Abstract
Neurodegeneration is a progressive loss of neuronal cells in certain regions of the brain. Most of the neurodegenerative disorders (NDDs) share the communal characteristic such as damage or reduction of various cell types typically including astrocytes and microglial activity. Several compounds are being trialed to treat NDDs but they possess solitary symptomatic advantages along with copious side effects. The finding of more enthralling and captivating compounds to suspend and standstill the pathology of NDDs will be considered as a hallmark of present times. Phytochemicals possess the potential to alternate the synthetic line of therapy against NDDs. The present review explores the potential efficacy of plant-derived flavonoids against most common NDDs including Alzheimer’s disease (AD) and Parkinson’s disease (PD). Flavonoids are biologically active phytochemicals which possess potential pharmacological effects, including antiviral, anti-allergic, antiplatelet, anti-inflammatory, anti-tumor, anti-apoptotic and anti-oxidant effects and are able to attenuate the pathology of various NDDs through down-regulating the nitric oxide (NO) production, by reducing the tumor necrosis factor-α (TNF-α), by reducing the excitotoxicity of superoxide as well as acting as tyrosine kinase (TK) and monoamine oxidase (MAO) inhibiting enzyme.
1. Introduction
Neurodegeneration is a composite progression of progressive loss of both the function and structure of neurons and involves the muscle weakening and deterioration of innumerable physiological functions of the body [1,2]. Deregulated lipid metabolism is one of the hallmarks of degeneration in the majority of neurodegenerative disorders [3,4,5,6]. During neurodegeneration, cell death is preeminent in post-mitotic cells, with an enormous number of neurons eliciting apoptotic signals, which might be the consequence of oxidative stress [7]. Both intrinsic and extrinsic pathways are two major aspects of apoptosis which are associated with mitochondrial and plasma membrane receptors, respectively [8]. Proteins of bcl-2 family play a crucial role in regulating the pathways of apoptosis involving mitochondria. They can be categorized into two functionally diverse groups as pro- and anti-apoptotic proteins [9]. Neurodegenerative diseases (NDDs) usually involve the discerning loss of neurons as well as engrossment of diversified functional systems describing their clinical presentation expanded by genetic, biochemical and molecular pathological factors. Enormous studies have revealed the deposition of proteins with transformed physiochemical properties within the human brains in NDDs [10]. Aggregation of interrelated proteins serves as a major hallmark of the NDDs, suggesting the same pathophysiology of the degenerative process. Recent studies state that such proteinopathies expose the contribution of the same protein in a number of diseases, thus signifying a common pathological progression [11].
Neurodegenerative diseases such as Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington disease (HD), Schizophrenia, Amyotrophic Lateral Sclerosis (ALS), seizure disorders, and head injuries are foremost health issues along with other systemic disorders [12,13,14]. Various studies also state the involvement of oxidative stress in the pathophysiology of NDDs. Oxidative stress causes the neuronal cell death by inducing the neuronal damage and modulating the intracellular signaling [15].
Natural products persist as a promising source of immense chemical diversity, biochemical specificity and various molecular characteristics which make them suitable for the modulation of multiple signaling pathways/cascades in various pathological conditions such as cancer and neurodegnerative diseases [16,17,18,19,20,21,22]. Currently, phytochemicals including flavonoids, alkaloids, terpenoids, and phenols are of considerable interest for the treatment of such diseases [12]. We have recently reviewed protective roles of plant-derived alkaloids in neurodegenerative diseases [23]. Flavonoids have been sanctioned to activate neuronal endogenous anti-oxidant status, thus, shielding them from neurodegeneration. Neuroprotective mechanism of flavonoids proceeds via suppression of lipid peroxidation, inhibition of inflammatory mediators, modulation of gene expressions and activation of anti-oxidant enzymes which makes them ideal therapeutic representative for the treatment of NDDs [24].
This review intends to emphasize the molecular mechanism of plant-derived flavonoids to diminish the risk of cellular degeneration and to enhance cell survivability. The scientific basis underlying the neuroprotective effect of this novel class of phytochemicals has been brought to light. This will facilitate the understanding of researchers regarding the pharmacological role of flavonoids in NDDs, thus, suggesting areas for further research. The literature was screened through various e-sites, including Springer Link, PubMed, Elsevier Science Direct Scopus and other relevant medical journals, highlighting the updates in this area of research. Key words used for searching are “Flavonoids”, “Neurodegenerative Diseases”, “Alzheimer’s disease (AD)”, and “Parkinson’s disease (PD)”.
2. Alzheimer’s Disease
Alzheimer’s disease (AD) is the progressive weakening of cognitive functions, memory, and learning [25], characterized by the aggregation of β-amyloid (Aβ) peptide, tau protein hyperphosphorylation, and amplified oxidative stress. However, the reasons for the massive majority of sporadic forms of AD remain un-demarcated [26]. Aβ peptides primarily form the senile plaques in the affected brain areas and these areas in turn exhibit a reduced number of synapses. These plaques usually contain scratched neurons, signifying the neuritis and synapse damage by Aβ. Aβ40/42 is generated by gamma-secretase-mediated sequential cleavages of the amyloid precursor protein (APP) and β-secreatase-(beta-site amyloid precursor protein cleaving enzyme, BACE) [27]. Hyperphosphorylated tau and Aβ accumulation in the brain are proposed to play an important role in the neurodegenerative process of AD [28] by activating the neuronal damage. Moreover, oxidative stress is another hallmark of AD along with the Aβ accumulation and hyperphosphorylation of tau [29]. In the pathogenesis of AD, oxidative stress may be the earliest change to occur [30]. Oxidative stress may be caused by hypercholesterolemia through forming reactive oxygen species (ROS) [31]. Endoplasmic reticulum (ER) stress can also be triggered by oxidative stress, and sustained ER stress can lead to the additional oxidative damage [32]. Currently, the global prevalence of dementia is as high as 36 million and is expected to reach 66 million by 2030 and 115 million by 2050, with almost two-third of the patients from the developed countries [33].
3. Parkinson’s Disease
Parkinson’s disease (PD) is characterized by the loss of dopaminergic neurons in the substantia nigra (SN) [34]. Initial symptoms of the disease include slowness of movement, shaking, rigidity, difficulty with walking, and behavioral problems [35]. It is a late onset disorder that occurs in 1–2% people over the age of 60 years [36]. The distinctive neuropathological changes in the brain include the abnormal formation of Lewy bodies. Degenerated dopaminergic nigrostriatal neurons with the Lewy bodies are major neuropathological correlation of motor damage in PD, but noradrenergic, adrenergic, glutamatergic, cholinergic, and GABAergic nerve cells also show identical damage in cytoskeleton [37]. Dopaminergic neurons of SN are progressively and selectively degenerated [38]. Neuroinflammation and particularly, microglial activation is associated with the pathogenesis of PD [35]. Microglial activation triggers the formation of a broad range of cytotoxic factors, including interleukin-1β (IL-1β), nitric oxide (NO), ROS, and tumor necrosis factor-α (TNF-α), causing neurodegeneration [39]. The adult hippocampal dentate gyrus (DG) receives inputs from dopaminergic neurons in the SN. So, deterioration of dopaminergic neurons may directly affect adult hippocampal neurogenesis [40].
4. Phytochemicals
Phytochemicals are a diversified group of bioactive compounds naturally occurring in plants. Various classes of phytochemicals including flavonoids, alkaloids, terpenoids, and phenols act as protective agents in nervous system disorders [41]. Phytochemical therapies have been extensively used against neural symptoms, but the underlying mechanism of action of phytomedicines is yet to be determined. One of the mechanistic approaches of phytomedicines is their potential efficacy to act as anti-oxidant and anti-inflammatory agents [42]. A copious number of phytochemicals are able to alter the neuronal excitability via inhibiting or activating the ion channels or specific receptors [41]. In this article, we have reviewed the potential efficacy of flavonoids as neuroprotective agents against NDDs by specifically focusing on molecular interactions of these compounds with various cellular targets.
5. Flavonoids
Flavonoids are naturally occurring, biologically active, and therapeutically effective polyphenols abundantly found in fruits and vegetables. They are classified in several categories including flavanols, flavonols, flavones, flavanones, isoflavones, anthocyanidins, and chalcones based on their chemical structure. To date, over 9000 flavonoids have been well-known, mainly found in fruits, vegetables, and beverages (tea, coffee, beer, wine and fruit drinks). Flavonoids and their metabolites exert countless health promoting effects both in human and animals. They possess multiple biological effects such as antiviral, anti-allergic, antiplatelet, anti-inflammatory, antitumor, and antioxidant activities [43]. Moreover, they can cross the blood-brain-barrier (BBB) and may exhibit neuropharmacological activities at the molecular level, influencing the protein function and gene expression. Importantly, dietary intake of flavonoids up-regulates the brain derived neurotrophic factor (BDNF) and thus improves the performance of spatial memory [44]. Extensive evidences have suggested their role in the attenuation of pathological pathways of NDDs [45,46]. Diet and lifestyle may play a potential role in the improvement of cognitive function and can also delay the onset of age related health disorders. Importantly, flavonoids enriched foods can induce memory and cognition improvements both in animals and humans. Similarly, it is proposed, by a growing number of studies, that dietary intervention with particularly diet rich in polyphenols exert neuroprotective effects in the brain, including the protection of neurons against neurotoxin-induced injury, suppression of neuroinflammation, and also has a potential to promote cognitive, learning, and memory functions [47]. Furthermore, flavonoids can modulate the immune system of brain, attenuate the neuroinflammation by inhibiting the production of nitric oxide and cytokines induced by activated microglia [48]. Thus, flavonoids signify their importance as potent molecules in the pursuit to develop a new group of drugs, having the ability to counteract the neuroinflammation and NDDs.
Hence, multiple effects of flavonoids have drawn the interests of scientists towards the investigation of neuroprotective role of flavonoids. Classifications of flavonoids with their dietary sources are represented in Table 1.

Table 1.
Classification of flavonoids and their dietary sources.
6. Classes of Flavonoids and Their Implications in Neurodegenerative Diseases
Classification of Flavonoids is based on their tertiary structure (Figure 1) and growing number of evidences have strengthened the idea that they may bestow attenuating effects against neurological, neurodegenerative, psychological and other diseases (Figure 2). In the present effort, we have reviewed the mechanisms and effects of flavonoids on Alzheimer’s disease and Parkinson’s disease based on the availability of published data.
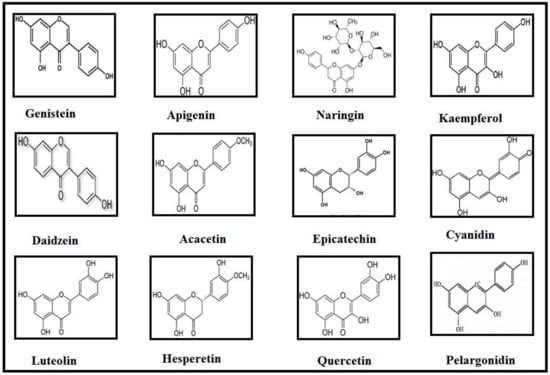
Figure 1.
Structures of compounds that discussed in this review.
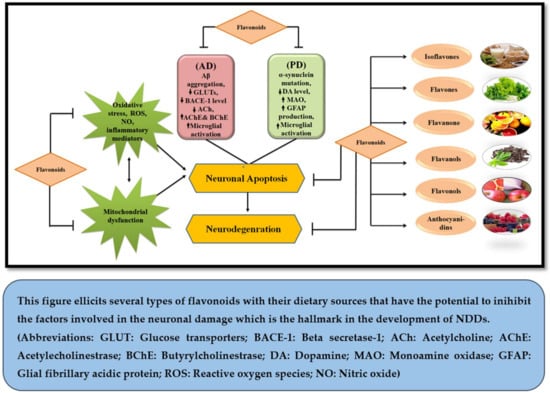
Figure 2.
Neuroprotective potential of flavonoids.
7. Isoflavones
7.1. Genistein
Genistein (Gen) is a primary soybean isoflavone, which exhibits numerous beneficial aptitudes for human health. It has structural similarity with endogenous steroid estrogen which enables it to mimic the pharmacological action of estrogen [67]. Gen may potentially reduce the process of neurodegeneration followed by inflammation by hindering the microglial inflammatory reactions in response to the exogenous stimulus [68]. A cumulative number of studies propose that Gen acts as a protective agent for neurons and thus it efficiently elicits a neuroprotective response in amyotrophic lateral sclerosis [69]. Importantly, it also shelters the cortical neurons of the human brain against free radical damage and thus portrays its anti-oxidative as well as anti-inflammatory property [70]. Moreover, its neuro-protective efficacy in NDDs has been discussed as follows.
