A Facile Route toward the Increase of Oxygen Content in Nanosized Zeolite by Insertion of Cerium and Fluorinated Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Nanosized Zeolite
2.2. Characterization of Nanosized Zeolite
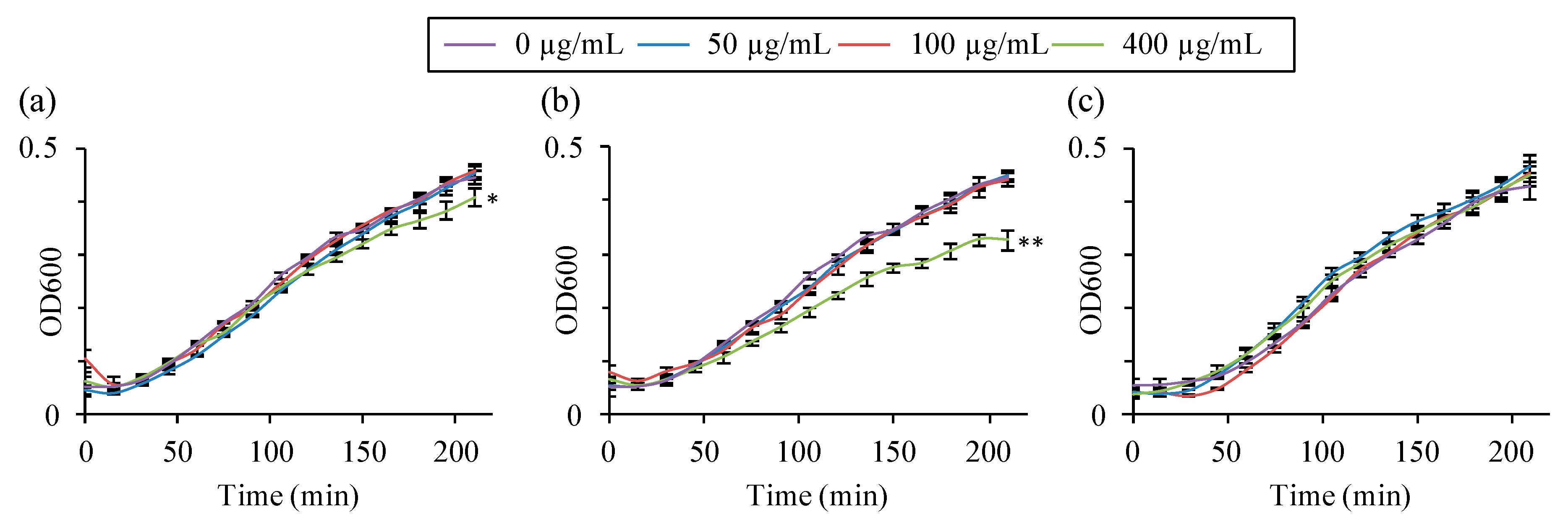
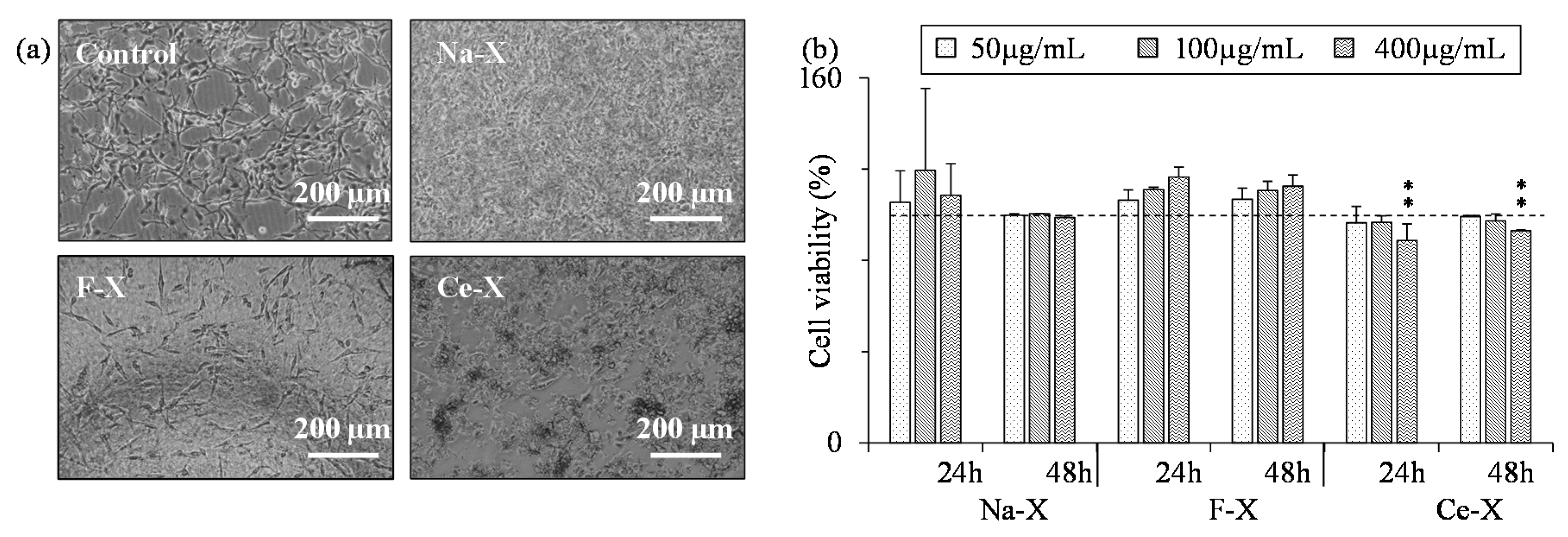
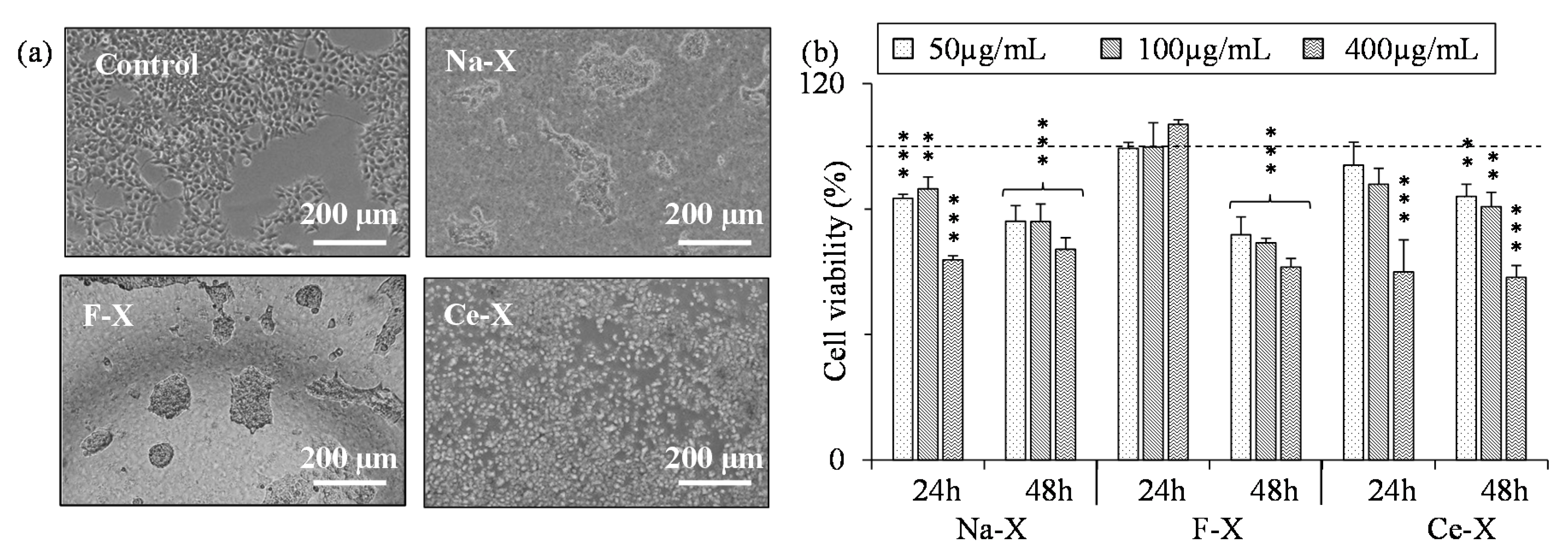
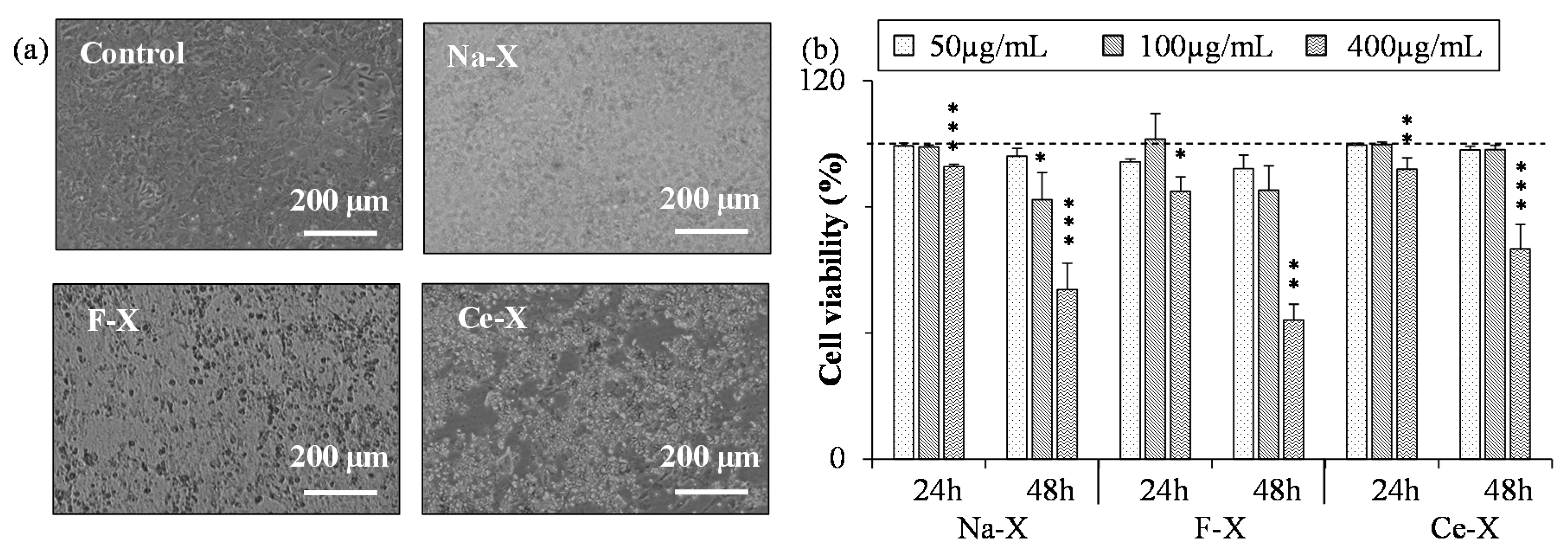
2.3. Toxicity Tests of Nanosized Zeolites
3. Results and Discussion
3.1. Structural Characterizations
3.2. Oxygen Adsorption Capacity of Nanosized Zeolites: In Situ IR Study
3.3. Toxicity Tests
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Peacock, A.J. ABC of oxygen: Oxygen at high altitude. BMJ 1998, 317, 1063–1066. [Google Scholar] [CrossRef] [PubMed]
- Litch, J.A.; Bishop, R.A. Oxygen concentrators for the delivery of supplemental oxygen in remote high-altitude areas. Wilderness Environ. Med. 2000, 11, 189–191. [Google Scholar] [CrossRef]
- Duke, T.; Wandi, F.; Jonathan, M.; Matai, S.; Kaupa, M.; Saavu, M.; Subhi, R.; Peel, D. Improved oxygen systems for childhood pneumonia: A multihospital effectiveness study in Papua New Guinea. Lancet 2008, 372, 1328–1333. [Google Scholar] [CrossRef]
- Friesen, R.M.; Raber, M.B.; Reimer, D.H. Oxygen concentrators: A primary oxygen supply source. Can. J. Anesth. 1999, 46, 1185–1190. [Google Scholar] [CrossRef] [PubMed]
