An Interaction of Rhamnolipids with Cu2+ Ions
Abstract
1. Introduction
2. Results and Discussion
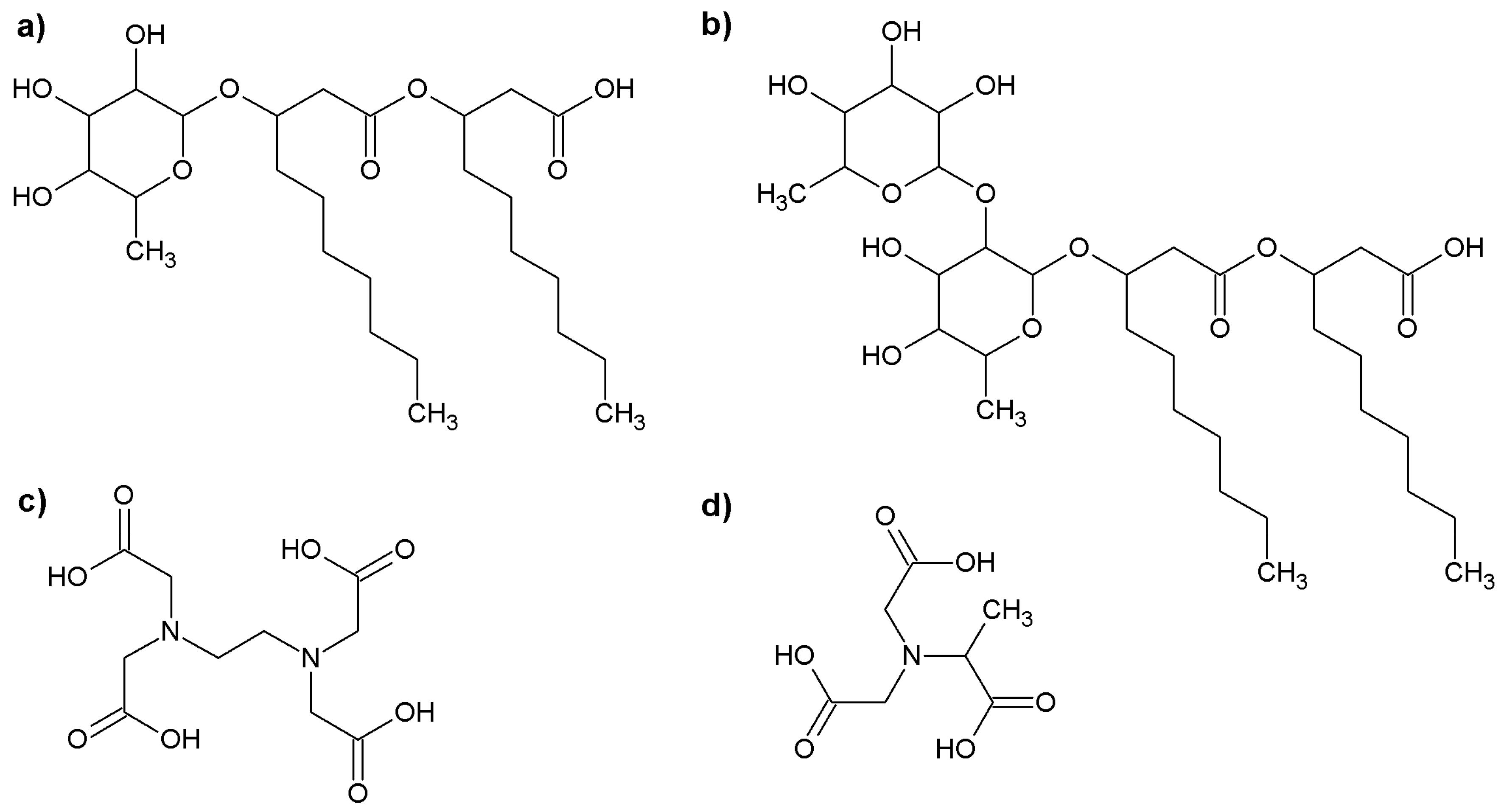
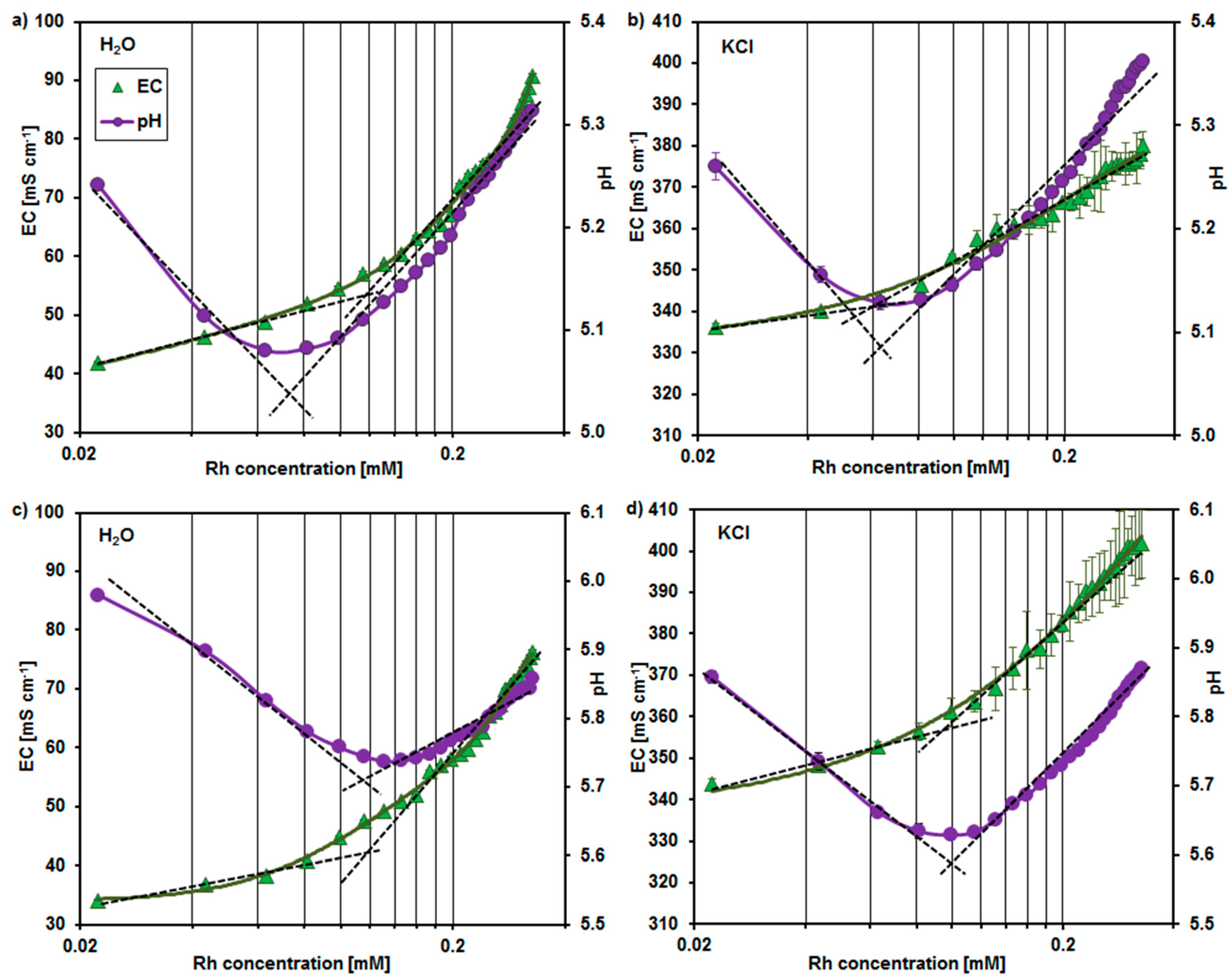
2.1. The Aggregation of the Rhamnolipid Molecules
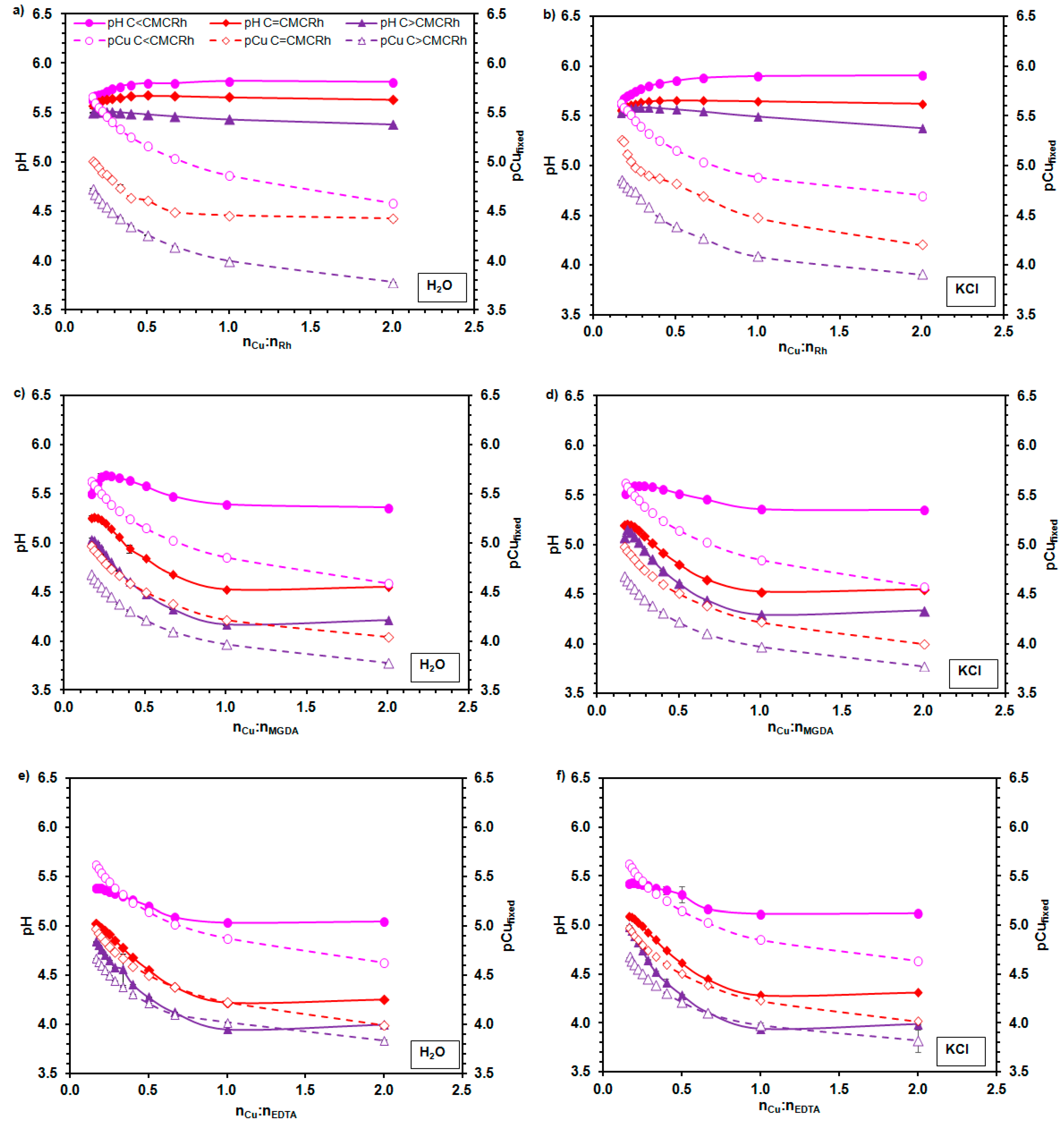
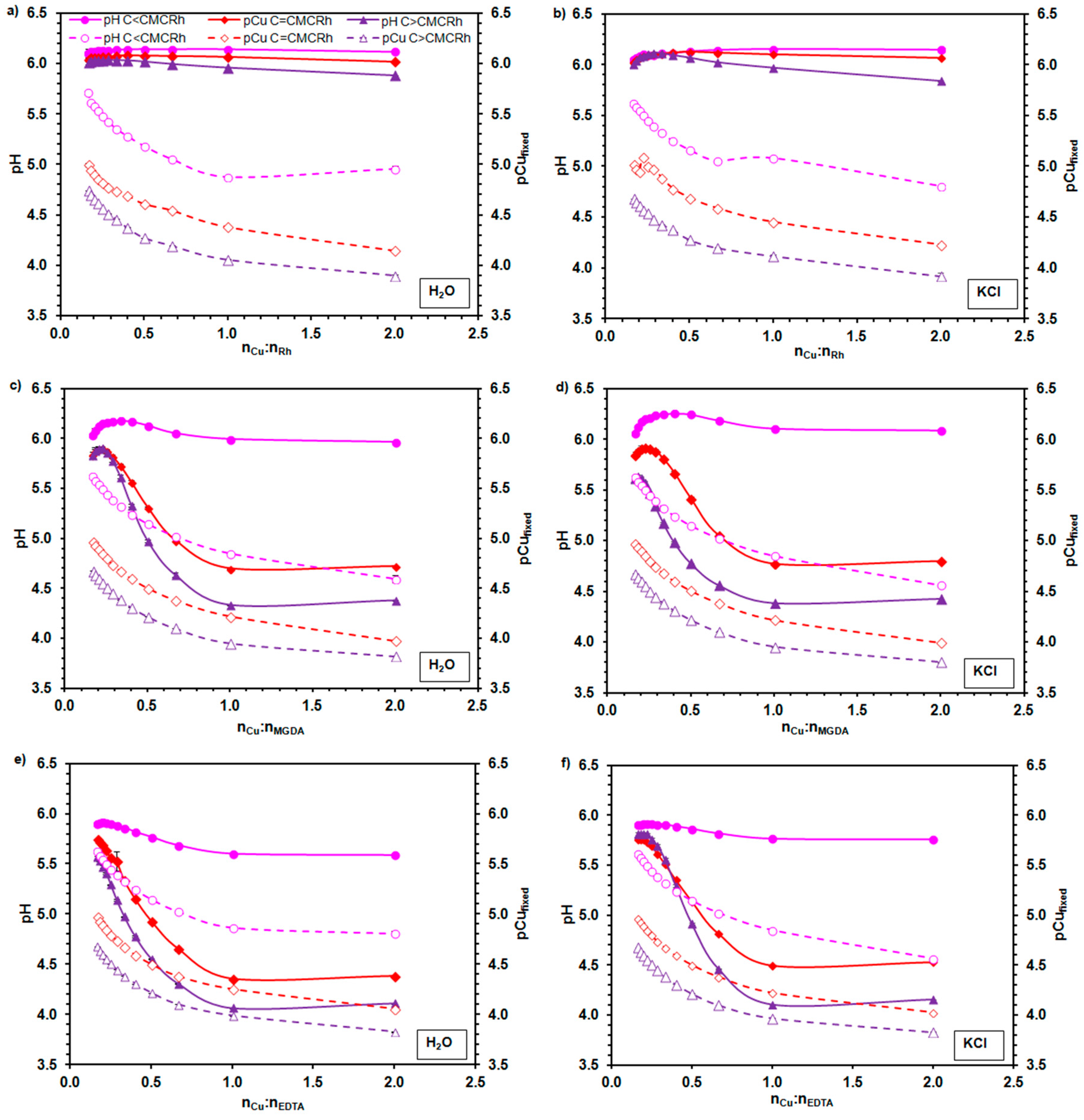
2.2. The Complexation of Cu2+ Ions by Rhamnolipids
3. Materials and Methods
3.1. The Chemicals Used
3.2. Estimation of the Rhamnolipid Critical Micelle Concentration (CMC)
3.3. Determination of the Particles Size and Electrophoretic Mobility in the Rhamnolipid Micellar Solutions
3.4. The Evaluation of Rhamnolipids’ Ability to Form Cu2+ Chelates
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hommel, R.K. Formation and function of biosurfactants for degradation of water-insoluble substrates. In Biochemistry of Microbial Degradation; Ratledge, C., Ed.; Springer: Dordrecht, The Netherlands, 1994; pp. 63–87. [Google Scholar]
- Bai, G.; Brusseau, M.L.; Miller, R.M. Influence of cation type, ionic strength, and pH on solubilization and mobilisation of residual hydrocarbon by a biosurfactant. J. Contam. Hydrol. 1998, 30, 265–279. [Google Scholar] [CrossRef]
- Müller, F.; Hönzke, S.; Luthardt, W.-O.; Wong, E.L.; Unbehauenb, M.; Bauer, J.; Haag, R.; Hedtrich, S.; Rühl, S.; Rademann, J. Rhamnolipids form drug-loaded nanoparticles for dermal drug delivery. Eur. J. Pharm. Biopharm. 2016, 116, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Sinumvayo, J.P.; Ishimwe, N. Agriculture and Food Applications of Rhamnolipids and its Production by Pseudomonas Aeruginosa. J. Chem. Eng. Process. Technol. 2015, 6, 1–8. [Google Scholar] [CrossRef]