7.1.1. Genistein in PD
PD is followed by the continuous damage of dopaminergic neurons in substantia nigra which ventures to the striatum [71]. Evidence from the imaging studies also report the minimized levels of dopamine in fronto-striatal circuit in PD patients [72]. Hence, it can be declared that the loss of dopaminergic neurons crucially upholds the underlying pathogenesis of PD. Gen has potential to protect the dopaminergic neurons in a dose-dependent manner against lipopolysaccharide (LPS)-induced neurotoxicity. It inhibits the production of NO, TNF-α, and superoxide in microglia as well as in mesencephalic neuron-glia cultures [70]. Moreover, the chief immune cells of brain microglia are eagerly activated in response to any infection or injury which leads to the release of pro-inflammatory factors [68] like NO and superoxide [73], which may form complexes with proteins causing the alteration of their functions and eventually causing cell death. Gen can attenuate the production and accumulation of superoxide and NO, thus delivering its neuro-protective efficiency to dopaminergic neurons and sheltering the dopaminergic neurons from a post injury response [70,73]. The least effective dose of Gen has been found as 0.25 µM while at 50 µM concentration, it is proposed to elucidate the toxicity in neuronal glia cultures. Interestingly, it fails to block the pro-inflammatory factors in glial cell cultures at the dose of 2.5 µM followed by the LPS induction in an animal model study [68]. Furthermore, it is obligatory to explore the other molecular targets involved in neuroprotection offered by Gen in the future.
7.1.2. Genistein in AD
Aggregation of Aβ proteins has been crucially involved in the pathogenesis of AD and it also acts as the foremost target for therapeutic development of the disease [74]. Aβ-induced neuronal cell death is also a leading cause of AD pathogenesis [75]. Gen is the foremost phytoestrogen in soybean and proficiently mimics the pharmacological functions of estrogen [67]. Estrogen possesses an affirmative potential of blocking the Aβ-induced neuronal cell death [75]. Gen possesses impartial neuroprotective potential because of its capability to act as estrogen receptors (ERs) agonist as they mediate the defensive cascade against Aβ-induced toxicity [76]. It also attenuates the crucial clinical outcome of memory impairment in AD patients by protecting the neuronal network of the brain. Hence, estrogen is also involved in memory and learning development in numerous brain regions (hippocampus, neo-cortex, nuclei of the basal forebrain) [77]. Moreover, Gen at 0.375 μg/mL dose protected the rat hippocampal neuronal cells by up-regulating the protein kinase signaling pathways [78]. It also has a capability to decrease the production of ROS at 50 μM and thus portrays its role as an anti-oxidant agent. The underlying mechanism to this protective feature of Gen involves the inhibition of mitochondrial transition pore opening, which ultimately prohibits the mitochondrial release of ROS in β-amyloid peptides 25–35-induced PC12 cells [79]. Importantly, it does not provide neuroprotection at the dose of 0.1 or 100 nM [77]. Several studies reveal that it does not elicit proliferative side effects on uterine endometrial cells along with the blocking of acetylcholine-induced neurotoxicity. Therefore, Gen may be a beneficial mediator for the treatment of AD [77]. Lastly, although Gen has been reported to possess neuroprotective activities, but still, there is a lack of clinical studies on its application as a therapeutic agent [80].
7.2. Daidzein
Daidzein belongs to the isoflavones class of flavonoids, naturally occurring entirely in legumes and soybeans [81]. Daidzein along with other isoflavones have been found in many plants like Kudzu (Pueraria lobata) and Kwao Krua (Pueraria mirifica) produced by the secondary metabolism of phenylpropanoid pathway [82]. Daidzein possesses diversified biological effects in various biological systems and may be able to serve as an agent to prove the therapeutic efficacy of flavonoids against several health issues [83] including improvement in blood cholesterol level, osteoporosis reduction [84], attenuating the risk of certain hormone related cancer, and coronary heart diseases [85]. Moreover, it also efficiently employs its action as neuroprotective agent via acting as agonist of estrogen [81]. It has the capability to bind with ERs in brain because of its structural similarity with estrogen. Thus, it elucidates ER-dependent activation of estrogen receptive promoters and DNA binding in numerous cell types [86]. ERα and ERβ are two prime types of estrogen receptors which are expressed in brain and daidzein parades more affinity of binding with ERβ as compared to ERα [87]. Therefore, it can portray a potential role in the attenuation of various NDDs as explained bellow.
Daidzein in PD
As the pro-inflammatory factors and microglia activation have been supposed to play a crucial role in neuronal cell death concomitant with PD [73]. Thus inhibition of both factors has been known to be associated with the possession of neuroprotective properties in PD [88]. Daidzein exhibits an effective property to diminish the release of inflammatory mediators in BV-2 microglial cells induced by lipopolysaccharide (LPS) [89]. An experimental investigation on male Sprague–Dawley rats reveals that daidzein exerts pro-oxidant activity as well as significantly attenuates malondialdehyde (MDA) content in the brain at an oral dose of 2 and 20 mg/day for 4 weeks dissolved in corn oil [90]. Whereas, another study suggests that daidzein may persuade detrimental effects at high concentration [91] and elucidates oxidant properties rather than anti-oxidant action by affecting the antioxidant enzyme defense system in rat hepatoma H4IIE cells [92]. Overload of free radicals followed by the oxidative stress is one of the most common features of neurodegenerative diseases such as PD and AD. It results in the production of ROS and NO which affect biosystem of body and has been found to affect the function and structure of neural cells. Thus it contributes to an extensive range of NDDs including AD and PD [93]. Daidzein inhibits oxidative stress associated production of NO and ROS [94]. Therefore, anti-oxidant agent which efficiently removes the ROS could portray potential therapeutic effect against PD [93]. Furthermore, studies on LPS-stimulated microglial cells suggest that it elucidates neuro-protective potential because of its efficiency to inhibit the microglia activation and ensuring the release of soluble pro-inflammatory factors [88]. Despite exhibiting the multiple aspects in neuroprotection there is still a dearth of clinically proven consideration and medication of daidzein.
8. Flavones
8.1. Luteolin
Luteolin (3′,4′,5,7-tetrahydroxyflavone) belongs to the flavone group of flavonoids which is abundantly found in the plant kingdom [95]. Chemical structure of luteolin comprises of C6-C3-C6 structure and contains an oxygen-containing ring, two benzene rings, and 2–3 carbon double bond [96]. It is abundantly found in fruits and vegetables such as chrysanthemum flowers, apple skins, cabbage, peppers, carrot, leaves of onion, broccoli, parsley, and celery [50,51,52]. It is well known for its potential anti-inflammatory and anti-oxidative properties and also exhibits phytoestrogen like activities [97,98]. Plants enriched with luteolin are utilized against cancer, inflammatory diseases and hypertension in Chinese traditional medicine [95].
8.1.1. Luteolin in AD
AD is the most common neurodegenerative disease which leads to the development of senile dementia. Cognitive dysfunction is particularly caused by AD. It is reported that cognitive dysfunction in cerebral hypoperfused rats can be protected by luteolin at a dose of 150 and 450 mg/kg [99]. The hallmark in the pathology of AD is Aβ plaques formation. To find out the Aβ reducing capability of luteolin, primary neuronal cells which are the SweAPP-overexpressing mice were treated with luteolin and it was seen that it momentously lessened the Aβ generation [100]. The mechanism behind the reduction of Aβ generation may encompass the GSK-3α isoform selective inactivation that enhances the p-PS1 levels which is the γ-secretase complex catalytic core [101]. Recently, it has been found that AD pathologies in mice, persuaded by traumatic brain injury can be reduced by luteolin at the dose of 20 mg/kg/day for 15 days [102]. Luteolin also possesses the protective effects on structure of hippocampus and learning flaws in streptozotocin-stimulated Alzheimer’s rat model. The administration of luteolin at10 and 20 mg/kg dose [101], shows significant results in this context.
8.1.2. Luteolin in PD
It is a well-known fact that the level of dopamine (DA) is reduced in SN in case of PD. In addition to the reduced level of DA, the inflammation in the brain accompanied by over-activation of microglia is also involved in the pathology of PD [103,104,105]. Unnecessary quantities of cytotoxic and pro-inflammatory factors produced by the activation of microglia in substantia nigra are lethal to neurons [106]. It was reported by an in vitro investigation on luteolin that its (5 µM) treatment may protect the LPS-induced dopaminergic neuronal degeneration by inhibiting the activation of microglia [107]. However, very limited work has been done to explore the beneficial effect of luteolin on CNS.
8.2. Apigenin
Apigenin (4′,5,7,-trihydroxyflavone), a naturally occurring phytochemical, belongs to flavone group of flavonoids. Naturally, it can be extracted from flowers and buds of Hypericum perforatum. It is copiously found in common vegetables and fruits such as onion, parsley, grapefruit, and orange [53,108]. It exhibits multiple pharmacological effects such as anti-inflammatory, anti-apoptotic, anti-oxidative, purgative, antiviral, and anti-mutagenic [109,110]. It has been shown that apigenin can reduce glutamate-induced Ca2+ signaling in murine cortical neurons [111].
8.2.1. Apigenin in AD
One of the pathogenic symbols of AD is Aβ generation, aggravated due to mutations in APP [112]. Furthermore, the buildup of Aβ leads to the microglial over-activation around Aβ plaques [113]. Neurotoxicity of Aβ can be induced by free radical production caused by transition metals like copper. Treatment with 10 µM apigenin can cease the enhanced expression of Aβ precursor protein caused by copper but it is not effective at any other concentration [114]. Apigenin also possesses the ability to improve the memory impairment associated with AD, to prevent oxidative stress and to decrease the burden of Aβ plaques. Numerous studies have demonstrated the anti-inflammatory [115] and anti-apoptotic effects of apigenin in various animal models [110] as well as in human [116]. It is reported that apigenin protects neurons against inflammatory stress and limits apoptotic cell death as well as reduces the neuronal hyper-excitability [53]. Furthermore, it was demonstrated that apigenin could inhibit the activation of pro-inflammatory cytokines and NO production, protecting AD neurons from inflammatory-induced stress. The concentration of apigenin 50 µM (IC50 value) can protect neurons against neurite shortening and neuronal death as well as reduce apoptosis [111]. Moreover, the IC50 values of apigenin ranging between 10 and 100 µM can be able to reduce the production of NO and pro-inflammatory cytokines [115]. It has been shown that 10 mg/kg and 20 mg/kg intraperitoneal administration of apigenin reduces the activity of AChE [117], which is a key enzyme involved in the development of AD. All these investigations suggest that apigenin has the ability to overcome the progression of AD. Hence, it needs to be introduced in clinical trials as well.
8.2.2. Apigenin in PD
The primary indications of PD are shakiness, postural abnormalities, bradykinesia, muscular rigidity, and tremor at rest [118]. A well-known hallmark in the pathology of PD is neuronal inflammation-induced glial cell activity in the SN [119]. The available treatment is DA agonist but the chronic administration of LDOPA or DA agonist can lead to severe non-motor and motor adverse effects [120]. The worse effect of LDOPA or DA agonist diverts the interest of scientists towards phytomedicines for treatment of PD to reduce or prevent the adverse effects. It was shown that apigenin enhanced the locomotor capability and proved to be very effective in a dose-dependent manner (5, 10 and 20 mg/kg) [121]. In vitro investigation has suggested that apigenin exerts inhibitory property against inflammatory mediators, proposes that it may possess neuroprotective potential against inflammation mediated diseases such as NDDs [122]. It was also reported that apigenin could secure the dopaminergic neuronal loss in Parkinson’s mice model by attenuating the microglial activation and neuroinflammation at dose of 10 and 20 mg/kg [121]. Importantly, administration of apigenin expressively prohibits the neuroinflammation in SN [117]. At present, the treatment of PD is dependent on DA agonists. There is a dire need to introduce apigenin in preclinical trials for the treatment of PD to overcome adverse effects of currently used medicines.
8.3. Acacetin
Besides luteolin and apigenin, there is another flavonoid compound known as acacetin (5,7-dihydroxy-4-methoxyflavone), which belongs to flavone group of flavonoids. It is extracted from Clerodendrum inerme (L.) Gaertn (CI) which possesses potential therapeutic efficacy against neuropsychiatric disorders [123]. It also exerts several biological actions including anticarcinogenic, anti-inflammatory, and antioxidant actions [124,125,126]. It can also be extracted from R. pseudoacacia [55]. The antioxidant and anti-inflammatory role of acacetin give the direction that it can be beneficial in the treatment of NDDs such as AD and PD.
8.3.1. Acacetin in AD
Neuroinflammation is one of the hallmark in the pathology of AD. Activation of microglia plays a crucial role in neurodegeneration mediated by inflammation. Microglial over-activation can lead to the neuronal cell death and CNS disorders through the production of several cytotoxic and pro-inflammatory factors such as IL-1β and TNF-α [127,128]. The transcription factor known as nuclear factor-κB (NF-κB) regulates the expression IL-1β, TNF-α, and iNOS [129]. Mitogen activated protein kinases (MAPKs) including JNK and p38 are also found to be involved in the microglial-induced inflammation [130,131]. It has been demonstrated that acacetin can inhibit the NO release and attenuates the IL-1β and TNF-α. Importantly, acacetin inhibits the p38 MAPK and NF-κB activation. Experimentation on the mouse model of lipopolysaccharide (LPS) mediated neuroinflammation indicated that acacetin expressively suppressed the activation of microglia in a dose-dependent manner [55]. Another factor which is involved in the neurodegeneration is excitotoxicity caused by excessive glutamatergic neurotransmission via NMDAR [132]. Glutamate is the chief excitatory neurotransmitter in the CNS and plays a crucial role in memory, learning, and cognition. In addition, excessive release of glutamate enhances the levels of intracellular Ca+ which in turn enhances the production of free radicals and mitochondrial dysfunction and eventually causes neuronal damage. It shows that acacetin inhibits the release of glutamate. So, the inhibition of glutamate ultimately stops the cascade of damaging cellular processes [133]. The inhibiting properties of acacetin indicate that it can be beneficial in the treatment of NDDs, particularly AD.
