Multifunctional Fischer Aminocarbene Complexes as Hole or Electron Transporting Layers in Organic Solar Cells
Abstract
1. Introduction
2. Results and Discussion
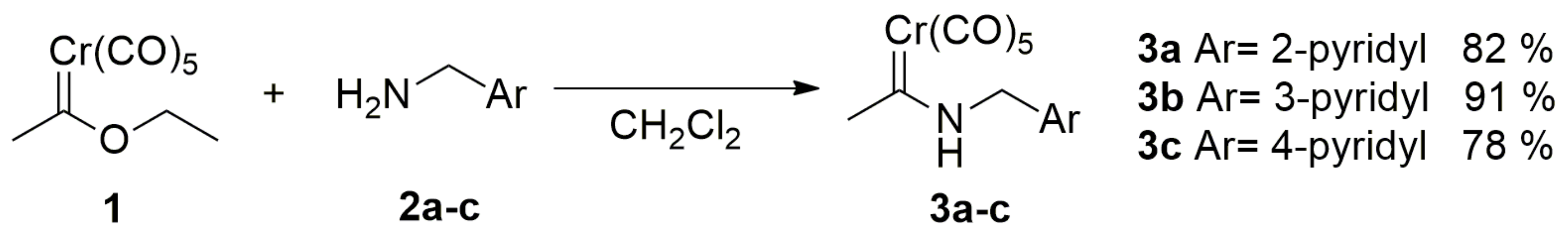
2.1. Synthesis of the Organometallic Precursors
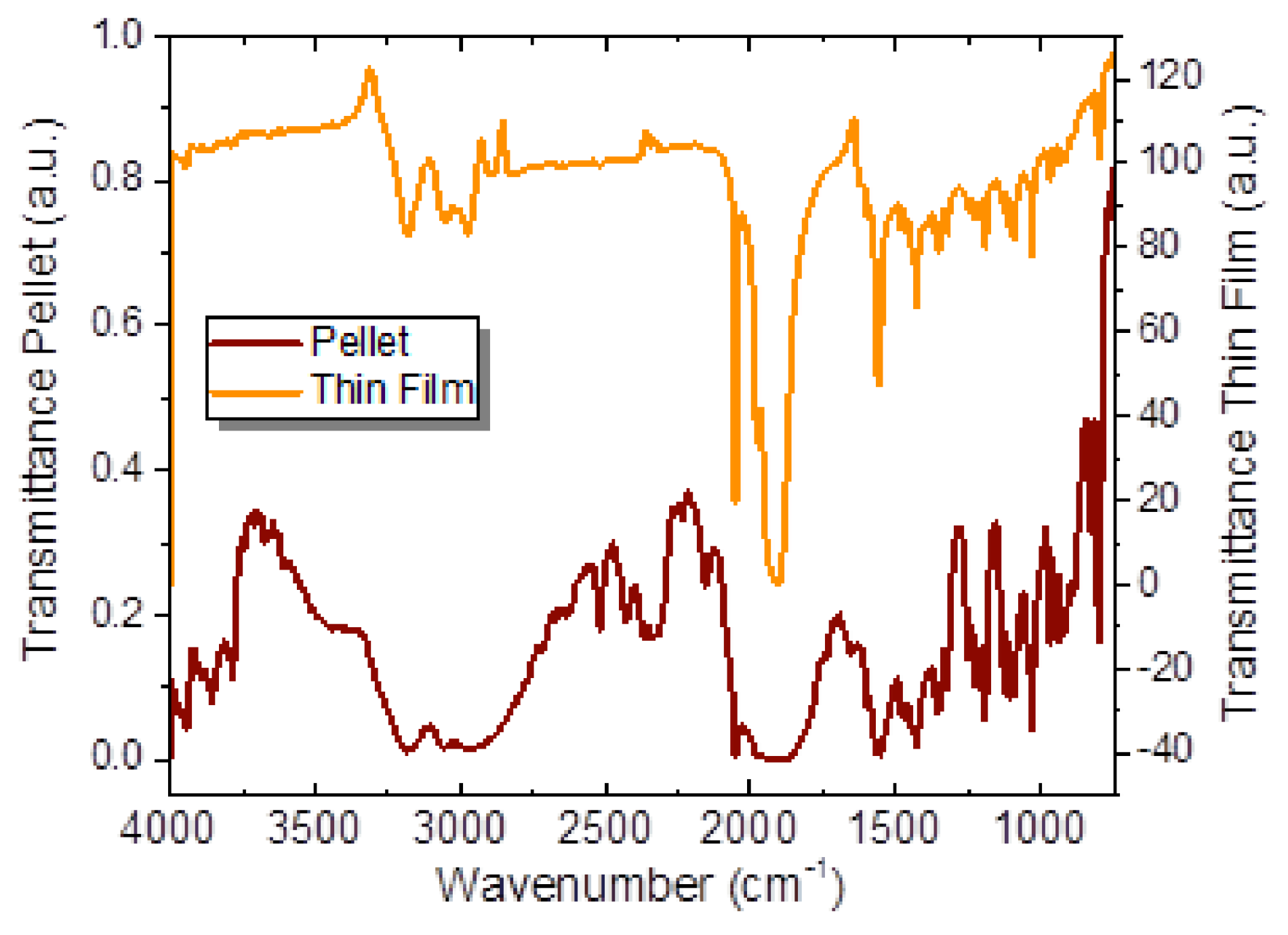
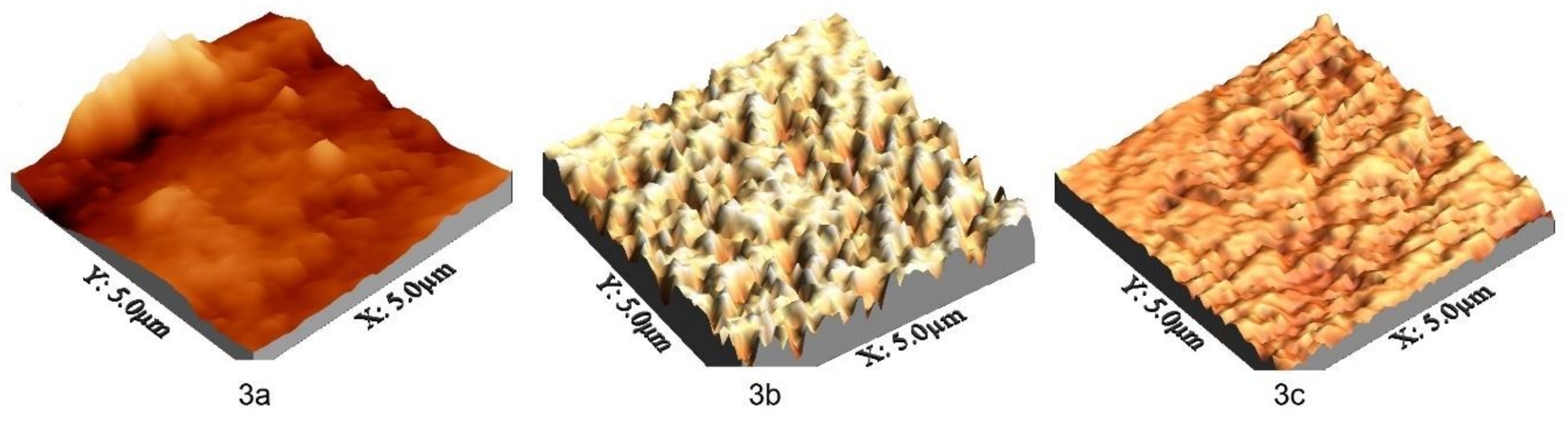
2.2. Preparation and Characterization of the Aminocarbene Thin Films
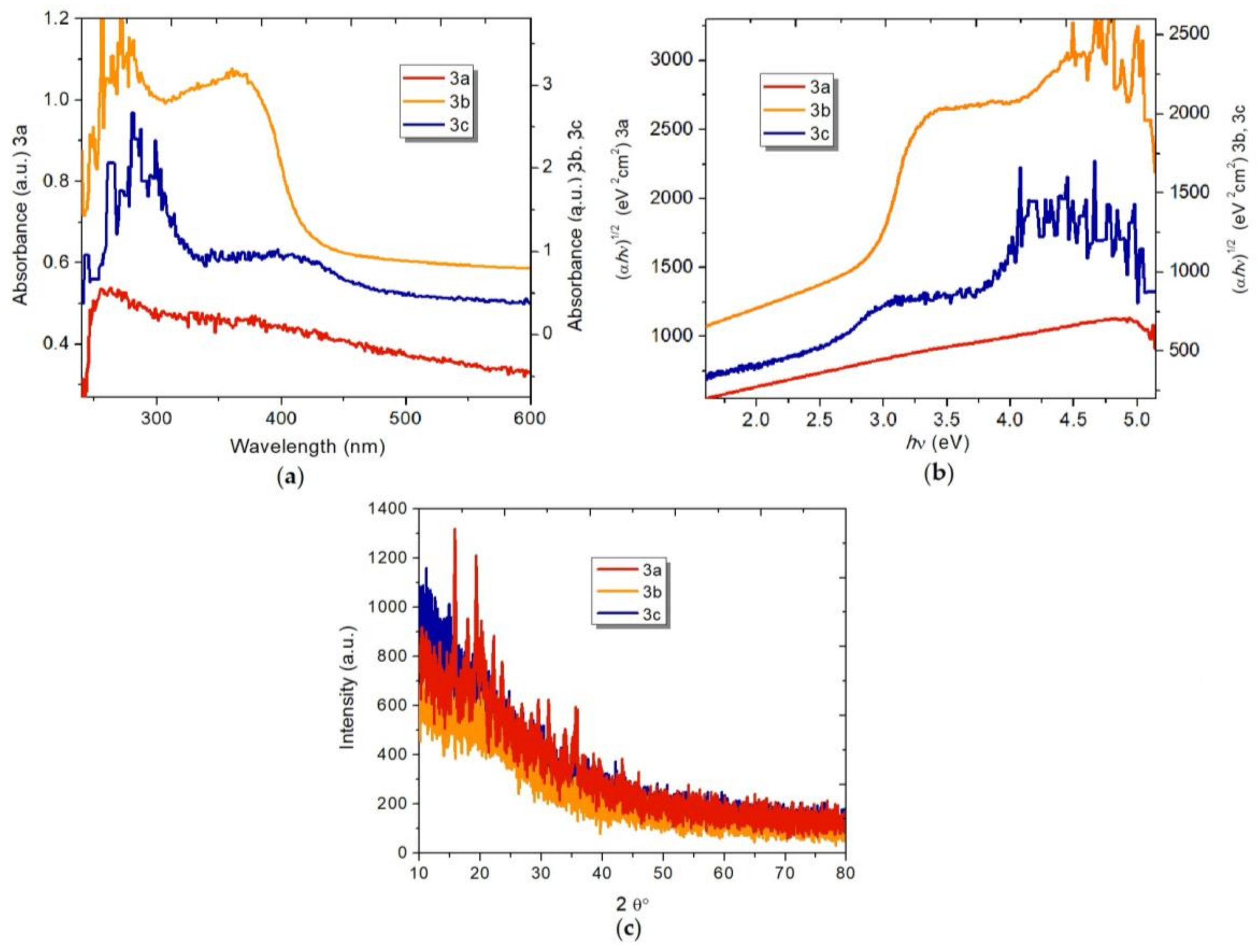
2.3. Optical Properties of the Fischer Aminocarbene Films
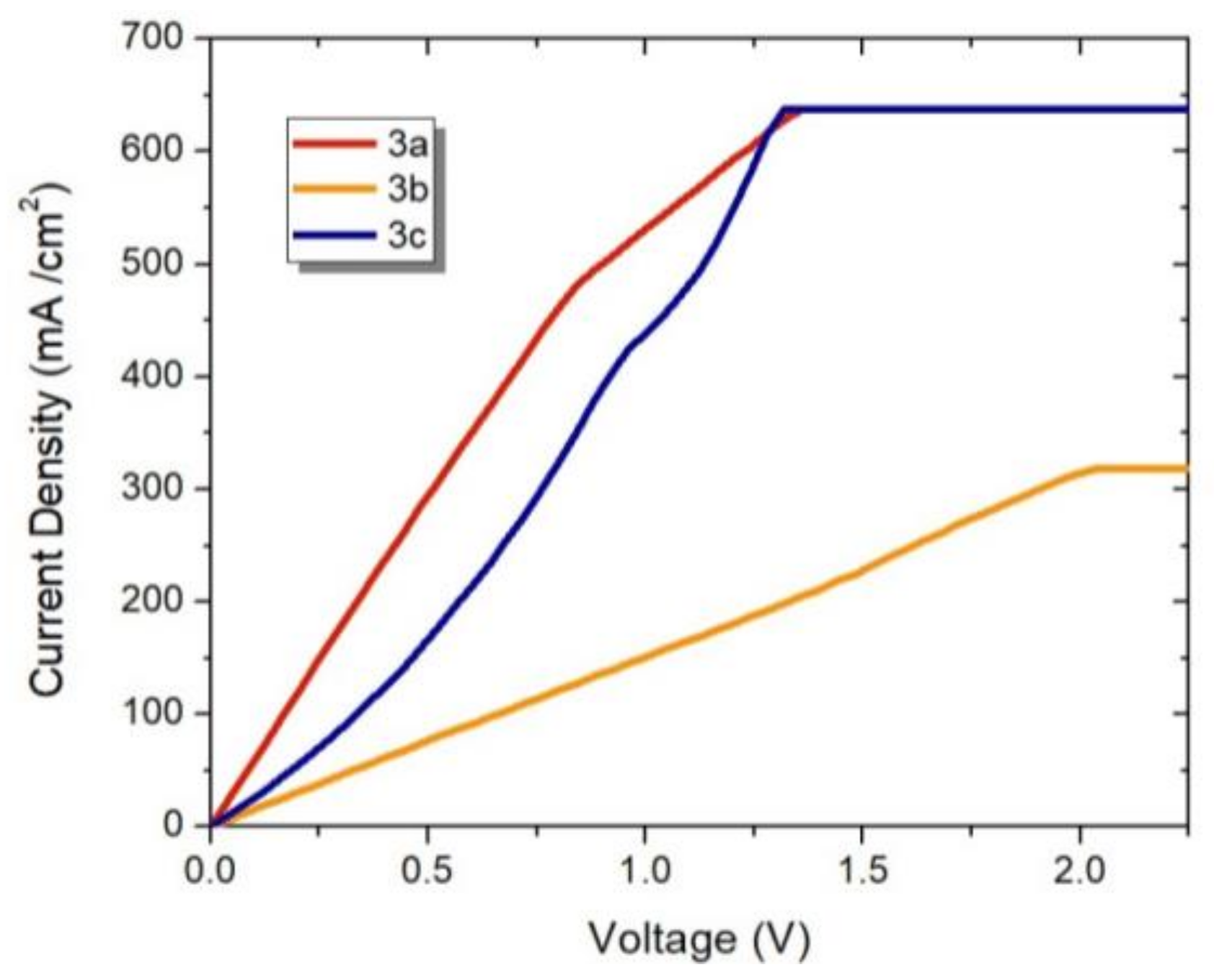
2.4. Electrical Properties of the Fischer Aminocarbene Films
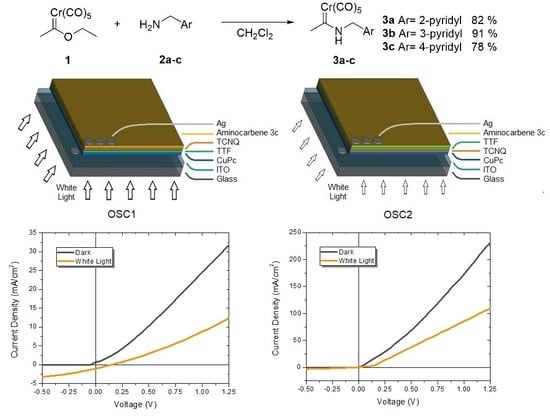
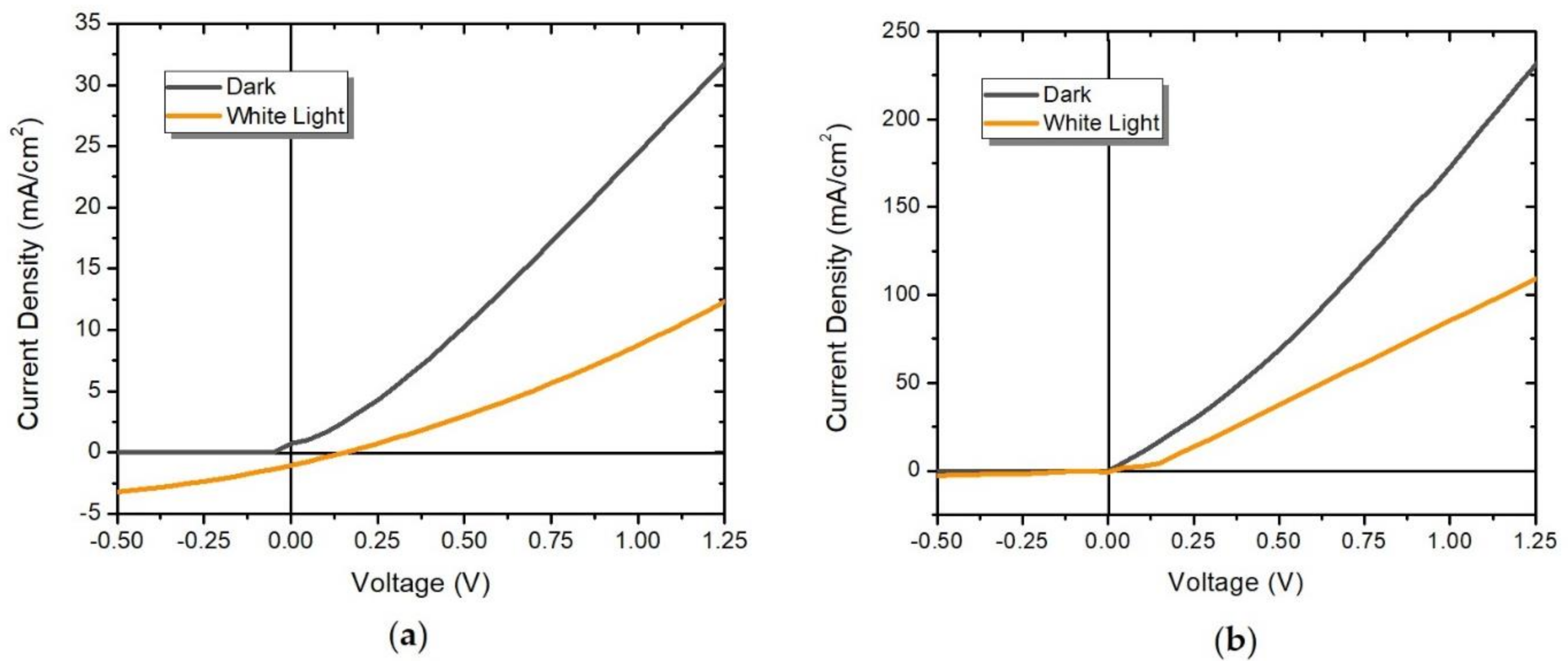
2.5. Electrical Properties of the OSCs
3. Experimental
3.1. General Information
3.2. Synthesis of Pyridyl(aminocarbene)chromium(0) Complexes 3a–c
3.2.1. [(Methyl)(pyridin-2-ylmethanamine)methylidene] Pentacarbonyl Chromium (0), 3a
3.2.2. [(Methyl)(pyridin-3-ylmethanamine)methylidene] Pentacarbonyl Chromium (0), 3b
3.2.3. [(Methyl)(pyridin-4-ylmethanamine)methylidene] Pentacarbonyl Chromium (0), 3c
3.3. Thin Film and Device Manufacture
3.4. Characterization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bajenescu, T.M.I. Present and Future of Photovoltaics. Electroteh. Electron. Autom. 2015, 63, 31–38. [Google Scholar]
- Más-Montoya, M. Synthesis of New Heteroacene Systems and Study of Their Properties as Organic Semiconductors for Their Application in Molecular Electronics. Master’s Thesis, Faculty of Chemistry, University of Murcia, Murcia, Spain, 2015. [Google Scholar]
