Mexican Propolis: A Source of Antioxidants and Anti-Inflammatory Compounds, and Isolation of a Novel Chalcone and ε-Caprolactone Derivative
Abstract
:1. Introduction
2. Results
2.1. Screening of Antioxidant Activity
2.2. Chemical Analysis of the Most Potent Extract
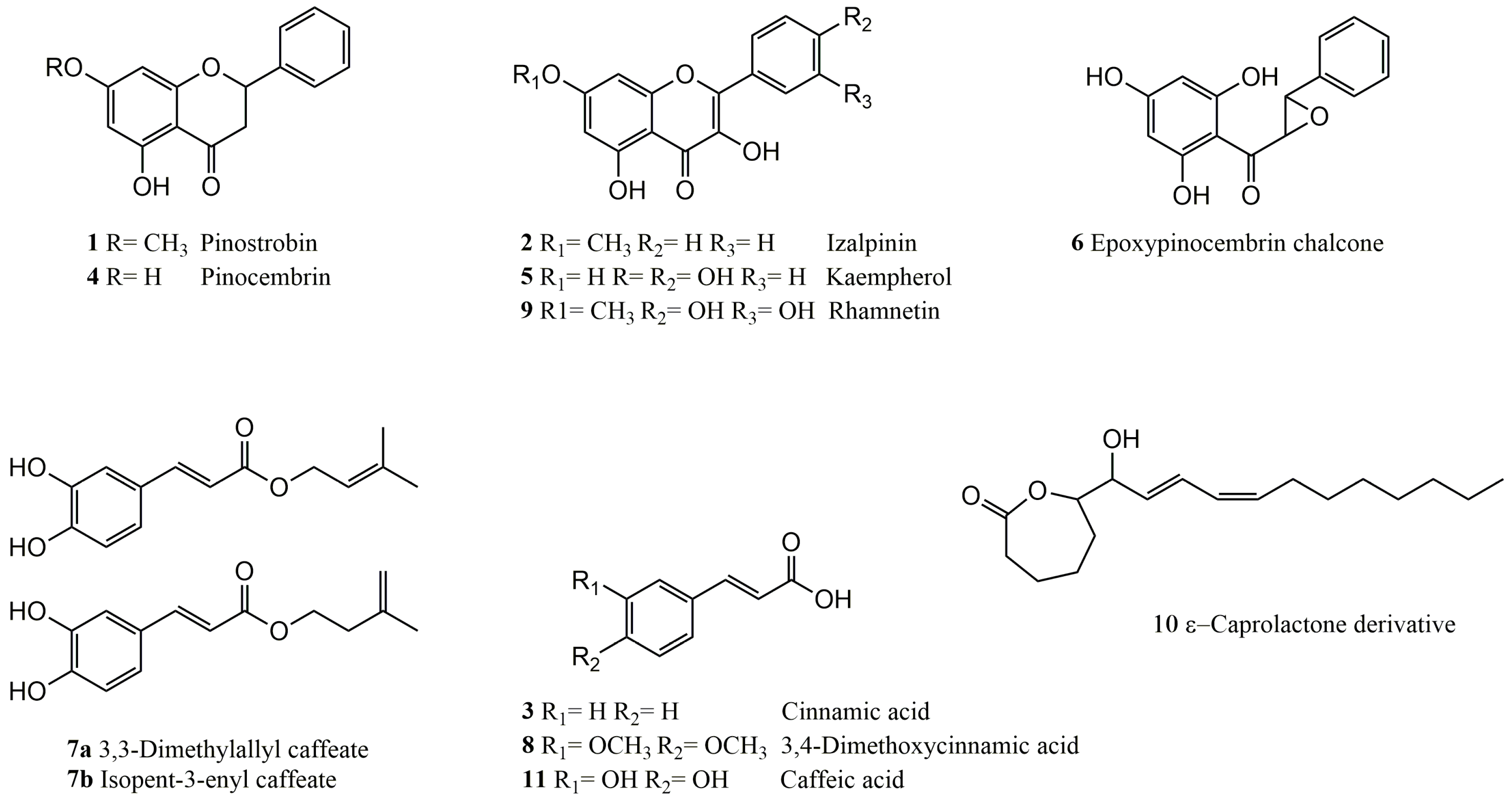
2.2.1. Structure Elucidation of Epoxypinocembrin Chalcone (6)
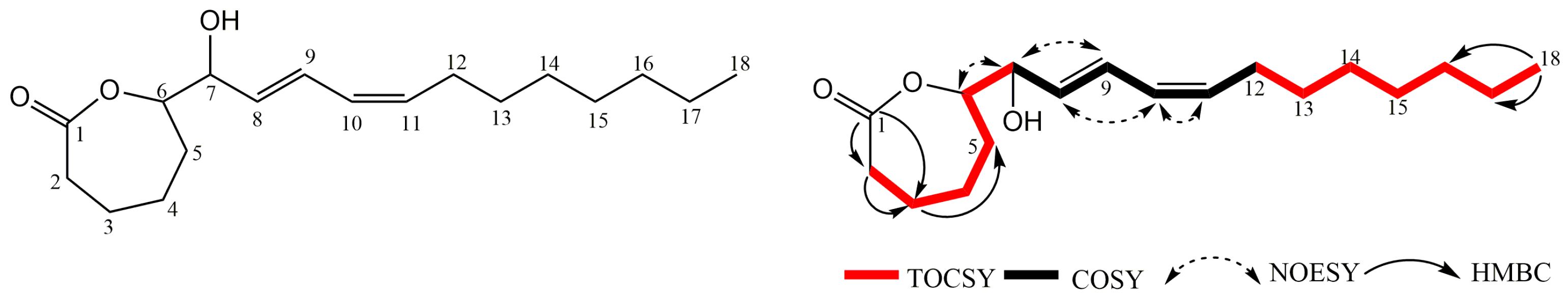
2.2.2. Structure Elucidation of the ε-Caprolactone Derivative 10
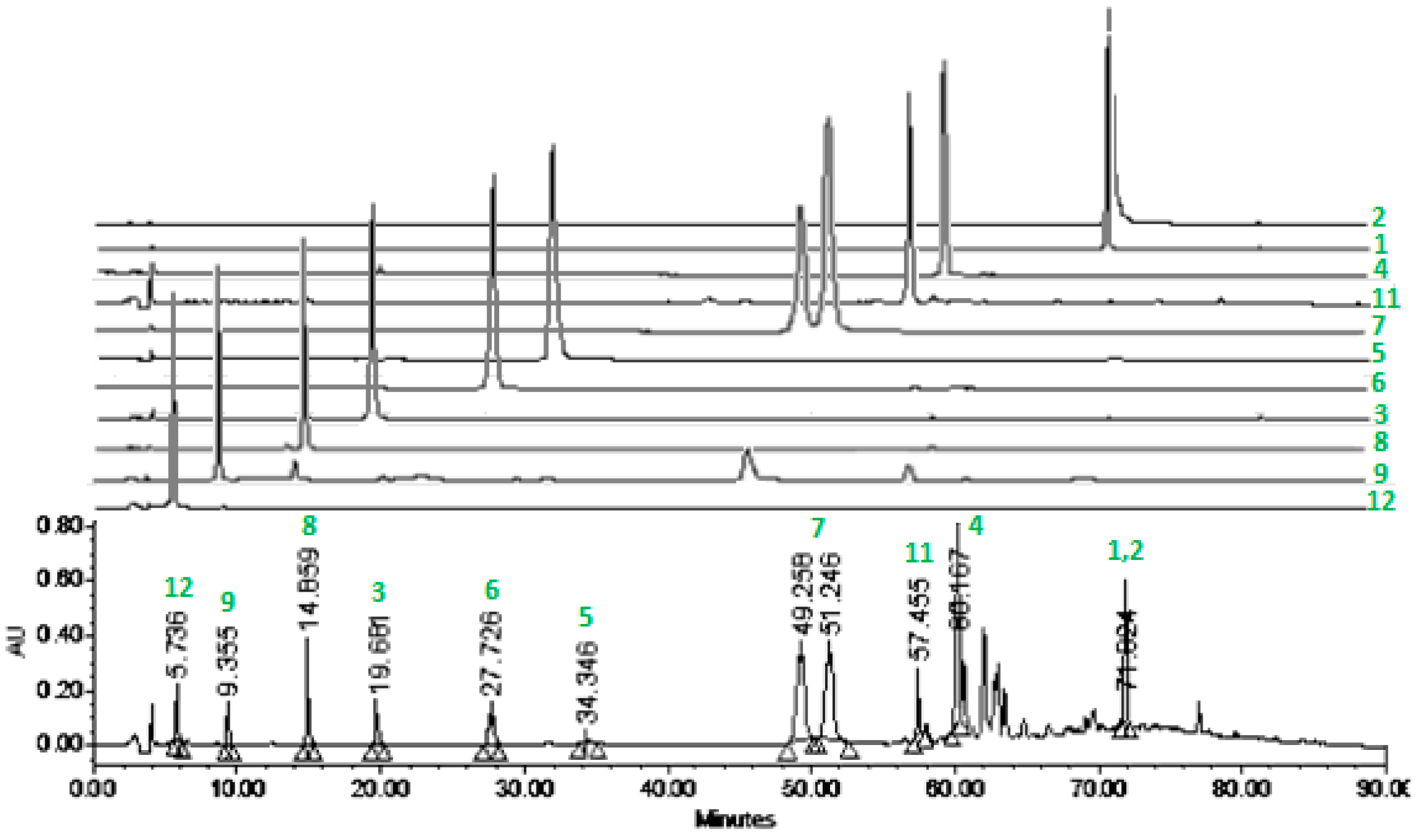
2.2.3. HPLC Profile of 4-EAEP
2.3. Biological Activity
2.3.1. TBARS
2.3.2. Determination of ROS in the Antigen-Induced Mast Cell Degranulation
2.3.3. DPPH Scavenging Capacity
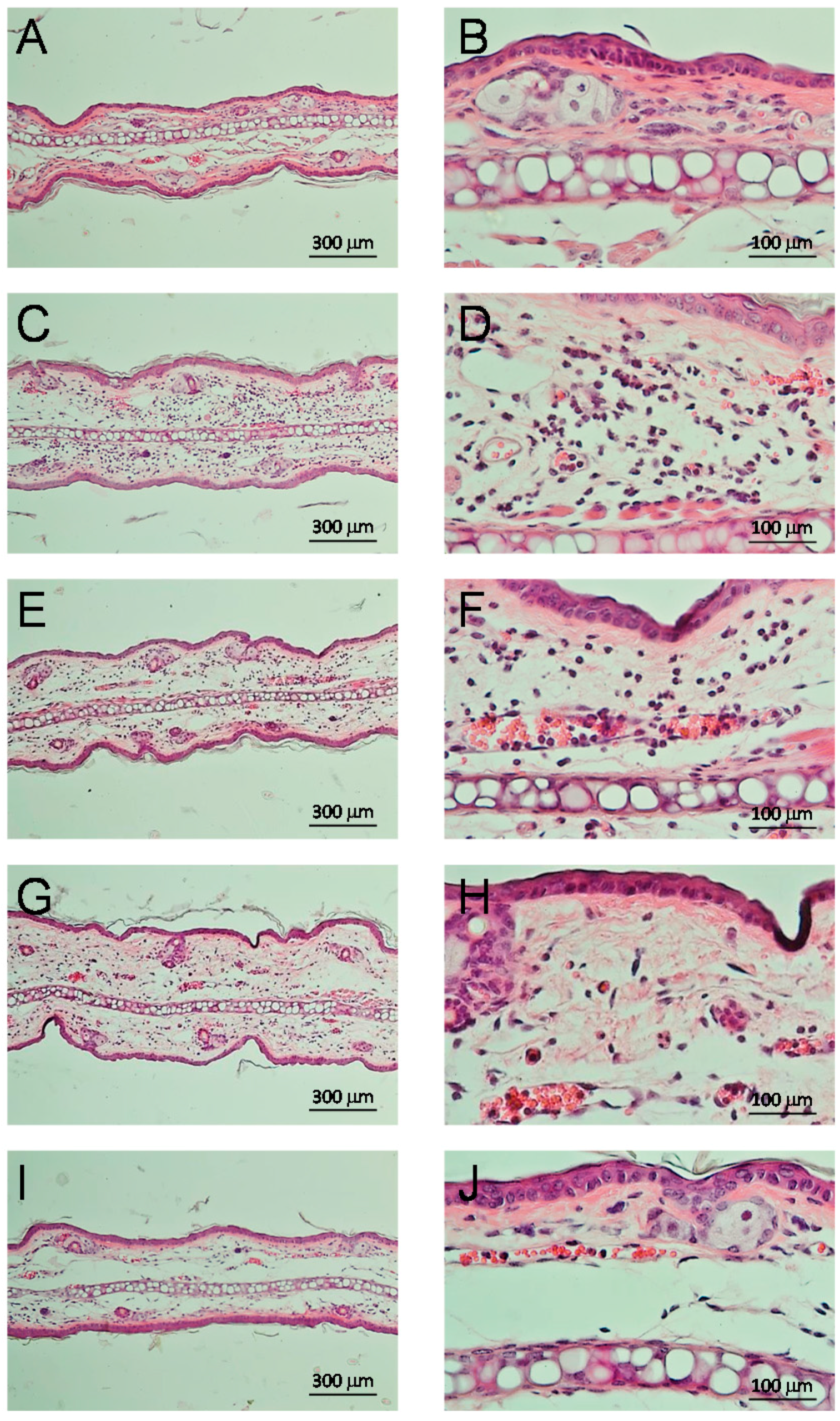
2.3.4. Anti-Inflammatory Effect and Inhibition of Myeloperoxidase
2.3.5. Anti-Mycobacterial Activity
3. Discussion
3.1. Screening
3.2. Chemical Analysis and HPLC Profile of 4-EAEP
3.3. Biological Activity
4. Materials and Methods
4.1. Propolis
4.2. General Experimental Procedures
4.3. Isolation Procedure of Compounds
4.4. HPLC Analysis of Propolis
4.5. Animals
4.6. Inhibition of Lipid Peroxidation Measured by TBARS Assay
4.7. Determination of Reactive Oxygen Species (ROS) in the Antigen-Induced Mast Cell Degranulation
4.8. DPPH Scavenging Capacity
4.9. Evaluation of Anti-Inflammatory Effect by TPA Assay
4.10. Myeloperoxidase (MPO) Assay
4.11. Histological Cuts
4.12. Evaluation of Anti-Mycobacterial Activity by REMA (Resazurin Microtiter Assay)
4.13. Evaluation of Cytotoxicity in Vero Cells
4.14. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bankova, V.S.; de Castro, S.L.; Marcucci, M.C. Propolis: Recent advances in chemistry and plant origin. Apidologie 2000, 31, 3–15. [Google Scholar] [CrossRef]
- Kubina, R.; Kabala-Dzik, A.; Dziedzic, A.; Bielec, B.; Wojtyczka, R.D.; Buldak, R.J.; Wyszynska, M.; Stawiarska-Pieta, B.; Szaflarska-Stojko, E. The Ethanol Extract of Polish Propolis Exhibits Anti-Proliferative and/or Pro-Apoptotic Effect on HCT 116 Colon Cancer and Me45 Malignant Melanoma Cells In Vitro Conditions. Adv. Clin. Exp. Med. 2015, 24, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Orsolic, N.; Saranovic, A.B.; Basic, I. Direct and indirect mechanism(s) of antitumour activity of propolis and its polyphenolic compounds. Planta Med. 2006, 72, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Bankova, V.; Boudourova-Krasteva, G.; Sforcin, J.M.; Frete, X.; Kujumgiev, A.; Maimoni-Rodella, R.; Popov, S. Phytochemical evidence for the plant origin of Brazilian propolis from Sao Paulo state. Z. Naturforsch. C 1999, 54, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Bueno-Silva, B.; Marsola, A.; Ikegaki, M.; Alencar, S.M.; Rosalen, P.L. The effect of seasons on Brazilian red propolis and its botanical source: Chemical composition and antibacterial activity. Nat. Prod. Res. 2017, 31, 1318–1324. [Google Scholar] [CrossRef] [PubMed]
- Toreti, V.C.; Sato, H.H.; Pastore, G.M.; Park, Y.K. Recent progress of propolis for its biological and chemical compositions and its botanical origin. Evid. Based Complement. Altern. Med. 2013, 2013, 697390. [Google Scholar] [CrossRef] [PubMed]
- Alday, E.; Valencia, D.; Carreno, A.L.; Picerno, P.; Piccinelli, A.L.; Rastrelli, L.; Robles-Zepeda, R.; Hernandez, J.; Velazquez, C. Apoptotic induction by pinobanksin and some of its ester derivatives from Sonoran propolis in a B-cell lymphoma cell line. Chem. Biol. Interact. 2015, 242, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Alday-Provencio, S.; Diaz, G.; Rascon, L.; Quintero, J.; Alday, E.; Robles-Zepeda, R.; Garibay-Escobar, A.; Astiazaran, H.; Hernandez, J.; Velazquez, C. Sonoran propolis and some of its chemical constituents inhibit in vitro growth of Giardia lamblia trophozoites. Planta Med. 2015, 81, 742–747. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, J.; Goycoolea, F.M.; Quintero, J.; Acosta, A.; Castaneda, M.; Dominguez, Z.; Robles, R.; Vazquez-Moreno, L.; Velazquez, E.F.; Astiazaran, H.; et al. Sonoran propolis: Chemical composition and antiproliferative activity on cancer cell lines. Planta Med. 2007, 73, 1469–1474. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Sanchez, R.D.; Torrescano-Urrutia, G.R.; Acedo-Felix, E.; Carvajal-Millan, E.; Gonzalez-Cordova, A.F.; Vallejo-Galland, B.; Torres-Llanez, M.J.; Sanchez-Escalante, A. Antioxidant and antimicrobial activity of commercial propolis extract in beef patties. J. Food Sci. 2014, 79, C1499–C1504. [Google Scholar] [CrossRef] [PubMed]
- Bankova, V.; Popova, M.; Bogdanov, S.; Sabatini, A.G. Chemical composition of European propolis: Expected and unexpected results. Z. Naturforsch. C 2002, 57, 530–533. [Google Scholar] [CrossRef] [PubMed]