8.3.2. Acacetin in PD
Neuroinflammation is considered to be the most prevalent factor involved in the pathology of PD. Studies show that acacetin inhibits the inflammatory factors production and hence protects the dopaminergic neurons, major targets in the development of PD [54]. More work is needed to be done to ensure the therapeutic role of acacetin in context of PD.
9. Flavanones
9.1. Hesperetin
Hesperetin (3′,5,7-trihydroxy-4-methoxyflavanone) belongs to the flavanone class of flavonoids, found in citrus fruits [56]. It is derived from the hydrolysis of aglycone, hesperidin (hesperetin 7-rhammnoglucoside) [134]. It exerts neuroprotective effects by acting as anti-inflammatory and anti-oxidative agent [135].
Hesperetin in AD
Aβ deposition results in prevention of insulin signaling in neurons and reduction in membrane insulin receptor (IR) activity which leads to the reduction in insulin levels and glucose transporters (GLUTs) in brains of AD patients [136]. Deposition of Aβ25–35 impairs glucose uptake and also leads to the neuronal damage by cellular autophagy. Hesperetin at a dose of 97.2 µM protects against Aβ25–35-stimulated neuronal damage [137]. It also possesses the ability to ameliorate the Aβ impaired glucose uptake moderately by impeding autophagy. The suggested dose of hesperetin which is very effective in attenuating the neuronal autophagy is 1–20 µM [138]. Importantly, like Aβ aggregation, oxidative damage which is induced by the lipid peroxidation is another feature involved in the pathophysiology of AD. It was demonstrated that with the IC50 values of 179.1 µM, hesperetin intensely inhibited the lipid peroxidation that could cause oxidative damage [137,138].
9.2. Naringin
Naringin is a flavanone glycoside derived from naringenin (a flavonoid) and it is one of the chief active constituents of Chinese herbal medicines including Citrus medica L. (CM), Citrus aurantium L. (CA), and Drynaria fortunei (Kunze) J. Sm. (DF) [139,140]. It is found in citrus fruits such as grapefruits [141] and bitter taste of citrus juices is dedicated to this flavonoid [142]. It executes several pharmacological and biological effects including anti-carcinogenic, anti-osteoporotic, anti-ulcer, anti-apoptotic [143], anti-inflammatory, cholesterol reducing and antioxidant effects [144].
Naringin in PD
PD is partially caused by microglial activation which are native immune cells of brain. Microglial activation is resultant of DA neuronal damage [145]. The activated microglia may also produce several neurotoxins such as pro-inflammatory cytokines and inducible nitric oxide synthase (iNOS) which leads to the nigrostriatal DA neuronal cell death [146,147]. Recently, it was reported that oral administration of naringin at the dose of 100 mg/kg reduced the microglial activation by decreasing the expression of glial fibrillary acidic protein (GFAP) [148]. GFAP expression is reported to be altered following brain damage as in case of PD [149]. It was mentioned that oxidative stress and neuroinflammation are involved in the pathology of PD. It was proposed that oral administration of 80 mg/kg naringin (dissolved in 0.5 mL of 0.25% Sodium carboxymethylcellulose) for two weeks in 3-nitropropionic acid-induced neurodegeneration rat models modulated the inflammatory reactions and oxidative stress, thereby, giving an idea of its neuroprotective effects against neurodegeneration [150]. Additionally, naringin also exerts neuroprotective effects by the initiation of neurotrophic factors [145,151,152]. Furthermore, it has been reported that naringin enhances the GDNF level in neurotoxin model of DA neurons and it also reduces the level of tumor necrosis factor-α in microglia. The effective dose reported in this aspect is 80 mg/kg (suspended in 0.25% sodium carboxymethylcellulose that was dissolved in 0.9% saline) [151]. Thus, these indications propose that naringin might be a possible natural compound involved in the treatment and anticipation of the NDDs.
10. Flavanols
10.1. (−) Epigallocatechingallate
(−) Epigallocatechin gallate (EGCG) contains 3 phenol ring structure and is one of the type of catechin. It is the main bioactive component of green tea leaves while it is also found in black tea in a minor quantity [43]. Polyphenols from green tea including ECCG have been reported to exert anti-oxidant [153], anti-carcinogenic [154] and anti-inflammatory effects [155]. EGCG is a major constituent of green tea that is responsible for its health-promoting potentials. The presence of two tri-phenolic groups in its structure is associated with its stronger activity [156]. Furthermore, its anti-oxidant activity has capability to attenuate neurotoxicity as well as neuronal damage resulting from the free radicals attack [157].
(−) Epigallocatechingallate in AD
Neurotoxicity of Aβ and neuronal cell death via an apoptotic procedure is a well-known hallmark of AD which is mediated by the production of free radicals and the state of pathogenesis could be accomplished through free radicals scavengers and anti-oxidants [158]. EGCG acts as a potent anti-oxidant agent and prevents the hippocampal neuronal cell death [153]. Programmed cell death, apoptosis, is reported as the distinct process of cell elimination from necrotic cell death. Caspase activation, most importantly, leads neuronal cells towards apoptosis [159]. Thus, caspase might play a crucial proliferative role in Aβ-induced neuronal cell death. Interestingly, EGCG obstructs the augmented caspase activity induced by Aβ25–35 and thus can attenuate apoptosis in neuronal cells via rummaging the ROS [160]. Moreover, it also attenuates the major hallmarks of AD pathology such as the interaction between ROS, apoptosis, and Aβ which chiefly contribute to the neuronal cell death. Most importantly, consumption of green tea may reduce the risk of AD [113] and its clinical significance has also been revealed by the animal model studies that EGCG can cross the blood-brain barrier (BBB) and can reach the brain parenchyma [156].
10.2. (−) Epicatechin
(−) Epicatechin (EC), a plant-derived flavanol, naturally found in blueberries, tea, cocoa, and grapes [59]. It has been recognized as a bioactive flavanol which can cross the BBB and absorbed into circulation after digestion of flavanol-rich foods [161,162]. EC has capability to enhance the cardiovascular function and the cortical blood flow especially in the hippocampus, thus, it may facilitate the neurogenesis [59]. Furthermore, its neuroprotective property in NDDs is discussed as follows.
(−) Epicatechin in PD
Neuroinflammation plays a very important role in the PD pathogenesis as supported by various human and animal studies which have enlighten the role of inflammatory cascade and oxidative stress in the progression of PD. Oxidative stress induced by the increased production of NO, ROS and thus can causes the nigral cell death [163]. A postmortem tissue study revealed that the oxidative stress-induced NO, ROS and decreased mitochondrial activity are chiefly involved in pathogenesis of PD [164] suggesting that the agents which hinder the production of NO and ROS and also able to favor the decreased mitochondrial activity might play a protective role in PD [165]. Green tea polyphenols (GTP) including EC moderately protected the dopaminergic neurons by modifying the NO and ROS levels, conserving the free radical as well as prevent an increase in nitrate/nitrite levels in rat model of PD [166]. ROS induce lipid peroxidation, damage to the mitochondrial membrane and thus disrupt the Ca2+ homeostasis [167]. Interestingly, GTP impedes the altitude of NO by stabilizing the Ca2+ homeostasis [166] and thus, it could serve as a potential marker to attenuate the pathogenesis of PD.
11. Flavonols
11.1. Quercetin
Quercetin (3,3′,4′,5,7-pentahydroxylflavone) belongs to the flavonol class of flavonoids [168], ubiquitously found in apples, onions, tea, red wines, and berries [60,61]. It is also present in medicinal plants such as Sambucus canadensis (Elder), Hypericum perforatum (St. John’s Wort), and Ginkgo biloba [169]. It possesses several pharmacological effects such as vasodilation, anti-inflammatory, and anti-oxidative properties [170]. It also exerts anticarcinogenic, antihypertensive, and antithrombic effects [171]. Moreover, it consistently promotes neuroprotective effects [172] and upsurges the hindrance of neurons to oxidative stress and excitotoxicity by tempering the cell death mechanisms [173,174]. It exerts valuable effects on CNS including cognition development and anti-anxiety effects by the inhibition or stimulation of signal transduction pathways or enzyme activities [175].
Quercetin in AD
AD is the most prevalent cause of dementia, characterized by the liberal deterioration in cognitive function. It is noteworthy that quercetin promotes the neuroprotective effects by ameliorating the memory impairment and neuronal cell death [176]. Quercetin also attenuates Aβ aggregation and declines the level of BACE-1 which mediates the cleavage of APP [177]. Furthermore, quercetin significantly protects the neuronal cells from neurotoxicity induced by oxidative stress in case of AD [178]. In vitro study reveals that quercetin acts as antioxidant at low doses (5 and 10 µM) while at high doses (20 and 40 µM) it can cause toxicity [179].
11.2. Kaempferol
Kaempferol (3,4,5,7,-tetrahydroxyflavone) is a phytoestrogen, and one of the most usual dietary flavonoids. It is frequently found in tea, broccoli, apples, beans, strawberries, and grapefruits [62,63]. It is known to possess potential anti-inflammatory and anti-oxidative effects [180]. It possesses efficient neuroprotective effects against numerous necrosis and apoptosis-inducing damages such as oxidizing low-density lipoproteins [181,182]. It effectively obstructs the upsurge in ROS which is linked to the oxidative stress [183].
Kaempferol in PD
Lipid peroxidation is the most common pathological symbol in the development of NDDs. It leads to the occurrence of oxidative damage which is caused by the generation of ROS. It was reported that kaempferol protected the brain against damage caused by ROS at the dose of 30 µM in rotenone-induced acute toxicity model [184]. Like lipid peroxidation, monoamine oxidase-A (MAO-A) also promotes the formation of ROS, causing neuronal cell death [185,186]. It is noteworthy that kaempferol possesses MAO-A inhibiting property at the IC50 value of 7 × 10−7 M which might be beneficial in the treatment of PD [187]. Additionally, it was proposed through experimentation that kaempferol administration amended motor synchronization, enhanced striatal DA in a dose-dependent manner (25, 50 and 100 mg/kg) [188]. Hence, it is proposed to have anti-parkinsonism properties.
12. Anthocyanidin
12.1. Cyanidin
Cyanidin-3-glucoside (C3G), is a naturally occurring anthocyanin, mainly found in enormous type of red berries including cranberry, blueberry, blackberry, mulberries, acai berry, and raspberry [64]. Out of which mulberries contain high concentration of anthocyanin and have been traditionally used to prevent and treat the diabetes. Importantly, its root bark has been used as an antitussive, anti-inflammatory, anti-pyretic and diuretic [189]. Furthermore, C3G extracted from mulberry fruit possesses neuroprotective property against glutamate-induced as well as oxygen-glucose deprived neuronal cell death [190,191]. Neuroprotective properties of C3G have been discussed as follows.
Cyanidin in AD
C3G is able to neutralize the level of Aβ1–42 peptides and minimize the H2O2-induced neurotoxicity [192,193,194]. More recently, it has also been shown that C3G significantly attenuates the Aβ25–35-induced expression of ER stress proteins, loss of cell viability and also tends to reduce the intracellular production of ROS in SK-N-SH cells [195]. It can cross the BBB and tempers the age-related deficits in neurons [194]. C3G, during an in vitro investigation, at 50 and 100 µM is reported to reduce the Aβ25–35 oligomer toxicity whereas at 100 µM it significantly decreases the necrotic cell (~44%) formation and apoptosis (~38%) induced by Aβ peptides [196]. Its polyphenolic ring structure seems to be fairly appropriate for precise aromatic connections with aromatic deposits of Aβ1–42 [197]. Furthermore, the property to block the Aβ1–42 interaction with the neuronal plasma membrane was also offset by the C3G. In this repute, several studies propose the adherence of soluble oligomeric Aβ1–42 peptides to plasma membrane causing lesions by a combination of impermeable pores formation and lipid peroxidation and thus finally leading to the cell death [198]. At membrane level, C3G inhibits oxidative stress-induced ROS formation and concentrates in several brain regions which are important for memory and learning such as hippocampus and cortex to protect the neurons [199]. Therefore, it is credible that C3G averts the oligomer-induced neuronal destabilization and lipid peroxidation [195]. Thus, it can serve as an alternate for the prevention of NDDs such as AD.