- Zaarour, M.; Dong, B.; Naydenova, I.; Retoux, R.; Mintova, S. Progress in zeolite synthesis promotes advanced applications. Microporous Mesoporous Mater. 2014, 189, 11–21. [Google Scholar] [CrossRef]
- Gribov, E.N.; Cocina, D.; Spoto, G.; Bordiga, S.; Ricchiardi, G.; Zecchina, A. Vibrational and thermodynamic properties of Ar, N2, O2, H2 and CO adsorbed and condensed into (H,Na)-Y zeolite cages as studied by variable temperature IR spectroscopy. Phys. Chem. Chem. Phys. 2006, 8, 1186–1196. [Google Scholar] [CrossRef] [PubMed]
- Tawari, A.; Einicke, W.-D.; Gläser, R. Photocatalytic Oxidation of NO over Composites of Titanium Dioxide and Zeolite ZSM-5. Catalysts 2016, 6, 31. [Google Scholar] [CrossRef]
- Dong, B.; Belkhair, S.; Zaarour, M.; Fisher, L.; Verran, J.; Tosheva, L.; Retoux, R.; Gilson, J.-P.; Mintova, S. Silver confined within zeolite EMT nanoparticles: Preparation and antibacterial properties. Nanoscale 2014, 6, 10859–10864. [Google Scholar] [CrossRef] [PubMed]
- Zaarour, M.; El Roz, M.; Dong, B.; Retoux, R.; Aad, R.; Cardin, J.; Dufour, C.; Gourbilleau, F.; Gilson, J.P.; Mintova, S. Photochemical preparation of silver nanoparticles supported on zeolite crystals. Langmuir 2014, 30, 6250–6256. [Google Scholar] [CrossRef] [PubMed]
