Effects of Phytochemical P-Glycoprotein Modulators on the Pharmacokinetics and Tissue Distribution of Doxorubicin in Mice
Abstract
:1. Introduction
2. Results
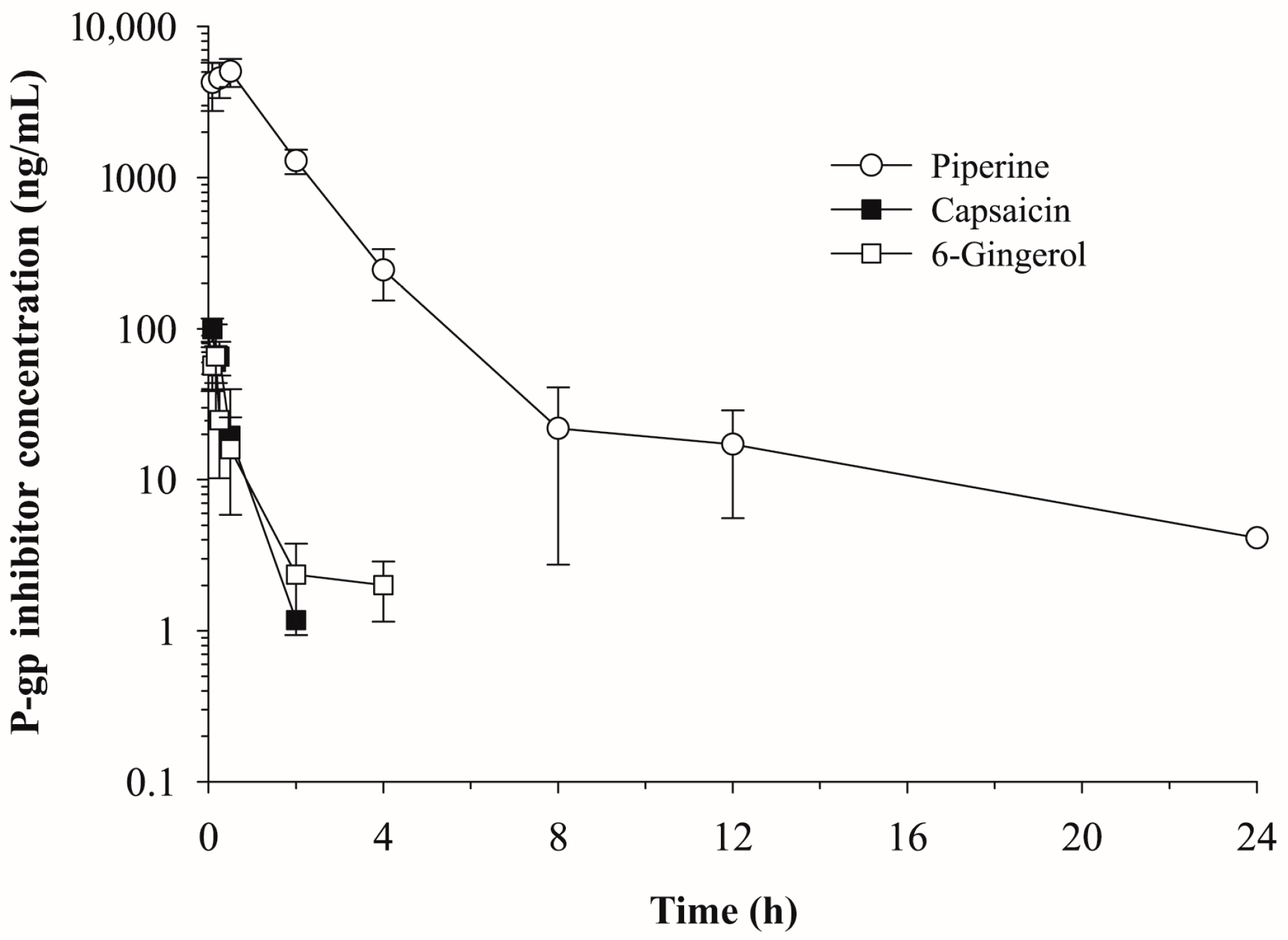
2.1. Pharmacokinetics of Piperine, Capsaicin and [6]-Gingerol
2.2. Effects of Piperine, Capsaicin and [6]-Gingerol on the Plasma Pharmacokinetics of Doxorubicin
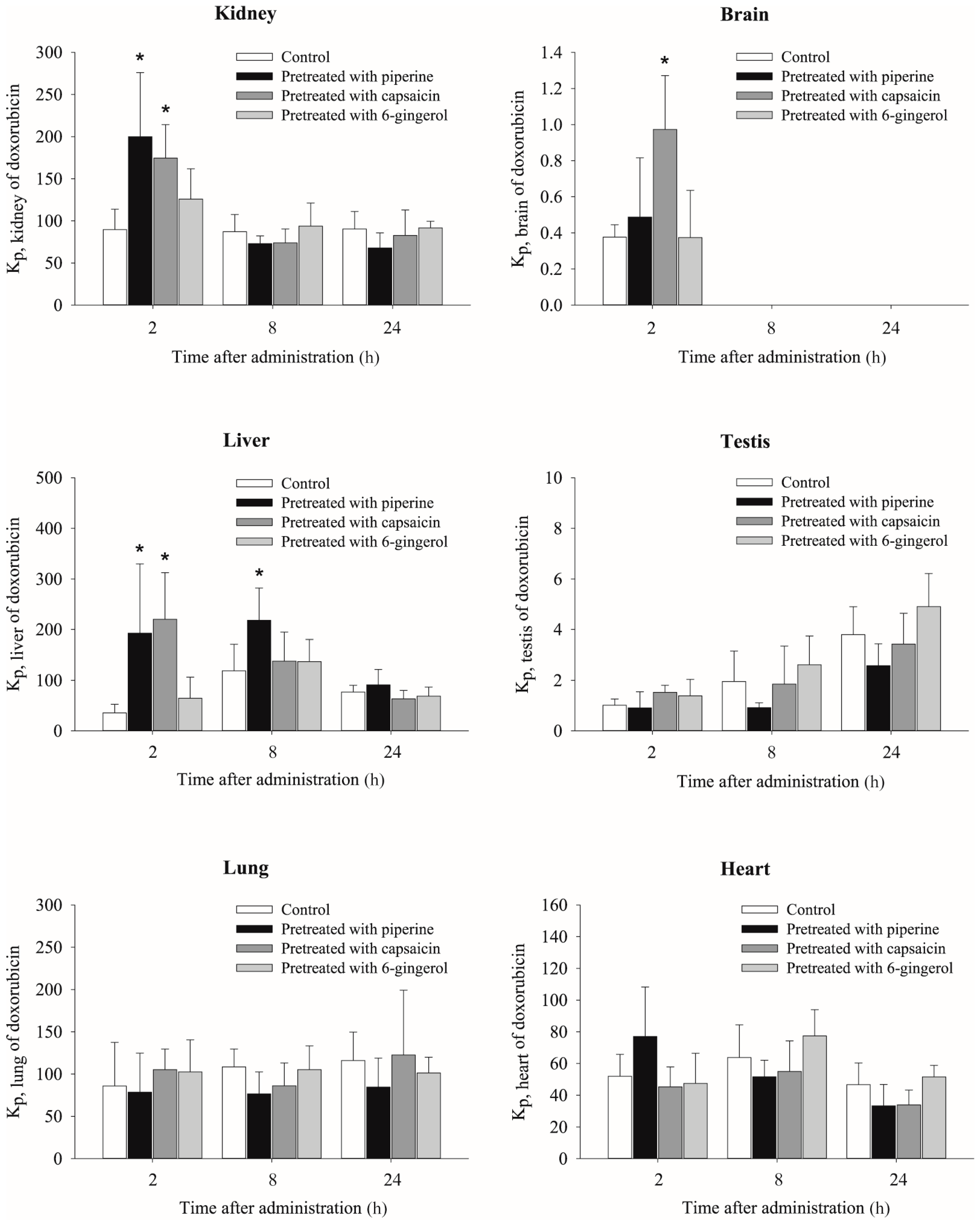
2.3. Effects of Piperine, Capsaicin and [6]-Gingerol on Tissue Distribution of Doxorubicin
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animal Study
4.3. LC-MS/MS
4.4. Data Analysis
4.5. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hester, R.E.; Harrison, R.M. Food Safety and Food Quality; Royal Society of Chemistry: Cambridge, UK, 2001. [Google Scholar]
- Govindarajan, V.S.; Sathyanarayana, M.N. Capsicum—Production, technology, chemistry and quality. Part V. Impact on physiology, pharmacology, nutrition and metabolism; structure, pungency, pain and desensitization sequences. Crit. Rev. Food Sci. Nutr. 1991, 29, 435–474. [Google Scholar] [CrossRef] [PubMed]
- Scientific Committee on Food. Opinion of the Scientific Committee on Food on Capsaicin; European Commission: Brussel, Belgium, 2002. [Google Scholar]
- Bhardwaj, R.K.; Glaeser, H.; Becquemont, L.; Klotz, U.; Gupta, S.K.; Fromm, M.F. Piperine, a major constituent of black pepper, inhibits human P-glycoprotein and cyp3a4. J. Pharmacol. Exp. Ther. 2002, 302, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Tan, T.M.C.; Lim, L.Y. In vitro and in vivo evaluation of the effects of piperine on P-gp function and expression. Toxicol. Appl. Pharmacol. 2008, 230, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Tan, T.M.; Lim, L.Y. Effects of capsaicin on P-gp function and expression in caco-2 cells. Biochem. Pharmacol. 2006, 71, 1727–1734. [Google Scholar] [CrossRef] [PubMed]
- Nabekura, T.; Kamiyama, S.; Kitagawa, S. Effects of dietary chemopreventive phytochemicals on p-glycoprotein function. Biochem. Biophys. Res. Commun. 2005, 327, 866–870. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Lim, L.Y. Effects of spice constituents on p-glycoprotein-mediated transport and cyp3a4-mediated metabolism in vitro. Drug Metab. Dispos. 2008, 36, 1283–1290. [Google Scholar] [CrossRef] [PubMed]
- Eichhorn, T.; Efferth, T. P-glycoprotein and its inhibition in tumors by phytochemicals derived from chinese herbs. J. Ethnopharmacol. 2012, 141, 557–570. [Google Scholar] [CrossRef] [PubMed]