- Rodrigues, L.; Banat, I.M.; Teixeira, J.; Oliveira, R. Biosurfactants: Potential applications in medicine. J. Antimicrob. Chemother. 2006, 57, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Asci, Y.; Nurbas, M.; Acikel, Y.S. A comparative study for the sorption of Cd(II) by soils with different clay contents and mineralogy and the recovery of Cd(II) using rhamnolipid biosurfactant. J. Hazard. Mater. 2008, 154, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Juwarkar, A.A.; Dubey, K.V.; Nair, A.; Singh, S.K. Bioremediation of multi-metal contaminated soil using biosurfactant—A novel approach. Indian J. Microbiol. 2008, 48, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Dahrazma, B.; Mulligan, C.N. Investigation of the removal of heavy metals from sediments using rhamnolipid in a continuous flow configuration. Chemosphere 2007, 69, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Mulligan, C.N. Rhamnolipid foam enhanced remediation of cadmium and nickel contaminated soil. Water Air Soil Pollut. 2004, 157, 315–330. [Google Scholar] [CrossRef]
- Nowack, B.; VanBriesen, J.M. Chelating agents in the environment. In Biogeochemistry of Chelating Agents; Nowack, B., VanBriesen, J.M., Eds.; ACS Symposium Series: Washington, DC, USA, 2005; pp. 1–18. [Google Scholar]
- Sillanpää, M.; Oikari, A. Assessing the impact of complexation by EDTA and DTPA on heavy metal toxicity using microtox bioassay. Chemosphere 1996, 32, 1485–1497. [Google Scholar] [CrossRef]
