Digestibility of Bovine Serum Albumin and Peptidomics of the Digests: Effect of Glycation Derived from α-Dicarbonyl Compounds
Abstract
1. Introduction
2. Results and Discussion
2.1. Glycation During Incubation
2.1.1. Loss of Primary Amino Group (−NH2)
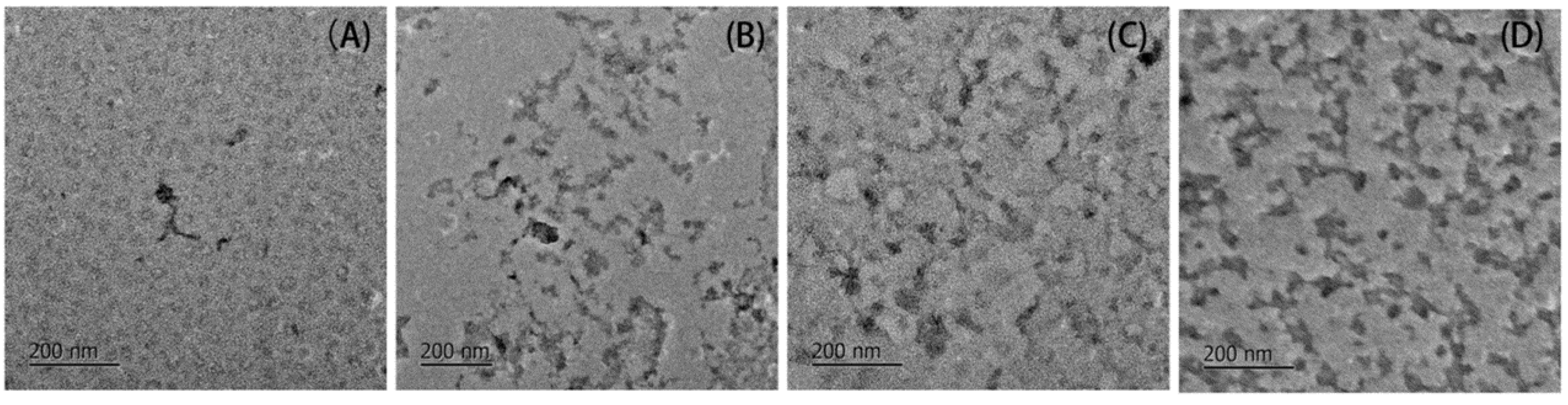
2.1.2. Changes in Aggregates Conformation
2.2. Changes in Digestibility
2.2.1. Change in Degree of Hydrolysis (DH) during Gastrointestinal Digestion
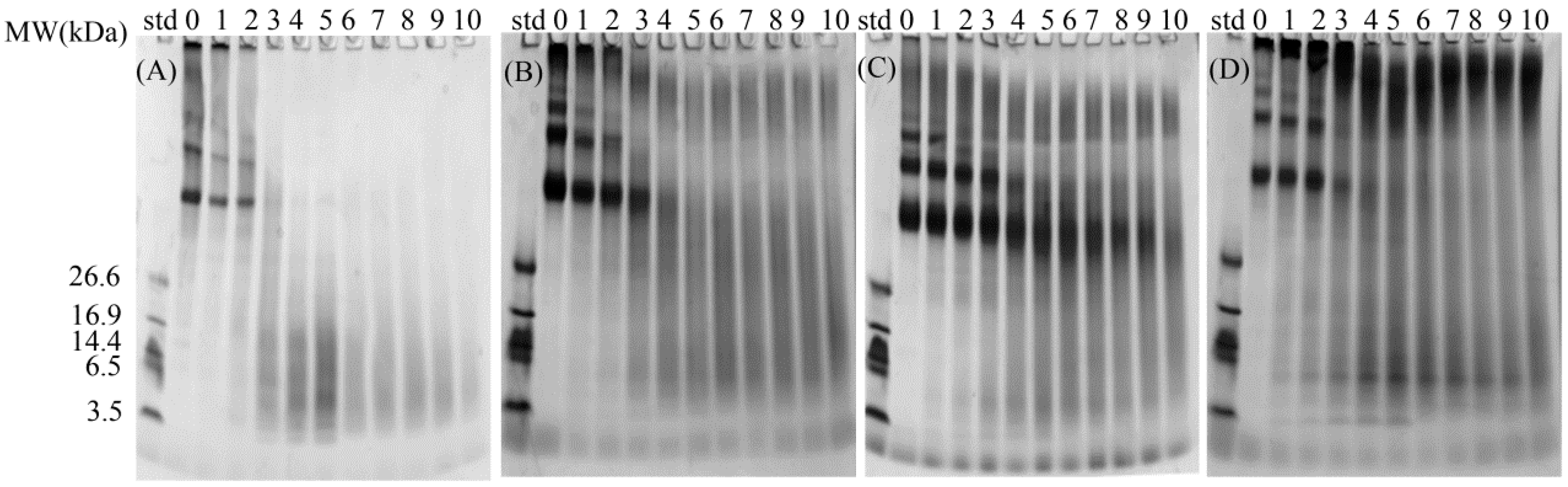
2.2.2. Dynamic Digestion Process Evaluated by SDS-PAGE
2.3. Peptides Analysis in Digests
2.3.1. Changes in Peptidomics of Digests
2.3.2. Peptide-AGEs in Digests
3. Materials and Methods
3.1. Materials
3.2. Glycation Model
3.3. In Vitro Digestion
3.4. Fluorescamine Assay
3.4.1. Loss of −NH2 during Glycation
3.4.2. DH of Digests
3.5. Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
3.5.1. SDS-PAGE of Aggregates
3.5.2. SDS-PAGE of Digests
3.6. Transmission Electron Microscopy (TEM)
3.7. LC-ESI-MS/MS
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hollnagel, A.; Kroh, L.W. Formation of alpha-dicarbonyl fragments from mono- and disaccharides under caramelization and Maillard reaction conditions. Z. Lebensmittel-Untersuch. Forsh. A 1998, 207, 50–54. [Google Scholar] [CrossRef]
- Thornalley, P.J. Dicarbonyl intermediates in the Maillard reaction. Ann. N. Y. Acad. Sci. 2005, 1043, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.P.; Hengel, M.; Pan, C.P.; Seiber, J.N.; Shibamoto, T. Determination of Toxic alpha-Dicarbonyl Compounds, Glyoxal, Methylglyoxal, and Diacetyl, Released to the Headspace of Lipid Commodities upon Heat Treatment. J. Agric. Food Chem. 2013, 61, 1067–1071. [Google Scholar] [CrossRef] [PubMed]
- Degen, J.; Hellwig, M.; Henle, T. 1,2-dicarbonyl compounds in commonly consumed foods. J. Agric. Food Chem. 2012, 60, 7071–7079. [Google Scholar] [CrossRef] [PubMed]
- Kokkinidou, S.; Peterson, D.G. Control of Maillard-type off-flavor development in ultrahigh-temperature-processed bovine milk by phenolic chemistry. J. Agric. Food Chem. 2014, 62, 8023–8033. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, K.; Shibamoto, T. Formation of genotoxic dicarbonyl compounds in dietary oils upon oxidation. Lipids 2004, 39, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Glomb, M.A.; Lang, G. Isolation and characterization of glyoxal-arginine modifications. J. Agric. Food Chem. 2001, 49, 1493–1501. [Google Scholar] [CrossRef] [PubMed]
- Lederer, M.O.; Klaiber, R.G. Cross-linking of proteins by Maillard processes: Characterization and detection of lysine-arginine cross-links derived from glyoxal and methylglyoxal. Bioorgan. Med. Chem. 1999, 7, 2499–2507. [Google Scholar] [CrossRef]