- Kemper, E.M.; Boogerd, W.; Thuis, I.; Beijnen, J.H.; van Tellingen, O. Modulation of the blood-brain barrier in oncology: Therapeutic opportunities for the treatment of brain tumours? Cancer Treat. Rev. 2004, 30, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Van Asperen, J.; Schinkel, A.H.; Beijnen, J.H.; Nooijen, W.J.; Borst, P.; van Tellingen, O. Altered pharmacokinetics of vinblastine in mdr1a P-glycoprotein-deficient mice. J. Natl. Cancer Inst. 1996, 88, 994–999. [Google Scholar] [CrossRef] [PubMed]
- Van Asperen, J.; van Tellingen, O.; Tijssen, F.; Schinkel, A.H.; Beijnen, J.H. Increased accumulation of doxorubicin and doxorubicinol in cardiac tissue of mice lacking mdr1a p-glycoprotein. Br. J. Cancer 1999, 79, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Mayer, U.; Wagenaar, E.; Beijnen, J.H.; Smit, J.W.; Meijer, D.K.; van Asperen, J.; Borst, P.; Schinkel, A.H. Substantial excretion of digoxin via the intestinal mucosa and prevention of long-term digoxin accumulation in the brain by the mdr 1a P-glycoprotein. Br. J. Pharmacol. 1996, 119, 1038–1044. [Google Scholar] [CrossRef] [PubMed]
- Sparreboom, A.; van Asperen, J.; Mayer, U.; Schinkel, A.H.; Smit, J.W.; Meijer, D.K.; Borst, P.; Nooijen, W.J.; Beijnen, J.H.; van Tellingen, O. Limited oral bioavailability and active epithelial excretion of paclitaxel (taxol) caused by P-glycoprotein in the intestine. Proc. Natl. Acad. Sci. USA 1997, 94, 2031–2035. [Google Scholar] [CrossRef] [PubMed]
- Glavinas, H.; Krajcsi, P.; Cserepes, J.; Sarkadi, B. The role of abc transporters in drug resistance, metabolism and toxicity. Curr. Drug Deliv. 2004, 1, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Sarisozen, C.; Pan, J.; Dutta, I.; Torchilin, V.P. Polymers in the co-delivery of sirna and anticancer drugs to treat multidrug-resistant tumors. J. Pharm. Investig. 2017, 47, 37–49. [Google Scholar] [CrossRef]
- Marchetti, S.; Mazzanti, R.; Beijnen, J.H.; Schellens, J.H. Concise review: Clinical relevance of drug drug and herb drug interactions mediated by the abc transporter abcb1 (mdr1, P-glycoprotein). Oncologist 2007, 12, 927–941. [Google Scholar] [CrossRef] [PubMed]
- Ecker, G.; Chiba, P. Structure-activity-relationship studies on modulators of the multidrug transporter P-glycoprotein—An overview. Wiener Klinische Wochenschrift 1995, 107, 681–686. [Google Scholar] [PubMed]