- Silaghi, M.-C.; Chizallet, C.; Raybaud, P. Challenges on molecular aspects of dealumination and desilication of zeolites. Microporous Mesoporous Mater. 2014, 191, 82–96. [Google Scholar] [CrossRef]
- Kubů, M.; Opanasenko, M.; Vitvarová, D. Desilication of SSZ-33 zeolite—Post-synthesis modification of textural and acidic properties. Catal. Today 2015, 243, 46–52. [Google Scholar] [CrossRef]
- Wilde, N.; Pelz, M.; Gebhardt, S.G.; Gläser, R. Highly efficient nano-sized TS-1 with micro-/mesoporosity from desilication and recrystallization for the epoxidation of biodiesel with H2O2. Green Chem. 2015, 17, 3378–3389. [Google Scholar] [CrossRef]
- Zheng, S.; Tanaka, H.; Jentys, A.; Lercher, J.A. Novel Model Explaining Toluene Diffusion in HZSM-5 after Surface Modification. J. Phys. Chem. B 2004, 108, 1337–1343. [Google Scholar] [CrossRef]
- Morris, R.E.; Wheatley, P.S. Gas storage in nanoporous materials. Angew. Chem. Int. Ed. Engl. 2008, 47, 4966–4981. [Google Scholar] [CrossRef] [PubMed]
- Mowbray, M.; Tan, X.; Wheatley, P.S.; Morris, R.E.; Weller, R.B. Topically Applied Nitric Oxide Induces T-Lymphocyte Infiltration in Human Skin, but Minimal Inflammation. J. Investig. Dermatol. 