Investigating the Interaction of Ascorbic Acid with Anthocyanins and Pyranoanthocyanins
Abstract
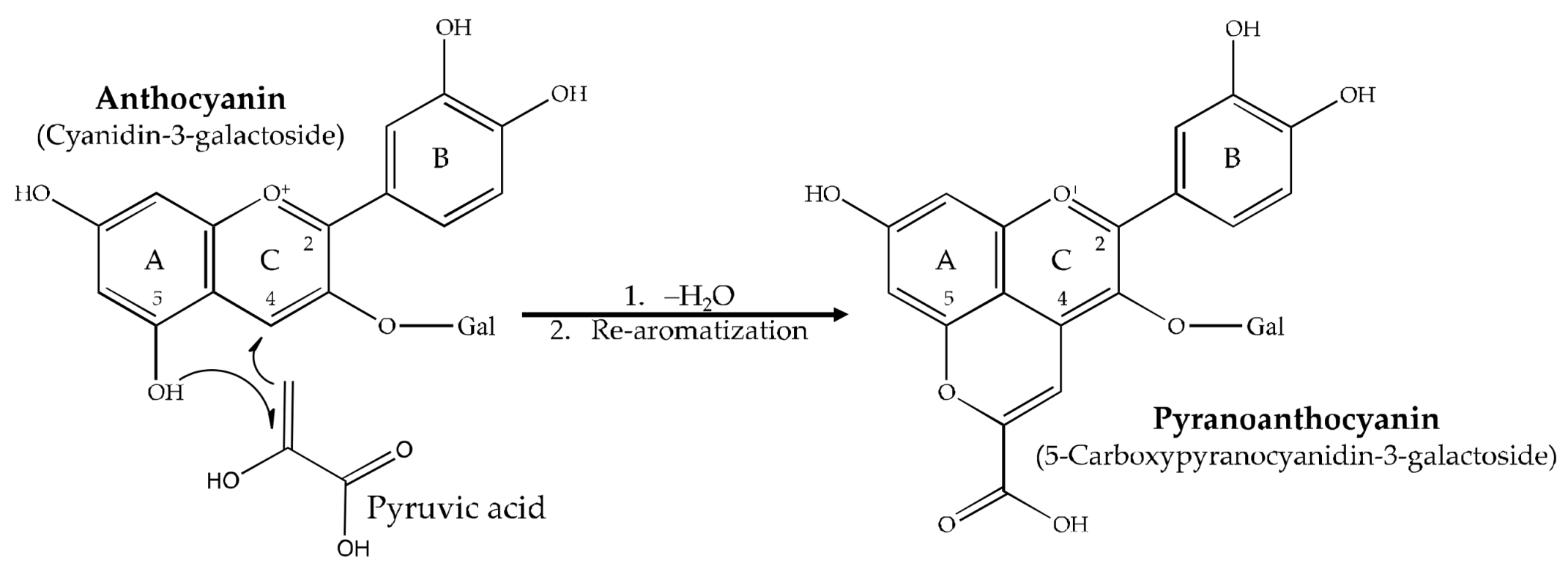
1. Introduction
2. Results and Discussion
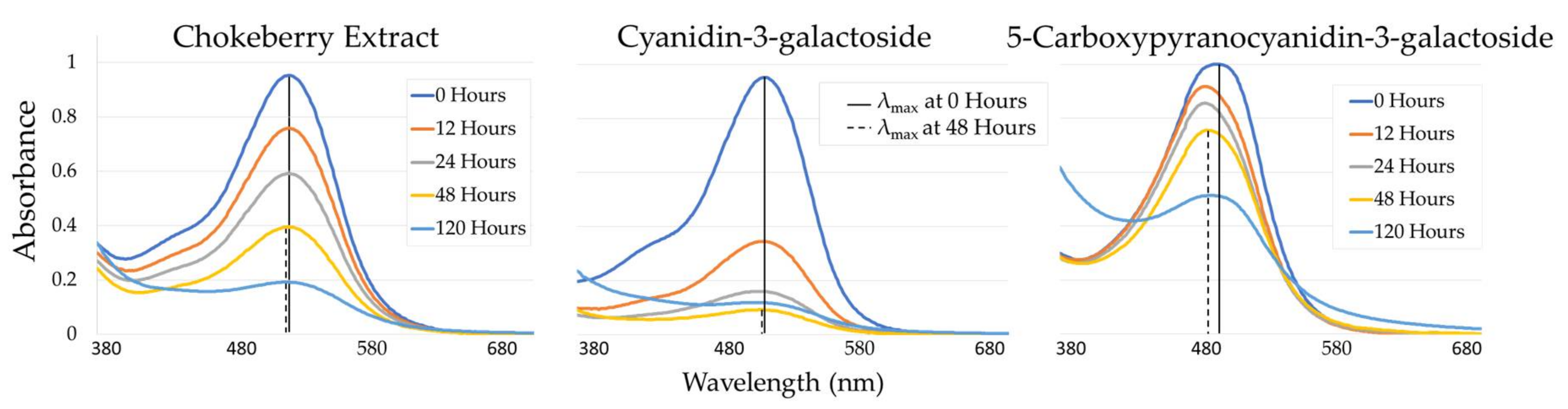
2.1. UV-Vis Spectrophotometry of Solutions
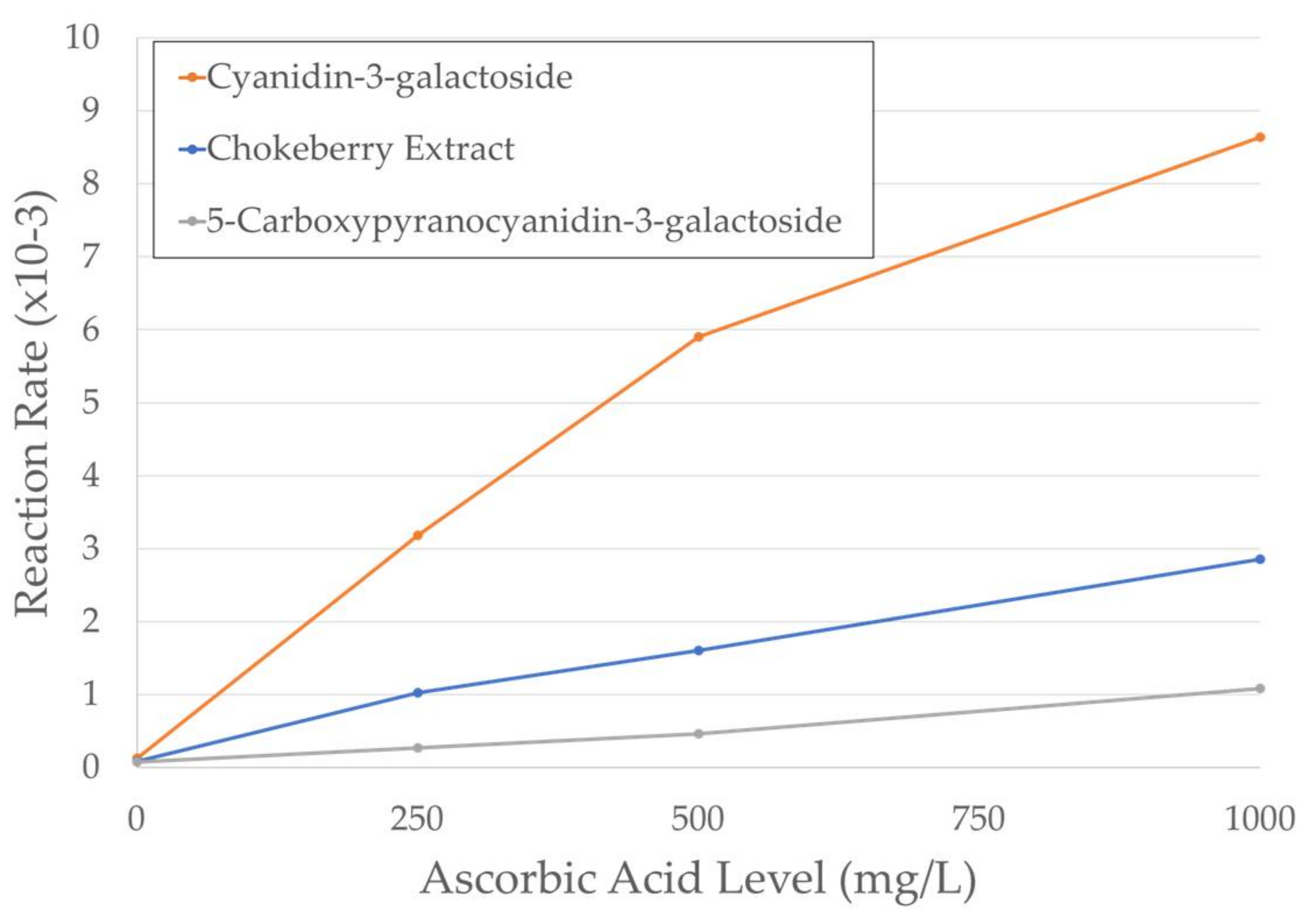
2.2. Kinetics of Degradation
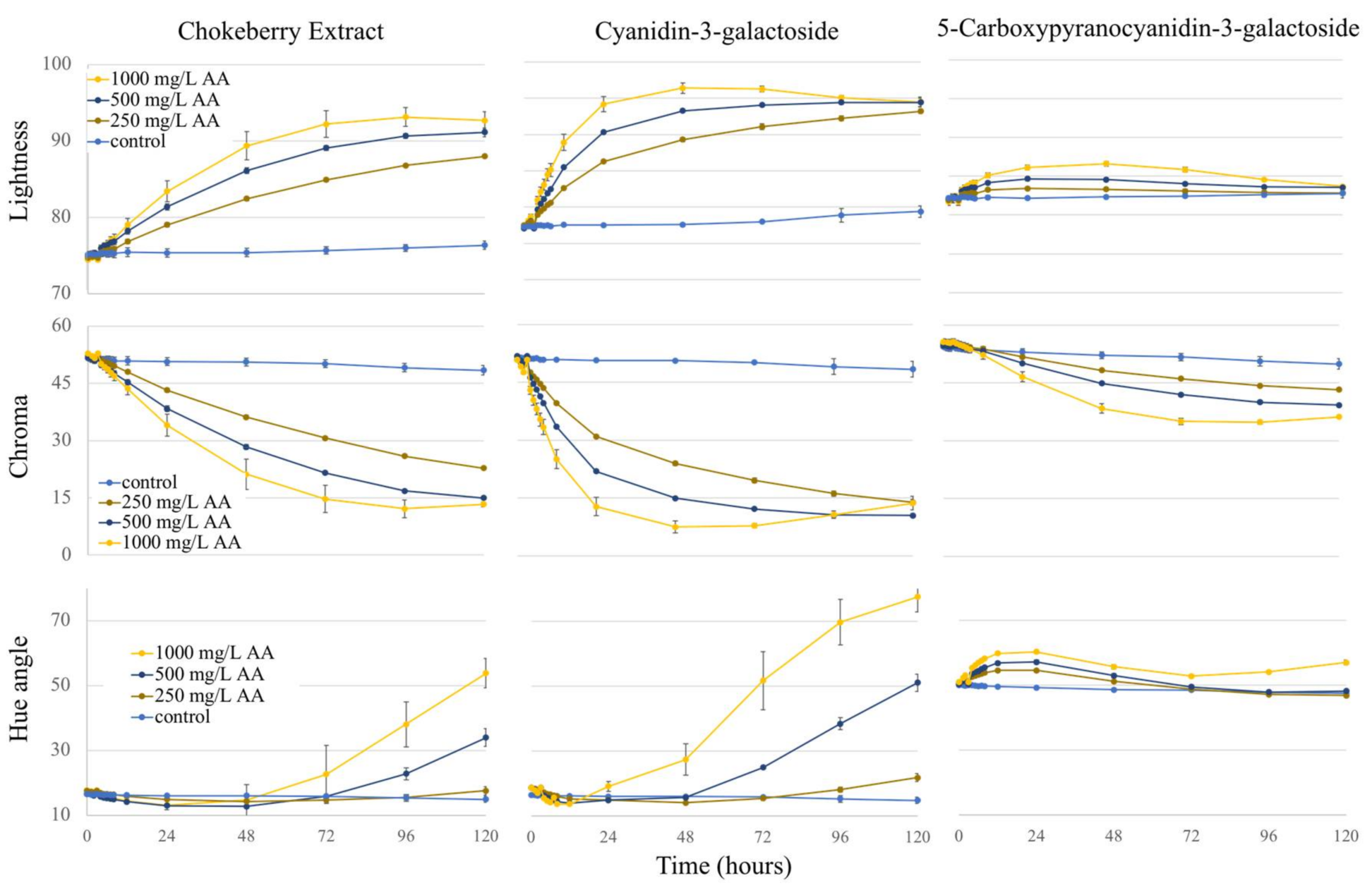
2.3. Colorimetry
2.3.1. Lightness
2.3.2. Chroma
2.3.3. Hue Angle
2.3.4. Total Color Change (ΔE)
2.4. HPLC and MS/MS Evaluation
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Anthocyanin Semi-Purification (SPE)
3.2.2. Pyranoanthocyanin Synthesis
3.2.3. Anthocyanin and Pyranoanthocyanin Purification
3.2.4. Anthocyanin and Pyranoanthocyanin Purity
3.2.5. Sample Preparation
3.2.6. UV-Vis Spectrophotometry of Samples
3.2.7. Color Analyses of Samples
3.2.8. HPLC Monitoring of Samples
3.2.9. MS/MS Evaluation of Pigments
3.2.10. Statistical Analysis of Data
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sharma, V.; McKone, H.T.; Markow, P.G. A Global Perspective on the History, Use, and Identification of Synthetic Food Dyes. J. Chem. Educ. 2010, 88, 24–28. [Google Scholar] [CrossRef]
- Kobylewski, S.; Jacobson, M.F. Food Dyes: A Rainbow of Risks. Decis. Sci. 2010, 30, 337–360. [Google Scholar]
- Martins, N.; Roriz, C.L.; Morales, P.; Barros, L.; Ferreira, I.C.F.R. Food colorants: Challenges, opportunities and current desires of agro-industries to ensure consumer expectations and regulatory practices. Trends Food Sci. Technol. 2016, 52, 1–15. [Google Scholar] [CrossRef]