- Marcucci, M.C.; Ferreres, F.; Garcia-Viguera, C.; Bankova, V.S.; De Castro, S.L.; Dantas, A.P.; Valente, P.H.; Paulino, N. Phenolic compounds from Brazilian propolis with pharmacological activities. J. Ethnopharmacol. 2001, 74, 105–112. [Google Scholar] [CrossRef]
- Bankova, V. Recent trends and important developments in propolis research. Evid. Based Complement. Alternat. Med. 2005, 2, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Cuesta-Rubio, O.; Frontana-Uribe, B.A.; Ramirez-Apan, T.; Cardenas, J. Polyisoprenylated benzophenones in cuban propolis; biological activity of nemorosone. Z. Naturforsch. C 2002, 57, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Valcic, S.; Montenegro, G.; Timmermann, B.N. Lignans from Chilean propolis. J. Nat. Prod. 1998, 61, 771–775. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J. Phase Ⅱ Study of Pinocembrin Injection to Treat Ischemic Stroke—Randomized, Double-blind, Placebo-Controlled, Multicenter Study. Available online: https://clinicaltrials.gov/ct2/show/NCT02059785?term=pinocembrin&rank=1 (accessed on 28 November 2017 ).
- Kabala-Dzik, A.; Rzepecka-Stojko, A.; Kubina, R.; Jastrzebska-Stojko, Z.; Stojko, R.; Wojtyczka, R.D.; Stojko, J. Comparison of Two Components of Propolis: Caffeic Acid (CA) and Caffeic Acid Phenethyl Ester (CAPE) Induce Apoptosis and Cell Cycle Arrest of Breast Cancer Cells MDA-MB-231. Molecules 2017, 22, 1554. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Awale, S.; Tezuka, Y.; Esumi, H.; Kadota, S. Study on the constituents of Mexican propolis and their cytotoxic activity against PANC-1 human pancreatic cancer cells. J. Nat. Prod. 2010, 73, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; He, Y.M.; Awale, S.; Kadota, S.; Tezuka, Y. Two new cytotoxic phenylallylflavanones from Mexican propolis. Chem. Pharm. Bull. 2011, 59, 1194–1196. [Google Scholar] [CrossRef] [PubMed]
- Lotti, C.; Campo Fernandez, M.; Piccinelli, A.L.; Cuesta-Rubio, O.; Márquez Hernández, I.; Rastrelli, L. Chemical Constituents of Red Mexican Propolis. J. Agric. Food Chem. 2010, 58, 2209–2213. [Google Scholar] [CrossRef] [PubMed]
- Colegate, S.M.; Din, L.B.; Ghisalberti, E.L.; Latiff, A. Tepanone, a retrochalcone from Ellipeia cuneifolia. Phytochemistry 1992, 31, 2123–2126. [Google Scholar] [CrossRef]