2008, 128, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Seifu, D.G.; Isimjan, T.T.; Mequanint, K. Tissue engineering scaffolds containing embedded fluorinated-zeolite oxygen vectors. Acta Biomater. 2011, 7, 3670–3678. [Google Scholar] [CrossRef] [PubMed]
- Lessi, S.; Schmutz, N. Carrier-Borne System for the Production of Oxygen-Enriched Gas Streams and Method for Supplying the Airways of the Occupants of an Aircraft. Patent No. CA 2520836 A1, 21 October 2004. [Google Scholar]
- Braschi, I.; Blasioli, S.; Gigli, L.; Gessa, C.E.; Alberti, A.; Martucci, A. Removal of sulfonamide antibiotics from water: Evidence of adsorption into an organophilic zeolite Y by its structural modifications. J. Hazard. Mater. 2010, 178, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Kim, Y.; Dutta, P.K. Controlled release of paraquat from surface-modified zeolite Y. Microporous Mesoporous Mater. 2006, 88, 312–318. [Google Scholar] [CrossRef]
- Redfern, J.; Goldyn, K.; Verran, J.; Retoux, R.; Tosheva, L.; Mintova, S. Application of Cu-FAU nanozeolites for decontamination of surfaces soiled with the ESKAPE pathogens. Microporous Mesoporous Mater. 2017, 253, 233–238. [Google Scholar] [CrossRef]