- Mirsafaei, M.; Fallahpour, A.H.; Lugli, P.; Rubahn, H.G.; Adam, J.; Madsen, M. The influence of electrical effects on device performance of organic solar cells with nano-structured electrodes. Sci. Rep. 2017, 7, 5300. [Google Scholar] [CrossRef] [PubMed]
- Polman, A.; Knight, M.; Garnett, E.C.; Ehrler, B.; Sinke, K.C. Photovoltaic materials: Present efficiencies and future challenges. Science 2016, 352, 307. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Lee, T.W.; Chin, B.D.; Wang, D.H.; Park, O.O. Roles of Interlayers in Efficient Organic Photovoltaic Devices. Macromol. Rapid Commun. 2010, 31, 2095–2108. [Google Scholar] [CrossRef] [PubMed]
- Yoo, I.; Lee, M.; Lee, C.; Kim, D.W.; Moon, I.S.; Hwang, D.H. The effect of a buffer layer on the photovoltaic properties of solar cells with P3OT: Fullerene composites. Synth. Met. 2005, 153, 97–100. [Google Scholar] [CrossRef]
- Sánchez-Vergara, M.E.; Monserrat, D.; Vidal-García, P.; Jiménez-Jarquín, C.; Hernandez-García, A.; Jiménez-Sandoval, O. Preparation of hybrid devices containing nylon/M(II)Pc-TTF (M = Cu, Zn) films with potential optical and electrical applications. Electron. Mater. Lett. 2017, 13, 191–200. [Google Scholar] [CrossRef]
- Wang, D.L.; Cui, H.J.; Hou, G.J.; Zhu, Z.G.; Yan, Q.B.; Su, G. Highly efficient light management for perovskite solar cells. Sci. Rep. 2016, 6, 18922. [Google Scholar] [CrossRef] [PubMed]
- Dibb, G.F.A.; Muth, M.A.; Kirchatrz, T.; Engmann, S.; Hoppe, H.; Gobsch, G.; Thelakkat, M.; Blouin, N.; Tierney, S.; Carrasco-Orozco, M.; et al. Influence of doping on charge carrier collection in normal and inverted geometry polymer: Fullerene solar cells. Sci. Rep. 2013, 3, 3335. [Google Scholar] [CrossRef]