12.2. Pelargonidin
Pelargonidin (Pel) is an anthocyanin derivative flavonoid and is an agonist of ER but it possesses minimal estrogen side effects [200]. It is one of the important flavonoid which is efficiently absorbed in the gastrointestinal tract and also has an accessibility to cross the BBB [201,202]. Pel exerts a vast number of beneficial effects on human health because of its proficient absorption and minimum side effects. Being the derivative of anthocyanin, it appears to elicits a potential efficacy as anti-oxidant, anti-inflammatory [203], antihyperglycemic [204], neural protection, non-genotoxicity responses [205], and anti-thrombosis activity [206]. The underlying mechanism of its anti-inflammatory property involves the modulation of interleukins-10 (IL-10), which contributes to the protective effects in inflammatory diseases but has no effect on the IL-6, IL-1β, and IL-8 [207]. Importantly, it would be one of the most valuable substitutes to avert the age-related memory and cognitive deficits [200]. Moreover, neuroprotective property of pel in NDDs is discussed as follows.
12.2.1. Pelargonidin in AD
In spite of conspicuous advances in pathophysiology and therapeutic knowledge about AD, there are only a minimum number of drugs which have been approved for symptomatic treatment due to the complex nature of the disease [208]. Anti-inflammatory agents can manage the state of disease because of their ability to modulate the underlying factors of inflammation. Likewise, Pel inhibits the inducible nitric oxide synthase (iNOS) protein and mRNA expression, NO production and NF-κB expression [209]. ERs are largely present in certain memory associated brain areas like frontal cortex, amygdala, and hippocampus [210] and they also possess neuroprotection in NDDs but the exact mechanism is not clarified yet [211]. Pel exerts its neuroprotective efficacy due to its ability to act as an agonist of ERs [212]. Blood flows in the hippocampal region may stimulate memory function and neurogenesis by its vasodilatory property. Moreover, memory concert and neuronal connectivity may also be amended by increased morphology repair and dendritic spine density in female rat models [213]. Studies have been reported that oral consumption (10 mg/kg) of Pel could converse the memory disturbance induced by Aβ25–35 via ERs independent pathways. Similarly, another study on rat model also depicts that it recovers the memory dysfunction in Morris water maze (MWM) test via improving the cholinergic dysfunction as well as down-regulating the glial fibrillary acidic protein (GFAP) [200]. Lastly, because of its diverse pathological mechanisms, it would be the valuable alternatives for estrogen to avert age-related memory deficit and cognitive changes in disorders like AD. However, additional studies should be done to define its precise mechanism and further explore the factors which could avert the pathogenesis of AD.
12.2.2. Pelargonidin in PD
Decreased glutamate levels, oxidative stress, increased lipid peroxidation, iron deposition, and DNA damage have been reported as the major pathological factors in PD [214]. Oxidative stress impairs the dopaminergic neurons and compromises the oxidative phosphorylation of mitochondria, leading to the cell death due to insufficient availability of energy [215]. Although inordinate advances have been made in the development of medicinal therapy for PD, but none of the agent addresses the associated problem i.e., the dopaminergic neuronal damage [216]. Thus, protection of dopaminergic neuronal damage and loss is the primary need to avert the pathogenesis of PD. Pel minimizes the neuronal loss and damage via inhibiting the formation of free radicals as well as modifying the antioxidant defensive system [217]. It also decreases the formation of thiobarbituric acid reactive substances (TBARS) at the oral dose of 20 mg/kg in a semi Parkinsonism rat model whereas it is unable to prevent the free radical generation significantly at the same dose [218]. Furthermore, Pel also mitigates the development of PD because of its anti-inflammatory efficiency [209]. However, further investigation pointing the mechanistic approach of its anti-inflammatory property has to be explored yet. It may possess neuroprotective activity because of its ability to prevent the dopamine oxidation mediated by peroxynitrite. Importantly, further studies are needed regarding to its toxicity. To date, it is suggested that Pel exhibits the neuromodulatory effects because of its ability to cross the BBB and accumulates in the brain at nanomolar concentrations [219,220].
13. Conclusions and Future Perspectives
Neuroprotective activity of natural flavonoids encompasses multiple effects within the brain, including their efficacy to shelter against neurotoxins-induced neuronal injury, to endorse learning, memory, cognitive functions, and to suppress the neuronal inflammation. Two common processes lay the foundation of such diversified neuroprotective effects of flavonoids. Firstly, they are reported to have various positive effects on the cerebral and peripheral vascular system, leading to the alterations in cerebrovascular blood flow. These alterations ultimately induce angiogenesis, neuronal cell growth in hippocampus, and improve neuronal morphology, all of which are crucial in regulating neuro-cognitive activities and maximal neuronal functions. Secondly, they interact with neuronal signaling networks within the brain leading to the inhibition of neurotoxin-induced apoptosis and promoting the differentiation and survival of neurons.
Dietary consumption of flavonoids rich foods such as cocoa and berries grasps the efficacy to attenuate neurodegeneration and averts or reverses the age-dependent deteriorations of cognitive function. However, definite temporal nature underlying neuroprotective effects of flavonoids is unclear at present. More work is needed to be done on flavonoids as a potential therapy for several untreatable NDDs. Most particularly, at present, there are inadequate data on the aspect of a causal relationship between the consumption of flavonoids and behavioral consequences. There should be more clinical and preclinical trials. The toxic values and availability of flavonoids in the market still needs to be explored.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81471152, 31771141 and 81701132).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mao, Z.; Zheng, Y.L.; Zhang, Y.Q.; Han, B.P.; Zhu, X.W.; Chang, Q.; Hu, X. Bin The anti-apoptosis effects of daidzein in the brain of d-galactose treated mice. Molecules 2007, 12, 1455–1470. [Google Scholar] [CrossRef] [PubMed]
- Hussain, G.; Schmitt, F.; Henriques, A.; Lequeu, T.; Rene, F.; Bindler, F.; Dirrig-Grosch, S.; Oudart, H.; Palamiuc, L.; Metz-Boutigue, M.-H.; et al. Systemic down-regulation of delta-9 desaturase promotes muscle oxidative metabolism and accelerates muscle function recovery following nerve injury. PLoS ONE 2013, 8, e64525. [Google Scholar] [CrossRef] [PubMed]
- Hussain, G.; Schmitt, F.; Loeffler, J.-P.; Gonzalez de Aguilar, J.-L. Fatting the brain: A brief of recent research. Front. Cell. Neurosci. 2013, 7, 144. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, F.; Hussain, G.; Dupuis, L.; Loeffler, J.-P.; Henriques, A. A plural role for lipids in motor neuron diseases: Energy, signaling and structure. Front. Cell. Neurosci. 2014, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Henriques, A.; Croixmarie, V.; Priestman, D.A.; Rosenbohm, A.; Dirrig-Grosch, S.; D’Ambra, E.; Huebecker, M.; Hussain, G.; Boursier-Neyret, C.; Echaniz-Laguna, A.; et al. Amyotrophic lateral sclerosis and denervation alter sphingolipids and up-regulate glucosylceramide synthase. Hum. Mol. Genet. 2015, 24, 7390–7405. [Google Scholar] [CrossRef] [PubMed]
- Bruneteau, G.; Bauché, S.; Gonzalez de Aguilar, J.L.; Brochier, G.; Mandjee, N.; Tanguy, M.-L.; Hussain, G.; Behin, A.; Khiami, F.; Sariali, E.; et al. Endplate denervation correlates with Nogo-A muscle expression in amyotrophic lateral sclerosis patients. Ann. Clin. Transl. Neurol. 2015, 2, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Su, J.; Head, E.; Cotman, C.W. Accumulation of caspase cleaved amyloid precursor protein represents an early neurodegenerative event in aging and in Alzheimer’s disease. Neurobiol. Dis. 2003, 14, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.H.; Zhang, Y.; Herman, B. Caspases, apoptosis and aging. Ageing Res. Rev. 2003, 2, 357–366. [Google Scholar] [CrossRef]
- Zhang, A.; Lorke, D.E.; Lai, H.W.L.; Chu, X.; Wu, Y.; Yew, D.T. Age-related alterations in cytochrome c-mediated caspase activation in rhesus macaque monkey (Macaca mulatta) brains. Mol. Brain Res. 2004, 123, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, G.G. Current concepts of neurodegenerative diseases. EMJ Neurol. 2014, 1, 78–86. [Google Scholar]
- Nieoullon, A. Neurodegenerative diseases and neuroprotection: Current views and prospects. J. Appl. Biomed. 2011, 9, 173–183. [Google Scholar] [CrossRef]
- Kumar, G.; Khanum, F. Neuroprotective potential of phytochemicals. Pharmacogn. Rev. 2012, 6, 81. [Google Scholar] [CrossRef] [PubMed]
- Hussain, G.; Shahzad, A.; Anwar, H.; Sohail, M.U.; Baig, S.M.; Shabbir, A.; de Aguilar, J.-L.G.; Iqbal, J. Neurological disorder burden in Faisalabad, Punjab-Pakistan: Data from the major tertiary care centers of the city. Pakistan J. Neurol. Sci. 2017, 12, 3–10. [Google Scholar]
- Hussain, G.; Rasul, A.; Anwar, H.; Sohail, M.U.; Kamran, S.K.S.; Baig, S.M.; Shabbir, A.; Iqbal, J. Epidemiological Data of Neurological Disorders in Pakistan and Neighboring Countries: A Review. Pakistan J. Neurol. Sci. 2017, 12, 52–70. [Google Scholar]
- Ramassamy, C. Emerging role of polyphenolic compounds in the treatment of neurodegenerative diseases: A review of their intracellular targets. Eur. J. Pharmacol. 2006, 545, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Rasul, A.; Di, J.; Millimouno, F.; Malhi, M.; Tsuji, I.; Ali, M.; Li, J.; Li, X. Reactive Oxygen Species Mediate Isoalantolactone-Induced Apoptosis in Human Prostate Cancer Cells. Molecules 2013, 18, 9382–9396. [Google Scholar] [CrossRef] [PubMed]
- Rasul, A.; Khan, M.; Yu, B.; Ma, T.; Yang, H. Xanthoxyletin, a coumarin induces S phase arrest and apoptosis in human gastric adenocarcinoma SGC-7901 cells. Asian Pac. J. Cancer Prev. 2011, 12, 1219–1223. [Google Scholar] [PubMed]
- Qin, H.; Rasul, A.; Li, X.; Masood, M.; Yang, G.; Wang, N.; Wei, W.; He, X.; Watanabe, N.; Li, J.; et al. CD147-induced cell proliferation is associated with Smad4 signal inhibition. Exp. Cell Res. 2017, 358, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Rasul, A.; Yu, B.; Zhong, L.; Khan, M.; Yang, H.; Ma, T. Cytotoxic effect of evodiamine in SGC-7901 human gastric adenocarcinoma cells via simultaneous induction of apoptosis and autophagy. Oncol. Rep. 2012, 27, 1481–1487. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Ding, C.; Rasul, A.; Yi, F.; Li, T.; Gao, H.; Gao, R.; Zhong, L.; Zhang, K.; Fang, X.; et al. Isoalantolactone induces reactive oxygen species mediated apoptosis in pancreatic carcinoma PANC-1 cells. Int. J. Biol. Sci. 2012, 8, 533–547. [Google Scholar] [CrossRef] [PubMed]
- Rasul, A.; Yu, B.; Khan, M.; Zhang, K.; Iqbal, F.; Ma, T.; Yang, H. Magnolol, a natural compound, induces apoptosis of SGC-7901 human gastric adenocarcinoma cells via the mitochondrial and PI3K/Akt signaling pathways. Int. J. Oncol. 2012, 40, 1153–1161. [Google Scholar] [CrossRef] [PubMed]
- Sarfraz, I.; Rasul, A.; Jabeen, F.; Younis, T.; Zahoor, M.K.; Arshad, M.; Ali, M. Fraxinus: A Plant with Versatile Pharmacological and Biological Activities. Evid. Based Complement. Altern. Med. 2017, 2017, 4269868. [Google Scholar] [CrossRef] [PubMed]