- Montesinos, R.N.; Moulari, B.; Gromand, J.; Beduneau, A.; Lamprecht, A.; Pellequer, Y. Coadministration of P-glycoprotein modulators on loperamide pharmacokinetics and brain distribution. Drug Metab. Dispos. 2014, 42, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Salama, N.N.; Kelly, E.J.; Bui, T.; Ho, R.J. The impact of pharmacologic and genetic knockout of P-glycoprotein on nelfinavir levels in the brain and other tissues in mice. J. Pharm. Sci. 2005, 94, 1216–1225. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.; Zeitlinger, M.; Todorut, D.; Bohmdorfer, M.; Muller, M.; Langer, O.; Jager, W. Pharmacokinetics of single ascending doses of the P-glycoprotein inhibitor tariquidar in healthy subjects. Pharmacology 2013, 91, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Dugasani, S.; Pichika, M.R.; Nadarajah, V.D.; Balijepalli, M.K.; Tandra, S.; Korlakunta, J.N. Comparative antioxidant and anti-inflammatory effects of [6]-gingerol, [8]-gingerol, [10]-gingerol and [6]-shogaol. J. Ethnopharmacol. 2010, 127, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S.; Kawada, T.; Kim, B.S.; Han, I.S.; Choe, S.Y.; Kurata, T.; Yu, R. Capsaicin exhibits anti-inflammatory property by inhibiting IkB-a degradation in LPS-stimulated peritoneal macrophages. Cell Signal. 2003, 15, 299–306. [Google Scholar] [CrossRef]
- Park, K.K.; Chun, K.S.; Lee, J.M.; Lee, S.S.; Surh, Y.J. Inhibitory effects of [6]-gingerol, a major pungent principle of ginger, on phorbol ester-induced inflammation, epidermal ornithine decarboxylase activity and skin tumor promotion in ICR mice. Cancer Lett. 1998, 129, 139–144. [Google Scholar] [CrossRef]
- Surh, Y.-J. Anti-tumor promoting potential of selected spice ingredients with antioxidative and anti-inflammatory activities: A short review. Food Chem. Toxicol. 2002, 40, 1091–1097. [Google Scholar] [CrossRef]
- Young, H.Y.; Luo, Y.L.; Cheng, H.Y.; Hsieh, W.C.; Liao, J.C.; Peng, W.H. Analgesic and anti-inflammatory activities of [6]-gingerol. J. Ethnopharmacol. 2005, 96, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.C.; Lokesh, B.R. Studies on spice principles as antioxidants in the inhibition of lipid peroxidation of rat liver microsomes. Mol. Cell. Biochem. 1992, 111, 117–124. [Google Scholar] [PubMed]
- Surh, Y.J. Cancer chemoprevention with dietary phytochemicals. Nat. Rev. Cancer 2003, 3, 768–780. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, H.; Ohshima, Y.; Nomoto, H.; Fujita, K.; Matsuda, E.; Iigo, M.; Takasuka, N.; Moore, M.A. Cancer prevention by natural compounds. Drug Metab. Pharmacokinet. 2004, 19, 245–263. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lei, Y.; Jia, Y.; Li, N.; Wink, M.; Ma, Y. Piperine, a piperidine alkaloid from piper nigrum re-sensitizes P-gp, MRP1 and BCRP dependent multidrug resistant cancer cells. Phytomedicine 2011, 19, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Van der Sandt, I.C.; Blom-Roosemalen, M.C.; de Boer, A.G.; Breimer, D.D. Specificity of doxorubicin versus rhodamine-123 in assessing p-glycoprotein functionality in the LLC-PK1, LLC-PK1:MDR1 and Caco-2 cell lines. Eur. J. Pharm. Sci. 2000, 11, 207–214. [Google Scholar] [CrossRef]
- Gustafson, D.L.; Long, M.E. Alterations in p-glycoprotein expression in mouse tissues by doxorubicin: Implications for pharmacokinetics in multiple dosing regimens. Chem. Biol. Interact. 2001, 138, 43–57. [Google Scholar] [CrossRef]
- Bano, G.; Amla, V.; Raina, R.K.; Zutshi, U.; Chopra, C.L. The effect of piperine on pharmacokinetics of phenytoin in healthy volunteers. Planta Med. 1987, 53, 568–569. [Google Scholar] [CrossRef] [PubMed]
- Bano, G.; Raina, R.K.; Zutshi, U.; Bedi, K.L.; Johri, R.K.; Sharma, S.C. Effect of piperine on bioavailability and pharmacokinetics of propranolol and theophylline in healthy volunteers. Eur. J. Clin. Pharmacol. 1991, 41, 615–617. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhai, X.; Zhu, C.; Lu, Y. Effects of capsaicin on pharmacokinetics of pitavastatin in rats. Xenobiotica 2015, 45, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.J.; Han, H.K. Effect of piperine, a major component of black pepper, on the intestinal absorption of fexofenadine and its implication on food-drug interaction. J. Food Sci. 2010, 75, H93–H96. [Google Scholar] [CrossRef] [PubMed]
- Okonta, J.M.; Uboh, M.; Obonga, W.O. Herb-drug interaction: A case study of effect of ginger on the pharmacokinetic of metronidazole in rabbit. Indian J. Pharm. Sci. 2008, 70, 230–232. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.J.; Chen, J.G.; Liu, J.M.; Shi, F.; Lu, Y.N. Food-drug interactions: Effect of capsaicin on the pharmacokinetics of simvastatin and its active metabolite in rats. Food Chem. Toxicol. 2013, 53, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.J.; Lu, Y.N. Food-drug interactions: Effect of capsaicin on the pharmacokinetics of galantamine in rats. Xenobiotica 2012, 42, 1151–1155. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.J.; Shi, F.; Chen, F.; Lu, Y.N. Capsaicin pretreatment increased the bioavailability of cyclosporin in rats: Involvement of P-glycoprotein and CYP 3a inhibition. Food Chem. Toxicol. 2013, 62, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Bhat, B.G.; Chandrasekhara, N. Studies on the metabolism of piperine: Absorption, tissue distribution and excretion of urinary conjugates in rats. Toxicology 1986, 40, 83–92. [Google Scholar] [CrossRef]
