for Carthamus lanatus biocontrol. J. Nat. Prod. 2012, 75, 1130–1137. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, A.; Andolfi, A.; Zonno, M.C.; Boari, A.; Troise, C.; Motta, A.; Vurro, M.; Ash, G.; Evidente, A. Phomentrioloxin, a fungal phytotoxin with potential herbicidal activity, and its derivatives: A structure-activity relationship study. J. Agric. Food Chem. 2013, 61, 9645–9649. [Google Scholar] [CrossRef] [PubMed]
- Evidente, A.; Andolfi, A.; Fiore, M.; Boari, A.; Vurro, M. Stimulation of Orobanche ramosa seed germination by fusicoccin derivatives: A structure-activity relationship study. Phytochemistry 2006, 67, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Aparicio, M.; Andolfi, A.; Cimmino, A.; Rubiales, D.; Evidente, A. Stimulation of seed germination of Orobanche species by ophiobolin a and fusicoccin derivatives. J. Agric. Food Chem. 2008, 56, 8343–8347. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Aparicio, M.; Masi, M.; Maddau, L.; Cimmino, A.; Evidente, M.; Rubiales, D.; Evidente, A. Induction of haustorium development by sphaeropsidones in radicles of the parasitic weeds Striga and Orobanche. A structure-activity relationship study. J. Agric. Food Chem. 2016, 64, 5188–5196. [Google Scholar] [CrossRef] [PubMed]
- Nickrent, D.L. Parasitic plants. Ecology 1997, 78, 1612–1613. [Google Scholar] [CrossRef]