- Sigurdson, G.T.; Tang, P.; Giusti, M.M. Natural Colorants: Food Colorants from Natural Sources. Annu. Rev. Food Sci. Technol. 2017, 8, 261–280. [Google Scholar] [CrossRef] [PubMed]
- Eiro, M.J.; Heinonen, M. Anthocyanin color behavior and stability during storage: Effect of intermolecular copigmentation. J. Agric. Food Chem. 2002, 50, 7461–7466. [Google Scholar] [CrossRef] [PubMed]
- Varvara, M.; Bozzo, G.; Disanto, C.; Pagliarone, C.N.; Celano, G.V. The use of the ascorbic acid as food additive and technical-legal issues. Ital. J. Food Saf. 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Sondheimer, E.; Kertesz, Z.I. Participation of ascorbic acid in the destruction of anthocyanin in strawberry juice and model systems. Biol. Chem. 1953, 18, 475–479. [Google Scholar] [CrossRef]
- Shriner, R.L.; Moffett, R.B. Benzopyrylium Salts. III. Syntheses from Substituted Coumarins and Chromones. J. Am. Chem. Soc. 1941, 63, 1694–1698. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Characterization and Measurement of Anthocyanins by UV-visible Spectroscopy. In Current Protocols in Food Analytical Chemistry; John Wiley & Sons: New York City, NY, USA, 2001; Volume 5, pp. 19–31. ISBN 9780471142911. [Google Scholar]
- Poei-Langston, M.S.; Wrolstad, R.E. Color Degradation in an Ascorbic Acid-Anthocyanin-Flavanol Model System. J. Food Sci. 1981, 46, 1218–1236. [Google Scholar] [CrossRef]
- Garcia-Viguera, C.; Bridle, P. Influence of structure on color stability of anthocyanins and flavylium salts. Food Chem. 1999, 64, 21–26. [Google Scholar] [CrossRef]