- Anfray, C.; Dong, B.; Komaty, S.; Mintova, S.; Valable, S. Acute Toxicity of Silver Free and Encapsulated in Nanosized Zeolite for Eukaryotic Cells. ACS Appl. Mater. Interfaces 2017, 9, 13849–13854. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.M.A.; Bonifacio, R.P.; Marrucho, I.M.; Padua, A.A.H.; Gomes, M.F.C. Solubility of oxygen in n-hexane and in n-perfluorohexane. Experimental determination and prediction by molecular simulation. Phys. Chem. Chem. Phys. 2003, 5, 543–549. [Google Scholar] [CrossRef]
- Riess, J.G. Oxygen Carriers (“Blood Substitutes”) Raison d’Etre, Chemistry, and Some Physiology Blut ist ein ganz besondrer Saft. Chem. Rev. 2001, 101, 2797–2920. [Google Scholar] [CrossRef] [PubMed]
- Gomes, L.; Gomes, E.R. Perfluorocarbons Compounts Used as Oxygen Carriers: From Liquid Ventilation to Blood Substitutes. Rev. Fac. Ciênc. Saúde. 2007, 4, 58–65. [Google Scholar]
- Dias, A.M.A.; Gonçalves, C.M.B.; Legido, J.L.; Coutinho, J.A.P.; Marrucho, I.M. Solubility of oxygen in substituted perfluorocarbons. Fluid Phase Equilib. 2005, 238, 7–12. [Google Scholar] [CrossRef]
- Saceda, J.J.F.; Rintramee, K.; Khabuanchalad, S.; Prayoonpokarach, S.; de Leon, R.L.; Wittayakun, J. Properties of zeolite Y in various forms and utilization as catalysts or supports for cerium oxide in ethanol oxidation. J. Ind. Eng. Chem. 2012, 18, 420–424. [Google Scholar] [CrossRef]