- Press, M.C.; Phoenix, G.K. Impacts of parasitic plants on natural communities. New Phytol. 2005, 166, 737–751. [Google Scholar] [CrossRef] [PubMed]
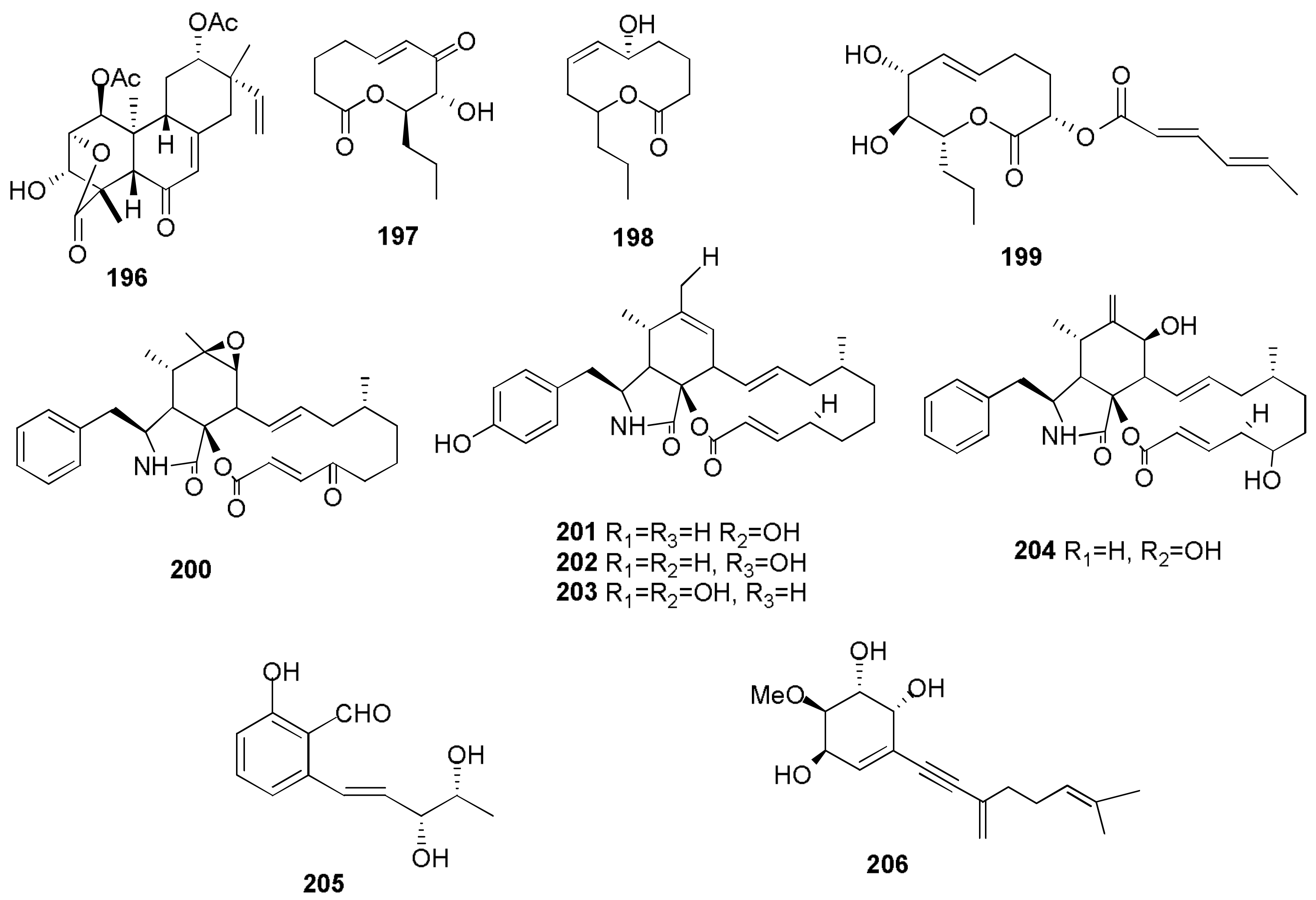
- Masi, M.; Meyer, S.; Clement, S.; Cimmino, A.; Cristofaro, M.; Evidente, A. Cochliotoxin, a dihydropyranopyran-4,5-dione, and its analogues produced by Cochliobolus australiensis display phytotoxic activity against buffelgrass (Cenchrus ciliaris). J. Nat. Prod. 2017, 80, 1241–1247. [Google Scholar] [CrossRef] [PubMed]
- Masi, M.; Meyer, S.; Clement, S.; Pescitelli, G.; Cimmino, A.; Cristofaro, M.; Evidente, A. Chloromonilinic acids C and D, phytotoxic