- Huang, W.-Z.; Zhang, C.-F.; Zhang, M.; Wang, Z.-T. A New Biphenylpropanoid from Alpinia Katsumadai. J. Chin. Chem. Soc. 2007, 54, 1553–1556. [Google Scholar] [CrossRef]
- Mazur, P.; Meyers, H.V.; Nakanishi, K.; El-Zayat, A.A.E.; Champe, S.P. Structural elucidation of sporogenic fatty acid metabolites from aspergillus nidulans. Tetrahedron Lett. 1990, 31, 3837–3840. [Google Scholar] [CrossRef]
- Řezanka, T.; Dembitsky, V.M. γ-Lactones from the soft corals Sarcophyton trocheliophorum and Lithophyton arboreum. Tetrahedron 2001, 57, 8743–8749. [Google Scholar] [CrossRef]
- Liu, R.; Gao, M.; Yang, Z.H.; Du, G.H. Pinocembrin protects rat brain against oxidation and apoptosis induced by ischemia-reperfusion both in vivo and in vitro. Brain Res. 2008, 1216, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Sala, A.; Recio, M.C.; Schinella, G.R.; Manez, S.; Giner, R.M.; Cerda-Nicolas, M.; Rosi, J.L. Assessment of the anti-inflammatory activity and free radical scavenger activity of tiliroside. Eur. J. Pharmacol. 2003, 461, 53–61. [Google Scholar] [CrossRef]
- Calderón-Cisneros, A.; Soto-Pinto, L. Transformaciones agrícolas en el contexto periurbano de la ciudad de San Cristóbal de Las Casas, Chiapas. LiminaR 2014, 12, 125–143. [Google Scholar]
- Popova, M.; Giannopoulou, E.; Skalicka-Wozniak, K.; Graikou, K.; Widelski, J.; Bankova, V.; Kalofonos, H.; Sivolapenko, G.; Gawel-Beben, K.; Antosiewicz, B.; et al. Characterization and Biological Evaluation of Propolis from Poland. Molecules 2017, 22, 1159. [Google Scholar] [CrossRef] [PubMed]
- García-Viguera, C.; Ferreres, F.; Tomás-Barberán, F.A. Study of Canadian Propolis by GC-MS and HPLC. Z. Naturforsh. 1993, 48c, 731–735. [Google Scholar]
- Uzel, A.; Sorkun, K.; Oncag, O.; Cogulu, D.; Gencay, O.; Salih, B. Chemical compositions and antimicrobial activities of four different Anatolian propolis samples. Microbiol. Res. 2005, 160, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Valle, V.; Méndez-Sánchez, N. Estrés oxidativo, antioxidantes y enfermedad. Rev. Investig. Med. Sur. 2013, 20, 161–168. [Google Scholar]
- Hausen, B.M.; Wollenweber, E. Propolis allergy. (III). Sensitization studies with minor constituents. Contact Dermat. 1988, 19, 296–303. [Google Scholar] [CrossRef]
