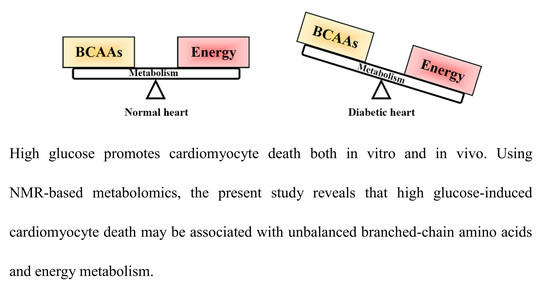
High Glucose-Induced Cardiomyocyte Death May Be Linked to Unbalanced Branched-Chain Amino Acids and Energy Metabolism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Streptozotocin-Induced Diabetic Rat Model
2.3. Heart Tissue Collection and Preparation
2.4. Histopathological Analysis
2.5. Cell Culture and Treatment
2.6. Flow Cytometry Analysis
2.7. Hoechst Staining Analysis
2.8. Intracellular Metabolite Extraction
2.9. Extracellular Metabolite Extraction
2.10. 1H-NMR-Based Metabolomic Analysis
2.11. Multivariate Analysis
2.12. Statistical Analysis
3. Results
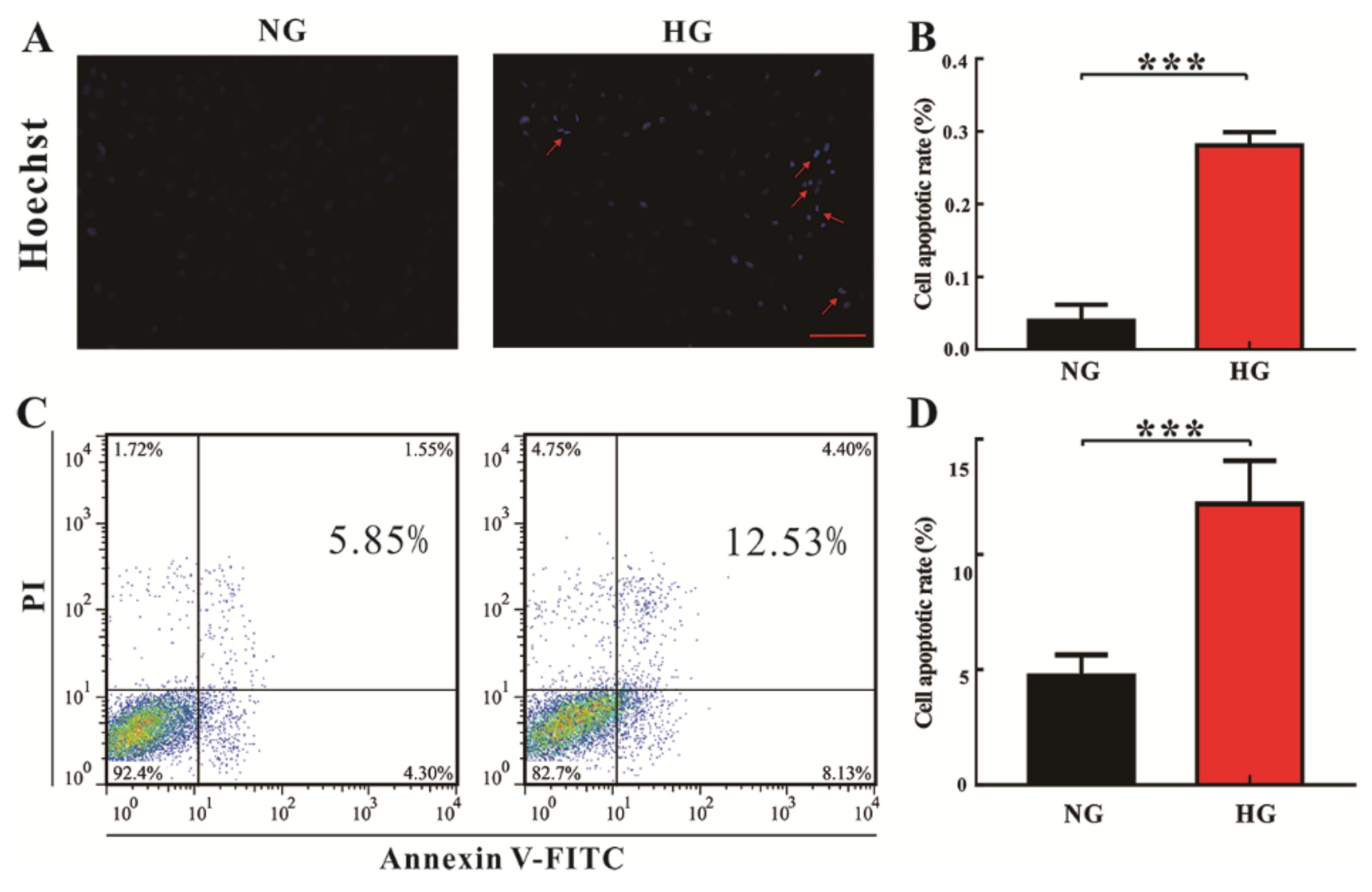
3.1. High Glucose Promotes Cardiomyocyte Death In Vitro and In Vivo
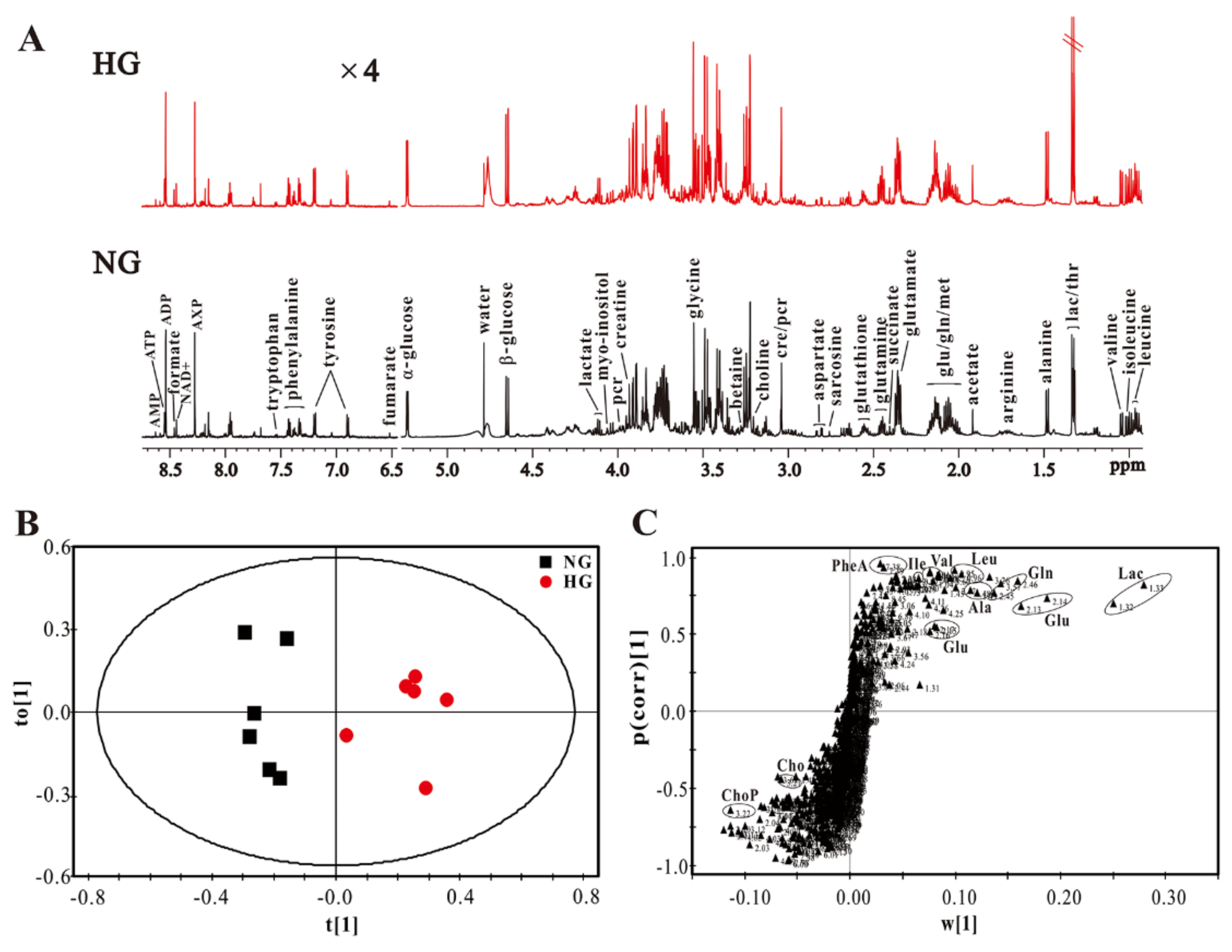
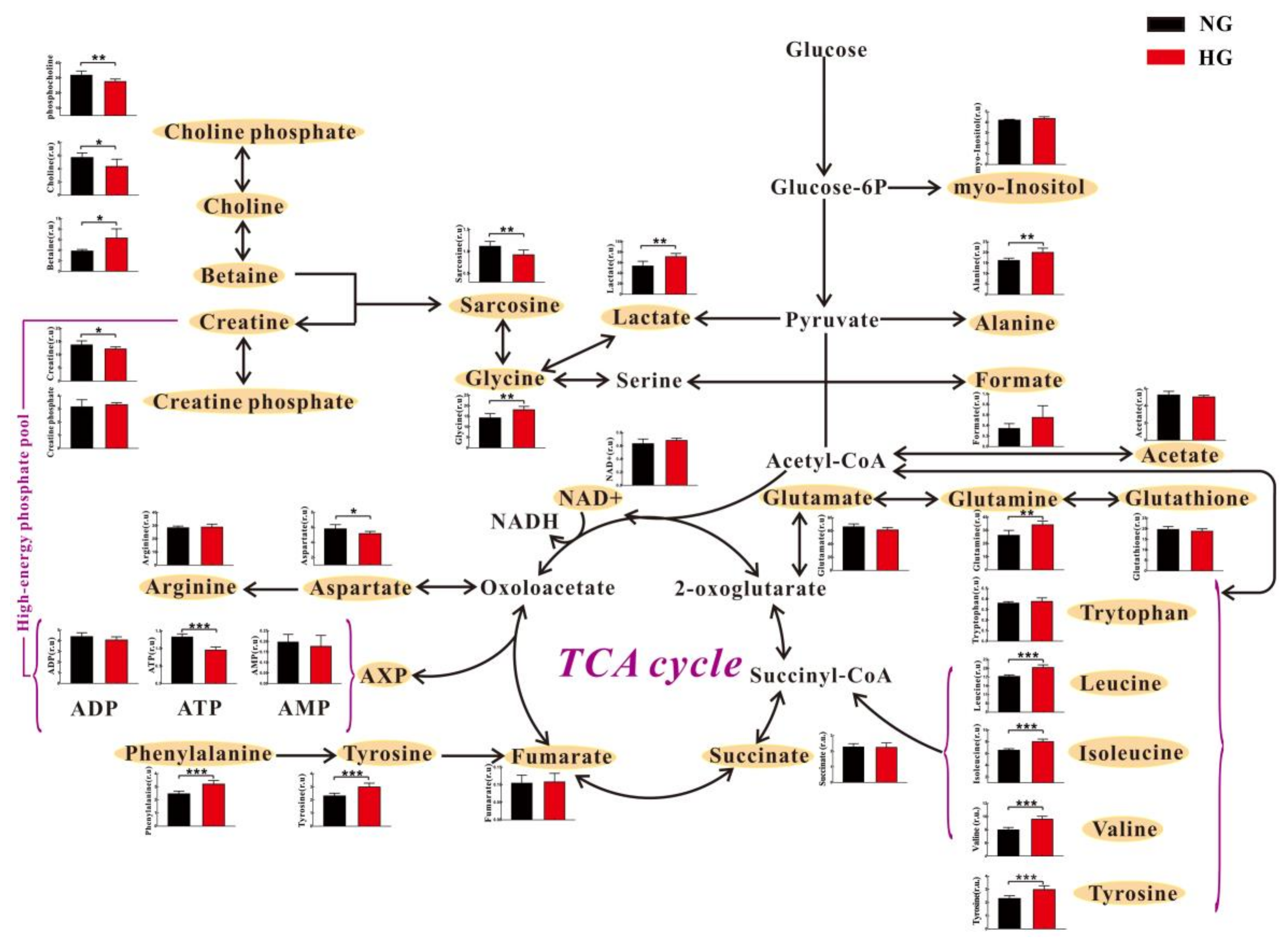
3.2. High Glucose Induces Metabolic Changes in Cardiomyocytes
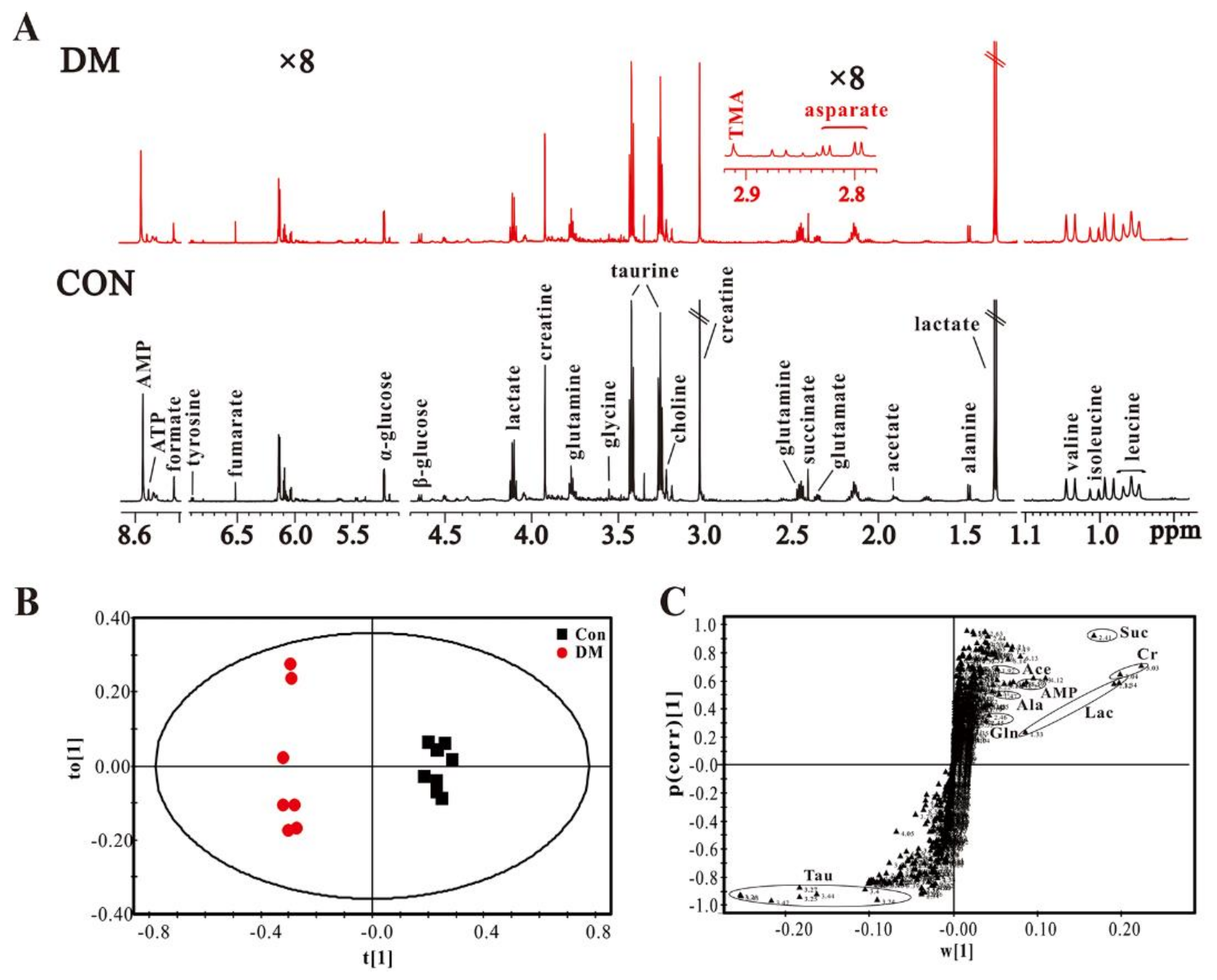
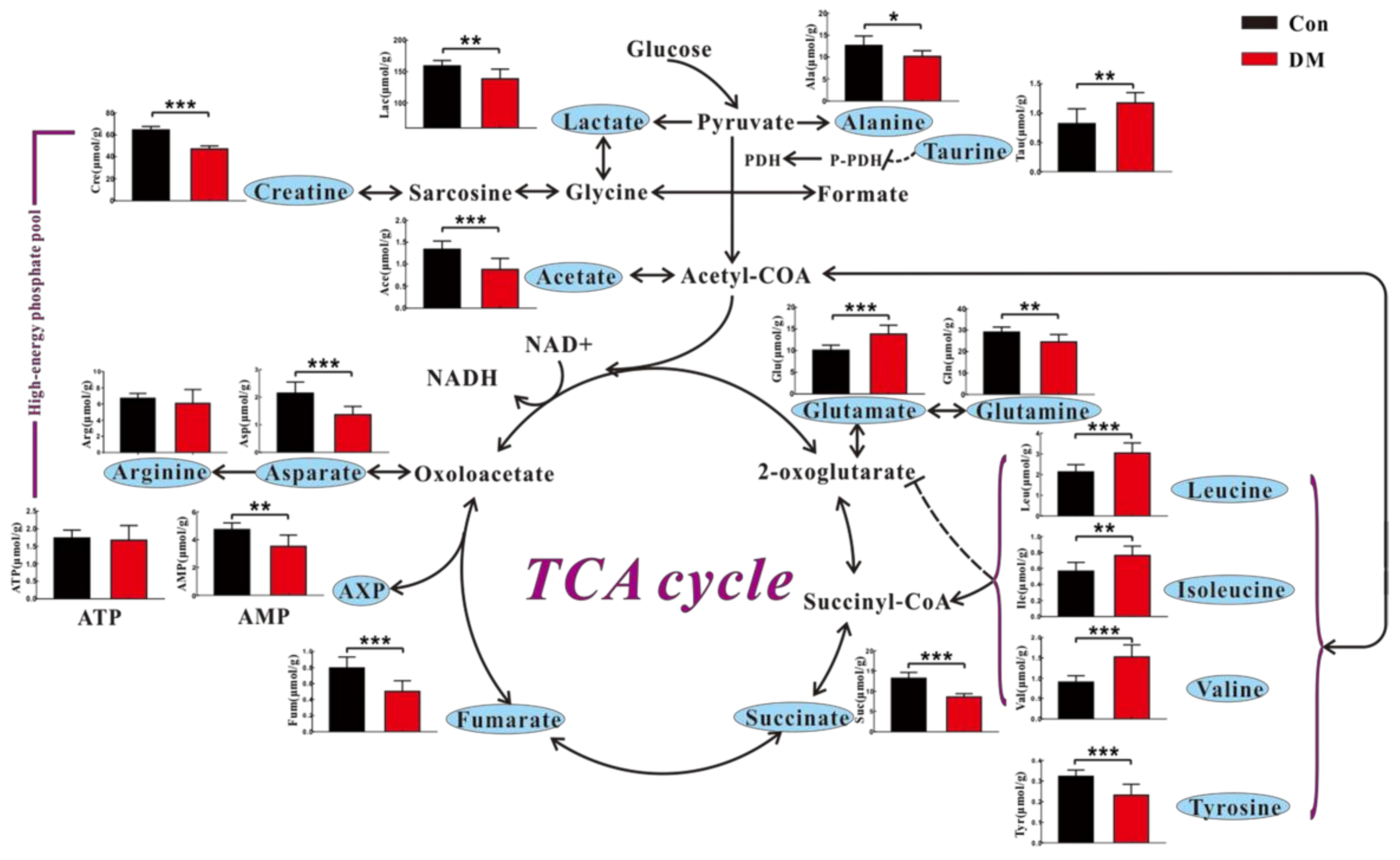
3.3. High Glucose Induces Metabolic Changes in the Heart of Diabetic Rats
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AAAs | aromatic amino acids |
| ADP | adenosine diphosphate |
| AMP | adenosine monophosphate |
| ATP | adenosine triphosphate |
| BCAAs | branch chain amino acids |
| DCM | diabetic cardiomyopathy |
| DM | diabetes mellitus |
| HE | haematoxylin-eosin |
| HG | high glucose |
| NAD+ | nicotinamide adenine dinucleotide |
| NG | normal glucose |
| NMR | nuclear magnetic resonance |
| OPLS-DA | orthogonal partial least squares-discriminant analysis |
| STZ | streptozotocin |
| TCA | tricarboxylic acid |
References
- Rachmiel, M.; Cohen, M.; Heymen, E.; Lezinger, M.; Inbar, D.; Gilat, S.; Bistritzer, T.; Leshem, G.; Kan-Dror, E.; Lahat, E.; et al. Hyperglycemia is associated with simultaneous alterations in electrical brain activity in youths with type 1 diabetes mellitus. Clin. Neurophysiol. 2016, 127, 1188–1195. [Google Scholar] [CrossRef] [PubMed]
- Guariguata, L.; Whiting, D.R.; Hambleton, I.; Beagley, J.; Linnenkamp, U.; Shaw, J.E. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res. Clin. Pract. 2014, 103, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Mathers, C.D.; Loncar, D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006, 3, e442. [Google Scholar] [CrossRef] [PubMed]