tetrasubstituted 3-chromanonacrylic acids isolated from Cochliobolus australiensis with potential herbicidal activity against buffelgrass (Cenchrus ciliaris). J. Nat. Prod. 2017, 80, 2771–2777. [Google Scholar] [CrossRef] [PubMed]
- Masi, M.; Meyer, S.; Górecki, M.; Mandoli, A.; Di Bari, L.; Pescitelli, G.; Cimmino, A.; Cristofaro, M.; Clement, S.; Evidente, A. Pyriculins A and B, two monosubstituted hex-4-ene-2,3-diols and other phytotoxic metabolites produced by Pyricularia grisea isolated from buffelgrass (Cenchrus ciliaris). Chirality 2017, 29, 726–736. [Google Scholar] [CrossRef] [PubMed]
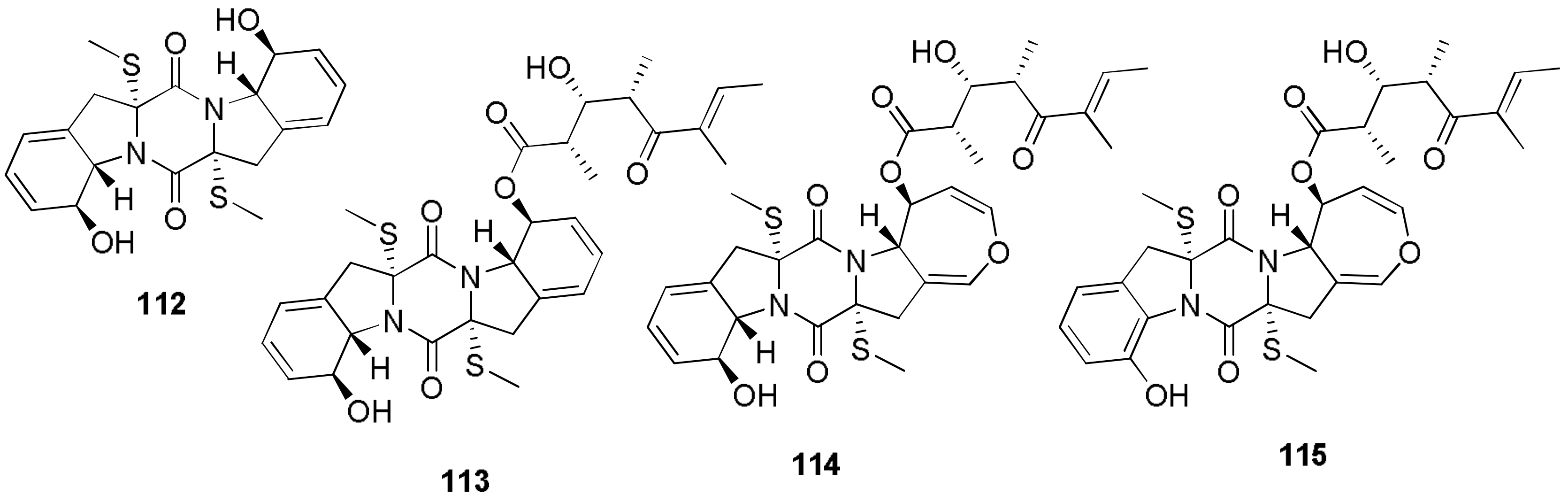