- Hausen, B.M.; Wollenweber, E.; Senff, H.; Post, B. Propolis allergy. (II). The sensitizing properties of 1,1-dimethylallyl caffeic acid ester. Contact Dermat. 1987, 17, 171–177. [Google Scholar] [CrossRef]
- Itoh, T.; Tsukane, M.; Koike, M.; Nakamura, C.; Ohguchi, K.; Ito, M.; Akao, Y.; Koshimizu, S.; Nozawa, Y.; Wakimoto, T.; et al. Inhibitory effects of whisky congeners on IgE-mediated degranulation in rat basophilic leukemia RBL-2H3 cells and passive cutaneous anaphylaxis reaction in mice. J. Agric. Food Chem. 2010, 58, 7149–7157. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, R.; Nakamura, R.; Watanabe, K.; Oka, K.; Ohta, S.; Mishima, S.; Teshima, R. Effects of propolis from different areas on mast cell degranulation and identification of the effective components in propolis. Int. Immunopharmacol. 2010, 10, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Khayyal, M.T.; El-Ghazaly, M.A.; El-Khatib, A.S.; Hatem, A.M.; de Vries, P.J.; el-Shafei, S.; Khattab, M.M. A clinical pharmacological study of the potential beneficial effects of a propolis food product as an adjuvant in asthmatic patients. Fundam. Clin. Pharmacol. 2003, 17, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Lan, X.; Wang, W.; Li, Q.; Wang, J. The Natural Flavonoid Pinocembrin: Molecular Targets and Potential Therapeutic Applications. Mol. Neurobiol. 2016, 53, 1794–1801. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.K.; Jaiswal, G.; Bhutani, K.K. A review on biological sources, chemistry and pharmacological activities of pinostrobin. Nat. Prod. Res. 2016, 30, 2017–2027. [Google Scholar] [CrossRef] [PubMed]
- Bertelli, D.; Papotti, G.; Bortolotti, L.; Marcazzan, G.L.; Plessi, M. (1) H-NMR simultaneous identification of health-relevant compounds in propolis extracts. Phytochem. Anal. 2012, 23, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, O.; Pena, R.C.; Ureta, E.; Montenegro, G.; Caldwell, C.; Timmermann, B.N. Phenolic compounds of propolis from Central Chilean matorral. Z. Naturforsch. C 2001, 56, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Kelley, C.J.; Harruff, R.C.; Carmack, M. Polyphenolic acids of Lithospermum ruderale. II. Carbon-13 nuclear magnetic resonance of lithospermic and rosmarinic acids. J. Org. Chem. 1976, 41, 449–455. [Google Scholar] [CrossRef]