- You, Q.; Wu, Z.J.; Wu, B.; Liu, C.; Huang, R.N.; Yang, L.; Guo, R.M.; Wu, K.; Chen, J.F. Naringin protects cardiomyocytes against hyperglycemia-induced injuries in vitro and in vivo. J. Endocrinol. 2016, 230, 197–214. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Mehmood, A.; Anjum, M.S.; Tarrar, M.N.; Khan, S.N.; Riazuddin, S. Diazoxide preconditioning of endothelial progenitor cells from streptozotocin-induced type 1 diabetic rats improves their ability to repair diabetic cardiomyopathy. Mol. Cell. Biochem. 2015, 410, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Dandamudi, S.; Slusser, J.; Mahoney, D.W.; Redfield, M.M.; Rodeheffer, R.J.; Chen, H.H. The prevalence of diabetic cardiomyopathy: A population-based study in olmsted county, minnesota. J. Card. Fail. 2014, 20, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Li, Y.; Wang, L.P.; Kang, Y. Suppression by metallothionein of early-phase myocardial cell death prevents the late development of diabetic cardiomyopathy. Diabetes 2003, 52, A172. [Google Scholar]
- Kobayashi, S.; Xu, X.M.; Chen, K.; Liang, Q.R. Suppression of autophagy is protective in high glucose-induced cardiomyocyte injury. Autophagy 2012, 8, 577–592. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, Y.W.; Feng, Q.P.; Arnold, M.; Peng, T.Q. Calpain activation contributes to hyperglycaemia-induced apoptosis in cardiomyocytes. Cardiovasc. Res. 2009, 84, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Younce, C.W.; Kolattukudy, P.E. MCP-1 causes cardiomyoblast death via autophagy resulting from ER stress caused by oxidative stress generated by inducing a novel zinc-finger protein, MCPIP. Biochem. J. 2010, 426, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.H.; Zhang, T.; Dai, H.Y.; Liu, G.H.; Wang, H.B.; Sun, Y.Y.; Zhang, Y.; Ge, Z.M. Endoplasmic reticulum stress is involved in myocardial apoptosis of streptozocin-induced diabetic rats (retracted article. See vol 207, pg 123, 2010). J. Endocrinol. 2008, 196, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Li, W.; Wang, G.W.; Guo, L.P.; Jiang, Y.C.; Kang, Y.J. Hyperglycemia-induced apoptosis in mouse myocardium—Mitochondrial cytochrome C-mediated caspase-3 activation pathway. Diabetes 2002, 51, 1938–1948. [Google Scholar] [CrossRef] [PubMed]
- Dyntar, D.; Eppenberger-Eberhardt, M.; Maedler, K.; Pruschy, M.; Eppenberger, H.M.; Spinas, G.A.; Donath, M.Y. Glucose and palmitic acid induce degeneration of myofibrils and modulate apoptosis in rat adult cardiomyocytes. Diabetes 2001, 50, 2105–2113. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.P.; Le, B.; Khode, R.; Baker, K.M.; Kumar, R. Intracellular angiotensin ii production in diabetic rats is correlated with cardiomyocyte apoptosis, oxidative stress, and cardiac fibrosis. Diabetes 2008, 57, 3297–3306. [Google Scholar] [CrossRef] [PubMed]