- Hussain, G.; Rasul, A.; Anwar, H.; Aziz, N.; Razzaq, A.; Wei, W.; Ali, M.; Li, J.; Li, X. Role of Plant Derived Alkaloids and Their Mechanism in Neurodegenerative Disorders. Int. J. Biol. Sci. 2018, 14, 341–357. [Google Scholar] [CrossRef] [PubMed]
- Magalingam, K.B.; Radhakrishnan, A.K.; Haleagrahara, N. Protective Mechanisms of Flavonoids in Parkinson’s Disease. Oxid. Med. Cell. Longev. 2015, 2015, 314560. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Wang, C.; Yang, W. Role of berberine in Alzheimer’s disease. Neuropsychiatr. Dis. Treat. 2016, 12, 2509–2520. [Google Scholar] [CrossRef] [PubMed]
- Prasanthi, J.R.P.; Dasari, B.; Marwarha, G.; Larson, T.; Chen, X.; Geiger, J.D.; Ghribi, O. Caffeine protects against oxidative stress and Alzheimer’s disease-like pathology in rabbit hippocampus induced by cholesterol-enriched diet. Free Radic. Biol. Med. 2010, 49, 1212–1220. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Wu, F.; Ma, Y.; Liu, G.; Li, Z.; Sun, Y.; Pei, Z. Decrease in the production of beta-amyloid by berberine inhibition of the expression of beta- secretase in HEK293 cells. BMC Neurosci. 2011, 12, 125. [Google Scholar] [CrossRef] [PubMed]
- Castellani, R.J.; Zhu, X.; Lee, H.; Moreira, P.I.; Perry, G.; Smith, M.A. Neuropathology and treatment of Alzheimer disease: Did we lose the forest for the trees? Expert Rev. Neurother. 2007, 7, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.A.; Taneda, S.; Richey, P.L.; Miyata, S.; Yant, S.-D.; Sternt, D.; Sayre, L.M.; Monnier, V.M.; Perry, G. Advanced Maillard reaction end products are associated with Alzheimer disease pathology. Neurobiology 1994, 91, 5710–5714. [Google Scholar] [CrossRef]
- Smith, M.A.; Zhu, X.; Tabaton, M.; Liu, G.; McKeel, D.W.; Cohen, M.L.; Wang, X.; Siedlak, S.L.; Dwyer, B.E.; Hayashi, T.; et al. Increased iron and free radical generation in preclinical Alzheimer disease and mild cognitive impairment. J. Alzheimers Dis. JAD 2010, 19, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Aytan, N.; Jung, T.; Tamtürk, F.; Grune, T.; Kartal-Ozer, N. Oxidative stress related changes in the brain of hypercholesterolemic rabbits. Biofactors 2008, 33, 225–236. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Yaung, J.; Kim, Y.H.; Barron, E.; Ryan, S.J.; Hinton, D.R. Endoplasmic reticulum stress induced by oxidative stress in retinal pigment epithelial cells. Graefes Arch. Clin. Exp. Ophthalmol. 2008, 246, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Prince, M.; Bryce, R.; Ferri, C. World Alzheimer Report—The Benefits of Early Diagnosis and Intervention World Alzheimer Report; Alzheimer’s Disease International: London, UK, 2011. [Google Scholar]
- Klemann, C.J.H.M.; Martens, G.J.M.; Sharma, M.; Martens, M.B.; Isacson, O.; Gasser, T.; Visser, J.E.; Poelmans, G. Integrated molecular landscape of Parkinson’s disease. NPJ Park. Dis. 2017, 3, 14. [Google Scholar] [CrossRef] [PubMed]
- Sveinbjornsdottir, S. The clinical symptoms of Parkinson’s disease. J. Neurochem. 2016, 1, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Sowell, R.A.; Owen, J.B.; Butterfield, D.A. Proteomics in animal models of Alzheimer’s and Parkinson’s diseases. Ageing Res. Rev. 2009, 8, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Braak, E. Pathoanatomy of Parkinson’s disease. J. Neurol. 2000, 247, II3–II10. [Google Scholar] [CrossRef] [PubMed]
- Shulman, J.M.; De Jager, P.L.; Feany, M.B. Parkinson’s disease: Genetics and pathogenesis. Annu. Rev. Pathol. 2011, 6, 193–222. [Google Scholar] [CrossRef] [PubMed]
- Minghetti, L.; Levi, G. Microglia as effector cells in brain damage and repair: Focus on prostanoids and nitric oxide. Prog. Neurobiol. 1998, 54, 99–125. [Google Scholar] [CrossRef]
- Park, J.H.; Enikolopov, G. Transient elevation of adult hippocampal neurogenesis after dopamine depletion. Exp. Neurol. 2010, 222, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am. J. Clin. Nutr. 2003, 78, 3–6. [Google Scholar] [CrossRef]
- Uriarte Pueyo, I.; Calvo, M.I. Phytochemical study and evaluation of antioxidant, neuroprotective and acetylcholinesterase inhibitor activities of Galeopsis ladanum L. extracts. Pharmacogn. Mag. 2009, 5, 287–290. [Google Scholar] [CrossRef]
- Gurung, R.B.; Kim, E.; Oh, T.; Sohng, J.K. Enzymatic Synthesis of Apigenin Glucosides by Glucosyltransferase (YjiC) from Bacillus licheniformis DSM 13. Mol. Cells 2013, 36, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Rendeiro, C.; Vauzour, D.; Rattray, M.; Waffo-Téguo, P.; Mérillon, J.M.; Butler, L.T.; Williams, C.M.; Spencer, J.P.E. Dietary Levels of Pure Flavonoids Improve Spatial Memory Performance and Increase Hippocampal Brain-Derived Neurotrophic Factor. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Solanki, I.; Parihar, P.; Mansuri, M.L.; Parihar, M.S. Flavonoid-Based Therapies in the Early Management of Neurodegenerative Diseases. Adv. Nutr. Int. Rev. J. 2015, 6, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Castellano, G.; González-santander, J.L.; Lara, A.; Torrens, F. Phytochemistry Classification of flavonoid compounds by using entropy of information theory. Phytochemistry 2013, 93, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Vauzour, D. Polyphenols and brain health. OCL 2017, 24, A202. [Google Scholar] [CrossRef]
- Spencer, J.P.E.; Vafeiadou, K.; Williams, R.J.; Vauzour, D. Neuroinflammation: Modulation by flavonoids and mechanisms of action. Mol. Aspects Med. 2012, 33, 83–97. [Google Scholar] [CrossRef] [PubMed]
- Kano, M.; Takayanagi, T.; Harada, K.; Sawada, S.; Ishikawa, F. Bioavailability of Isoflavones after Ingestion of Soy Beverages in Healthy Adults. J. Nutr. 2006, 136, 2291–2296. [Google Scholar] [CrossRef] [PubMed]
- Neuhouser, M.L. Dietary flavonoids and cancer risk: Evidence from human population studies. Nutr. Cancer 2004, 50, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Shi, R.; Wang, X.; Shen, H.-M. Luteolin, a flavonoid with potential for cancer prevention and therapy. Curr. Cancer Drug Targets 2008, 8, 634–646. [Google Scholar] [CrossRef] [PubMed]
- Miean, K.H.; Mohamed, S. Flavonoid (myrcetin, quercetin, kaempferol, luteolin, and apigein) content of edible tropical plants. J. Agric. Food Chem. 2001, 49, 3106–3112. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Gupta, S. Apigenin: A promising molecule for cancer prevention. Pharm. Res. 2010, 27, 962–978. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.G.; Ju, M.S.; Ha, S.K.; Lee, H.; Lee, H.; Kim, S.Y.; Oh, M.S. Acacetin Protects Dopaminergic Cells against 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine-Induced Neuroinflammation in Vitro and in Vivo. Biol. Pharm. Bull. 2012, 35, 1287–1294. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.K.; Moon, E.; Lee, P.; Ryu, J.H.; Oh, M.S.; Kim, S.Y. Acacetin attenuates neuroinflammation via regulation the response to LPS stimuli in Vitro and in Vivo. Neurochem. Res. 2012, 37, 1560–1567. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Garg, S.; Zaneveld, L.J.D.; Singla, A.K. Chemistry and pharmacology of the Citrus bioflavonoid hesperidin. Phyther. Res. 2001, 15, 655–669. [Google Scholar] [CrossRef] [PubMed]
- Jagetia, G.C.; Reddy, T.K. The grapefruit flavanone naringin protects against the radiation-induced genomic instability in the mice bone marrow: A micronucleus study. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2002, 519, 37–48. [Google Scholar] [CrossRef]
- Scholey, A.; Downey, L.A.; Ciorciari, J.; Pipingas, A.; Nolidin, K.; Finn, M.; Wines, M.; Catchlove, S.; Terrens, A.; Barlow, E.; et al. Acute neurocognitive effects of epigallocatechin gallate (EGCG). Appetite 2012, 58, 767–770. [Google Scholar] [CrossRef] [PubMed]
- Van Praag, H.; Lucero, M.J.; Yeo, G.W.; Stecker, K.; Heivand, N.; Zhao, C.; Yip, E.; Afanador, M.; Schroeter, H.; Hammerstone, J.; et al. Plant-Derived Flavanol (−)Epicatechin Enhances Angiogenesis and Retention of Spatial Memory in Mice. J. Neurosci. 2007, 27, 5869–5878. [Google Scholar] [CrossRef] [PubMed]
- Edwards, R.L.; Lyon, T.; Litwin, S.E.; Rabovsky, A.; Symons, J.D.; Jalili, T. Quercetin reduces blood pressure in hypertensive subjects. J. Nutr. 2007, 137, 2405–2411. [Google Scholar] [CrossRef] [PubMed]
- Egert, S.; Wolffram, S.; Bosy-Westphal, A.; Boesch-Saadatmandi, C.; Wagner, A.E.; Frank, J.; Rimbach, G.; Mueller, M.J. Daily quercetin supplementation dose-dependently increases plasma quercetin concentrations in healthy humans. J. Nutr. 2008, 138, 1615–1621. [Google Scholar] [CrossRef] [PubMed]
- Bhathena, S.J.; Velasquez, M.T. Beneficial role of dietary phytoestrogens in obesity and diabetes. Am. J. Clin. Nutr. 2002, 76, 1191–1201. [Google Scholar] [CrossRef] [PubMed]
- Somerset, S.M.; Johannot, L. Dietary flavonoid sources in Australian adults. Nutr. Cancer 2008, 60, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Hamuel, J. Phytochemicals: Extraction Methods, Basic Structures and Mode of Action as Potential Chemotherapeutic Agents. In Phytochemicals—A Global Perspective of Their Role in Nutrition and Health; InTech: London, UK, 2012; ISBN 978-953-51-029600. [Google Scholar]
- Mazza, G. Compositional and Functional Properties of Saskatoon Berry and Blueberry. Int. J. Fruit Sci. 2005, 5, 101–120. [Google Scholar] [CrossRef]
- Lin, L.-Z.; Harnly, J.M.; Pastor-Corrales, M.S.; Luthria, D.L. The polyphenolic profiles of common bean (Phaseolus vulgaris L.). Food Chem. 2008, 107, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Vegeto, E.; Bonincontro, C.; Pollio, G.; Sala, A.; Viappiani, S.; Nardi, F.; Brusadelli, A.; Viviani, B.; Ciana, P.; Maggi, A. Estrogen prevents the lipopolysaccharide-induced inflammatory response in microglia. J. Neurosci. 2001, 21, 1809–1818. [Google Scholar] [PubMed]
- Wang, X.; Chen, S.; Ma, G.; Ye, M.; Lu, G. Genistein protects dopaminergic neurons by inhibiting microglial activation. Neuroreport 2005, 16, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Trieu, V.N.; Uckun, F.M. Genistein Is Neuroprotective in Murine Models of Familial Amyotrophic Lateral Sclerosis and Stroke. Biochem. Biophys. Res. Commun. 1999, 258, 685–688. [Google Scholar] [CrossRef] [PubMed]
- Sonee, M.; Sum, T.; Wang, C.; Mukherjee, S.K. The soy isoflavone, genistein, protects human cortical neuronal cells from oxidative stress. Neurotoxicology 2004, 25, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Sawamoto, N.; Piccini, P.; Hotton, G.; Pavese, N.; Thielemans, K.; Brooks, D.J. Cognitive deficits and striato-frontal dopamine release in Parkinson’s disease. Brain 2008, 131, 1294–1302. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.M.; Jiang, J.; Wilson, B.; Zhang, W.; Hong, J.S.; Liu, B. Microglial activation-mediated delayed and progressive degeneration of rat nigral dopaminergic neurons: Relevance to Parkinson’s disease. J. Neurochem. 2002, 81, 1285–1297. [Google Scholar] [CrossRef] [PubMed]
- Wildburger, N.C.; Esparza, T.J.; Leduc, R.D.; Fellers, R.T.; Thomas, P.M.; Cairns, N.J.; Kelleher, N.L.; Bateman, R.J.; Brody, D.L. Diversity of Amyloid-beta Proteoforms in the Alzheimer’s Disease Brain. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J. The Amyloid Hypothesis of Alzheimer’s Disease: Progress and Problems on the Road to Therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Bang, O.Y.; Jung, M.W.; Ha, S.D.; Hong, H.S.; Huh, K.; Kim, S.U.; Mook-Jung, I. Neuroprotective effects of estrogen against beta-amyloid toxicity are mediated by estrogen receptors in cultured neuronal cells. Neurosci. Lett. 2001, 302, 58–62. [Google Scholar] [CrossRef]