- Mattarei, A.; Biasutto, L.; Rastrelli, F.; Garbisa, S.; Marotta, E.; Zoratti, M.; Paradisi, C. Regioselective O-derivatization of quercetin via ester intermediates. An improved synthesis of rhamnetin and development of a new mitochondriotropic derivative. Molecules 2010, 15, 4722–4736. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, N.H.; Salim, F.; Ahmad, R. Chemical Constituents of Malaysian U. cordata var. ferruginea and Their in Vitro alpha-Glucosidase Inhibitory Activities. Molecules 2016, 21, 525. [Google Scholar] [CrossRef] [PubMed]
- Kartal, M.; Yildiz, S.; Kaya, S.; Kurucu, S.; Topcu, G. Antimicrobial activity of propolis samples from two different regions of Anatolia. J. Ethnopharmacol. 2003, 86, 69–73. [Google Scholar] [CrossRef]
- Jeong, C.-H.; Jeong, H.R.; Choi, G.N.; Kim, D.-O.; Lee, U.; Heo, H.J. Neuroprotective and anti-oxidant effects of caffeic acid isolated from Erigeron annuus leaf. Chin. Med. 2011, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Ching, A.Y.L.; Wang, T.S.; Sukari, M.A.; Lian, G.E.C.; Rahmani, M.; Khalid, K. Characterization of flavonoid derivatives from Boesenbergia rotunda (L.). Malays. J. Anal. Sci. 2007, 11, 154–159. [Google Scholar]
- Palomino, J.C.; Martin, A.; Camacho, M.; Guerra, H.; Swings, J.; Portaels, F. Resazurin microtiter assay plate: Simple and inexpensive method for detection of drug resistance in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2002, 46, 2720–2722. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.M.; Liu, Y.; Shi, Y.P. Phenolic derivatives with free-radical-scavenging activities from Ixeridium gracile (DC.) Shih. Chem. Biodivers. 2007, 4, 2172–2181. [Google Scholar] [CrossRef] [PubMed]
- de Aguiar, S.C.; Zeoula, L.M.; Franco, S.L.; Peres, L.P.; Arcuri, P.B.; Forano, E. Antimicrobial activity of Brazilian propolis extracts against rumen bacteria in vitro. World J. Microbiol. Biotechnol. 2013, 29, 1951–1959. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, M.; Nieto, A.; Marin, J.C.; Keck, A.S.; Jeffery, E.; Cespedes, C.L. Antioxidant activities of extracts from Barkleyanthus salicifolius (Asteraceae) and Penstemon gentianoides (Scrophulariaceae). J. Agric. Food Chem. 2005, 53, 5889–5895. [Google Scholar] [CrossRef] [PubMed]
- Esterbauer, H.; Cheeseman, K.H. Determination of aldehydic lipid peroxidation products: Malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990, 186, 407–421. [Google Scholar] [PubMed]