- Terasaki, T.; Iga, T.; Sugiyama, Y.; Hanano, M. Pharmacokinetic study on the mechanism of tissue distribution of doxorubicin: Interorgan and interspecies variation of tissue-to-plasma partition coefficients in rats, rabbits and guinea pigs. J. Pharm. Sci. 1984, 73, 1359–1363. [Google Scholar] [CrossRef] [PubMed]
- Ballet, F.; Vrignaud, P.; Robert, J.; Rey, C.; Poupon, R. Hepatic extraction, metabolism and biliary excretion of doxorubicin in the isolated perfused rat liver. Cancer Chemother. Pharmacol. 1987, 19, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Tavoloni, N.; Guarino, A.M. Bile secretory function: A determinant of adriamycin disposition. Arch. Int. Pharmacodyn. Ther. 1980, 245, 180–197. [Google Scholar] [PubMed]
- Tavoloni, N.; Guarino, A.M. Biliary and urinary excretion of adriamycin in anesthetized rats. Pharmacology 1980, 20, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Thiebaut, F.; Tsuruo, T.; Hamada, H.; Gottesman, M.M.; Pastan, I.; Willingham, M.C. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc. Natl. Acad. Sci. USA 1987, 84, 7735–7738. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, E.; Demeule, M.; Ghitescu, L.; Beliveau, R. P-glycoprotein is strongly expressed in the luminal membranes of the endothelium of blood vessels in the brain. Biochem. J. 1997, 326 Pt 2, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Van Asperen, J.; van Tellingen, O.; Beijnen, J.H. The role of mdr1a P-glycoprotein in the biliary and intestinal secretion of doxorubicin and vinblastine in mice. Drug Metab. Dispos. 2000, 28, 264–267. [Google Scholar] [PubMed]
- Han, H.K. The effects of black pepper on the intestinal absorption and hepatic metabolism of drugs. Expert Opin. Drug Metab. Toxicol. 2011, 7, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Melaine, N.; Lienard, M.O.; Dorval, I.; Le Goascogne, C.; Lejeune, H.; Jegou, B. Multidrug resistance genes and p-glycoprotein in the testis of the rat, mouse, guinea pig and human. Biol. Reprod. 2002, 67, 1699–1707. [Google Scholar] [CrossRef] [PubMed]
- Croop, J.M.; Raymond, M.; Haber, D.; Devault, A.; Arceci, R.J.; Gros, P.; Housman, D.E. The three mouse multidrug resistance (mdr) genes are expressed in a tissue-specific manner in normal mouse tissues. Mol. Cell. Biol. 1989, 9, 1346–1350. [Google Scholar] [CrossRef] [PubMed]