- Bang, O.Y.; Hong, H.S.; Kim, D.H.; Kim, H.; Boo, J.H.; Huh, K.; Mook-Jung, I. Neuroprotective effect of genistein against beta amyloid-induced neurotoxicity. Neurobiol. Dis. 2004, 16, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Jin, G.; Zhao, M.; Yang, H. The Effect of Genistein on the Content and Activity of α- and β-Secretase and Protein Kinase C in Aβ-Injured Hippocampal Neurons. Basic Clin. Pharmacol. Toxicol. 2013, 112, 182–185. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Yuan, L.; Yu, H.; Ding, B.; Xi, Y.; Feng, J.; Xiao, R. Genistein as a neuroprotective antioxidant attenuates redox imbalance induced by β-amyloid peptides 25–35 in PC12 cells. Int. J. Dev. Neurosci. 2010, 28, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Devi, K.P.; Shanmuganathan, B.; Manayi, A.; Nabavi, S.F.; Nabavi, S.M. Molecular and Therapeutic Targets of Genistein in Alzheimer’s Disease. Mol. Neurobiol. 2016, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.; Yu, O.; Lau, S.-M.C.; O’Keefe, D.P.; Odell, J.; Fader, G.; McGonigle, B. Identification and expression of isoflavone synthase, the key enzyme for biosynthesis of isoflavones in legumes. Nat. Biotechnol. 2000, 18, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Fedoreyev, S.A.; Pokushalova, T.V.; Veselova, M.V.; Glebko, L.I.; Kulesh, N.I.; Muzarok, T.I.; Seletskaya, L.D.; Bulgakov, V.P.; Zhuravlev, Y.N. Isoflavonoid production by callus cultures of Maackia amurensis. Fitoterapia 2000, 71, 365–372. [Google Scholar] [CrossRef]
- Choi, R.C.Y.; Zhu, J.T.T.; Yung, A.W.Y.; Lee, P.S.C.; Xu, S.L.; Guo, A.J.Y.; Zhu, K.Y.; Dong, T.T.X.; Tsim, K.W.K. Synergistic Action of Flavonoids, Baicalein, and Daidzein in Estrogenic and Neuroprotective Effects: A Development of Potential Health Products and Therapeutic Drugs against Alzheimer‘s Disease. Hindawi 2013, 2013, 635694. [Google Scholar] [CrossRef] [PubMed]
- Civitelli, R. In vitro and in vivo effects of ipriflavone on bone formation and bone biomechanics. Calcif. Tissue Int. 1997, 61, 12–14. [Google Scholar] [CrossRef]
- Peterson, G.; Barnes, S. Genistein inhibition of the growth of human breast cancer cells: Independence from estrogen receptors and the multi-drug resistance gene. Biochem. Biophys. Res. Commun. 1991, 179, 661–667. [Google Scholar] [CrossRef]
- Schreihofer, D.A. Transcriptional regulation by phytoestrogens in neuronal cell lines. Mol. Cell. Endocrinol. 2005, 231, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, G.G.; Lemmen, J.G.; Carlsson, B.; Corton, J.C.; Safe, S.H.; van der Saag, P.T.; van der Burg, B.; Gustafsson, J.A. Interaction of estrogenic chemicals and pytoestrogens with estrogen receptor beta. Endocrinology 1998, 139, 4252–4263. [Google Scholar] [CrossRef] [PubMed]
- Chinta, S.J.; Ganesan, A.; Reis-Rodrigues, P.; Lithgow, G.J.; Andersen, J.K. Anti-inflammatory role of the isoflavone diadzein in lipopolysaccharide- stimulated microglia: Implications for parkinson’s disease. Neurotox. Res. 2013, 23, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Occhiuto, F.; Zangla, G.; Samperi, S.; Palumbo, D.R.; Pino, A.; De Pasquale, R.; Circosta, C. The phytoestrogenic isoflavones from Trifolium pratense L. (Red clover) protects human cortical neurons from glutamate toxicity. Phytomedicine 2008, 15, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.J. The prooxidant, rather than antioxidant, acts of daidzein in vivo and in vitro: Daidzein suppresses glutathione metabolism. Eur. J. Pharmacol. 2006, 542, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Röhrdanz, E.; Ohler, S.; Tran-Thi, Q.-H.; Kahl, R. The phytoestrogen daidzein affects the antioxidant enzyme system of rat hepatoma H4IIE cells. J. Nutr. 2002, 132, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Kulling, S.E.; Honig, D.M.; Simat, T.J.; Metzler, M. Oxidative in vitro metabolism of the soy phytoestrogens daidzein and genistein. J. Agric. Food Chem. 2000, 48, 4963–4972. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Sun, Q.; Chen, S. Oxidative stress: A major pathogenesis and potential therapeutic target of antioxidative agents in Parkinson’s disease and Alzheimer’s disease. Prog. Neurobiol. 2016, 147, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Neumann, J.; Sauerzweig, S.; Ronicke, R.; Gunzer, F.; Dinkel, K.; Ullrich, O.; Gunzer, M.; Reymann, K.G. Microglia Cells Protect Neurons by Direct Engulfment of Invading Neutrophil Granulocytes: A New Mechanism of CNS Immune Privilege. J. Neurosci. 2008, 28, 5965–5975. [Google Scholar] [CrossRef] [PubMed]
- Harborne, J.B.; Williams, C.A. Advances in flavonoid research since 1992. Phytochemistry 2000, 55, 481–504. [Google Scholar] [CrossRef]
- Ross, J.A.; Kasum, C.M. Dietary flavonoids: Bioavailability, metabolic effects, and safety. Annu. Rev. Nutr. 2002, 22, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Dirscherl, K.; Karlstetter, M.; Ebert, S.; Kraus, D.; Hlawatsch, J.; Walczak, Y.; Moehle, C.; Fuchshofer, R.; Langmann, T. Luteolin triggers global changes in the microglial transcriptome leading to a unique anti-inflammatory and neuroprotective phenotype. J. Neuroinflamm. 2010, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Gao, M.; Qiang, G.F.; Zhang, T.T.; Lan, X.; Ying, J.; Du, G.H. The anti-amnesic effects of luteolin against amyloid β25–35 peptide-induced toxicity in mice involve the protection of neurovascular unit. Neuroscience 2009, 162, 1232–1243. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Zhang, J.; Guo, L.; Xu, Y.; Sun, L.; Wang, S.; Feng, Y.; Gou, L.; Zhang, L.; Liu, Y. Protective role of luteolin against cognitive dysfunction induced by chronic cerebral hypoperfusion in rats. Pharmacol. Biochem. Behav. 2014, 126, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Rezai-Zadeh, K.; Douglas Shytle, R.; Bai, Y.; Tian, J.; Hou, H.; Mori, T.; Zeng, J.; Obregon, D.; Town, T.; Tan, J. Flavonoid-mediated presenilin-1 phosphorylation reduces Alzheimer’s disease β-amyloid production. J. Cell. Mol. Med. 2009, 13, 574–588. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, H.; Cheng, H.; Che, Z. Ameliorating effect of luteolin on memory impairment in an Alzheimer’s disease model. Mol. Med. Rep. 2016, 13, 4215–4220. [Google Scholar] [CrossRef] [PubMed]
- Sawmiller, D.; Li, S.; Shahaduzzaman, M.; Smith, A.J.; Obregon, D.; Giunta, B.; Borlongan, C.V.; Sanberg, P.R.; Tan, J. Luteolin reduces Alzheimer’s disease pathologies induced by traumatic brain injury. Int. J. Mol. Sci. 2014, 15, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Hong, J.-S. Role of microglia in inflammation-mediated neurodegenerative diseases: Mechanisms and strategies for therapeutic intervention. J. Pharmacol. Exp. Ther. 2003, 304, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, S.; Ma, G.; Ye, M.; Lu, G. Involvement of proinflammatory factors, apoptosis, caspase-3 activation and Ca2+ disturbance in microglia activation-mediated dopaminergic cell degeneration. Mech. Ageing Dev. 2005, 126, 1241–1254. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Yan, Z.Q.; Lu, G.Q.; Stuart, S.; Chen, S. Di Parkinson disease IgG and C5a-induced synergistic dopaminergic neurotoxicity: Role of microglia. Neurochem. Int. 2007, 50, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.G.; Mohney, R.P.; Wilson, B.; Jeohn, G.H.; Liu, B.; Hong, J.S. Regional difference in susceptibility to lipopolysaccharide-induced neurotoxicity in the rat brain: Role of microglia. J. Neurosci. 2000, 20, 6309–6316. [Google Scholar] [PubMed]
- Chen, H.Q.; Jin, Z.Y.; Wang, X.J.; Xu, X.M.; Deng, L.; Zhao, J.W. Luteolin protects dopaminergic neurons from inflammation-induced injury through inhibition of microglial activation. Neurosci. Lett. 2008, 448, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Cheung, Z.H.; Leung, M.C.P.; Yip, H.K.; Wu, W.; Siu, F.K.W.; So, K.F. A neuroprotective herbal mixture inhibits caspase-3-independent apoptosis in retinal ganglion cells. Cell. Mol. Neurobiol. 2008, 28, 137–155. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Landau, J.M.; Huang, M.T.; Newmark, H.L. Inhibition of carcinogenesis by dietary polyphenolic compounds. Annu. Rev. Nutr. 2001, 21, 381–406. [Google Scholar] [CrossRef] [PubMed]
- Choi, A.Y.; Choi, J.H.; Lee, J.Y.; Yoon, K.S.; Choe, W.; Ha, J.; Yeo, E.J.; Kang, I. Apigenin protects HT22 murine hippocampal neuronal cells against endoplasmic reticulum stress-induced apoptosis. Neurochem. Int. 2010, 57, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Balez, R.; Steiner, N.; Engel, M.; Muñoz, S.S.; Lum, J.S.; Wu, Y.; Wang, D.; Vallotton, P.; Sachdev, P.; Connor, M.O.; et al. Neuroprotective effects of apigenin against inflammation, neuronal excitability and apoptosis in an induced pluripotent stem cell model of Alzheimer’s disease. Sci. Rep. 2016, 1–16. [Google Scholar] [CrossRef] [PubMed]
- De Strooper, B.; Iwatsubo, T.; Wolfe, M.S. Presenilins and gamma-secretase: Structure, function, and role in Alzheimer Disease. Cold Spring Harb. Perspect. Med. 2012, 2, a006304. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, H.; Barger, S.; Barnum, S.; Bradt, B.; Bauer, J.; Cole, G.M.; Cooper, N.R.; Eikelenboom, P.; Emmerling, M.; Fiebich, B.L.; et al. Inflammation and Alzheimer’s disease. Neurobiol. Aging 2000, 21, 383–421. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, J.L.; Wang, Y.R.; Fa, X.Z. Apigenin attenuates copper-mediated β-amyloid neurotoxicity through antioxidation, mitochondrion protection and MAPK signal inactivation in an AD cell model. Brain Res. 2013, 1492, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, G.; Gurley, E.C.; Zhou, H. Flavonoid apigenin inhibits lipopolysaccharide-induced inflammatory response through multiple mechanisms in Macrophages. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.S.; Lee, J.Y.; Choi, Y.K.; Kim, G.S.; Han, B.H. Neuroprotective effects of flavones on hydrogen peroxide-induced apoptosis in SH-SY5Y neuroblostoma cells. Bioorgan. Med. Chem. Lett. 2004, 14, 2261–2264. [Google Scholar] [CrossRef] [PubMed]
- Anusha, C.; Sumathi, T.; Joseph, L.D. Protective role of apigenin on rotenone induced rat model of Parkinson’s disease: Suppression of neuroinflammation and oxidative stress mediated apoptosis. Chem. Biol. Interact. 2017, 269, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, J. Parkinson’s disease: Clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatry 2008, 79, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.M.; Main, B.S.; Crack, P.J. Neuroinflammation and oxidative stress: Co-conspirators in the pathology of Parkinson’s disease. Neurochem. Int. 2013, 62, 803–819. [Google Scholar] [CrossRef] [PubMed]
- Meissner, W.G.; Frasier, M.; Gasser, T.; Goetz, C.G.; Lozano, A.; Piccini, P.; Obeso, J.A.; Rascol, O.; Schapira, A.; Voon, V.; et al. Priorities in Parkinson’s disease research. Nat. Rev. Drug Discov. 2011, 10, 377–393. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.P.; Jain, P.D.; Sancheti, J.S.; Ghumatkar, P.J.; Tambe, R.; Sathaye, S. Neuroprotective and neurotrophic effects of Apigenin and Luteolin in MPTP induced parkinsonism in mice. Neuropharmacology 2014, 86, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Rezai-Zadeh, K.; Ehrhart, J.; Bai, Y.; Sanberg, P.R.; Bickford, P.; Tan, J.; Shytle, R.D. Apigenin and luteolin modulate microglial activation via inhibition of STAT1-induced CD40 expression. J. Neuroinflamm. 2008, 5, 41. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.L.; Lee, H.J.; Huang, W.J.; Chou, J.F.; Fan, P.C.; Du, J.C.; Ku, Y.L.; Chiou, L.C. Clerodendrum inerme leaf extract alleviates animal behaviors, hyperlocomotion, and prepulse inhibition disruptions, mimicking tourette syndrome and schizophrenia. Evid. Based Complement. Altern. Med. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.-H.H.; Hung, S.-H.H.; Yin, L.-T.T.; Huang, C.-S.S.; Chao, C.-H.H.; Liu, C.-L.L.; Shih, Y.-W.W. Acacetin, a flavonoid, inhibits the invasion and migration of human prostate cancer DU145 cells via inactivation of the p38 MAPK signaling pathway. Mol. Cell Biochem. 2010, 333, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Shim, H.-Y.; Park, J.-H.; Paik, H.-D.; Nah, S.-Y.; Kim, D.S.H.L.; Han, Y.S. Acacetin-induced apoptosis of human breast cancer MCF-7 cells involves caspase cascade, mitochondria-mediated death signaling and SAPK/JNK1/2-c-Jun activation. Mol. Cells 2007, 24, 95–104. [Google Scholar] [PubMed]
