Recent Advances in Detecting Mitochondrial DNA Heteroplasmic Variations
Abstract
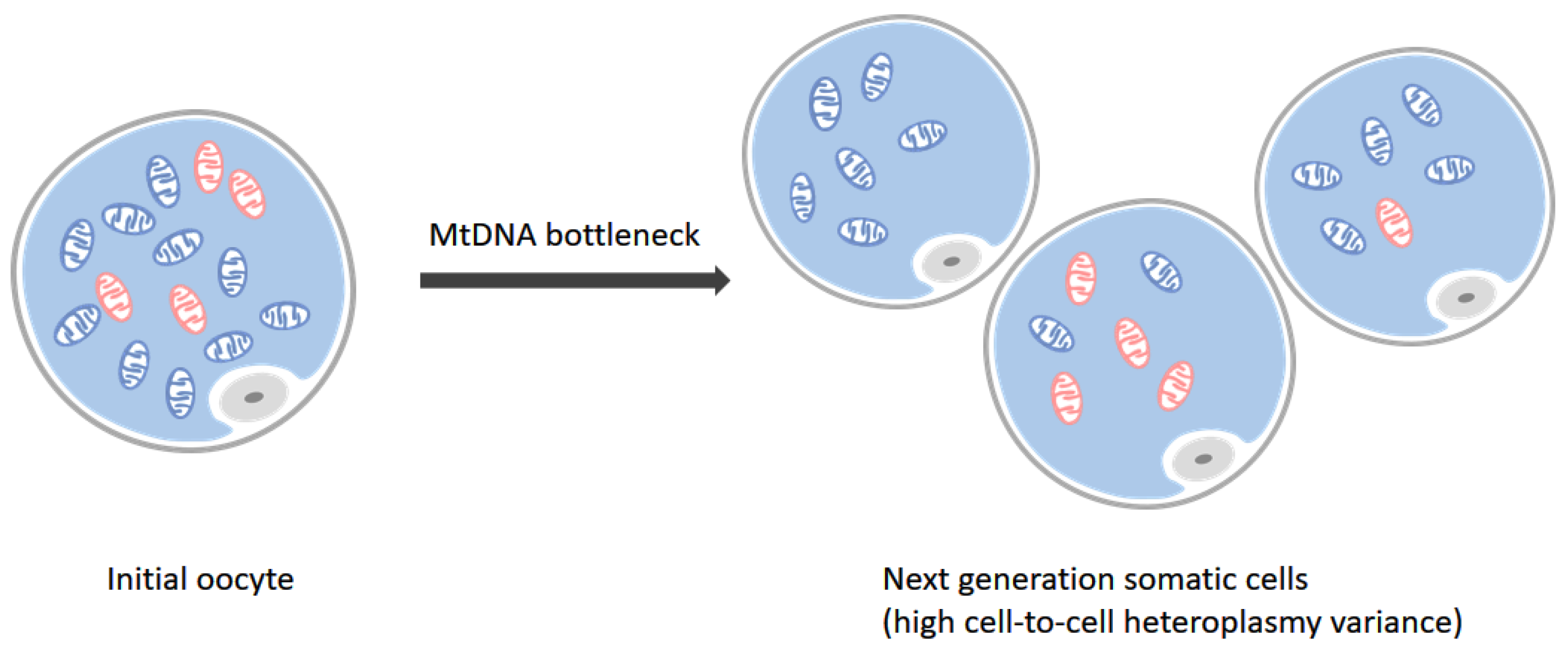
1. Introduction
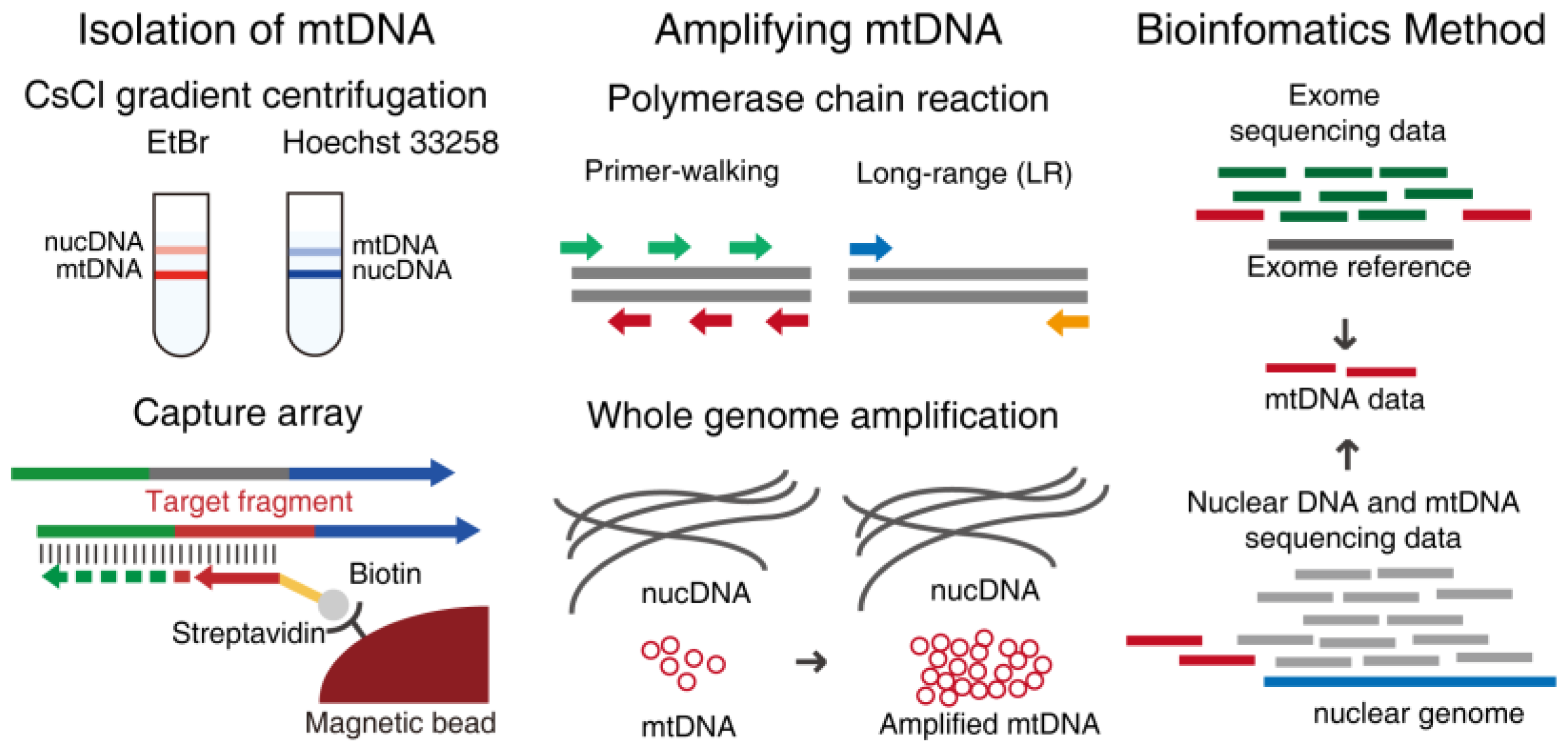
2. Methods of Enriching mtDNA
2.1. Isolation of mtDNA
2.1.1. Density Gradient Centrifugation
2.1.2. Capture Array
2.2. Amplifying mtDNA
2.2.1. Polymerase Chain Reaction
2.2.2. Whole Genome Amplification