- Cao, A.; Carucci, A.; Lai, T.; La Colla, P.; Tamburini, E. Effect of biodegradable chelating agents on heavy metals phytoextraction with Mirabilis jalapa and on its associated bacteria. Eur. J. Soil Biol. 2007, 43, 200–206. [Google Scholar] [CrossRef]
- Kos, B.; Lestan, D. Influence of a biodegradable ([S,S]-EDDS) and nondegradable (EDTA) chelate and hydrogel modified soil water sorption capacity on Pb phytoextraction and leaching. Plant Soil 2003, 253, 403–411. [Google Scholar] [CrossRef]
- Jachuła, J.; Kołodyńska, D.; Hubicki, Z. Methylglycinediacetic acid as a new complexing agent for removal of heavy metal ions from industrial wastewater. Solvent Extr. Ion Exch. 2012, 30, 181–196. [Google Scholar] [CrossRef]
- Jessop, P.G.; Ahmadpour, F.; Buczynski, M.A.; Burns, T.J.; Green, N.B., II; Korwin, R.; Long, D.; Massad, S.K.; Manley, J.B.; Omidbakhsh, N.; et al. Opportunities for greener alternatives in chemical formulations. Green Chem. 2015, 17, 2664–2678. [Google Scholar] [CrossRef]
- Deshpande, P.; Dapkekar, A.; Oak, M.D.; Paknikar, K.M.; Rajwade, J.M. Zinc complexed chitosan/TPP nanoparticles: A promising micronutrient nanocarrier suited for foliar application. Carbohydr. Polym. 2017, 165, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, T.; Rothmaier, M.; Zander, H.; Ring, J.; Gutermuth, J.; Anliker, M.D. Acid-coated Textiles (pH 5.5–6.5)—A New Therapeutic Strategy for Atopic Eczema? Acta Derm. Venereol. 2015, 95, 659–663. [Google Scholar] [CrossRef] [PubMed]