- Sánchez-Vergara, M.E.; Leyva-Esqueda, E.A.; Alvarez, C.; López, M.; Miralrio, A.; Salcedo, R. Influence of TCNQ acceptor on optical and electrical properties of tetrasubstituted allenes films fabricated by vacuum thermal evaporation. J. Mater. Sci. Mater. Electron. 2016, 27, 9900–9910. [Google Scholar] [CrossRef]
- Li, N.; Baran, D.; Forberich, K.; Machui, F.; Ameri, T.; Turbiez, M.; Carrasco-Orozco, M.; Drees, M.; Facchetti, A.; Krebs, F.C.; et al. Towards 15% energy conversion efficiency: A systematic study of the solution-processed organic tandem solar cells based on commercially available materials. Energy Environ. Sci. 2013, 6, 3407–3413. [Google Scholar] [CrossRef]
- Feng, Z.; Hou, Y.; Lei, D. The influence of electrode buffer layers on the performance of polymer photovoltaic devices. Renew. Energy 2010, 35, 1175–1178. [Google Scholar] [CrossRef]
- Chaudhary, S.; Lu, H.; Müller, A.M.; Bardeen, C.J.; Ozkan, M. Hierarchical Placement and Associated Optoelectronic Impact of Carbon Nanotubes in Polymer-Fullerene Solar Cells. Nano Lett. 2007, 7, 1973–1979. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Yu, Z.; Zhang, Y.; Lai, J.; Li, J.; Gurzadyan, G.G.; Yang, X.; Sun, L. High-Performance Regular Perovskite Solar Cells Employing Low-Cost Poly(ethylenedioxythiophene) as a Hole-Transporting Material. Sci. Rep. 2017, 7, 42564. [Google Scholar] [CrossRef] [PubMed]
- Gommans, H.; Verreet, B.; Rand, B.P.; Muller, R.; Poortmans, J.; Heremans, P.; Genoe, J. On the Role of Bathocuproine in Organic Photovoltaic Cells. Adv. Funct. Mater. 2008, 18, 3686–3691. [Google Scholar] [CrossRef]
- Peumans, P.; Bulović, V.; Forrest, S.R. Efficient photon harvesting at high optical intensities in ultrathin organic double-heterostructure photovoltaic diodes. Appl. Phys. Lett. 2000, 76, 2650–2652. [Google Scholar] [CrossRef]