- Singal, P.K.; Iliskovic, N. Doxorubicin-induced cardiomyopathy. N. Engl. J. Med. 1998, 339, 900–905. [Google Scholar] [CrossRef] [PubMed]
- Bellamy, W.T.; Peng, Y.M.; Odeleye, A.; Ellsworth, L.; Xu, M.J.; Grogan, T.M.; Weinstein, R.S. Cardiotoxicity in the scid mouse following administration of doxorubicin and cyclosporin a. Anticancer Drugs 1995, 6, 736–743. [Google Scholar] [CrossRef] [PubMed]
- Darvari, R.; Boroujerdi, M. Investigation of the influence of modulation of P-glycoprotein by a multiple dosing regimen of tamoxifen on the pharmacokinetics and toxicodynamics of doxorubicin. Cancer Chemother. Pharmacol. 2005, 56, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Candussio, L.; Decorti, G.; Crivellato, E.; Granzotto, M.; Rosati, A.; Giraldi, T.; Bartoli, F. Toxicologic and pharmacokinetic study of low doses of verapamil combined with doxorubicin. Life Sci. 2002, 71, 3109–3119. [Google Scholar] [CrossRef]
- Piyachaturawat, P.; Glinsukon, T.; Toskulkao, C. Acute and subacute toxicity of piperine in mice, rats and hamsters. Toxicol. Lett. 1983, 16, 351–359. [Google Scholar] [CrossRef]
- Nagabhushan, M.; Bhide, S.V. Mutagenicity of chili extract and capsaicin in short-term tests. Environ. Mutagen. 1985, 7, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.; Yamamoto, M. Acute oral toxicity of capsaicin in mice and rats. J. Toxicol. Sci. 1996, 21, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Suekawa, M.; Ishige, A.; Yuasa, K.; Sudo, K.; Aburada, M.; Hosoya, E. Pharmacological studies on ginger. I. Pharmacological actions of pungent constitutents, (6)-gingerol and (6)-shogaol. J. Pharmacobiodyn. 1984, 7, 836–848. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |


| Phytochemical | Cell Line | Substrate | Effects | Reference |
|---|---|---|---|---|
| Piperine | Caco-2 | Digoxin Cyclosporin A | Inhibited P-gp mediated efflux transport | [4] |
| Caco-2 L-MDR1 | [3H] Digoxin | Inhibited P-gp mediated efflux transport | [5] | |
| MCF-7/DOX | Rhodamine 123 Mitoxantrone | Inhibited efflux activity of P-gp, MRP1 and BCRP | [30] | |
| A-549/DDP | Doxorubicin | Inhibited efflux activity of P-gp, MRP1 and BCRP | [30] | |
| MCF-7/DOX A-549/DDP | Doxorubicin | Increased cytotoxicity by reversing transporter mediated doxorubicin and mitoxantrone resistance | [30] | |
| MCF-7/DOX | Mitoxantrone | Increased cytotoxicity by reversing transporter mediated doxorubicin and mitoxantrone resistance | [30] | |
| MCF-7/DOX A-549/DDP | - | Inhibited transcription of the corresponding ABC transporter genes | [30] | |