- Maupin, I.; Mijoin, J.; Barbier, J.; Bion, N.; Belin, T.; Magnoux, P. Improved oxygen storage capacity on CeO2/zeolite hybrid catalysts. Application to VOCs catalytic combustion. Catal. Today 2011, 176, 103–109. [Google Scholar] [CrossRef]
- Dai, Q.; Wang, X.; Lu, G. Low-temperature catalytic destruction of chlorinated VOCs over cerium oxide. Catal. Commun. 2007, 8, 1645–1649. [Google Scholar] [CrossRef]
- Awala, H.; Gilson, J.-P.; Retoux, R.; Boullay, P.; Goupil, J.-M.; Valtchev, V.; Mintova, S. Template-free nanosized faujasite-type zeolites. Nat. Mater. 2015, 14, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Santra, S.; Archipov, T.; Ene, A.B.; Komnik, H.; Stoll, H.; Roduner, E.; Rauhut, G. Adsorption of dioxygen to copper in CuHY zeolite. Phys. Chem. Chem. Phys. 2009, 11, 8855–8866. [Google Scholar] [CrossRef] [PubMed]
- Ene, A.B.; Matthias, B.; Archipov, T.; Roduner, E. Hydroxyapatite as a key biomaterial: Quantum-mechanical simulation of its surfaces in interaction with biomolecules. Phys. Chem. Chem. Phys. 2010, 12, 6520–6531. [Google Scholar] [CrossRef] [PubMed]
- De Lara, E.R.C. Experimental and theoretical determination of vibrational frequency shifts of diatomic molecules adsorbed in NaA zeolite. Mol. Phys. 1989, 66, 479–492. [Google Scholar] [CrossRef]