- Rosen, M.J.; Kunjappu, J.T. Micelle Formation by Surfactants. In Surfactants and Interfacial Phenomena, 4th ed.; Rosen, M.J., Kunjappu, J.T., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 123–201. [Google Scholar]
- Sánchez, M.; Aranda, F.J.; Espuny, M.J.; Marqués, A.; Teruel, J.A.; Manresa, A.; Ortiz, A. Aggregation behaviour of a dirhamnolipid biosurfactant secreted by Pseudomonas aeruginosa in aqueous media. J. Colloid Interface Sci. 2007, 307, 246–253. [Google Scholar]
- Özdemir, G.; Peker, S.; Helvaci, S.S. Effect of pH on the surface and interfacial behavior of rhamnolipids R1 and R2. Colloids Surf. A Physicochem. Eng. Asp. 2004, 234, 135–143. [Google Scholar] [CrossRef]
- Helvaci, S.S.; Peker, S.; Özdemir, G. Effect of electrolytes on the surface behavior of rhamnolipids R1 and R2. Colloids Surf. B Biointerfaces 2004, 35, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Cieśla, J.; Bieganowski, A.; Narkiewicz-Michałek, J.; Szymula, M. Use of dynamic light scattering technique for SDS/water/pentanol studies. J. Disper. Sci. Technol. 2013, 34, 566–574. [Google Scholar] [CrossRef]
- Zhang, W.; Li, G.; Mu, J.; Shen, Q.; Zheng, L.; Liang, H.; Wu, C. Effect of KBr on the micellar properties of CTAB. Chin. Sci. Bull. 2000, 45, 1854–1857. [Google Scholar] [CrossRef]
- Dahrazma, B.; Mulligan, C.N.; Nieh, M.-P. Effects of additives on the structure of rhamnolipid (biosurfactant): A small-angle neutron scattering (SANS) study. J. Colloid Interface Sci. 2008, 319, 590–593. [Google Scholar] [CrossRef] [PubMed]