- Martin-Avila, A.; Medina-Tamayo, J.; Ibarra-Sanchez, A.; Vazquez-Victorio, G.; Castillo-Arellano, J.I.; Hernandez-Mondragon, A.C.; Rivera, J.; Madera-Salcedo, I.K.; Blank, U.; Macias-Silva, M.; et al. Protein Tyrosine Kinase Fyn Regulates TLR4-Elicited Responses on Mast Cells Controlling the Function of a PP2A-PKCalpha/beta Signaling Node Leading to TNF Secretion. J. Immunol. 2016, 196, 5075–5088. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.K.; Fang, S.H.; Tzeng, Y.M. Anti-inflammatory activities of flavonoids isolated from Caesalpinia pulcherrima. J. Ethnopharmacol. 2005, 100, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Marin-Loaiza, J.C.; Nieto-Camacho, A.; Cespedes, C.L. Antioxidant and anti-inflammatory activities of Pittocaulon species from Mexico. Pharm. Biol. 2013, 51, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Mescher, A.L. Junqueira’s Basic Histology: Text and Atlas, 13th ed.; McGraw-Hill Education: New York, NY, USA, 2013; p. 520. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
Sample Availability: Not available. |



| Propolis Sample/Location (Altitude), State | Extract | %Inhibition (10 µg/mL) | IC50 (μg/mL) |
|---|---|---|---|
| 1 Tapachula (177 m), Chiapas | Hexane | 1.32 | |
| Ethyl acetate | 5.28 | ||
| Methanol | 9.23 | ||
| 2 La Trinitaria (1558 m), Chiapas | Hexane | 52.58 | 46.17 ± 0.83 |
| Ethyl acetate | 94.68 | 8.11 ± 0.16 | |
| Methanol | 96.11 | 11.17 ± 0.61 | |
| 3 Pantelhó (1056 m), Chiapas | Hexane | 21.37 | |
| Ethyl acetate | 35.28 | 25 ± 1.39 | |
| Methanol | 64.00 | 11.59 ± 88 | |
| 4 San Cristóbal de las Casas (2200 m), Chiapas | Ethyl acetate (4-EAEP) | 99.28 | 1.42 ± 0.07 |
| 5 Rancho San Pedro Chenchelá (227 m), Yucatán | Hexane | 12.06 | |
| Ethyl acetate | 23.33 | ||
| Methanol | 20.47 |
| Position | δH (J in Hz) | δC | Position | δH (J in Hz) | δC |
|---|---|---|---|---|---|
| 1 | 138.55 | 1′ | 101.81 | ||
| 2 | 7.53 dd (7,2.1) | 128.92 | 2′ | 165.34 a | |
| 3 | 7.41 m | 129.4 | 3′ | 5.94 d (2.1) b | 97.45 b |
| 4 | 7.39 m | 129.9 | 4′ | 168.84 | |
| 5 | 7.41 m | 129.4 | 5′ | 5.90 d (2.1) b | 96.35 b |
| 6 | 7.53 dd (7,2.1) | 128.92 | 6′ | 164.38 a | |
| 7 | 5.07 d (11.5) | 85.04 | |||
| 8 | 4.54 d (11.5) | 73.71 | |||
| 9 | 198.18 |
| Position | δH (J in Hz) | δC | Position | δH (J in Hz) | δC |