- Fischer, E.O.; Maasböl, A. On the Existence of a Tungsten Carbonyl Carbene Complex. Angew. Chem. Int. Ed. 1964, 3, 580–581. [Google Scholar] [CrossRef]
- Raubenheimer, H.G. Fischer carbene complexes remain favourite targets, and vehicles for new discoveries. Dalton Trans. 2014, 43, 16959–16973. [Google Scholar] [CrossRef] [PubMed]
- Dötz, K.H.; Stendel, J. Fischer Carbene Complexes in Organic Synthesis: Metal-Assisted and Metal-Templated Reactions. Chem. Rev. 2009, 109, 3227–3274. [Google Scholar] [CrossRef] [PubMed]
- Barluenga, J.; Santamaría, J.; Tomás, M. Synthesis of Heterocycles via Group VI Fischer Carbene Complexes. Chem. Rev. 2004, 104, 2259–2284. [Google Scholar] [CrossRef] [PubMed]
- Barluenga, J.; Aguilar, E. Chapter One-Group 6 Metal Fischer Carbene Complexes: Versatile Synthetic Building Blocks. Adv. Organomet. Chem. 2017, 67, 1–150. [Google Scholar] [CrossRef]
- Sierra, M.A. Di- and Polymetallic Heteroatom Stabilized (Fischer) Metal Carbene Complexes. Chem. Rev. 2000, 100, 3591–3638. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Gallego, M.; Mancheño, M.J.; Sierra, M.A. Catalytic Transmetalation from Group 6 Fischer Carbene Complexes: An Emerging Powerful Tool in Organic Synthesis. Acc. Chem. Res. 2005, 38, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Fernández, I.; Cossío, F.P.; Sierra, M.A. Photochemistry of Group 6 Fischer Carbene Complexes: Beyond the Photocarbonylation Reaction. Acc. Chem. Res. 2011, 44, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Chu, G.M.; Fernández, I.; Sierra, M.A. Control over the E/Z Selectivity of the Catalytic Dimerization of Group 6 (Fischer) Metal