- Gomez, L.; Chavanis, N.; Argaud, L.; Chalabreysse, L.; Gateau-Roesch, O.; Ninet, J.; Ovize, M. Fas-independent mitochondrial damage triggers cardiomyocyte death after ischemia-reperfusion. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H2153–H2158. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.Z.; Sheu, S.S.; Robotham, J.L.; Yoon, Y.S. Mitochondrial fission mediates high glucose-induced cell death through elevated production of reactive oxygen species. Cardiovasc. Res. 2008, 79, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Isfort, M.; Stevens, S.C.W.; Schaffer, S.; Jong, C.J.; Wold, L.E. Metabolic dysfunction in diabetic cardiomyopathy. Heart Fail. Rev. 2014, 19, 35–48. [Google Scholar] [CrossRef] [PubMed]
- An, D.; Rodrigues, B. Role of changes in cardiac metabolism in development of diabetic cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, 1489–1506. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.C.; Chang, C.K.; Ou, H.Y.; Cheng, K.C.; Cheng, J.T. Decrease of peroxisome proliferator-activated receptor delta expression in cardiomyopathy of streptozotocin-induced diabetic rats. Cardiovasc. Res. 2008, 80, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Ussher, J.R. The role of cardiac lipotoxicity in the pathogenesis of diabetic cardiomyopathy. Expert Rev. Cardiovasc. Ther. 2014, 12, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Jang, C.; Oh, S.F.; Wada, S.; Rowe, G.C.; Liu, L.; Chan, M.C.; Rhee, J.; Hoshino, A.; Kim, B.; Ibrahim, A.; et al. A branched-chain amino acid metabolite drives vascular fatty acid transport and causes insulin resistance. Nat. Med. 2016, 22, 421. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhou, M.Y.; Sun, H.P.; Wang, Y.B. Branched-chain amino acid metabolism in heart disease: An epiphenomenon or a real culprit? Cardiovasc. Res. 2011, 90, 220–223. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, M.; Lewis, G.D.; Ericson, U.; Orho-Melander, M.; Hedblad, B.; Engstrom, G.; Ostling, G.; Clish, C.; Wang, T.J.; Gerszten, R.E.; et al. A diabetes-predictive amino acid score and future cardiovascular disease. Eur. Heart J. 2013, 34, 1982–1989. [Google Scholar] [CrossRef] [PubMed]