- Bier, K.D.; Jodl, H.J. Influence of temperature on elementary excitations in solid oxygen by Raman studies. J. Chem. Phys. 1984, 81, 1192–1197. [Google Scholar] [CrossRef]
- Benaliouche, F.; Boucheffa, Y.; Ayrault, P.; Mignard, S.; Magnoux, P. NH3-TPD and FTIR spectroscopy of pyridine adsorption studies for characterization of Ag- and Cu-exchanged X zeolites. Microporous Mesoporous Mater. 2008, 111, 80–88. [Google Scholar] [CrossRef]
- Baur, G.B.; Héroguel, F.; Spring, J.; Luterbacher, J.S.; Kiwi-Minsker, L. Hydrothermally-treated Na-X as efficient adsorbents for butadiene removal. Chem. Eng. J. 2016, 288, 19–27. [Google Scholar] [CrossRef]
Sample Availability: The samples Na-X, Ce-X, and F-X are available in LCS. |
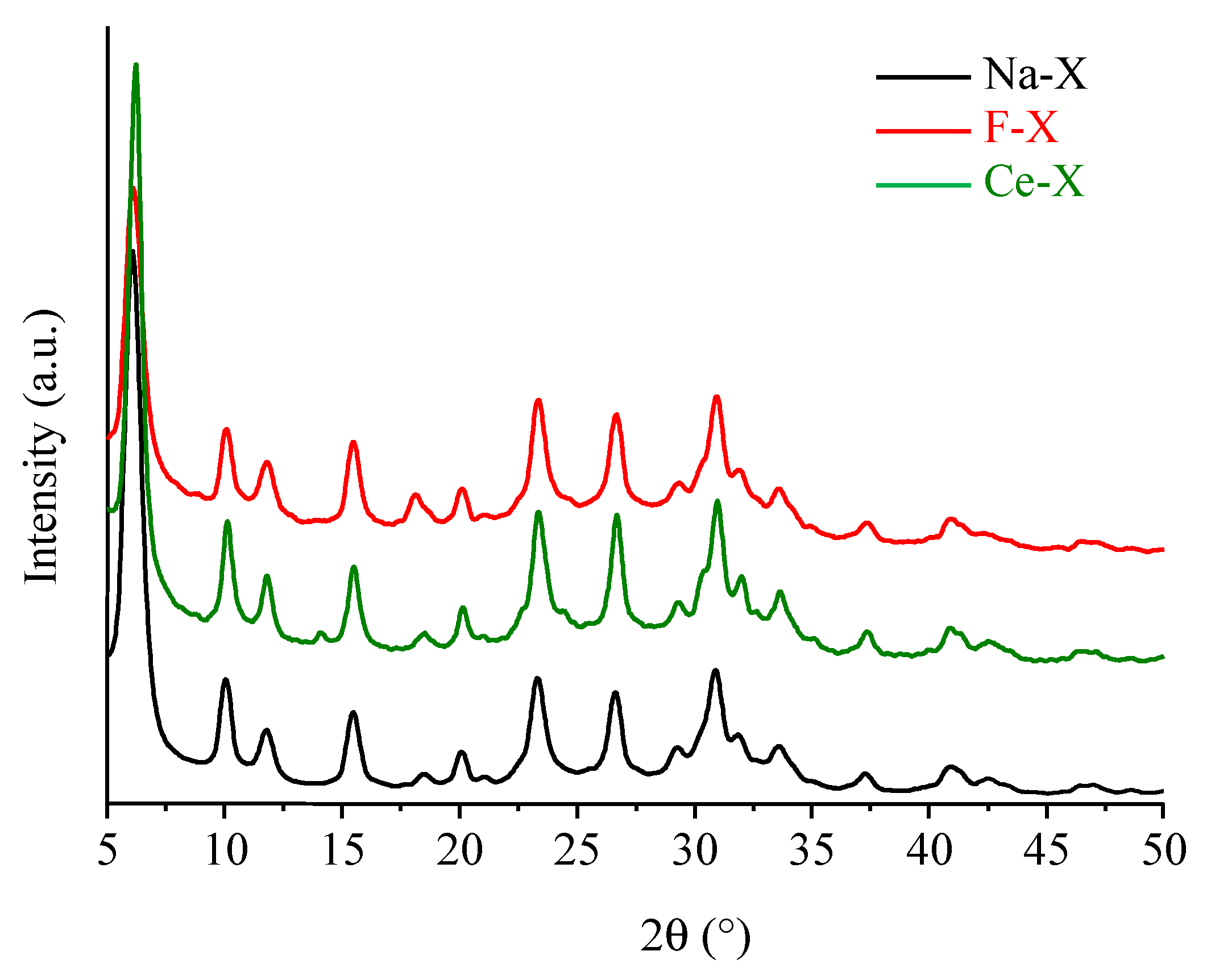
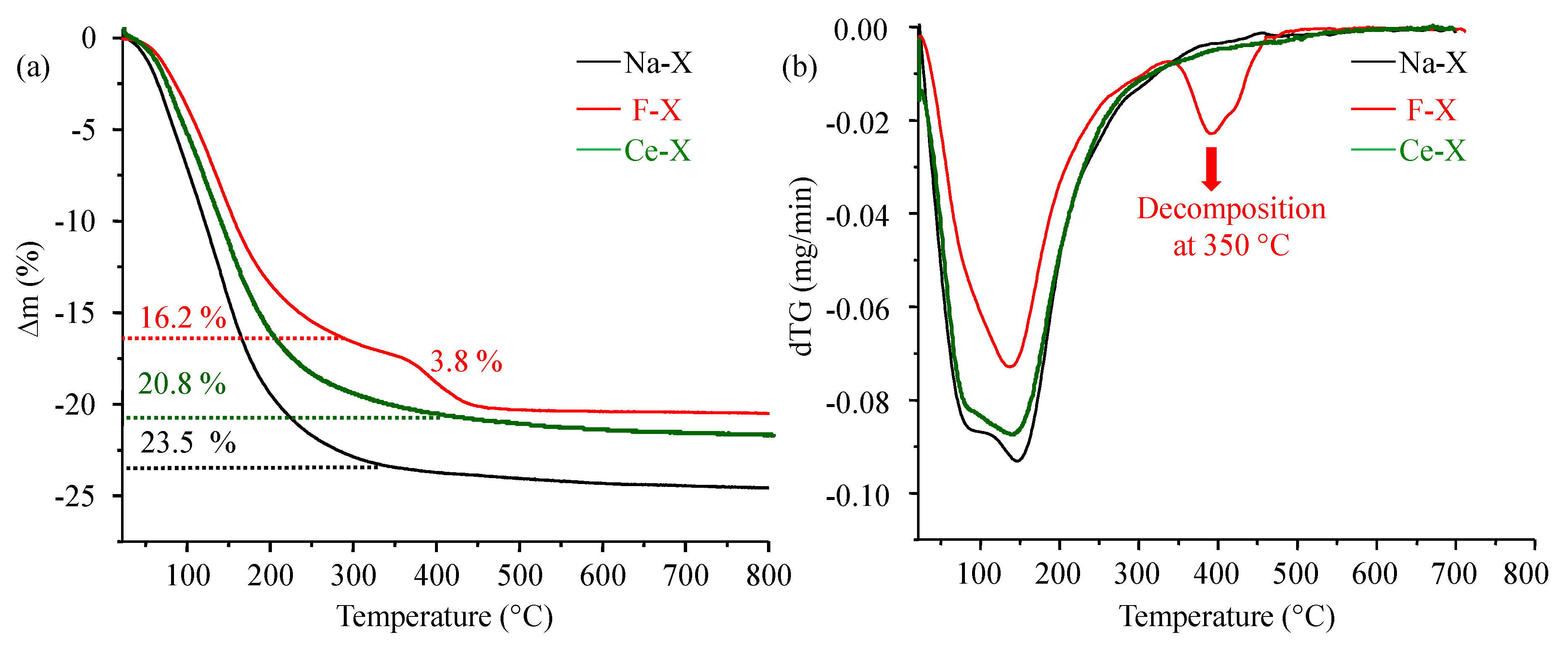

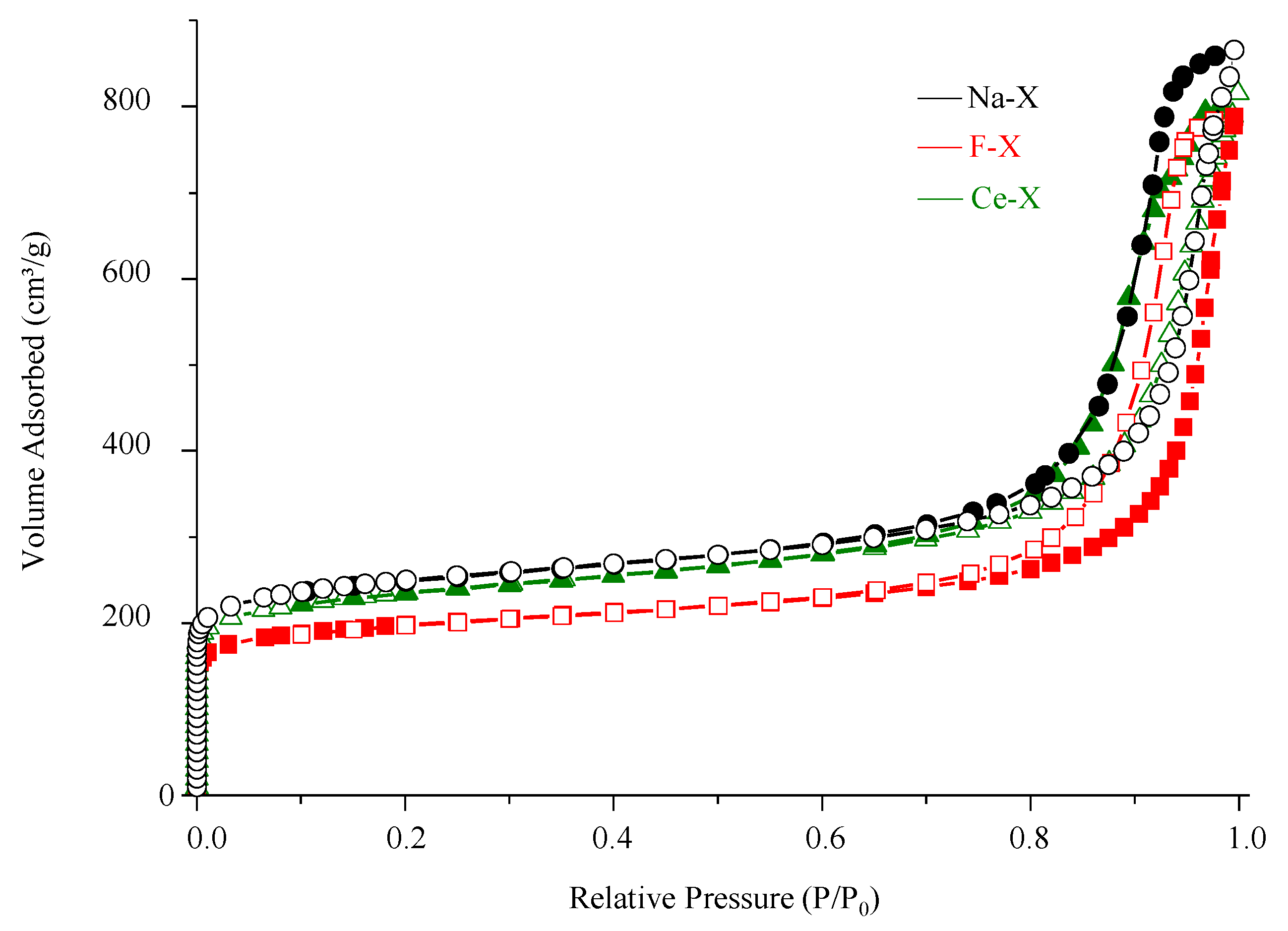
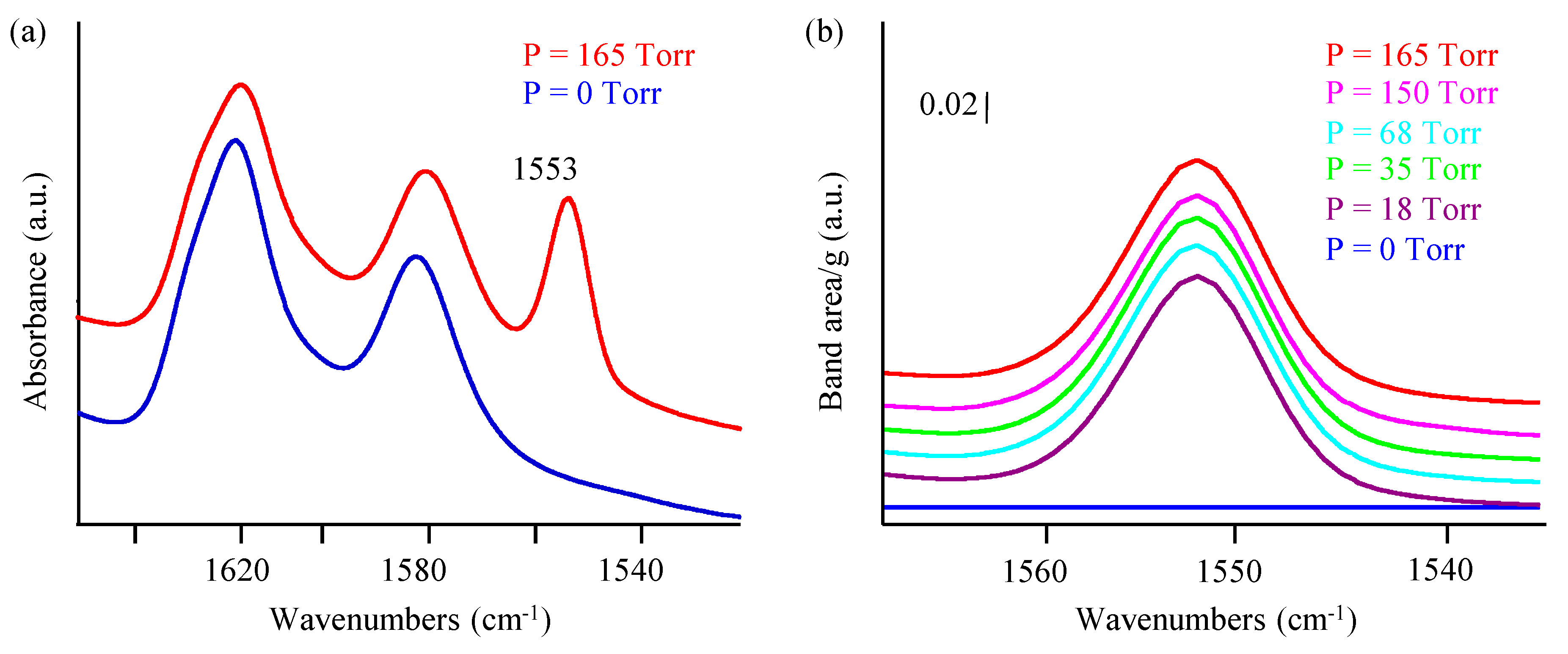

| SBET (m2∙g−1) | Vmic (cm3∙g−1) | Vtotal (cm3∙g−1) | |
|---|---|---|---|
| Na-X | 880 | 0.30 | 1.40 |
| F-X | 825 | 0.27 | 1.28 |
| Ce-X | 875 | 0.28 | 1.35 |
| Zeolite | Astrocytes | HEK 293T | U87-MG | |||
|---|---|---|---|---|---|---|
| 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | |
| Na-X | N/A | 422 ± 100 | 569 ± 61 | 768 ± 210 | N/A | N/A |
| F-X | N/A | 359 ± 34 | N/A | 528 ± 75 | N/A | N/A |