|---|---|---|---|---|---|
| 1 | 179.9 | 10 | 6.00 dd (11.1, 11.1) | 129.43 | |
| 2 | 2.21 t (7.6) | 36.70 | 11 | 5.41 dt (11, 7.7) | 133.08 |
| 3 | 1.59 q (7.1) | 26.75 | 12 | 2.19 td (8.05,7.6) | 28.63 |
| 4 | 1.53 m | 33.75 | 13 | 1.39 q (7.14) | 30.55 |
| 5 | 1.34 m | 30.5 | 14 | 1.28–1.36 m | 30.48 |
| 6 | 3.48 m | 75.69 | 15 | 1.28–1.36 m | 26.83 |
| 7 | 3.96 dd (7,6) | 76.9 | 16 | 1.28–1.36 m | 32.51 |
| 8 | 5.73 dd (15, 7) | 133.53 | 17 | 1.28–1.36 m | 23.61 |
| 9 | 6.56 ddt (15, 11.1, 1.1) | 128.37 | 18 | 0.9 t (7) | 14.4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guzmán-Gutiérrez, S.L.; Nieto-Camacho, A.; Castillo-Arellano, J.I.; Huerta-Salazar, E.; Hernández-Pasteur, G.; Silva-Miranda, M.; Argüello-Nájera, O.; Sepúlveda-Robles, O.; Espitia, C.I.; Reyes-Chilpa, R. Mexican Propolis: A Source of Antioxidants and Anti-Inflammatory Compounds, and Isolation of a Novel Chalcone and ε-Caprolactone Derivative. Molecules 2018, 23, 334. https://doi.org/10.3390/molecules23020334
Guzmán-Gutiérrez SL, Nieto-Camacho A, Castillo-Arellano JI, Huerta-Salazar E, Hernández-Pasteur G, Silva-Miranda M, Argüello-Nájera O, Sepúlveda-Robles O, Espitia CI, Reyes-Chilpa R. Mexican Propolis: A Source of Antioxidants and Anti-Inflammatory Compounds, and Isolation of a Novel Chalcone and ε-Caprolactone Derivative. Molecules. 2018; 23(2):334. https://doi.org/10.3390/molecules23020334
Chicago/Turabian StyleGuzmán-Gutiérrez, Silvia Laura, Antonio Nieto-Camacho, Jorge Ivan Castillo-Arellano, Elizabeth Huerta-Salazar, Griselda Hernández-Pasteur, Mayra Silva-Miranda, Omar Argüello-Nájera, Omar Sepúlveda-Robles, Clara Inés Espitia, and Ricardo Reyes-Chilpa. 2018. "Mexican Propolis: A Source of Antioxidants and Anti-Inflammatory Compounds, and Isolation of a Novel Chalcone and ε-Caprolactone Derivative" Molecules 23, no. 2: 334. https://doi.org/10.3390/molecules23020334
APA StyleGuzmán-Gutiérrez, S. L., Nieto-Camacho, A., Castillo-Arellano, J. I., Huerta-Salazar, E., Hernández-Pasteur, G., Silva-Miranda, M., Argüello-Nájera, O., Sepúlveda-Robles, O., Espitia, C. I., & Reyes-Chilpa, R. (2018). Mexican Propolis: A Source of Antioxidants and Anti-Inflammatory Compounds, and Isolation of a Novel Chalcone and ε-Caprolactone Derivative. Molecules, 23(2), 334. https://doi.org/10.3390/molecules23020334