- Kaddurah-Daouk, R.; Weinshilboum, R.M.; Network, P.R. Pharmacometabolomics: Implications for clinical pharmacology and systems pharmacology. Clin. Pharmacol. Ther. 2014, 95, 154–167. [Google Scholar] [CrossRef] [PubMed]
- Hunter, W.G.; Kelly, J.P.; McGarrah, R.W., 3rd; Kraus, W.E.; Shah, S.H. Metabolic dysfunction in heart failure: Diagnostic, prognostic, and pathophysiologic insights from metabolomic profiling. Curr. Heart Fail. Rep. 2016, 13, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Heather, L.C.; Wang, X.Z.; West, J.A.; Griffin, J.L. A practical guide to metabolomic profiling as a discovery tool for human heart disease. J. Mol. Cell. Cardiol. 2013, 55, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.T.; Zhao, L.C.; Jia, J.M.; Xia, H.H.; Du, Y.; Lin, Q.T.; Lin, X.D.; Ye, X.J.; Yan, Z.H.; Gao, H.C. Metabonomic analysis of potential biomarkers and drug targets involved in diabetic nephropathy mice. Sci. Rep. 2015, 5, 11998. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Zheng, Y.Q.; Zhao, L.C.; Chen, M.J.; Bai, G.H.; Hu, Y.S.; Hu, W.Y.; Yan, Z.H.; Gao, H.C. Cognitive decline in type 2 diabetic db/db mice may be associated with brain region-specific metabolic disorders. BBA Mol. Basis Dis. 2017, 1863, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Lin, Q.T.; Wang, D.; Xu, P.T.; Zhao, L.C.; Hu, W.Y.; Bai, G.H.; Yan, Z.H.; Gao, H.C. NMR-based metabolomics reveals brain region-specific metabolic alterations in streptozotocin-induced diabetic rats with cognitive dysfunction. Metab. Brain Dis. 2017, 32, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.Z.; Tian, X.Q.; Zhang, M.; Cai, L.; Ru, A.; Shen, X.T.; Jiang, X.; Jin, R.R.; Zheng, L.; Hawkins, K.; et al. Functional and pathological improvements of the hearts in diabetes model by the combined therapy of bFGF-loaded nanoparticles with ultrasound-targeted microbubble destruction. J. Control. Release 2014, 186, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.T.; Zhen, J.; Yang, Y.; Gu, J.N.; Wu, S.S.; Liu, Q. Ginsenoside Rg1 ameliorates diabetic cardiomyopathy by inhibiting endoplasmic reticulum stress-induced apoptosis in a streptozotocin-induced diabetes rat model. J. Cell. Mol. Med. 2016, 20, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.; Dludla, P.; Joubert, E.; February, F.; Mazibuko, S.; Ghoor, S.; Muller, C.; Louw, J. Aspalathin, a dihydrochalcone C-glucoside, protects H9c2 cardiomyocytes against high glucose induced shifts in substrate preference and apoptosis. Mol. Nutr. Food Res. 2016, 60, 922–934. [Google Scholar] [CrossRef] [PubMed]