- Mathews, J.M.; Watson, S.L.; Snyder, R.W.; Burgess, J.P.; Morgan, D.L. Reaction of the Butter Flavorant Diacetyl (2,3-Butanedione) with N-alpha-Acetylarginine: A Model for Epitope Formation with Pulmonary Proteins in the Etiology of Obliterative Bronchiolitis. J. Agric. Food Chem. 2010, 58, 12761–12768. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schleicher, E.D.; Wagner, E.; Nerlich, A.G. Increased accumulation of the glycoxidation product N-epsilon(carboxymethyl)lysine in human tissues in diabetes and aging. J. Clin. Investig. 1997, 99, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Murata, T.; Nagai, R.; Ishibashi, T.; Inomata, H.; Ikeda, K.; Horiuchi, S. The relationship between accumulation of advanced glycation end products and expression of vascular endothelial growth factor in human diabetic retinas. Diabetologia 1997, 40, 764–769. [Google Scholar] [CrossRef] [PubMed]
- Nagaraj, R.H.; Linetsky, M.; Stitt, A.W. The pathogenic role of Maillard reaction in the aging eye. Amino Acids 2012, 42, 1205–1220. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zeng, M.; He, Z.; Zheng, Z.; Qin, F.; Tao, G.; Zhang, S.; Chen, J. Increased accumulation of protein-bound Nε-(Carboxymethyl) lysine in tissues of healthy rats after chronic oral Nε-(Carboxymethyl) lysine. J. Agric. Food Chem. 2015, 63, 1658–1663. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, M.W.; Hedegaard, R.V.; Andersen, J.M.; de Courten, B.; Bügel, S.; Nielsen, J.; Skibsted, L.H.; Dragsted, L.O. Advanced glycation endproducts in food and their effects on health. Food Chem. Toxicol. 2013, 60, 10–37. [Google Scholar] [CrossRef] [PubMed]
- Grossin, N.; Auger, F.; Niquet-Leridon, C.; Durieux, N.; Montaigne, D.; Schmidt, A.M.; Susen, S.; Jacolot, P.; Beuscart, J.B.; Tessier, F.J.; et al. Dietary CML-enriched protein induces functional arterial aging in a RAGE-dependent manner in mice. Mol. Nutr. Food Res. 2015, 59, 927–938. [Google Scholar] [CrossRef] [PubMed]
- Moscovici, A.M.; Joubran, Y.; Briard-Bion, V.; Mackie, A.; Dupont, D.; Lesmes, U. The impact of the Maillard reaction on the in vitro proteolytic breakdown of bovine lactoferrin in adults and infants. Food Funct. 2014, 5, 1898–1908. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Li, L.; Le, T.T.; Larsen, L.B.; Su, G.Y.; Liang, Y.; Li, B. Digestibility of Glyoxal-Glycated β-Casein and β-Lactoglobulin and Distribution of Peptide-Bound Advanced Glycation End Products in Gastrointestinal Digests. J. Agric. Food Chem. 2017, 65, 5778–5788. [Google Scholar] [CrossRef] [PubMed]
- Milkovska-Stamenova, S.; Hoffmann, R. Identification and quantification of bovine protein lactosylation sites in different milk products. J. Proteomics 2016, 134, 112–126. [Google Scholar] [CrossRef] [PubMed]