Carbene Complexes. J. Org. Chem. 2013, 78, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Chu, G.M.; Guerrero-Martínez, A.; Ramírez de Arellano, C.; Fernández, I.; Sierra, M.A. Remote Control by π-Conjugation of the Emissive Properties of Fischer Carbene-BODIPY Dyads. Inorg. Chem. 2016, 55, 2737–2747. [Google Scholar] [CrossRef] [PubMed]
- Chu, G.M.; Guerrero-Martínez, A.; Fernandez, I.; Sierra, M.A. Tuning the Photophysical Properties of BODIPY Molecules by π-Conjugation with Fischer Carbene Complexes. Chem. Eur. J. 2014, 20, 1367–1375. [Google Scholar] [CrossRef] [PubMed]
- Sierra, M.A.; Gómez-Gallego, M.; Martínez-Álvarez, A. Fischer Carbene Complexes: Beautiful Playgrounds to Study Single Electron Transfer (SET) Reactions. Chem. Eur. J. 2007, 13, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Landman, M.; Fraser, R.; Twigge, L.; Conradie, J. Fischer aminocarbene conformers containing a 2-thienyl or 2-furyl ring: A crystallographic, NMR, and DFT study. Coord. Chem. 2015, 68, 2388–2408. [Google Scholar] [CrossRef]
- Landman, M. A DFT and structural investigation of the conformations of Fischer carbene complexes. J. Phys. Conf. Ser. 2015, 633, 012051. [Google Scholar] [CrossRef]
- Sánchez-Vergara, M.E.; Ortiz, A.; Álvarez-Toledano, C.; Moreno, A.; Alvarez, J.R. Thin films of molecular materials synthesized from fisher’s carbene ferrocenyl: Film formation and electrical properties. Thin Solid Films 2008, 516, 6382–6387. [Google Scholar] [CrossRef]
- Denise, B.; Dubost, P.; Parlier, A.; Rudler, M.; Rudler, H.; Daran, J.C.; Vaissermann, J.; Delgado, F.; Arevalo, A.R.; Toscano, R.A.; et al. Preparation, structure and reactivity of aminocarbene complexes of chromium and molybdenum derived from primary amines. J. Organomet. Chem. 1991, 418, 377–393. [Google Scholar] [CrossRef]