- Raza, Z.A.; Khalid, Z.M.; Khan, M.S.; Banat, I.M.; Rehman, A.; Naeem, A.; Saddique, M.T. Surface properties and sub-surface aggregate assimilation of rhamnolipid surfactants in different aqueous systems. Biotechnol. Lett. 2010, 32, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Hunter, R.J. The Calculation of Zeta Potential. In Zeta Potential in Colloid Science, Principles and Applications, 1st ed.; Academic Press: London, UK, 1988; pp. 59–124. [Google Scholar]
- Pinto, I.S.; Neto, I.F.; Soares, H.M. Biodegradable chelating agents for industrial, domestic, and agricultural applications—A review. Environ. Sci. Pollut. Res. 2014, 21, 11893–11906. [Google Scholar] [CrossRef] [PubMed]
- BASF. Technical Information Ti/EVD 1418 e—Trilon® M Types; BASF: Ludwigshafen, Germany, 2007. [Google Scholar]
- Hogan, D.E.; Curry, J.E.; Pemberton, J.E.; Maier, R.M. Rhamnolipid biosurfactant complexation of rare earth elements. J. Hazard. Mater. 2017, 340, 171–178. [Google Scholar] [CrossRef] [PubMed]
- ISO 13321. Particle Size Analysis—Photon Correlation Spectroscopy, International Organization for Standardization; ISO: Geneva, Switzerland, 1996. [Google Scholar]
- Kaszuba, M.; McKnight, D.; Connah, M.T.; McNeil-Watson, F.K.; Nobbmann, U. Measuring sub nanometre sizes using dynamic light scattering. J. Nanopart. Res. 2008, 10, 823–829. [Google Scholar] [CrossRef]
- Pecora, R. Dynamic Light Scattering Measurement of Nanometer Particles in Liquids. J. Nanopart. Res. 2000, 2, 123–131. [Google Scholar] [CrossRef]
- Malvern Instruments. Zetasizer Nano Series User Manual, MAN0317; Issue 1.1; Malvern Instruments Ltd.: Malvern, UK, 2004. [Google Scholar]