- Berké, B.; Chèze, C.; Vercauteren, J.; Deffieux, G. Bisulfite addition to anthocyanins: Revisited structures of colourless adducts. Tetrahedron Lett. 1998, 39, 5771–5774. [Google Scholar] [CrossRef]
- Brenes, C.H.; Del Pozo-Insfran, D.; Talcott, S.T. Stability of copigmented anthocyanins and ascorbic acid in a grape juice model system. J. Agric. Food Chem. 2005, 53, 49–56. [Google Scholar] [CrossRef] [PubMed]
- De Freitas, V.; Mateus, N. Formation of pyranoanthocyanins in red wines: A new and diverse class of anthocyanin derivatives. Anal. Bioanal. Chem. 2011, 401, 1467–1477. [Google Scholar] [CrossRef] [PubMed]
- Fossen, T.; Andersen, Ø.M. Anthocyanins from red onion, Allium cepa, with novel aglycone. Phytochemistry 2003, 62, 1217–1220. [Google Scholar] [CrossRef]
- Andersen, Ø.M.; Fossen, T.; Torskangerpoll, K.; Fossen, A.; Hauge, U. Anthocyanin from strawberry (Fragaria ananassa) with the novel aglycone, 5-carboxypyranopelargonidin. Phytochemistry 2004, 65, 405–410. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Carvalho, A.R.F.; Mateus, N.; De Freitas, V. Spectral Features and Stability of Oligomeric Pyranoanthocyanin-flavanol Pigments Isolated from Red Wines. J. Agric. Food Chem 2010, 58, 9249–9258. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.; Fernandes, V.; Miranda, C.C.C.C.; Santos-Buelga, C.; Silva, A.; De Freitas, V.; Mateus, N. Color properties of four cyanidin-pyruvic acid adducts. J. Agric. Food Chem. 2006, 54, 6894–6903. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Alonso, S.; Blanco-Vega, D.; Gómez, M.V.; Hermosín-Gutiérrez, I. Synthesis, isolation, structure elucidation, and color properties of 10-Acetyl-pyranoanthocyanins. J. Agric. Food Chem. 2012, 60, 12210–12223. [Google Scholar] [CrossRef] [PubMed]