- Meade, S.J.; Gerrard, J.A. The structure-activity relationships of dicarbonyl compounds and their role in the Maillard reaction. Int. Congr. Ser. 2002, 1245, 455–456. [Google Scholar] [CrossRef]
- Bouma, B.; Kroon-Batenburg, L.M.J.; Wu, Y.P.; Brünjes, B.; Posthuma, G.; Kranenburg, O.; de Groot, P.G.; Voest, E.E.; Gebbink, M.F.B.G. Glycation induces formation of amyloid cross-beta structure in albumin. J. Biol. Chem. 2003, 278, 41810–41819. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.D.; Bouhallab, S.; de Carvalho, A.F.; Henry, G.; Putaux, J.L.; Leonil, J. Glucose Slows Down the Heat-Induced Aggregation of beta-Lactoglobulin at Neutral pH. J. Agric. Food Chem. 2012, 60, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhong, Q.X. Glycation of whey protein to provide steric hindrance against thermal aggregation. J. Agric. Food Chem. 2012, 60, 9754–9762. [Google Scholar] [CrossRef] [PubMed]
- Wada, Y.; Lonnerdal, B. Effects of different industrial heating processes of milk on site-specific protein modifications and their relationship to in vitro and in vivo digestibility. J. Agric. Food Chem. 2014, 62, 4175–4185. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.S.; Leonil, J.; Henry, G.; Cauty, C.; Carvalho, A.F.; Bouhallab, S. Heating and glycation of beta-lactoglobulin and beta-casein: Aggregation and in vitro digestion. Food Res. Int. 2014, 55, 70–76. [Google Scholar] [CrossRef]
- Dunn, B.M. Overview of pepsin-like aspartic peptidases. Curr. Protoc. Protein Sci. 2001. [Google Scholar] [CrossRef]
- Broer, S. Amino acid transport across mammalian intestinal and renal epithelia. Physiol. Rev. 2008, 88, 249–286. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.P.N.; Ritari, J.; Boeren, S.; de Waard, P.; Plugge, C.M.; de Vos, W.M. Production of butyrate from lysine and the Amadori product fructoselysine by a human gut commensal. Nat. Commun. 2015, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hellwig, M.; Bunzel, D.; Huch, M.; Franz, C.M.; Kulling, S.E.; Henle, T. Stability of individual Maillard reaction products in the presence of the human colonic microbiota. J. Agric. Food Chem. 2015, 63, 6723–6730. [Google Scholar] [CrossRef] [PubMed]
- Tuohy, K.M.; Hinton, D.J.S.; Davies, S.J.; Crabbe, M.J.C.; Gibson, G.R.; Ames, J.M. Metabolism of Maillard reaction products by the human gut microbiota - implications for health. Mol. Nutr. Food Res. 2006, 50, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Mills, D.J.S.; Tuohy, K.M.; Booth, J.; Buck, M.; Crabbe, M.J.C.; Gibson, G.; Ames, J.M. Dietary glycated protein modulates the colonic microbiota towards a more detrimental composition in ulcerative colitis patients and non-ulcerative colitis subjects. J. Appl. Microbiol. 