- Kim, S.J.; Matsumoto, M.; Shigehara, K. Synthesis and electrical properties of one-dimensional octacyanometallophthalocyanine (M ≡ Fe, Co.) polymers. J. Porphyr. Phthalocyanines 2000, 4, 136–144. [Google Scholar] [CrossRef]
- Hünig, S. N,N′-dicyanoquinone diimines (DCNQIs): Unique acceptors for conducting materials. J. Mater. Chem. 1995, 5, 1469–1479. [Google Scholar] [CrossRef]
- Simon, J.; Tournilhac, F.; André, J.J. Molecular materials. II. Towards electronics finalities. New J. Chem. 1987, 11, 383–399. [Google Scholar]
- Kayunkid, N.; Tammarugwattana, N.; Mano, K.; Rangkasikorn, A.; Nukeaw, J. Growth and characterizations of tin-doped nickel-phthalocyanine thin film prepared by thermal co-evaporation as a novel nanomaterial. Surf. Coat. Technol. 2016, 306, 101–105. [Google Scholar] [CrossRef]
- Thomas, G. Materials science: Invisible circuits. Nature 1997, 389, 907–908. [Google Scholar] [CrossRef]
- Facchetti, A.; Marks, T.J. Transparent Electronics: From Synthesis to Applications; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2010. [Google Scholar]
- Cody, G.D. Hydrogenated amorphous silicon, Part B: Optical Properties. In Semiconductors and Semimetals, 1st ed.; Pankove, J.I., Ed.; Academic Press: Orlando, FL, USA, 1984; Volume 21, p. 299. ISBN 0-12-752150-X. [Google Scholar]
- Qi, B.; Wang, J. Fill factor in organic solar cells. Phys. Chem. Chem. Phys. 2013, 15, 8972–8982. [Google Scholar] [CrossRef] [PubMed]
- Gebeyehu, D.; Maennig, B.; Drechsel, J.; Leo, K.; Pfeiffer, M. Bulk-heterojunction photovoltaic devices based on donor–acceptor organic small molecule blends. Sol. Energy Mater. Sol. Cells 2003, 79, 81–92. [Google Scholar] [CrossRef]