- Trouillas, P.; Sancho-García, J.C.; De Freitas, V.; Gierschner, J.; Otyepka, M.; Dangles, O. Stabilizing and Modulating Color by Copigmentation: Insights from Theory and Experiment. Chem. Rev. 2016, 116, 4937–4982. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.; Rojanasasithara, T.; Mutilangi, W.; McClements, D.J. Stabilization of natural colors and nutraceuticals: Inhibition of anthocyanin degradation in model beverages using polyphenols. Food Chem. 2016, 212, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Choe, E.; Min, D.B. Mechanisms of Antioxidants in the Oxidation of Foods. Compr. Rev. Food Sci. Food Saf. 2009, 8, 345–358. [Google Scholar] [CrossRef]
- Kulling, S.E.; Rawel, H.M. Chokeberry (Aronia melanocarpa)—A review on the characteristic components and potential health effects. Planta Med. 2008, 74, 1625–1634. [Google Scholar] [CrossRef] [PubMed]
- Sondheimer, E.; Kertesz, Z.I. The kinetics of the oxidation of strawberry anthocyanin by hydrogen peroxide. J. Food Sci. 1952, 17, 288–298. [Google Scholar] [CrossRef]
- Ozkan, M.; Yemenicioglu, A.; Asefi, N.; Cemeroglu, B. Degradation Kinetics of Anthocyanins from Sour Cherry, Pomegranate, and Strawberry Juices by Hydrogen Peroxide. J. Food Sci. 2002, 67, 525–529. [Google Scholar] [CrossRef]
- Häkkinen, S.H.; Kärenlampi, S.O.; Heinonen, I.M.; Mykkänen, H.M.; Törrönen, A.R. Content of the Flavonols Quercetin, Myricetin, and Kaempferol in 25 Edible Berries. J. Agric. Food Chem. 1999, 47, 2274–2279. [Google Scholar] [CrossRef] [PubMed]