| Capsaicin | KB-C2 | Daunorubicin | Increased cellular accumulation | [7] |
| KB-C2 | Rhodamine 123 | Inhibited efflux transport | [7] | |
| KB-C2 | Vinblastine | Increased vinblastine cytotoxicity by inhibition of efflux transporter | [7] | |
| Caco-2 | [3H] Digoxin | Inhibited P-gp mediated efflux transport | [6] | |
| [6]-Gingerol | KB-C2 | Daunorubicin | Increased cellular accumulation | [7] |
| KB-C2 | Rhodamine 123 | Inhibited efflux transport | [7] | |
| KB-C2 | Vinblastine | Increased vinblastine cytotoxicity by inhibition of efflux transporter | [7] | |
| Caco-2 L-MDR1 | [3H] Digoxin | Inhibited P-gp mediated efflux transport | [8] |
| Parameters | Piperine (10 mg/kg) | Capsaicin (5 mg/kg) | [6]-Gingerol (5 mg/kg) |
|---|---|---|---|
| t1/2 (h) | 6.30 | 0.33 | 1.36 |
| Tmax (h) | 0.75 | 0.17 | 0.17 |
| Cmax (ng/mL) | 5050.97 | 118.90 | 153.37 |
| AUCinf (ng·h/mL) | 9956.92 | 66.83 | 66.34 |
| CL/F (mL/min/kg) | 16.74 | 1247.02 | 1256.20 |
| Vz/F (L/kg) | 9.12 | 35.66 | 147.54 |
| Parameters | Pretreatment | |||
|---|---|---|---|---|
| Control | Piperine | Capsaicin | [6]-Gingerol | |
| t1/2 (h) | 17.39 | 15.68 | 13.27 | 15.48 |
| C0 (ng/mL) | 3984.48 | 3404.57 | 4428.01 | 4036.96 |
| AUCinf (ng·h/mL) | 537.08 | 651.03 | 661.02 | 579.44 |
| CL (mL/min/kg) | 31.03 | 25.60 | 25.21 | 28.76 |
| Vz (L/kg) | 46.72 | 34.75 | 28.96 | 38.53 |
| Control | Piperine | Capsaicin | [6]-Gingerol | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Concentration (ng/mL or ng/g) | Kp | Concentration (ng/mL or ng/g) | Kp | Concentration (ng/mL or ng/g) | Kp | Concentration (ng/mL or ng/g) | Kp | ||
| 2 h | Plasma | 19.4 ± 5.0 | - | 18.2 ± 8.1 | - | 18.5 ± 4.8 | - | 23.4 ± 5.3 | - |
| Kidney | 2303 ± 686.9 | 89.6 ± 24.3 | 2840.8 ± 431.2 | 199.8 ± 76.3 * | 3131.9 ± 255.6 * | 174.5 ± 39.9 * | 2756.7 ± 536.7 | 125.8 ± 36.2 | |
| Brain | 8.2 ± 2.4 | 0.3 ± 0.1 | 7.0 ± 3.5 | 0.5 ± 0.3 | 18.1 ± 7.7 * | 1.0 ± 0.3 * | 7.9 ± 4.2 | 0.4 ± 0.3 | |
| Liver | 909.7 ± 458.5 | 35.4 ± 17.0 | 2488.1 ± 1120.7 | 192.5 ± 137.1 * | 4106.1 ± 2248 * | 220 ± 92.6 * | 1450.1 ± 924.9 | 64.5 ± 41.9 | |
| Testis | 25.2 ± 8.2 | 1.0 ± 0.2 | 11.9 ± 5.1 | 0.9 ± 0.6 | 28.1 ± 6.7 | 1.5 ± 0.3 | 30.3 ± 12.8 | 1.4 ± 0.6 | |
| Lung | 2356.3 ± 1647.2 | 85.9 ± 51.5 | 932.8 ± 350.6 | 78.6 ± 46.2 | 1916.4 ± 386.2 | 105.3 ± 24.2 | 2247.3 ± 620.8 | 102.5 ± 37.9 | |
| Heart | 1302.9 ± 246.6 | 51.9 ± 13.9 | 1097.4 ± 246.3 | 77.0 ± 31.3 | 815.4 ± 138.4 * | 45.3 ± 12.5 | 1021.1 ± 261.8 | 47.5 ± 19.0 | |