- Masi, M.; Meyer, S.; Clement, S.; Andolfi, A.; Cimmino, A.; Evidente, A. Spirostaphylotrichin W, a spirocyclic γ-lactam isolated from liquid culture of Pyrenophora semeniperda, a potential mycoherbicide for cheatgrass (Bromus tectorum) biocontrol. Tetrahedron 2014, 70, 1497–1501. [Google Scholar] [CrossRef]
- Masi, M.; Meyer, S.; Cimmino, A.; Andolfi, A.; Evidente, A. Pyrenophoric acid, a phytotoxic sesquiterpenoid penta-2,4-dienoic acid produced by a potential mycoherbicide Pyrenophora semeniperda. J. Nat. Prod. 2014, 77, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Meyer, S.E.; Masi, M.; Clement, S.; Davis, T.L.; Beckstead, J. Mycelial growth rate and toxin production in the seed pathogen Pyrenophora semeniperda: Resource trade-offs and temporally varying selection. Plant Pathol. 2015, 64, 1450–1460. [Google Scholar] [CrossRef]
- Cimmino, A.; Masi, M.; Evidente, M.; Superchi, S.; Evidente, A. Application of Mosher’s method for absolute configuration assignment to bioactive plants and fungi metabolites. J. Pharm. Biomed. Anal. 2017, 144, 59–89. [Google Scholar] [CrossRef] [PubMed]
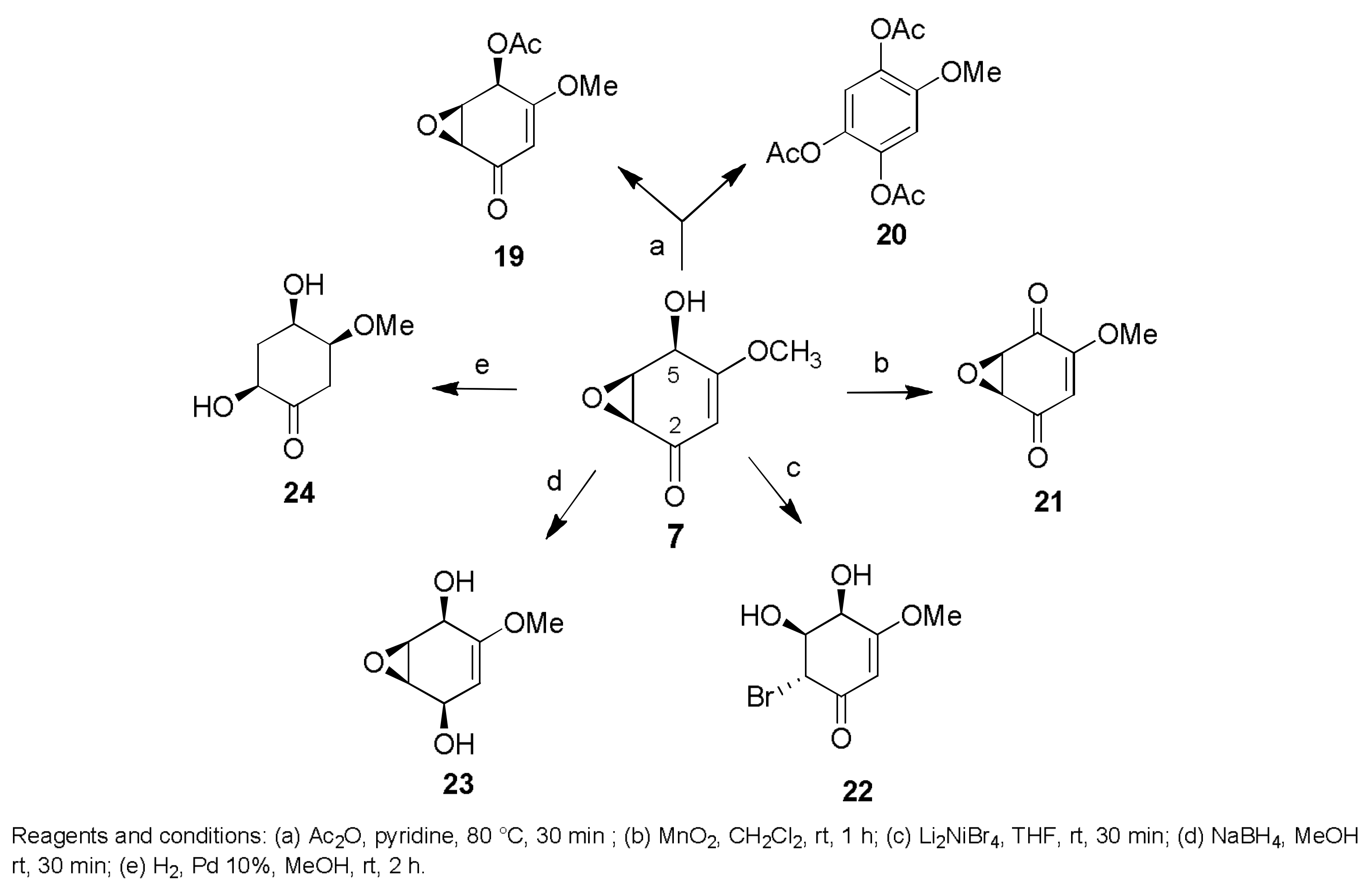
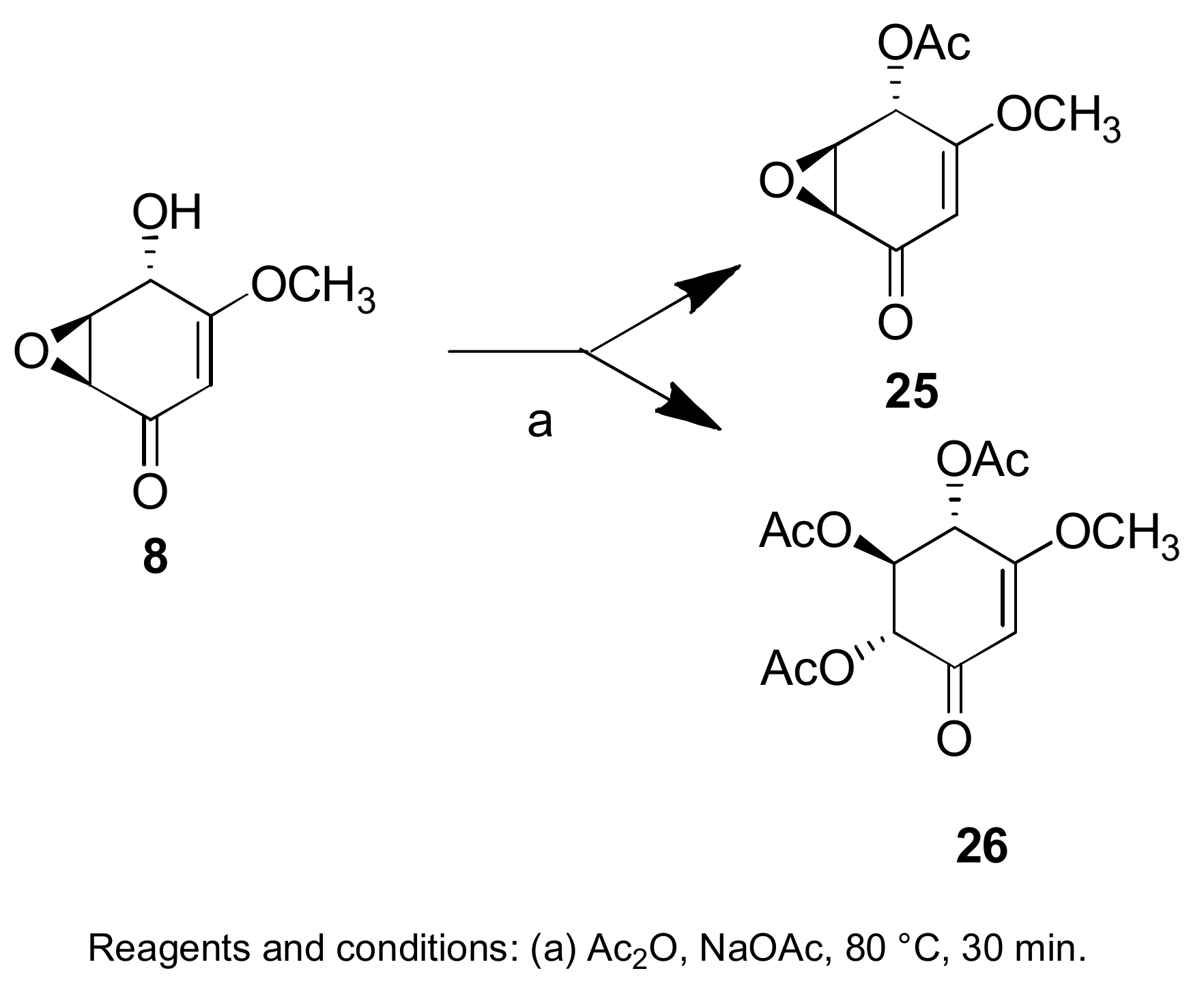
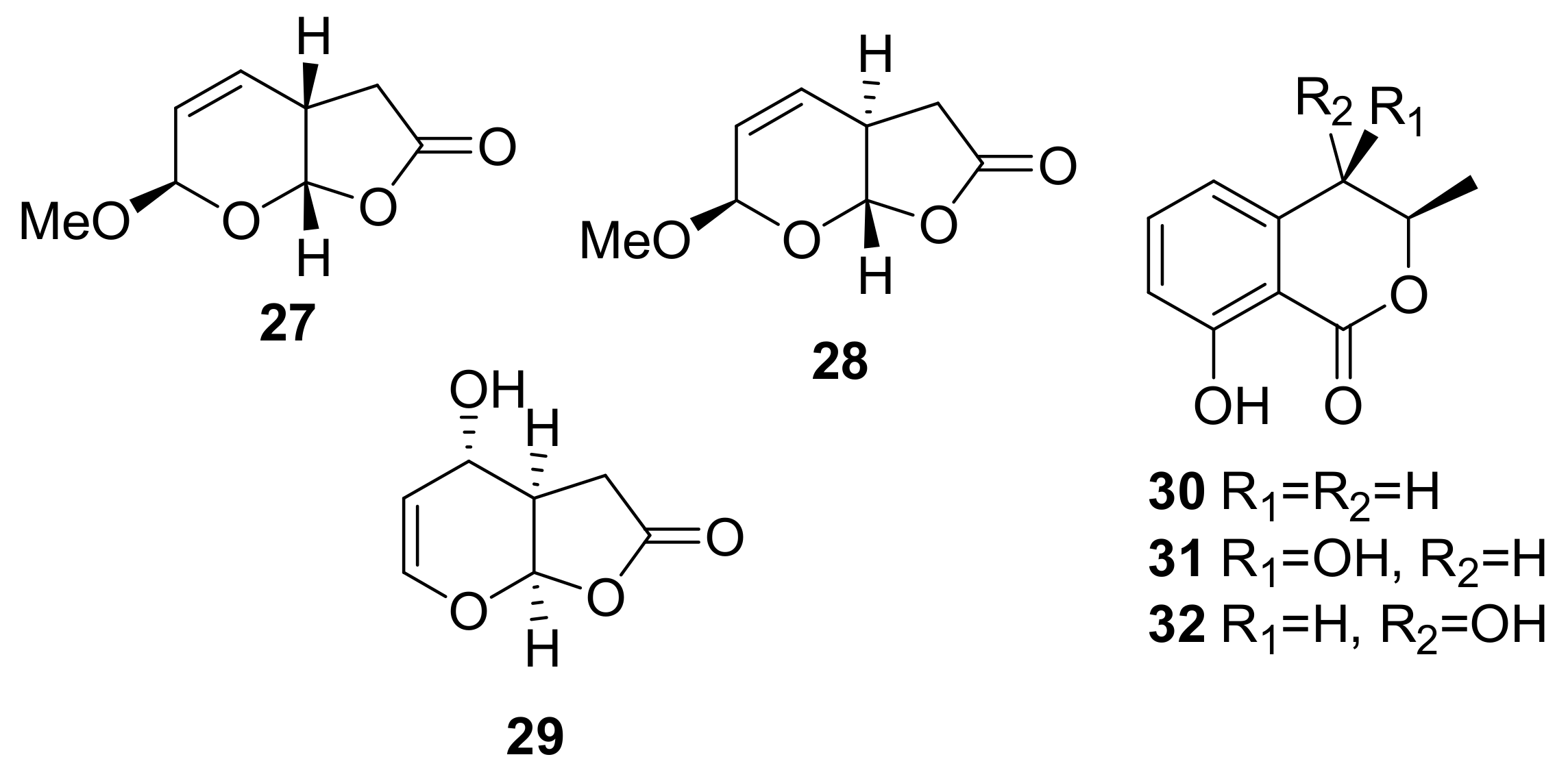
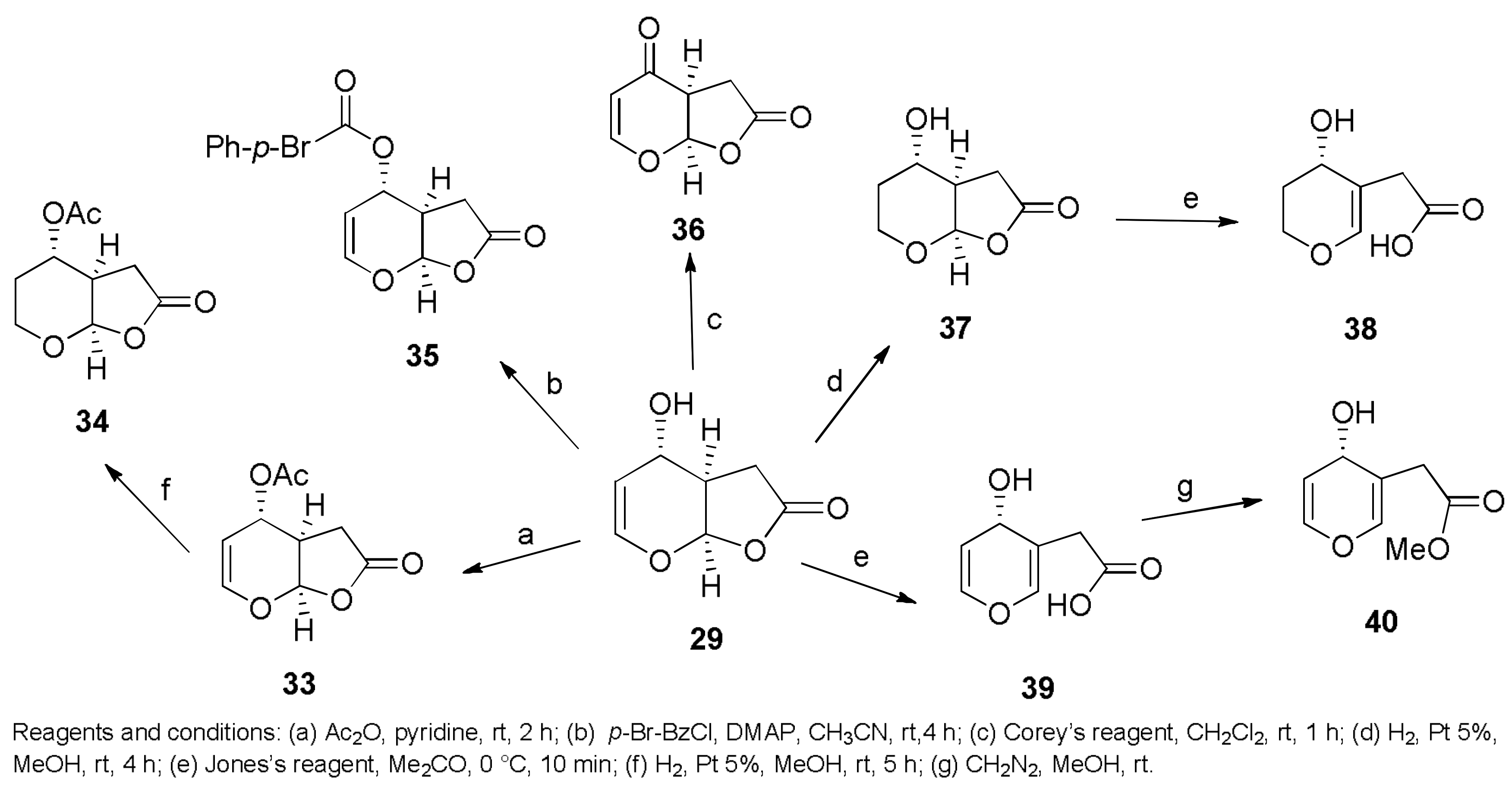
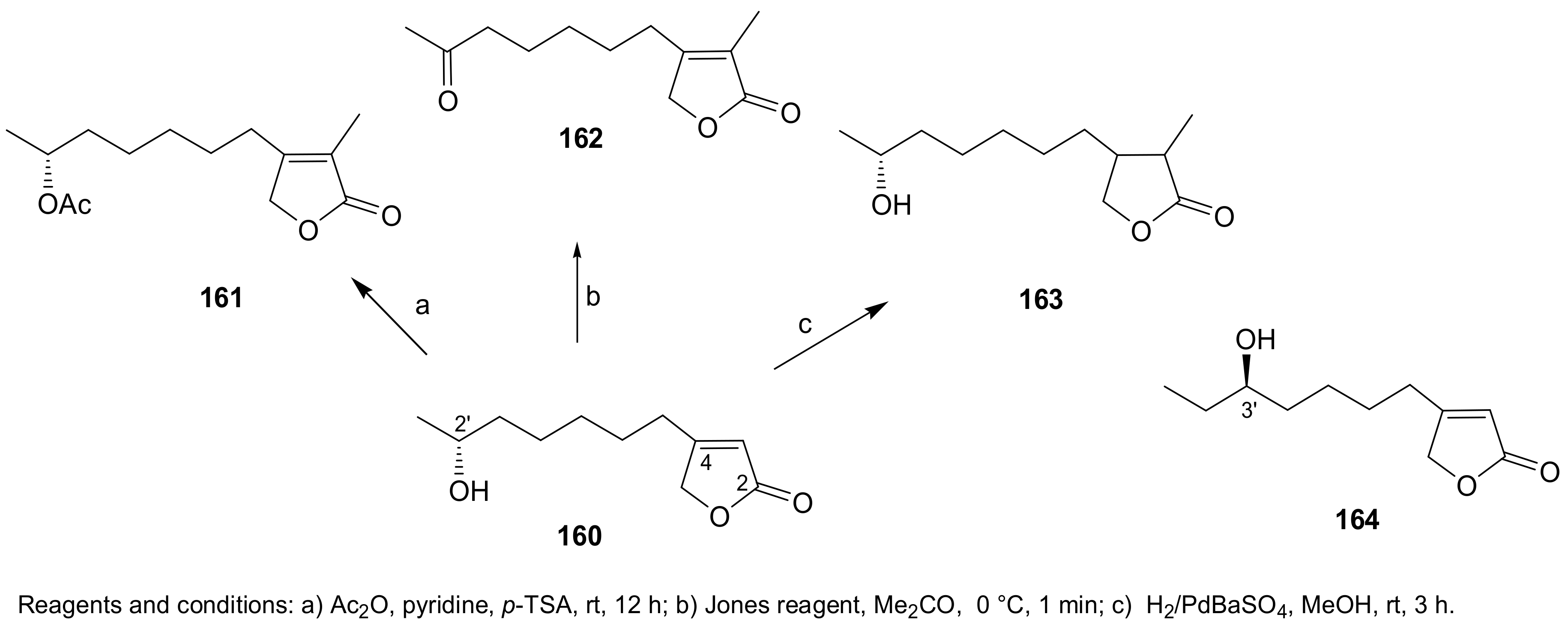
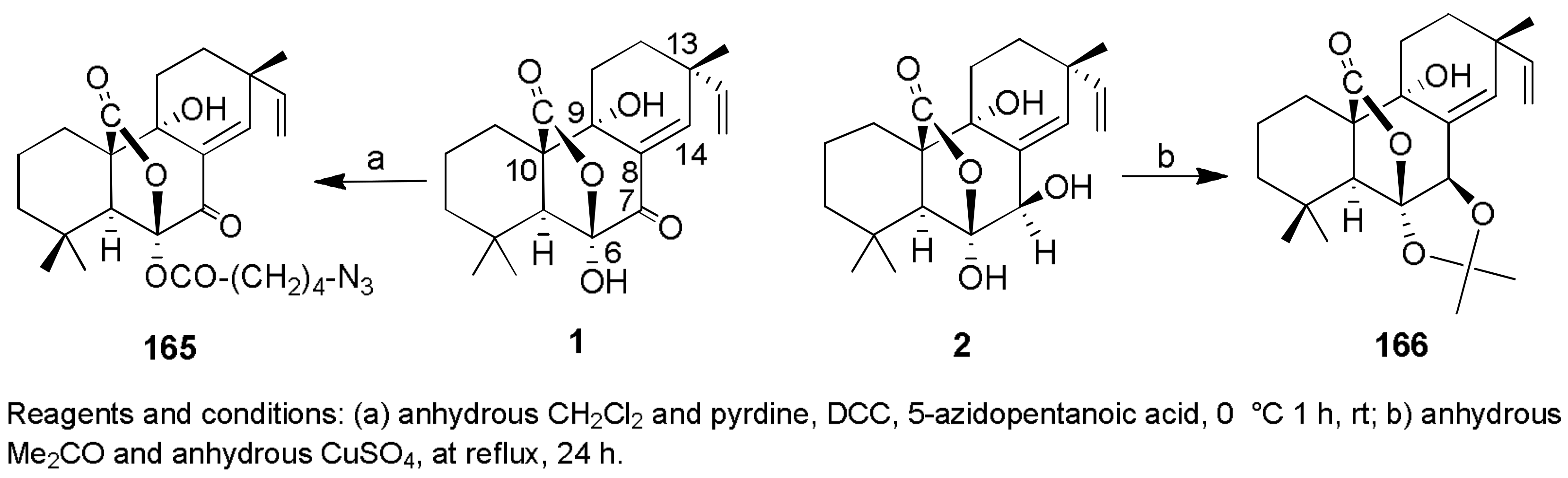