- Diao, J.; Wei, J.; Yan, R.; Liu, X.; Li, Q.; Lin, L.; Zhu, Y.; Li, H. Rosmarinic acid suppressed high glucose-induced apoptosis in H9c2 cells by ameliorating the mitochondrial function and activating STAT3. Biochem. Biophys. Res. Commun. 2016, 477, 1024–1030. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, C.; Wang, T.; Xuan, J.; Su, C.; Wang, Y. Heme oxygenase 1 induction protects myocardiac cells against hypoxia/reoxygenation-induced apoptosis: The role of JNK/c-JUN/caspase-3 inhibition and akt signaling enhancement. Herz 2016, 41, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.J.; Zheng, H.; Wei, T.T.; Wang, D.; Xia, H.H.; Zhao, L.C.; Ji, J.S.; Gao, H.C. High glucose-induced PC12 cell death by increasing glutamate production and decreasing methyl group metabolism. BioMed Res. Int. 2016. [Google Scholar] [CrossRef] [PubMed]
- Miccheli, A.; Ricciolini, R.; Piccolella, E.; Delfini, M.; Conti, F. Modulation of human lymphoblastoid B cell line by phorbol ester and sphingosine. A 31P-NMR study. Biochim. Biophys. Acta 1991, 1093, 29–35. [Google Scholar] [CrossRef]
- Liu, K.; Ye, X.J.; Hu, W.Y.; Zhang, G.Y.; Bai, G.H.; Zhao, L.C.; He, J.W.; Zhu, H.; Shao, J.B.; Yan, Z.H.; et al. Neurochemical changes in the rat occipital cortex and hippocampus after repetitive and profound hypoglycemia during the neonatal period: An ex vivo 1H magnetic resonance spectroscopy study. Mol. Neurobiol. 2013, 48, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Savorani, F.; Tomasi, G.; Engelsen, S.B. Icoshift: A versatile tool for the rapid alignment of 1D NMR spectra. J. Magn. Reson. 2010, 202, 190–202. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Jewison, T.; Guo, A.C.; Wilson, M.; Knox, C.; Liu, Y.F.; Djoumbou, Y.; Mandal, R.; Aziat, F.; Dong, E.; et al. HMDB 3.0—The human metabolome database in 2013. Nucleic Acids Res. 2013, 41, D801–D807. [Google Scholar] [CrossRef] [PubMed]
- Bollard, M.E.; Murray, A.J.; Clarke, K.; Nicholson, J.K.; Griffin, J.L. A study of metabolic compartmentation in the rat heart and cardiac mitochondria using high-resolution magic angle spinning 1H-NMR spectroscopy. FEBS. Lett. 2003, 553, 73–78. [Google Scholar] [CrossRef]
- Eriksson, L.; Trygg, J.; Wold, S. CV-ANOVA for significance testing of PLS and OPLS® models. J. Chemom. 2008, 22, 594–600. [Google Scholar] [CrossRef]