- Giusti, M.M.; Rodríguez-Saona, L.E.; Griffin, D.; Wrolstad, R.E. Electrospray and Tandem Mass Spectroscopy As Tools for Anthocyanin Characterization. J. Agric. Food Chem. 1999, 47, 4657–4664. [Google Scholar] [CrossRef] [PubMed]
- Jansson, P.J.; Jung, H.R.; Lindqvist, C.; Nordström, T. Oxidative decomposition of vitamin C in drinking water. Free Radic. Res. 2004, 38, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.T.; Martell, A.E. Metal Ion and Metal Chelate Catalyzed Oxidation of Ascorbic Acid. J. Am. Chem. Soc. 1967, 89, 4176–4185. [Google Scholar] [CrossRef] [PubMed]
- Buettner, G.R.; Jurkiewicz, B.A. Catalytic metals, ascorbate and free radicals: Combinations to avoid. Radiat. Res. 1996, 145, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Stebbins, N.B.; Howarda, L.R.; Prior, R.L.; Brownmiller, C.; Liyanage, R.; Lay, J.O.; Yang, X.; Qian, S.Y. Ascorbic acid-catalyzed degradation of cyanidin-3-O-β-glucoside: Proposed mechanism and identification of a novel hydroxylated product. J. Berry Res. 2016, 6, 175–187. [Google Scholar] [CrossRef]
- Farr, J.E.; Giusti, M.M. ColorBySpectra. Available online: https://buckeyevault.com/products/colorbyspectra (accessed on 18 October 2017).
- CIE. CIE 015:2004 Colorimetry; CIE: Vienna, Austria, 2004; ISBN 978-3-901-90633-6. [Google Scholar]
Sample Availability: Samples of the pyranoanthocyanin evaluated may be requested by email contact with the corresponding author. |

| Ascorbic Acid Level | Pigment | k (hour−1) | t1/2 (h) | R2 |