- Socol, M.; Preda, N.; Rasoga, O.; Breazu, C.; Stavareche, I.; Stanculescu, F.; Socol, G.; Gherendi, F.; Grumezescu, V.; Popescu-Pelin, G.; et al. Flexible heterostructures based on metal phthalocyanines thin films obtained by MAPLE. Appl. Surf. Sci. 2016, 374, 403–410. [Google Scholar] [CrossRef]
- Ali, H.E.A.; Altındal, A.; Altun, S.; Odabaş, Z. Highly efficient dye-sensitized solar cells based on metal-free and copper(II) phthalocyanine bearing 2-phenylphenoxy moiety. Dyes Pigment. 2016, 124, 180–187. [Google Scholar] [CrossRef]
- Sánchez-Vergara, M.E.; Leyva-Esqueda, M.; Alvarez-Vada, J.R.; García-Montalvo, V.; Rojas-Montoya, I.D.; Jiménez-Sandoval, O. Optical and Electrical Properties of TTF-MPcs (M = Cu, Zn) Interfaces for Optoelectronic Applications. Molecules 2015, 20, 21037–21049. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available. |
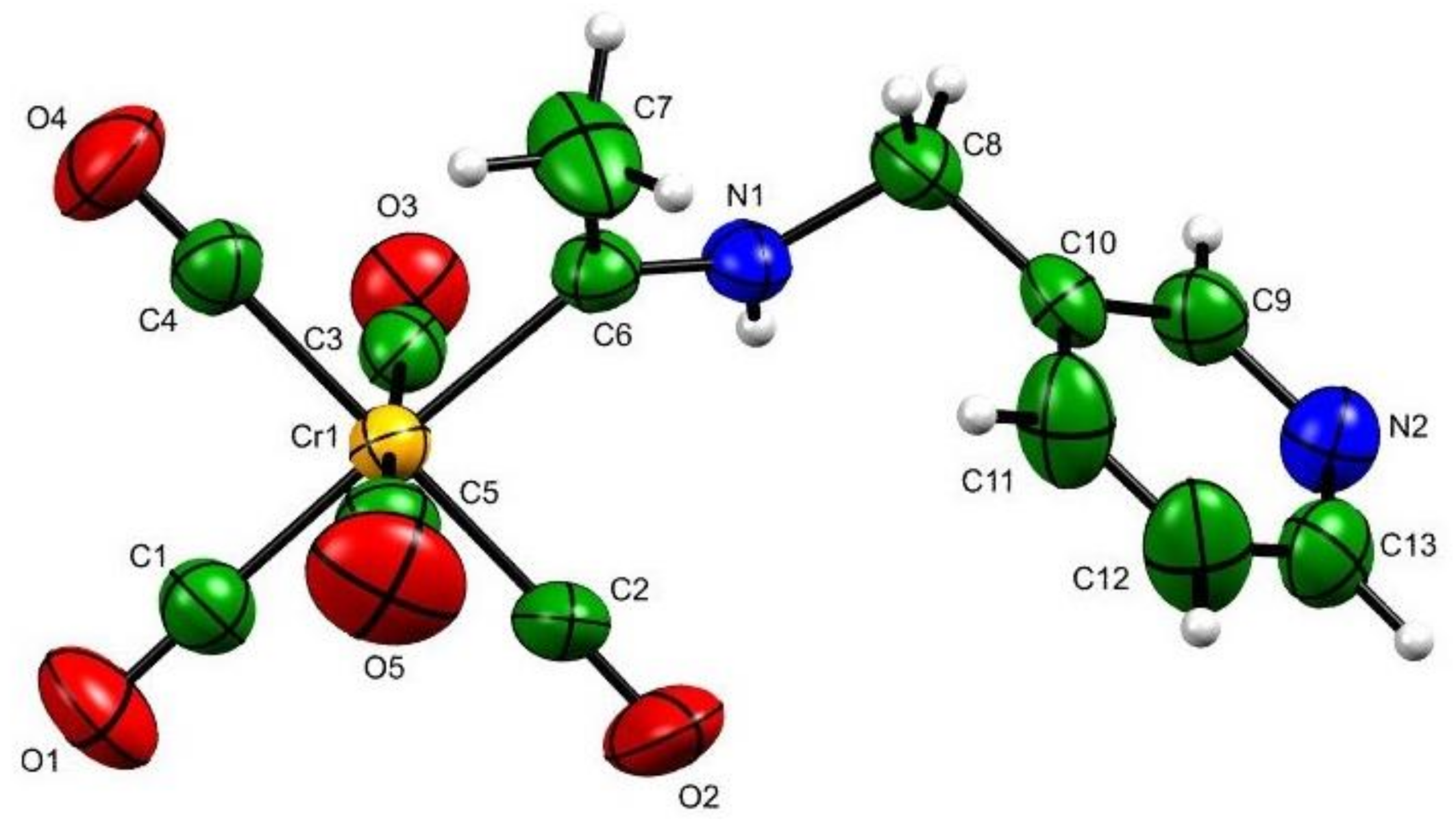


| Compound | 3b |
|---|---|
| Empirical Formula | C13H10CrN2O5 |
| Formula Weight (g mol−1) | 326.23 |
| Crystal size (nm) | 0.471 × 0.323 × 0.21 |
| Color | Yellow |
| Crystal system | Monoclinic |
| Space Group | P 21/n |
| a (Å) | 7.4665(4) |
| b (Å) | 16.6098(8) |
| c (Å) | 12.4271(6) |
| α (°) | 90 |
| β (°) | 105.505(3) |
| γ (°) | 90 |
| V (Å3) | 1485.08(13) |
| Z | 4 |
| Dcalc (g cm3) | 1.459 |
| Number of collected reflections | 31279 |
| Number of independent reflections (Rint) | 4342, Rint = 0.0479 |
| Maximum and minimum transmission | 0.707 and 0.851 |
| Data/restraints/parameters | 4342/0/195 |
| Final R indices [l > 2σ(l)] | R = 0.0369, wR2 0.0872 |