| 8 h | Plasma | 8.4 ± 2.0 | - | 12.4 ± 2.1 * | - | 12.3 ± 2.6 * | - | 9.9 ± 3.2 | - |
| Kidney | 673.5 ± 104.5 | 87.2 ± 20.4 | 811.4 ± 168.8 | 72.9 ± 9.3 | 777.9 ± 210.1 | 74.0 ± 16.4 | 694.6 ± 188.8 | 93.9 ± 27.3 | |
| Brain | - | - | - | - | - | - | - | - | |
| Liver | 891.1 ± 276.6 | 118.6 ± 52.7 | 2413.8 ± 690.6 * | 218.0 ± 63.9 * | 1357.8 ± 287 | 137.7 ± 57.0 | 999 ± 234.5 | 137.0 ± 43.5 | |
| Testis | 15.4 ± 10.9 | 2.0 ± 1.2 | 10.0 ± 1.7 | 0.9 ± 0.2 | 19.9 ± 18.4 | 1.9 ± 1.5 | 19.3 ± 8.7 | 2.6 ± 1.1 | |
| Lung | 851.2 ± 164 | 108.4 ± 21.2 | 821 ± 163.7 | 76.6 ± 25.9 | 888.6 ± 218.3 | 86.1 ± 27.0 | 779.7 ± 184.7 | 105.2 ± 28.2 | |
| Heart | 531.7 ± 315.6 | 63.8 ± 20.6 | 572.4 ± 133.6 | 51.7 ± 10.4 | 544.6 ± 65.2 | 55.0 ± 19.3 | 577.2 ± 132 | 77.5 ± 16.4 | |
| 24 h | Plasma | 4.6 ± 0.8 | - | 6.1 ± 1.5 | - | 5.6 ± 2.1 | - | 4.9 ± 0.6 | - |
| Kidney | 425.6 ± 78.7 | 90.3 ± 20.9 | 394.4 ± 26.5 | 67.8 ± 17.9 | 424 ± 57 | 82.6 ± 30.4 | 442.2 ± 47.4 | 91.6 ± 8.0 | |
| Brain | - | - | - | - | - | - | - | - | |
| Liver | 364.5 ± 58.1 | 76.6 ± 13.6 | 524.1 ± 118.7 | 91.0 ± 30.5 | 362.3 ± 208.4 | 63.1 ± 16.7 | 334.4 ± 94.6 | 68.7 ± 17.9 | |
| Testis | 17.8 ± 3.8 | 3.8 ± 1.1 | 14.8 ± 2.3 | 2.6 ± 0.9 | 17.5 ± 3.1 | 3.4 ± 1.2 | 23.4 ± 5.4 | 4.9 ± 1.3 | |
| Lung | 546.6 ± 128.6 | 116.0 ± 33.7 | 485.5 ± 141.8 | 84.7 ± 34.2 | 647.4 ± 362.1 | 122.7 ± 76.6 | 499.2 ± 152 | 101.3 ± 18.6 | |
| Heart | 218.2 ± 43.8 | 46.7 ± 13.6 | 190.8 ± 46.8 | 33.3 ± 13.5 | 178.2 ± 32.3 | 33.9 ± 9.4 | 253.4 ± 66.2 | 51.5 ± 7.3 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, T.H.; Shin, S.; Yoo, S.D.; Shin, B.S. Effects of Phytochemical P-Glycoprotein Modulators on the Pharmacokinetics and Tissue Distribution of Doxorubicin in Mice. Molecules 2018, 23, 349. https://doi.org/10.3390/molecules23020349
Kim TH, Shin S, Yoo SD, Shin BS. Effects of Phytochemical P-Glycoprotein Modulators on the Pharmacokinetics and Tissue Distribution of Doxorubicin in Mice. Molecules. 2018; 23(2):349. https://doi.org/10.3390/molecules23020349
Chicago/Turabian StyleKim, Tae Hwan, Soyoung Shin, Sun Dong Yoo, and Beom Soo Shin. 2018. "Effects of Phytochemical P-Glycoprotein Modulators on the Pharmacokinetics and Tissue Distribution of Doxorubicin in Mice" Molecules 23, no. 2: 349. https://doi.org/10.3390/molecules23020349
APA StyleKim, T. H., Shin, S., Yoo, S. D., & Shin, B. S. (2018). Effects of Phytochemical P-Glycoprotein Modulators on the Pharmacokinetics and Tissue Distribution of Doxorubicin in Mice. Molecules, 23(2), 349. https://doi.org/10.3390/molecules23020349