- Ventura-Clapier, R.; Garnier, A.; Veksler, V. Energy metabolism in heart failure. J. Physiol. Lond. 2004, 555, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Stanley, W.C.; Chandler, M.P.; Huang, H.; McElfresh, T. Dobutamine plus flow restriction stimulates nonoxidative glycolysis despite no change in myocardial oxygen consumption or fatty acid uptake. FASEB J. 2002, 16, A32. [Google Scholar]
- Chatham, J.C.; Gao, Z.P.; Bonen, A.; Forder, J.R. Preferential inhibition of lactate oxidation relative to glucose oxidation in the rat heart following diabetes. Cardiovasc. Res. 1999, 43, 96–106. [Google Scholar] [CrossRef]
- Pointon, A.V.; Walker, T.M.; Phillips, K.M.; Luo, J.; Riley, J.; Zhang, S.D.; Parry, J.D.; Lyon, J.J.; Marczylo, E.L.; Gant, T.W. Doxorubicin in vivo rapidly alters expression and translation of myocardial electron transport chain genes, leads to ATP loss and caspase 3 activation. PLoS ONE 2010, 5, e12733. [Google Scholar] [CrossRef] [PubMed]
- Beer, M.; Seyfarth, T.; Sandstede, J.; Landschutz, W.; Lipke, C.; Kostler, H.; von Kienlin, M.; Harre, K.; Hahn, D.; Neubauer, S. Absolute concentrations of high-energy phosphate metabolites in normal, hypertrophied, and failing human myocardium measured noninvasively with 31P-SLOOP magnetic resonance spectroscopy. J. Am. Coll. Cardiol. 2002, 40, 1267–1274. [Google Scholar] [CrossRef]
- Gowda, G.A.N.; Abell, L.; Lee, C.F.; Tian, R.; Raftery, D. Simultaneous analysis of major coenzymes of cellular redox reactions and energy using ex vivo 1H-NMR spectroscopy. Anal. Chem. 2016, 88, 4817–4824. [Google Scholar] [CrossRef] [PubMed]
- Zervou, S.; Whittington, H.J.; Russell, A.J.; Lygate, C.A. Augmentation of creatine in the heart. Mini Rev. Med. Chem. 2016, 16, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Nascimben, L.; Ingwall, J.S.; Pauletto, P.; Friedrich, J.; Gwathmey, J.K.; Saks, V.; Pessina, A.C.; Allen, P.D. Creatine kinase system in failing and nonfailing human myocardium. Circulation 1996, 94, 1894–1901. [Google Scholar] [CrossRef] [PubMed]
- Neubauer, S. Mechanisms of disease—The failing heart—An engine out of fuel. N. Engl. J. Med. 2007, 356, 1140–1151. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.D.; Zhao, L.C.; Tang, S.L.; Zhou, Q.; Lin, Q.T.; Li, X.K.; Zheng, H.; Gao, H.C. Metabolic effects of basic fibroblast growth factor in streptozotocin-induced diabetic rats: A 1H-NMR-based metabolomics investigation. Sci. Rep. 2016, 6, 36474. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, J.; Tatsumi, T.; Keira, N.; Akashi, K.; Mano, A.; Yamanaka, S.; Matoba, S.; Asayama, J.; Yaoi, T.; Fushiki, S.; et al. Important role of energy-dependent mitochondrial pathways in cultured rat cardiac myocyte apoptosis. Am. J. Physiol. Heart Circ. Physiol. 2001, 281, H1637–H1647. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, Y.; Shimizu, S.; Tsujimoto, Y. Intracellular ATP levels determine cell death fate by apoptosis or necrosis. Cancer Res. 1997, 57, 1835–1840. [Google Scholar] [PubMed]
- Leist, M.; Single, B.; Castoldi, A.F.; Kuhnle, S.; Nicotera, P. Intracellular adenosine triphosphate (ATP) concentration: A switch in the decision between apoptosis and necrosis. J. Exp. Med. 1997, 185, 1481–1486. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.G.; Buel, G.R.; Blenis, J. Nutrient regulation of the mTOR complex 1 signaling pathway. Mol. Cells 2013, 35, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Birsoy, K.; Wang, T.; Chen, W.W.; Freinkman, E.; Abu-Remaileh, M.; Sabatini, D.M. An essential role of the mitochondrial electron transport chain in cell proliferation is to enable aspartate synthesis. Cell 2015, 162, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, R.; Babu, P.V.A.; Shyamaladevi, C.S. Aspartate and glutamate prevents isoproterenol-induced cardiac toxicity by alleviating oxidative stress in rats. Exp. Toxicol. Pathol. 2011, 63, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, R.; Babu, P.V.A.; Shyamaladevi, C.S. Protective effect of aspartate and glutamate on cardiac mitochondrial function during myocardial infarction in experimental rats. Chem. Biol. Interact. 2008, 176, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Wurtz, P.; Soininen, P.; Kangas, A.J.; Ronnemaa, T.; Lehtimaki, T.; Kahonen, M.; Viikari, J.S.; Raitakari, O.T.; Ala-Korpela, M. Branched-chain and aromatic amino acids are predictors of insulin resistance in young adults. Diabetes Care 2013, 36, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Ridgway, N.D. The role of phosphatidylcholine and choline metabolites to cell proliferation and survival. Crit. Rev. Biochem. Mol. 2013, 48, 20–38. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.F.; Lin, H.X.; Xu, C.Q.; Liu, Y.; Wang, H.Z.; Han, H.; Wang, Z.G. Choline produces cytoprotective effects against ischemic myocardial injuries: Evidence for the role of cardiac M3 subtype muscarinic acetylcholine receptors. Cell. Physiol. Biochem. 