- Pan, M.H.; Lai, C.S.; Wang, Y.J.; Ho, C.T. Acacetin suppressed LPS-induced up-expression of iNOS and COX-2 in murine macrophages and TPA-induced tumor promotion in mice. Biochem. Pharmacol. 2006, 72, 1293–1303. [Google Scholar] [CrossRef] [PubMed]
- Hanisch, U.-K. Microglia as a source and target of cytokines. Glia 2002, 40, 140–155. [Google Scholar] [CrossRef] [PubMed]
- González-Scarano, F.; Baltuch, G. Microglia as Mediators of Inflammatory and Degenerative Diseases. Annu. Rev. Neurosci. 1999, 22, 219–240. [Google Scholar] [CrossRef] [PubMed]
- Tak, P.P.; Firestein, G.S.; Tak, P.P.; Firestein, G.S. NF-kappaB: A key role in inflammatory diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Waetzig, V.; Czeloth, K.; Hidding, U.; Mielke, K.; Kanzow, M.; Brecht, S.; Goetz, M.; Lucius, R.; Herdegen, T.; Hanisch, U.R. c-Jun N-terminal kinases (JNKs) mediate pro-inflammatory actions of microglia. Glia 2005, 50, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Schieven, G.L. The biology of p38 kinase: A central role in inflammation. Curr. Top. Med. Chem. 2005, 5, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Reddy, P.H. Role of Glutamate and NMDA Receptors in Alzheimer’s Disease. J. Alzheimers Dis. 2017, 57, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.Y.; Huang, W.J.; Wu, C.C.; Lu, C.W.; Wang, S.J. Acacetin inhibits glutamate release and prevents kainic acid-induced neurotoxicity in rats. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Jung, K.J.; Choi, J.S.; Chung, H.Y. Hesperetin: A potent antioxidant against peroxynitrite. Free Radic. Res. 2004, 38, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Parhiz, H.; Roohbakhsh, A.; Soltani, F.; Rezaee, R.; Iranshahi, M. Antioxidant and anti-inflammatory properties of the citrus flavonoids hesperidin and hesperetin: An updated review of their molecular mechanisms and experimental models. Phyther. Res. 2015, 29, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.-L.; Yang, F.; Rosario, E.R.; Ubeda, O.J.; Beech, W.; Gant, D.J.; Chen, P.P.; Hudspeth, B.; Chen, C.; Zhao, Y.; et al. Amyloid Oligomers Induce Phosphorylation of Tau and Inactivation of Insulin Receptor Substrate via c-Jun N-Terminal Kinase Signaling: Suppression by Omega-3 Fatty Acids and Curcumin. J. Neurosci. 2009, 29, 9078–9089. [Google Scholar] [CrossRef] [PubMed]
- Cho, J. Antioxidant and neuroprotective effects of hesperidin and its aglycone hesperetin. Arch. Pharm. Res. 2006, 29, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.M.; Tsai, S.Y.; Lin, J.A.; Wu, C.H.; Yen, G.C. Cytoprotective effects of hesperetin and hesperidin against amyloid β-induced impairment of glucose transport through downregulation of neuronal autophagy. Mol. Nutr. Food Res. 2012, 56, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Cheng, W.; Qin, Z.; Yu, H.; Yu, Z.; Zhong, M.; Sun, K.; Zhang, W. Effects of Naringin on Proliferation and Osteogenic Differentiation of Human Periodontal Ligament Stem Cells In Vitro and In Vivo. Stem Cells Int. 2015, 2015, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Gao, W.; Liu, Z.; Zhang, Z.; Liu, C. Systematic analysis of main constituents in rat biological samples after oral administration of the methanol extract of fructus aurantii by HPLC-ESI-MS/MS. Iran. J. Pharm. Res. 2014, 13, 493–503. [Google Scholar] [PubMed]
- Wong, K.C.; Pang, W.Y.; Wang, X.L.; Mok, S.K.; Lai, W.P.; Chow, H.K.; Leung, P.C.; Yao, X.S.; Wong, M.S. Drynaria fortunei-derived total flavonoid fraction and isolated compounds exert oestrogen-like protective effects in bone. Br. J. Nutr. 2013, 110, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Chtourou, Y.; Gargouri, B.; Kebieche, M.; Fetoui, H. Naringin Abrogates Cisplatin-Induced Cognitive Deficits and Cholinergic Dysfunction Through the Down-Regulation of AChE Expression and iNOS Signaling Pathways in Hippocampus of Aged Rats. J. Mol. Neurosci. 2015, 56, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.M.; Yang, Y.J.; Zhang, L.; Zhang, X.; Guan, F.F.; Zhang, L.F. Naringin enhances CaMKII activity and improves long-term memory in a mouse model of Alzheimer’s disease. Int. J. Mol. Sci. 2013, 14, 5576–5586. [Google Scholar] [CrossRef] [PubMed]
- Chanet, A.; Milenkovic, D.; Manach, C.; Mazur, A.; Morand, C. Citrus flavanones: What is their role in cardiovascular protection? J. Agric. Food Chem. 2012, 60, 8809–8822. [Google Scholar] [CrossRef] [PubMed]
- Jung, U.J.; Leem, E.; Kim, S.R. Naringin: A Protector of the Nigrostriatal Dopaminergic Projection. Exp. Neurobiol. 2014, 23, 124. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Chung, E.S.; Bok, E.; Baik, H.H.; Chung, Y.C.; Won, S.Y.; Joe, E.; Kim, T.H.; Kim, S.S.; Jin, M.Y.; et al. Prothrombin Kringle-2 induces death of mesencephalic dopaminergic neurons in vivo and in vitro via microglial activation. J. Neurosci. Res. 2010, 88, 1537–1548. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.H.; Leem, E.; Jeon, M.-T.; Kim, Y.-J.; Jung, U.J.; Choi, M.-S.; Maeng, S.; Jin, B.K.; Kim, S.R. Inhibition of prothrombin kringle-2-induced inflammation by minocycline protects dopaminergic neurons in the substantia nigra in vivo. Neuroreport 2014, 25, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Vinayagam, M.M.; Sadiq, A.M. Flavonoid naringin inhibits microglial activation and exerts neuroprotection against deltamethrin induced neurotoxicity through Nrf2/ARE signaling in the cortex and hippocampus of rats. World J. Pharm. Sci. 2015, 3, 2410–2426. [Google Scholar]
- Clairembault, T.; Kamphuis, W.; Leclair-Visonneau, L.; Rolli-Derkinderen, M.; Coron, E.; Neunlist, M.; Hol, E.M.; Derkinderen, P. Enteric GFAP expression and phosphorylation in Parkinson’s disease. J. Neurochem. 2014, 130, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, K.; Sudhandiran, G. Naringin modulates oxidative stress and inflammation in 3-nitropropionic acid-induced neurodegeneration through the activation of nuclear factor-erythroid 2-related factor-2 signalling pathway. Neuroscience 2012, 227, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Leem, E.; Nam, J.H.; Jeon, M.T.; Shin, W.H.; Won, S.Y.; Park, S.J.; Choi, M.S.; Jin, B.K.; Jung, U.J.; Kim, S.R. Naringin protects the nigrostriatal dopaminergic projection through induction of GDNF in a neurotoxin model of parkinson’s disease. J. Nutr. Biochem. 2014, 25, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Dogra, S.; Prakash, A. Protective effect of naringin, a citrus flavonoid, against colchicine-induced cognitive dysfunction and oxidative damage in rats. J. Med. Food 2010, 13, 976–984. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Bae, J.H.; Lee, S.-R. Protective effect of green tea polyphenol EGCG against neuronal damage and brain edema after unilateral cerebral ischemia in gerbils. J. Neurosci. Res. 2004, 77, 892–900. [Google Scholar] [CrossRef] [PubMed]
- Gensler, H.L.; Timmermann, B.N.; Valcic, S.; Wachter, G.A.; Dorr, R.; Dvorakova, K.; Alberts, D.S. Prevention of photocarcinogenesis by topical administration of pure epigallocatechin gallate isolated from green tea. Nutr. Cancer Int. J. 1996, 26, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Stoner, G.D.; Mukhtar, H. Polyphenols as cancer chemopreventive agents. J. Cell. Biochem. 1995, 59, 169–180. [Google Scholar] [CrossRef]
- Suganuma, M.; Okabe, S.; Oniyama, M.; Tada, Y.; Ito, H.; Fujiki, H. Wide distribution of [3H](−)-epigallocatechin gallate, a cancer preventive tea polyphenol, in mouse tissue. Carcinogenesis 1998, 19, 1771–1776. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.M.Y.; Chyi, B.Y.; Wu, L.Y.; Hwang, L.S.; Ho, L.T. The antioxidative property of green tea against iron-induced oxidative stress in rat brain. Chin. J. Physiol. 1998, 41, 189–194. [Google Scholar] [PubMed]
- Miranda, S.; Opazo, C.; Larrondo, L.F.; Munoz, F.J.; Ruiz, F.; Leighton, F.; Inestrosa, N.C. The role of oxidative stress in the toxicity induced by amyloid beta-peptide in Alzheimer’s disease. Prog. Neurobiol. 2000, 62, 633–648. [Google Scholar] [CrossRef]
- Marín, N.; Romero, B.; Bosch-Morell, F.; Llansola, M.; Felipo, V.; Romá, J.; Romero, F.J. β-Amyloid-induced activation of Caspase-3 in primary cultures of rat neurons. Mech. Ageing Dev. 2000, 119, 63–67. [Google Scholar] [CrossRef]
- Choi, Y.T.; Jung, C.H.; Lee, S.R.; Bae, J.H.; Baek, W.K.; Suh, M.H.; Park, J.; Park, C.W.; Suh, S. The green tea polyphenol (−)-epigallocatechin gallate attenuates β-amyloid-induced neurotoxicity in cultured hippocampal neurons. Life Sci. 2001, 70, 603–614. [Google Scholar] [CrossRef]
- Schroeter, H.; Heiss, C.; Balzer, J.; Kleinbongard, P.; Keen, C.L.; Hollenberg, N.K.; Sies, H.; Kwik-Uribe, C.; Schmitz, H.H.; Kelm, M. (-)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc. Natl. Acad. Sci. USA 2006, 103, 1024–1029. [Google Scholar] [CrossRef] [PubMed]
- Abd El Mohsen, M.M.; Kuhnle, G.; Rechner, A.R.; Schroeter, H.; Rose, S.; Jenner, P.; Rice-Evans, C.A. Uptake and metabolism of epicatechin and its access to the brain after oral ingestion. Free Radic. Biol. Med. 2002, 33, 1693–1702. [Google Scholar] [CrossRef]
- Niranjan, R. The Role of inflammatory and oxidative stress mechanisms in the pathogenesis of parkinson’s disease: Focus on astrocytes. Mol. Neurobiol. 2014, 49, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Gaki, G.S.; Papavassiliou, A.G. Oxidative stress-induced signaling pathways implicated in the pathogenesis of Parkinson’s disease. Neuromol. Med. 2014, 16, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Park, C.S.; Kim, D.J.; Cho, M.H.; Jin, B.K.; Pie, J.E.; Chung, W.G. Prevention of nitric oxide-mediated 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinson’s disease in mice by tea phenolic epigallocatechin 3-gallate. Neurotoxicology 2002, 23, 367–374. [Google Scholar] [CrossRef]
- Guo, S.; Yan, J.; Yang, T.; Yang, X.; Bezard, E.; Zhao, B. Protective Effects of Green Tea Polyphenols in the 6-OHDA Rat Model of Parkinson’s Disease Through Inhibition of ROS-NO Pathway. Biol. Psychiatry 2007, 62, 1353–1362. [Google Scholar] [CrossRef] [PubMed]
- Park, E.S.; Park, C.; Kim, D.Y.; Kim, Y.R. The effect of spasticity on cortical somatosensory-evoked potentials: Changes of cortical somatosensory-evoked potentials after botulinum toxin type A injection. Arch. Phys. Med. Rehabil. 2002, 83, 1592–1596. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhou, J.; Ji, C. Quercetin: A potential drug to reverse multidrug resistance. Life Sci. 2010, 87, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Chalcone, Q. Quercetin. Altern. Med. Rev. Monogr. 2002, 10, 361–366. [Google Scholar]
- Erlund, I. Review of the flavonoids quercetin, hesperetin, and naringenin. Dietary sources, bioactivities, bioavailability, and epidemiology. Nutr. Res. 2004, 24, 851–874. [Google Scholar] [CrossRef]
- Ruiz, P.A.; Braune, A.; Hö, G.; Quintanilla-Fend, L.; Haller, D. Quercetin Inhibits TNF-Induced NF-kB Transcription Factor Recruitment to Proinflammatory Gene Promoters in Murine Intestinal Epithelial Cells 1,2. J. Nutr. 2007, 137, 1208–1215. [Google Scholar] [CrossRef] [PubMed]