|---|---|---|---|---|
| Control | Chokeberry Extract | 8.08 × 104 | 858 | 0.947 |
| Cyanidin-3-galactoside | 1.27 × 103 | 546 | 0.957 | |
| 5-Carboxypyranocyanidin-3-galactoside | 7.08 × 104 | 978 | 0.977 | |
| 250 mg/L AA | Chokeberry Extract | 1.02 × 102 | 68 | 0.991 |
| Cyanidin-3-galactoside | 3.18 × 102 | 22 | 0.996 | |
| 5-Carboxypyranocyanidin-3-galactoside | 2.69 × 103 | 258 | 0.992 | |
| 500 mg/L AA | Chokeberry Extract | 1.60 × 102 | 43 | 0.991 |
| Cyanidin-3-galactoside | 5.90 × 102 | 12 | 0.998 | |
| 5-Carboxypyranocyanidin-3-galactoside | 4.61 × 103 | 150 | 0.965 | |
| 1000 mg/L AA | Chokeberry Extract | 2.85 × 102 | 24 | 0.999 |
| Cyanidin-3-galactoside | 8.64 × 102 | 8 | 0.996 | |
| 5-Carboxypyranocyanidin-3-galactoside | 1.08 × 102 | 64 | 0.998 |
| Treatment | Lightness | Chroma | Hue Angle | ΔE | ||||
|---|---|---|---|---|---|---|---|---|
| Day 0 | Day 5 | Day 0 | Day 5 | Day 0 | Day 5 | Over 5 Days | ||
| Chokeberry Extract | Control | 75.1 (0.5) | 76.3 (0.5) | 51.6 (0.8) | 48.4 (1.2) | 16.6 (0.5) | 14.9 (0.8) | 3.3 (0.4) |
| 250 mg/L AA | 74.7 (0.2) | 88.0 (0.2) | 52.3 (0.4) | 22.8 (0.3) | 17.4 (0.2) | 17.5 (0.5) | 29.9 (0.4) | |
| 500 mg/L AA | 75.0 (0.3) | 91.1 (0.6) | 51.8 (0.6) | 15.0 (0.4) | 16.6 (0.3) | 34.0 (3.1) | 36.9 (0.2) | |
| 1000 mg/L AA | 74.4 (0.2) | 92.7 (1.1) | 52.9 (0.2) | 13.3 (0.6) | 17.6 (0.2) | 53.8 (3.1) | 42.0 (2.2) | |
| Cyanidin-3-galactoside | Control | 77.3 (0.1) | 79.4 (0.8) | 51.2 (0.2) | 46.5 (2.1) | 18.6 (0.1) | 15.9 (1.0) | 2.6 (1.1) |