| R indices (all data) | R = 0.0612, wR2 = 0.1008 |
| GoF (F2) | 1.026 |
| Absorption correction method | Multi-scan |
| Device | Vmax (V) | Jmax (mA/cm2) | VOC (V) | JSC (mA/cm2) | FF |
|---|---|---|---|---|---|
| OSC1 | 0.050 | 0.717 | 0.150 | 1.037 | 0.23 |
| OSC2 | 0.010 | 0.121 | 0.025 | 0.694 | 0.07 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vidal-García, P.; Sánchez-Vergara, M.E.; Corona-Sánchez, R.; Jiménez-Sandoval, O.; Mercado, E.G.-R.; Toscano, R.A.; Álvarez-Toledano, C. Multifunctional Fischer Aminocarbene Complexes as Hole or Electron Transporting Layers in Organic Solar Cells. Molecules 2018, 23, 751. https://doi.org/10.3390/molecules23040751
Vidal-García P, Sánchez-Vergara ME, Corona-Sánchez R, Jiménez-Sandoval O, Mercado EG-R, Toscano RA, Álvarez-Toledano C. Multifunctional Fischer Aminocarbene Complexes as Hole or Electron Transporting Layers in Organic Solar Cells. Molecules. 2018; 23(4):751. https://doi.org/10.3390/molecules23040751
Chicago/Turabian StyleVidal-García, Pablo, María Elena Sánchez-Vergara, Ricardo Corona-Sánchez, Omar Jiménez-Sandoval, Efraín Gutiérrez-Rivas Mercado, Rubén A. Toscano, and Cecilio Álvarez-Toledano. 2018. "Multifunctional Fischer Aminocarbene Complexes as Hole or Electron Transporting Layers in Organic Solar Cells" Molecules 23, no. 4: 751. https://doi.org/10.3390/molecules23040751
APA StyleVidal-García, P., Sánchez-Vergara, M. E., Corona-Sánchez, R., Jiménez-Sandoval, O., Mercado, E. G.-R., Toscano, R. A., & Álvarez-Toledano, C. (2018). Multifunctional Fischer Aminocarbene Complexes as Hole or Electron Transporting Layers in Organic Solar Cells. Molecules, 23(4), 751. https://doi.org/10.3390/molecules23040751