- Kanter, M.; Unsal, C.; Aktas, C.; Erboga, M. Neuroprotective effect of quercetin against oxidative damage and neuronal apoptosis caused by cadmium in hippocampus. Toxicol. Ind. Health 2016, 32, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.M.; Kim, B.C.; Cho, Y.H.; Choi, K.H.; Chang, J.; Park, M.S.; Kim, M.K.; Cho, K.H.; Kim, J.K. Effects of Flavonoid Compounds on beta-amyloid-peptide-induced Neuronal Death in Cultured Mouse Cortical Neurons. Chonnam Med. J. 2014, 50, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.M.; Zheng, G.H.; Cheng, C.; Sun, J.M. Quercetin protects mouse brain against lead-induced neurotoxicity. J. Agric. Food Chem. 2013, 61, 7630–7635. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.J.; Spencer, J.P.E.; Rice-Evans, C. Flavonoids: Antioxidants or signalling molecules? Free Radic. Biol. Med. 2004, 36, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Pu, F.; Mishima, K.; Irie, K.; Motohashi, K.; Tanaka, Y.; Orito, K.; Egawa, T.; Kitamura, Y.; Egashira, N.; Iwasaki, K.; et al. Neuroprotective effects of quercetin and rutin on spatial memory impairment in an 8-arm radial maze task and neuronal death induced by repeated cerebral ischemia in rats. J. Pharmacol. Sci. 2007, 104, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Sabogal-Guáqueta, A.M.; Muñoz-Manco, J.I.; Ramírez-Pineda, J.R.; Lamprea-Rodriguez, M.; Osorio, E.; Cardona-Gómez, G.P. The Flavonoid Quercetin Ameliorates Alzheimer’s Disease Pathology and Protects Cognitive and Emotional Function in Aged Triple Transgenic Alzheimer’s Disease Model Mice; Elsevier Ltd.: Amsterdam, The Netherlands, 2015; ISBN 5742196458. [Google Scholar]
- Heo, H.J.; Lee, C.Y. Protective effects of quercetin and vitamin C against oxidative stress-induced neurodegeneration. J. Agric. Food Chem. 2004, 52, 7514–7517. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.A.; Abdul, H.M.; Joshi, G.; Opii, W.O.; Butterfield, D.A. Protective effect of quercetin in primary neurons against Aβ(1–42): Relevance to Alzheimer’s disease. J. Nutr. Biochem. 2009, 20, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Zuk, M.; Kulma, A.; Dymińska, L.; Szołtysek, K.; Prescha, A.; Hanuza, J.; Szopa, J. Flavonoid engineering of flax potentiate its biotechnological application. BMC Biotechnol. 2011, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Schroeter, H.; Williams, R.J.; Matin, R.; Iversen, L.; Rice-Evans, C.A. Phenolic antioxidants attenuate neuronal cell death following uptake of oxidized low-density lipoprotein. Free Radic. Biol. Med. 2000, 29, 1222–1233. [Google Scholar] [CrossRef]
- Schroeter, H.; Spencer, J.P.; Rice-Evans, C.; Williams, R.J. Flavonoids protect neurons from oxidized low-density-lipoprotein-induced apoptosis involving c-Jun N-terminal kinase (JNK), c-Jun and caspase-3. Biochem. J. 2001, 358, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Ishige, K.; Schubert, D.; Sagara, Y. Flavonoids protect neuronal cells from oxidative stress by three distinct mechanisms. Free Radic. Biol. Med. 2001, 30, 433–446. [Google Scholar] [CrossRef]
- Filomeni, G.; Graziani, I.; de Zio, D.; Dini, L.; Centonze, D.; Rotilio, G.; Ciriolo, M.R. Neuroprotection of kaempferol by autophagy in models of rotenone-mediated acute toxicity: Possible implications for Parkinson’s disease. Neurobiol. Aging 2012, 33, 767–785. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Himeda, T.; Araki, T. Mechanisms of MPTP toxicity and their implications for therapy of Parkinson’s disease. Med. Sci. Monit. 2005, 11, RA17–RA23. [Google Scholar] [PubMed]
- Olanow, C.W.; Obeso, J.A.; Stocchi, F. Continuous dopamine-receptor treatment of Parkinson’s disease: Scientific rationale and clinical implications. Lancet Neurol. 2006, 5, 677–687. [Google Scholar] [CrossRef]
- Sloley, B.D.; Urichuk, L.J.; Morley, P.; Durkin, J.; Shan, J.J.; Pang, P.K.T.; Coutts, R.T. Identification of Kaempferol as a Monoamine Oxidase Inhibitor and Potential Neuroprotectant in Extracts of Ginkgo Biloba Leaves. J. Pharm. Pharmacol. 2000, 52, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.I. Neuroprotective Effect of Kaempferol against a 1-Methyl-4-phenyl-1, 2, 3, 6- tetrahydropyridine-Induced Mouse Model of Parkinson’s Disease. Biol. Pharm. Bull. 2011, 34, 1291–1296. [Google Scholar]
- Asano, N.; Yamashita, T.; Yasuda, K.; Ikeda, K.; Kizu, H.; Kameda, Y.; Kato, A.; Nash, R.J.; Lee, H.S.; Ryu, K.S. Polyhydroxylated alkaloids isolated from mulberry trees (Morus alba L.) and silkworms (Bombyx mori L.). J. Agric. Food Chem. 2001, 49, 4208–4213. [Google Scholar] [CrossRef] [PubMed]
- Bhuiyan, M.I.H.; Kim, H.B.; Kim, S.Y.; Cho, K.O. The neuroprotective potential of cyanidin-3-glucoside fraction extracted from mulberry following oxygen-glucose deprivation. Korean J. Physiol. Pharmacol. 2011, 15, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Guan, R.; Shen, H.; Xia, Q.; Liu, M. Optimization of conditions for cyanidin-3-O-glucoside (C3G) nanoliposome production by response surface methodology and cellular uptake studies in caco-2 cells. Molecules 2017, 22. [Google Scholar] [CrossRef] [PubMed]
- Tarozzi, A.; Merlicco, A.; Morroni, F.; Franco, F.; Cantelli-Forti, G.; Teti, G.; Falconi, M.; Hrelia, P. Cyanidin 3-O-glucopyranoside protects and rescues SH-SY5Y cells against amyloid-beta peptide-induced toxicity. Neuroreport 2008, 19, 1483–1486. [Google Scholar] [CrossRef] [PubMed]
- Tarozzi, A.; Morroni, F.; Hrelia, S.; Angeloni, C.; Marchesi, A.; Cantelli-Forti, G.; Hrelia, P. Neuroprotective effects of anthocyanins and their in vivo metabolites in SH-SY5Y cells. Neurosci. Lett. 2007, 424, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Shin, W.H.; Park, S.J.; Kim, E.J. Protective effect of anthocyanins in middle cerebral artery occlusion and reperfusion model of cerebral ischemia in rats. Life Sci. 2006, 79, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Thummayot, S.; Tocharus, C.; Suksamrarn, A.; Tocharus, J. Neuroprotective effects of cyanidin against Aβ-induced oxidative and ER stress in SK-N-SH cells. Neurochem. Int. 2016, 101, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Tarozzi, A.; Morroni, F.; Merlicco, A.; Bolondi, C.; Teti, G.; Falconi, M.; Cantelli-Forti, G.; Hrelia, P. Neuroprotective effects of cyanidin 3-O-glucopyranoside on amyloid β25–35 oligomer-induced toxicity. Neurosci. Lett. 2010, 473, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Porat, Y.; Abramowitz, A.; Gazit, E. Inhibition of amyloid fibril formation by polyphenols: Structural similarity and aromatic interactions as a common inhibition mechanism. Chem. Biol. Drug Des. 2006, 67, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Rauk, A. Why is the amyloid beta peptide of Alzheimer’s disease neurotoxic? Dalton Trans. 2008, 1273–1282. [Google Scholar] [CrossRef] [PubMed]
- Andres-Lacueva, C.; Shukitt-Hale, B.; Galli, R.L.; Jauregui, O.; Lamuela-Raventos, R.M.; Joseph, J.A. Anthocyanins in aged blueberry-fed rats are found centrally and may enhance memory. Nutr. Neurosci. 2005, 8, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Sohanaki, H.; Baluchnejadmojarad, T.; Nikbakht, F.; Roghani, M. Pelargonidin improves passive avoidance task performance in a rat amyloid beta25-35 model of Alzheimer’s disease via estrogen receptor independent pathways. Acta Med. Iran. 2016, 54, 245–250. [Google Scholar] [PubMed]
- Carkeet, C.; Clevidence, B.A.; Novotny, J.A. Anthocyanin Excretion by Humans Increases Linearly with Increasing Strawberry Dose 1. J. Nutr. 2008, 138, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.P.E. Food for thought: The role of dietary flavonoids in enhancing human memory, learning and neuro-cognitive performance. Proc. Nutr. Soc. 2008, 67, 238–252. [Google Scholar] [CrossRef] [PubMed]
- Bowen-Forbes, C.S.; Zhang, Y.; Nair, M.G. Anthocyanin content, antioxidant, anti-inflammatory and anticancer properties of blackberry and raspberry fruits. J. Food Compos. Anal. 2010, 23, 554–560. [Google Scholar] [CrossRef]
- Mirshekar, M.; Roghani, M.; Khalili, M.; Baluchnejadmojarad, T.; Moazzen, S.A. Chronic oral pelargonidin alleviates streptozotocin-induced diabetic neuropathic hyperalgesia in rat: Involvement of oxidative stress. Iran. Biomed. J. 2010, 14, 33–39. [Google Scholar] [PubMed]
- Nabavi, S.F.; Habtemariam, S.; Daglia, M.; Shafighi, N.; Barber, A.J.; Nabavi, S.M. Anthocyanins as a potential therapy for diabetic retinopathy. Curr. Med. Chem. 2015, 22, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Rechner, A.R.; Kroner, C. Anthocyanins and colonic metabolites of dietary polyphenols inhibit platelet function. Thromb. Res. 2005, 116, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Amini, A.M.; Muzs, K.; Spencer, J.P.E.; Yaqoob, P. ScienceDirect Pelargonidin-3-O-glucoside and its metabolites have modest anti-inflammatory effects in human whole blood cultures. Nutr. Res. 2017, 46, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Bianchetti, A.; Ranieri, P.; Margiotta, A.; Trabucchi, M. Pharmacological treatment of Alzheimer’s Disease. Aging Clin. Exp. Res. 2006, 18, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Hämäläinen, M.; Nieminen, R.; Vuorela, P.; Heinonen, M.; Moilanen, E. Anti-inflammatory effects of flavonoids: Genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-κB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-κB activation along with their inhibitory effect on i. Mediat. Inflamm. 2007, 2007. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, M.; Haque, A.; Banik, N.L.; Nagarkatti, P.; Nagarkatti, M.; Ray, S.K. Estrogen receptor agonists for attenuation of neuroinflammation and neurodegeneration. Brain Res. Bull. 2014, 109, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Dhandapani, K.M.; Brann, D.W. Protective effects of estrogen and selective estrogen receptor modulators in the brain. Biol. Reprod. 2002, 67, 1379–1385. [Google Scholar] [CrossRef] [PubMed]
- Sohanaki, H.; Baluchnejadmojarad, T.; Nikbakht, F.; Roghani, M. Pelargonidin improves memory deficit in amyloid β25–35 rat model of Alzheimer’s disease by inhibition of glial activation, cholinesterase, and oxidative stress. Biomed. Pharmacother. 2016, 83, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Fader, A.J.; Johnson, P.E.M.; Dohanich, G.P. Estrogen improves working but not reference memory and prevents amnestic effects of scopolamine on a radial-arm maze. Pharmacol. Biochem. Behav. 1999, 62, 711–717. [Google Scholar] [CrossRef]
- Schroeter, H.; Boyd, C.; Spencer, J.P.E.; Williams, R.J.; Cadenas, E.; Rice-Evans, C. MAPK signaling in neurodegeneration: Influences of flavonoids and of nitric oxide. Neurobiol. Aging 2002, 23, 861–880. [Google Scholar] [CrossRef]
- Dauer, W.; Przedborski, S. Parkinson’s Disease: Mechanisms and Models. Neuron 2003, 39, 889–909. [Google Scholar] [CrossRef]
- Wu, S.S.; Frucht, S.J. Treatment of Parkinson’s disease: What’s on the Horizon? CNS Drugs 2005, 19, 723–743. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Ichiyanagi, T.; Komiyama, T.; Sato, S.; Konishi, T. Effects of anthocyanins on psychological stress-induced oxidative stress and neurotransmitter status. J. Agric. Food Chem. 2008, 56, 7545–7550. [Google Scholar] [CrossRef] [PubMed]
- Roghani, M.; Niknam, A.; Jalali-Nadoushan, M.R.; Kiasalari, Z.; Khalili, M.; Baluchnejadmojarad, T. Oral pelargonidin exerts dose-dependent neuroprotection in 6-hydroxydopamine rat model of hemi-parkinsonism. Brain Res. Bull. 2010, 82, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Youdim, K.A.; Qaiser, M.Z.; Begley, D.J.; Rice-Evans, C.A.; Abbott, N.J. Flavonoid permeability across an in situ model of the blood-brain barrier. Free Radic. Biol. Med. 2004, 36, 592–604. [Google Scholar] [CrossRef] [PubMed]
- El Mohsen, M.A.; Marks, J.; Kuhnle, G.; Moore, K.; Debnam, E.; Srai, S.K.; Rice-Evans, C.; Spencer, J.P.E. Absorption, tissue distribution and excretion of pelargonidin and its metabolites following oral administration to rats. Br. J. Nutr. 2007, 95, 51–58. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).