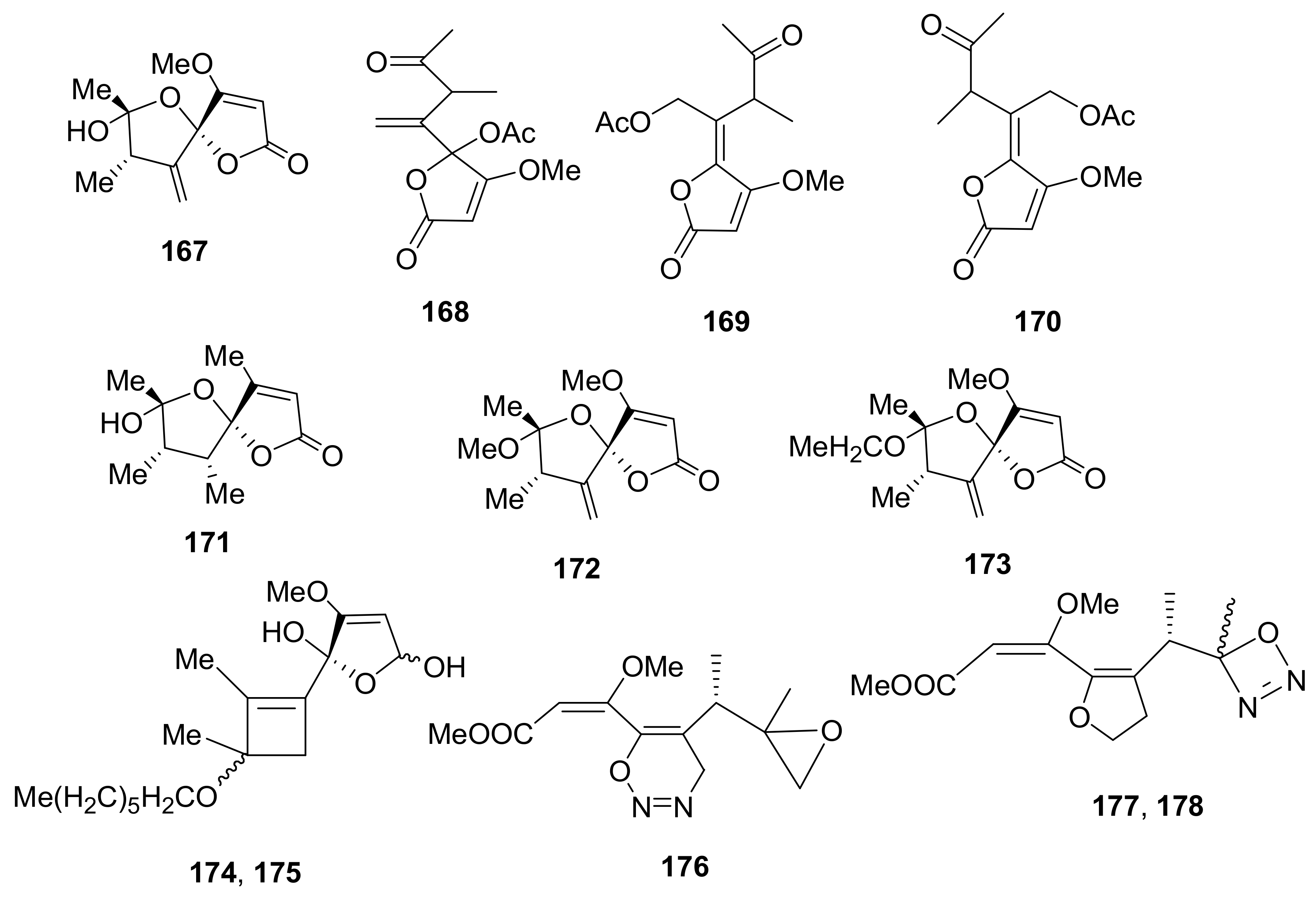
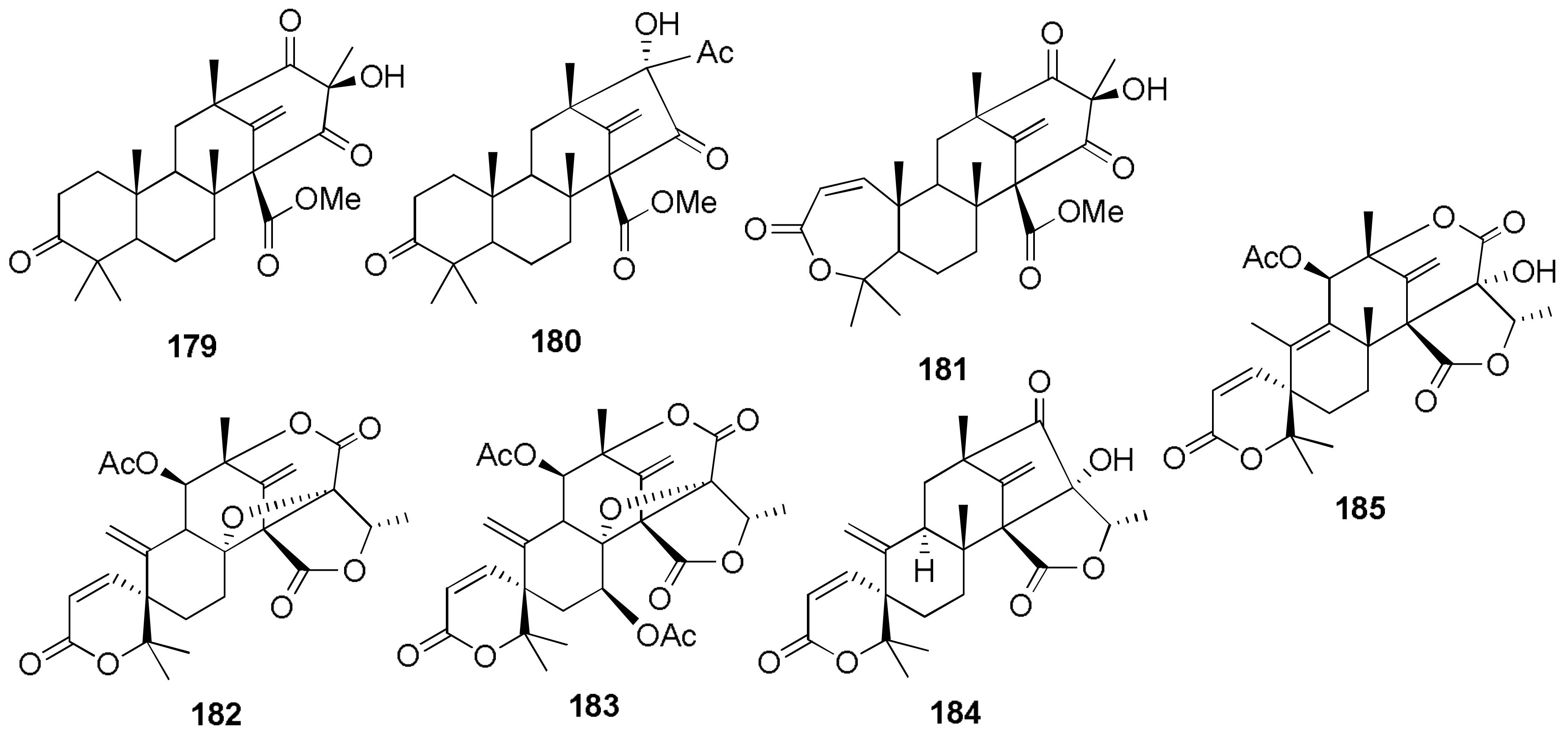
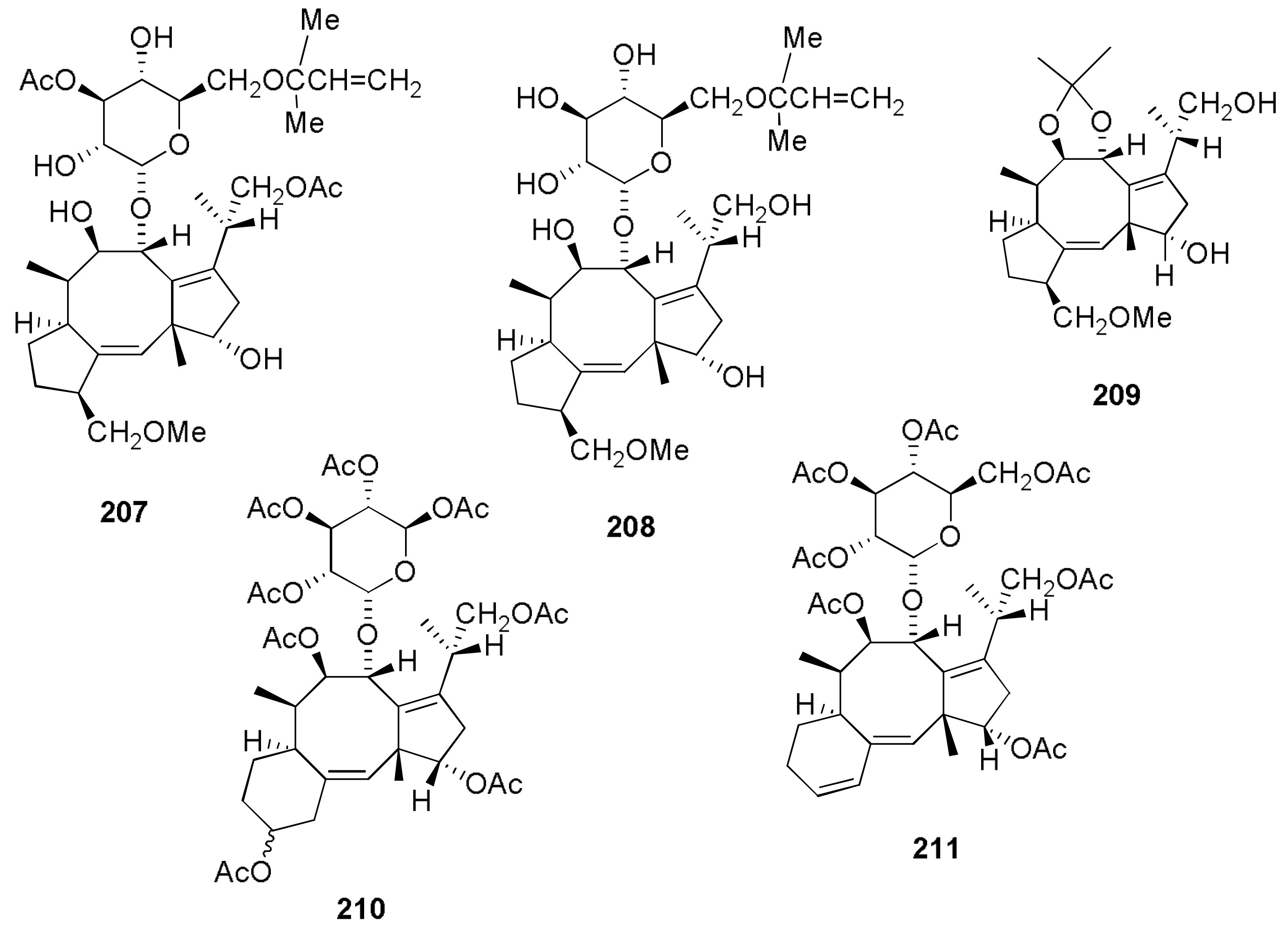
Sample Availability: Samples of the compounds 1, 2, 7, 8, 29–32, 43, 44, 150, 160, 167, 207, 216–224 are available from the authors. |





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masi, M.; Nocera, P.; Reveglia, P.; Cimmino, A.; Evidente, A. Fungal Metabolites Antagonists towards Plant Pests and Human Pathogens: Structure-Activity Relationship Studies. Molecules 2018, 23, 834. https://doi.org/10.3390/molecules23040834
Masi M, Nocera P, Reveglia P, Cimmino A, Evidente A. Fungal Metabolites Antagonists towards Plant Pests and Human Pathogens: Structure-Activity Relationship Studies. Molecules. 2018; 23(4):834. https://doi.org/10.3390/molecules23040834
Chicago/Turabian StyleMasi, Marco, Paola Nocera, Pierluigi Reveglia, Alessio Cimmino, and Antonio Evidente. 2018. "Fungal Metabolites Antagonists towards Plant Pests and Human Pathogens: Structure-Activity Relationship Studies" Molecules 23, no. 4: 834. https://doi.org/10.3390/molecules23040834
APA StyleMasi, M., Nocera, P., Reveglia, P., Cimmino, A., & Evidente, A. (2018). Fungal Metabolites Antagonists towards Plant Pests and Human Pathogens: Structure-Activity Relationship Studies. Molecules, 23(4), 834. https://doi.org/10.3390/molecules23040834