2005, 16, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Repetto, M.G.; Ossani, G.; Monserrat, A.J.; Boveris, A. Oxidative damage: The biochemical mechanism of cellular injury and necrosis in choline deficiency. Exp. Mol. Pathol. 2010, 88, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, B.; Buddhan, S.; Anandan, R.; Sivakumar, R.; AnbinEzhilan, R. Antioxidant defense of betaine against isoprenaline-induced myocardial infarction in rats. Mol. Biol. Rep. 2010, 37, 1319–1327. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H. Metabolic crosstalk between choline/1-carbon metabolism and energy homeostasis. Clin. Chem. Lab. Med. 2013, 51, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, S.W.; Jong, C.J.; Ramila, K.C.; Azuma, J. Physiological roles of taurine in heart and muscle. J. Biomed. Sci. 2010, 17, S2. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.G.; Li, W.; Lu, X.H.; Zhao, X.; Xu, L. Taurine attenuates oxidative stress and alleviates cardiac failure in type I diabetic rats. Croat. Med. J. 2013, 54, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Cao, L.S.; Zeng, Q.T.; Liu, X.Q.; Zhang, Y.Z.; Dai, T.R.; Hu, D.N.; Huang, K.; Wang, Y.; Wang, X.; et al. Taurine may prevent diabetic rats from developing cardiomyopathy also by downregulating angiotensin II type2 receptor expression. Cardiovasc. Drug Ther. 2005, 19, 105–112. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Lin, Q.; Chen, J.; Wei, T.; Li, C.; Zhao, L.; Gao, H.; Zheng, H. High Glucose-Induced Cardiomyocyte Death May Be Linked to Unbalanced Branched-Chain Amino Acids and Energy Metabolism. Molecules 2018, 23, 807. https://doi.org/10.3390/molecules23040807
Zhang X, Lin Q, Chen J, Wei T, Li C, Zhao L, Gao H, Zheng H. High Glucose-Induced Cardiomyocyte Death May Be Linked to Unbalanced Branched-Chain Amino Acids and Energy Metabolism. Molecules. 2018; 23(4):807. https://doi.org/10.3390/molecules23040807
Chicago/Turabian StyleZhang, Xi, Qiuting Lin, Jiuxia Chen, Tingting Wei, Chen Li, Liangcai Zhao, Hongchang Gao, and Hong Zheng. 2018. "High Glucose-Induced Cardiomyocyte Death May Be Linked to Unbalanced Branched-Chain Amino Acids and Energy Metabolism" Molecules 23, no. 4: 807. https://doi.org/10.3390/molecules23040807
APA StyleZhang, X., Lin, Q., Chen, J., Wei, T., Li, C., Zhao, L., Gao, H., & Zheng, H. (2018). High Glucose-Induced Cardiomyocyte Death May Be Linked to Unbalanced Branched-Chain Amino Acids and Energy Metabolism. Molecules, 23(4), 807. https://doi.org/10.3390/molecules23040807