- Mayinger, F. (Ed.) Optical Measurements Techniques and Applications; Springer: Berlin, Germany, 1994. [Google Scholar]
- Malvern Instruments. Zetasizer Nano Application Note, Enhanced Protein Aggregation Detection Using Dual Angle Dynamic Light Scattering, MRK1441-01; Malvern Instruments Ltd.: Malvern, UK, 2010. [Google Scholar]
Sample Availability: Samples of the compounds rhamnolipids, methylglycinediacetic acid and ethylenediaminetetraacetic acid are not available from the authors. |

| pH | Analyzed Property | The CMC Determined in: | |||
|---|---|---|---|---|---|
| H2O | 20 mM KCl | ||||
| mg L–1 | mM | mg L–1 | mM | ||
| 5.5 | pH | 42.46 | 0.074 | 32.14 | 0.056 |
| EC ## | 66.39 | 0.115 | 34.04 | 0.059 | |
| 54.42 * | 0.094 * | 33.09 * | 0.057 * | ||
| 6.0 | pH | 69.64 | 0.112 | 58.28 | 0.101 |
| EC ## | 68.09 | 0.118 | 55.39 | 0.096 | |
| 68.86 * | 0.115 * | 56.83 * | 0.098 * | ||
| pH | Substance | H2O | 20 mM KCl | ||||
|---|---|---|---|---|---|---|---|
| C < CMCRh | C = CMCRh | C > CMCRh | C < CMCRh | C = CMCRh | C > CMCRh | ||
| 5.5 | Rhamnolipids | 95.82 ± 0.12 | 53.61 ± 1.37 | 77.63 ± 0.44 | 91.37 ± 0.51 | 51.72 ± 0.84 | 63.13 ± 1.34 |
| MGDA | 98.24 ± 0.03 | 93.50 ± 0.03 | 83.08 ± 0.09 | 98.58 ± 0.01 | 93.86 ± 0.01 | 82.63 ± 0.17 | |
| EDTA | 92.37 ± 0.20 | 91.28 ± 0.28 | 73.34 ± 0.21 | 98.44 ± 0.03 | 91.54 ± 0.15 | 81.65 ± 6.60 | |
| 6.0 | Rhamnolipids | 93.66 ± 0.18 | 64.06 ± 0.98 | 68.08 ± 0.58 | 58.08 ± 0.67 | 54.46 ± 0.47 | 58.83 ± 1.27 |
| MGDA | 98.47 ± 0.03 | 92.83 ± 0.02 | 86.32 ± 0.09 | 98.58 ± 0.00 | 93.85 ± 0.01 | 85.96 ± 0.18 | |