| Ce-X | N/A | 622 ± 141 | 504 ± 71 | 474 ± 41 | N/A | N/A |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komaty, S.; Anfray, C.; Zaarour, M.; Awala, H.; Ruaux, V.; Valable, S.; Mintova, S. A Facile Route toward the Increase of Oxygen Content in Nanosized Zeolite by Insertion of Cerium and Fluorinated Compounds. Molecules 2018, 23, 37. https://doi.org/10.3390/molecules23020037
Komaty S, Anfray C, Zaarour M, Awala H, Ruaux V, Valable S, Mintova S. A Facile Route toward the Increase of Oxygen Content in Nanosized Zeolite by Insertion of Cerium and Fluorinated Compounds. Molecules. 2018; 23(2):37. https://doi.org/10.3390/molecules23020037
Chicago/Turabian StyleKomaty, Sarah, Clément Anfray, Moussa Zaarour, Hussein Awala, Valérie Ruaux, Samuel Valable, and Svetlana Mintova. 2018. "A Facile Route toward the Increase of Oxygen Content in Nanosized Zeolite by Insertion of Cerium and Fluorinated Compounds" Molecules 23, no. 2: 37. https://doi.org/10.3390/molecules23020037
APA StyleKomaty, S., Anfray, C., Zaarour, M., Awala, H., Ruaux, V., Valable, S., & Mintova, S. (2018). A Facile Route toward the Increase of Oxygen Content in Nanosized Zeolite by Insertion of Cerium and Fluorinated Compounds. Molecules, 23(2), 37. https://doi.org/10.3390/molecules23020037