| 250 mg/L AA | 77.5 (0.2) | 93.2 (0.3) | 50.8 (0.2) | 13.9 (0.7) | 18.7 (0.1) | 21.9 (1.2) | 19.3 (0.3) | |
| 500 mg/L AA | 77.0 (0.1) | 94.4 (0.6) | 51.8 (0.0) | 10.5 (0.4) | 18.8 (0.0) | 51.1 (2.7) | 23.6 (0.2) | |
| 1000 mg/L AA | 77.2 (0.3) | 94.5 (0.7) | 50.9 (0.1) | 13.7 (1.8) | 18.7 (0.1) | 77.5 (4.5) | 27.6 (0.7) | |
| 5-Carboxypyranocyanidin-3-galactoside | Control | 82.2 (0.2) | 82.8 (0.6) | 54.3 (0.7) | 49.9 (1.3) | 50.0 (0.2) | 47.5 (0.6) | 2.1 (0.4) |
| 250 mg/L AA | 81.9 (0.1) | 82.8 (0.3) | 55.2 (0.4) | 43.2 (0.2) | 50.7 (0.2) | 46.9 (0.3) | 4.5 (0.2) | |
| 500 mg/L AA | 82.1 (0.3) | 83.6 (0.4) | 54.7 (0.3) | 39.2 (0.3) | 50.4 (0.1) | 48.3 (0.4) | 5.0 (0.2) | |
| 1000 mg/L AA | 81.7 (0.5) | 83.7 (0.2) | 55.6 (0.7) | 36.2 (0.3) | 51.0 (0.1) | 57.1 (0.7) | 5.2 (0.5) | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farr, J.E.; Giusti, M.M. Investigating the Interaction of Ascorbic Acid with Anthocyanins and Pyranoanthocyanins. Molecules 2018, 23, 744. https://doi.org/10.3390/molecules23040744
Farr JE, Giusti MM. Investigating the Interaction of Ascorbic Acid with Anthocyanins and Pyranoanthocyanins. Molecules. 2018; 23(4):744. https://doi.org/10.3390/molecules23040744
Chicago/Turabian StyleFarr, Jacob E., and M. Monica Giusti. 2018. "Investigating the Interaction of Ascorbic Acid with Anthocyanins and Pyranoanthocyanins" Molecules 23, no. 4: 744. https://doi.org/10.3390/molecules23040744
APA StyleFarr, J. E., & Giusti, M. M. (2018). Investigating the Interaction of Ascorbic Acid with Anthocyanins and Pyranoanthocyanins. Molecules, 23(4), 744. https://doi.org/10.3390/molecules23040744