2008, 105, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Corzo-Martinez, M.; Avila, M.; Moreno, F.J.; Requena, T.; Villamiel, M. Effect of milk protein glycation and gastrointestinal digestion on the growth of bifidobacteria and lactic acid bacteria. Int. J. Food Microbiol. 2012, 153, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Hellwig, M.; Geissler, S.; Peto, A.; Knutter, I.; Brandsch, M.; Henle, T. Transport of free and peptide-bound pyrraline at intestinal and renal epithelial cells. J. Agric. Food Chem. 2009, 57, 6474–6480. [Google Scholar] [CrossRef] [PubMed]
- Hellwig, M.; Geissler, S.; Matthes, R.; Peto, A.; Silow, C.; Brandsch, M.; Henle, T. Transport of free and peptide-bound glycated amino acids: synthesis, transepithelial flux at Caco-2 cell monolayers, and interaction with apical membrane transport proteins. ChemBioChem 2011, 12, 1270–1279. [Google Scholar] [CrossRef] [PubMed]
- Pohl, J.; Dunn, B.M. Secondary enzyme-substrate interactions: kinetic evidence for ionic interactions between substrate side chains and the pepsin active site. Biochemistry 1988, 27, 4827–4834. [Google Scholar] [CrossRef] [PubMed]
- Boudier, C.; Jung, M.L.; Stambolieva, N.; Bieth, J.G. Importance of secondary enzyme-substrate interactions in human cathepsin G and chymotrypsin II catalysis. Arch. Biochem. Biophys. 1981, 210, 790–793. [Google Scholar] [CrossRef]
- Morihara, K.; Oka, T. Effect of secondary interaction on the enzymatic activity of trypsin-like enzymes from Streptomyces. Arch. Biochem. Biophys. 1973, 156, 764–771. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Balance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Yaylayan, V.A.; Huyghues-Despointes, A.; Polydorides, A. A fluorescamine-based assay for the degree of glycation in bovine serum albumin. Food Res. Int. 1992, 25, 269–275. [Google Scholar] [CrossRef]
- Petrat-Melin, B.; Andersen, P.; Rasmussen, J.T.; Poulsen, N.A.; Larsen, L.B.; Young, J.F. In vitro digestion of purified beta-casein variants A(1), A(2), B, and I: effects on antioxidant and angiotensin-converting enzyme inhibitory capacity. J. Dairy Sci. 2015, 98, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680. [Google Scholar] [CrossRef] [PubMed]
- Schagger, H.; von Jagow, G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 1987, 166, 368–379. [Google Scholar] [CrossRef]
- Le, T.T.; Nielsen, S.D.; Villumsen, N.S.; Kristiansen, G.H.; Nielsen, L.R.; Nielsen, S.B.; Hammershøj, M.; Larsen, L.B. Using proteomics to characterise storage-induced aggregates in acidic whey protein isolate drinks. Int. Dairy J. 2016, 60, 39–46. [Google Scholar] [CrossRef]