| EDTA | 95.13 ± 0.34 | 85.76 ± 0.14 | 79.23 ± 0.20 | 98.58 ± 0.00 | 92.52 ± 0.08 | 82.78 ± 0.12 | |
| pH | Substance | H2O | 20 mM KCl | ||||
|---|---|---|---|---|---|---|---|
| C < CMCRh | C = CMCRh | C > CMCRh | C < CMCRh | C = CMCRh | C > CMCRh | ||
| 5.5 | Rhamnolipids | 11.10 ± 0.01 | 8.50 ± 0.02 | 8.38 ± 0.01 | 10.75 ± 0.03 | 8.48 ± 0.01 | 8.07 ± 0.03 |
| MGDA | 14.86 ± 0.01 | 12.30 ± 0.00 | 10.93 ± 0.00 | 14.95 ± 0.00 | 12.33 ± 0.00 | 10.92 ± 0.00 | |
| EDTA | 18.22 ± 0.01 | 15.53 ± 0.01 | 13.75 ± 0.00 | 18.93 ± 0.01 | 15.57 ± 0.01 | 13.95 ± 0.15 | |
| 6.0 | Rhamnolipids | 10.51 ± 0.01 | 8.29 ± 0.02 | 7.77 ± 0.01 | 9.50 ± 0.01 | 8.12 ± 0.01 | 7.60 ± 0.02 |
| MGDA | 14.91 ± 0.03 | 12.24 ± 0.00 | 11.03 ± 0.00 | 14.94 ± 0.00 | 12.31 ± 0.00 | 11.02 ± 0.00 | |
| EDTA | 18.26 ± 0.01 | 15.12 ± 0.00 | 13.72 ± 0.00 | 18.81 ± 0.00 | 15.43 ± 0.00 | 13.82 ± 0.00 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cieśla, J.; Koczańska, M.; Bieganowski, A. An Interaction of Rhamnolipids with Cu2+ Ions. Molecules 2018, 23, 488. https://doi.org/10.3390/molecules23020488
Cieśla J, Koczańska M, Bieganowski A. An Interaction of Rhamnolipids with Cu2+ Ions. Molecules. 2018; 23(2):488. https://doi.org/10.3390/molecules23020488
Chicago/Turabian StyleCieśla, Jolanta, Magdalena Koczańska, and Andrzej Bieganowski. 2018. "An Interaction of Rhamnolipids with Cu2+ Ions" Molecules 23, no. 2: 488. https://doi.org/10.3390/molecules23020488
APA StyleCieśla, J., Koczańska, M., & Bieganowski, A. (2018). An Interaction of Rhamnolipids with Cu2+ Ions. Molecules, 23(2), 488. https://doi.org/10.3390/molecules23020488