- Rauh, V.M.; Johansen, L.B.; Ipsen, R.; Paulsson, M.; Larsen, L.B.; Hammershoj, M. Plasmin activity in UHT Milk: relationship between proteolysis, age gelation, and bitterness. J. Agric. Food Chem. 2014, 62, 6852–6860. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds before and after digestion are available from the authors. |




| Sample ID | Loss of −NH2 (%) |
|---|---|
| BSA | 1.7 ± 0.1 |
| BSA + GO (10 mM) | 28.2 ± 1.9 |
| BSA + MGO (10 mM) | 24.4 ± 2.2 |
| BSA + DA (10 mM) | 23.9 ± 1.2 |
| No. | Identified Mass (Da) | Theoretical Mass (Da) | Sequence | Modification | Origin |
|---|---|---|---|---|---|
| Gastric digested peptides | |||||
| 1 | 1151.572 | 1151.586 | PKAFDEK(508)LF | CML | BSA + GO |
| 2 | 991.037 | 991.465 | LYYANK(163)Y | CML | BSA + GO |
| 3 | 940.531 | 940.516 | LILNR(462)LC | G-H1 | BSA + GO |
| 4 | 770.343 | 770.408 | WSVAR(221)L | G-H1 | BSA + GO |
| 5 | 666.545 | 666.443 | VLLR(351)L | MG-H1 | BSA + MGO |
| 6 | 784.404 | 784.423 | WSVAR(221)L | MG-H1 | BSA + MGO |
| 7 | 1396.587 | 1396.714 | YGFQNALIVR(413)Y | MG-H1 | BSA + MGO |
| 8 | 1685.861 | 1685.868 | IARR(148)HPYFYAPEL | MG-H1 | BSA + MGO |
| Gastrointestinal digested peptides | |||||
| 9 | 1926.005 | 1925.962 | VEK(298)DAIPENLPPLTADF | CML | BSA + GO |
| 10 | 1099.576 | 1099.660 | QEAK(351)DAFLG | CEL | BSA + MGO |
| 11 | 1122.581 | 1122.712 | AVSVLLR(351)LAK | MG-H1 | BSA + MGO |
| 12 | 1284.632 | 1284.704 | VEVSR(431)SLGKVGT | MG-H1 | BSA + MGO |
| 13 | 729.470 | 729.366 | VEVSR(431)S | MG-H1 | BSA + MGO |
| 14 | 632.342 | 632.292 | FGER(212)A | MG-H1 | BSA + MGO |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheng, B.; Larsen, L.B.; Le, T.T.; Zhao, D. Digestibility of Bovine Serum Albumin and Peptidomics of the Digests: Effect of Glycation Derived from α-Dicarbonyl Compounds. Molecules 2018, 23, 712. https://doi.org/10.3390/molecules23040712
Sheng B, Larsen LB, Le TT, Zhao D. Digestibility of Bovine Serum Albumin and Peptidomics of the Digests: Effect of Glycation Derived from α-Dicarbonyl Compounds. Molecules. 2018; 23(4):712. https://doi.org/10.3390/molecules23040712
Chicago/Turabian StyleSheng, Bulei, Lotte Bach Larsen, Thao T. Le, and Di Zhao. 2018. "Digestibility of Bovine Serum Albumin and Peptidomics of the Digests: Effect of Glycation Derived from α-Dicarbonyl Compounds" Molecules 23, no. 4: 712. https://doi.org/10.3390/molecules23040712
APA StyleSheng, B., Larsen, L. B., Le, T. T., & Zhao, D. (2018). Digestibility of Bovine Serum Albumin and Peptidomics of the Digests: Effect of Glycation Derived from α-Dicarbonyl Compounds. Molecules, 23(4), 712. https://doi.org/10.3390/molecules23040712