2.2.3. Bioinformatics Methods
3. Methods of Detecting Heteroplasmy
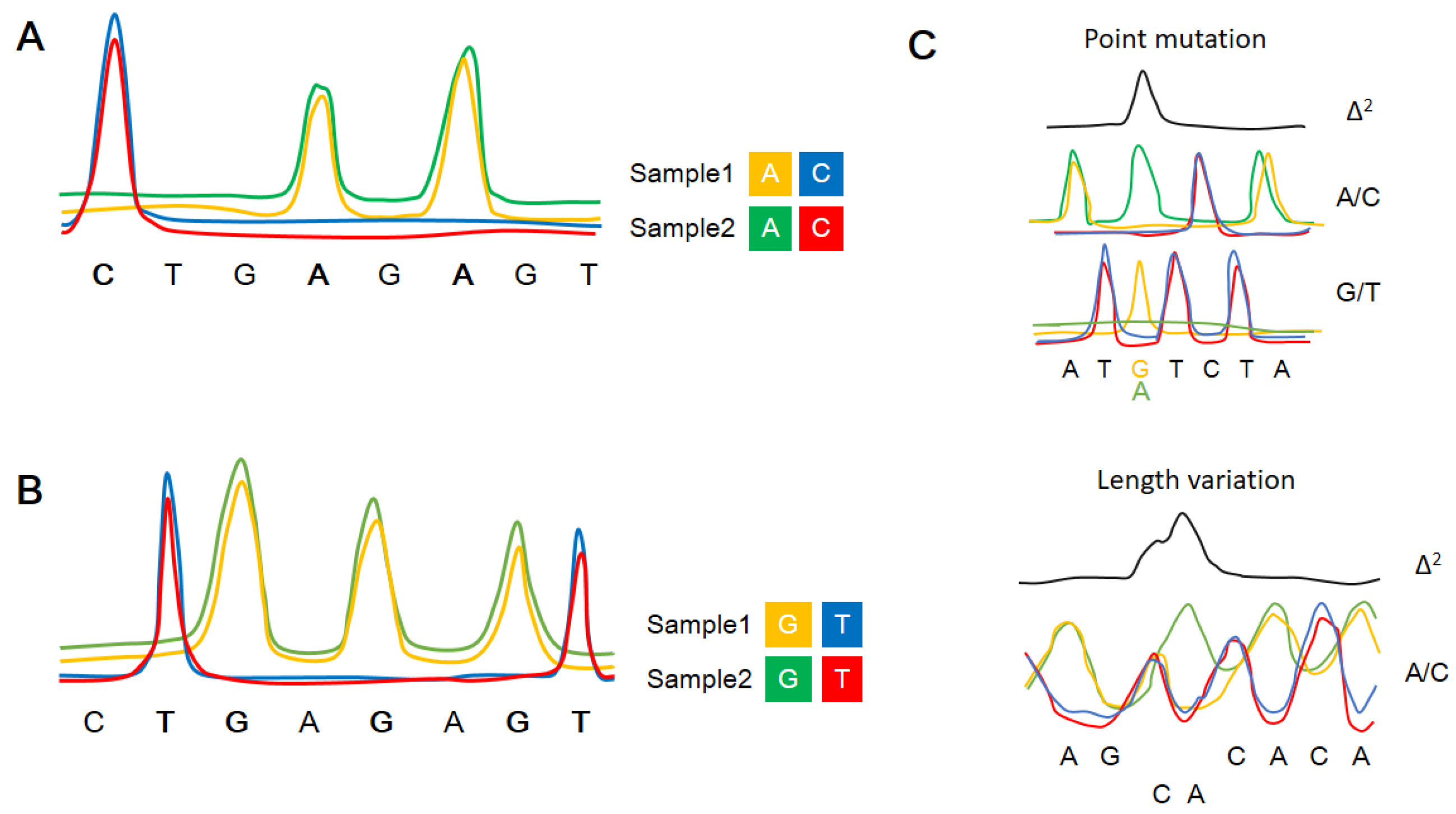
3.1. Sanger Sequencing
3.2. Microarray-Based Methods
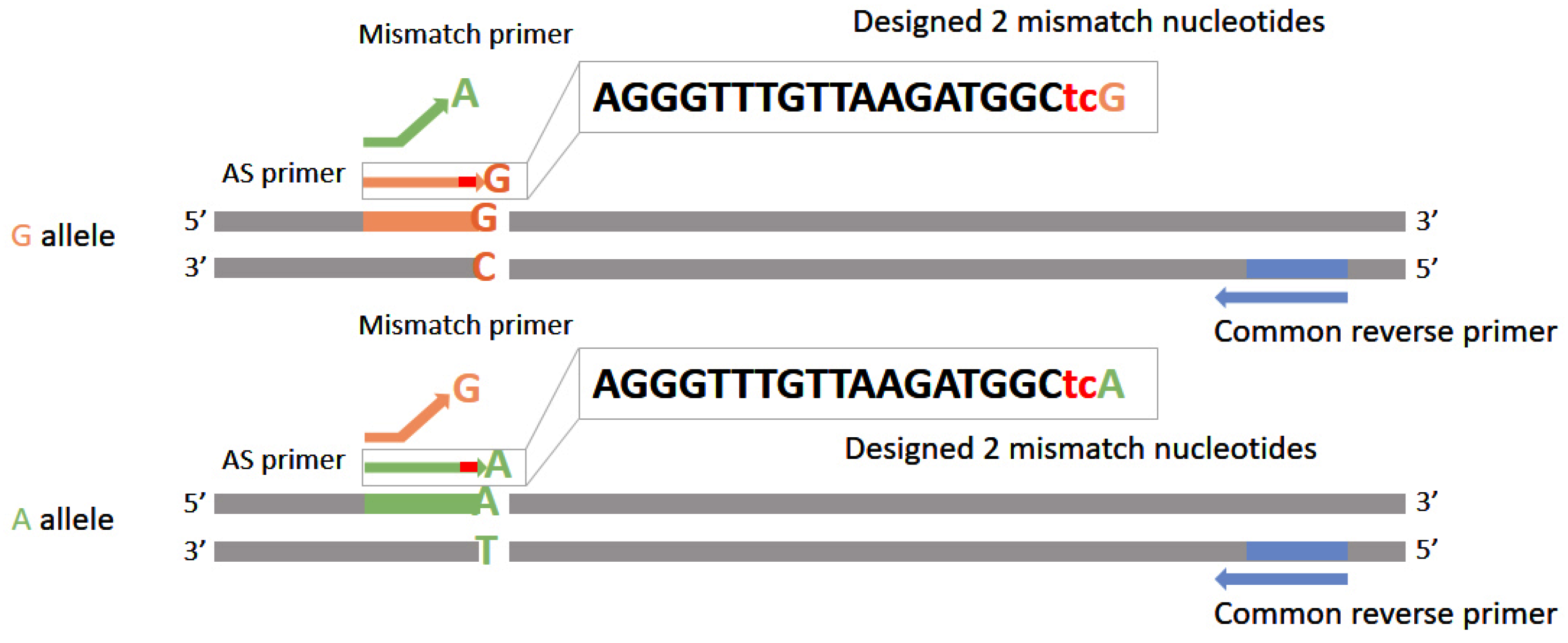
3.3. PCR-Based Methods
3.4. Next Generation Sequencing
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Anderson, S.; Bankier, A.T.; Barrell, B.G.; De Bruijn, M.; Coulson, A.R.; Drouin, J.; Eperon, I.; Nierlich, D.; Roe, B.A.; Sanger, F. Sequence and organization of the human mitochondrial genome. Nature 1981, 290, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Mambo, E.; Sidransky, D. Mitochondrial DNA mutations in human cancer. Oncogene 2006, 25, 4663–4674. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.C.; Singh, G.; Lott, M.T.; Hodge, J.A.; Schurr, T.G.; Lezza, A.M.S.; Elsas, L.J.; Nikoskelainen, E. Mitochondrial DNA mutation associated with leber’s hereditary optic neuropathy. Science 1988, 242, 1427–1430. [Google Scholar] [CrossRef] [PubMed]
- Lightowlers, R.N.; Chinnery, P.F.; Turnbull, D.M.; Howell, N. Mammalian mitochondrial genetics: Heredity, heteroplasmy and disease. Trends Genet. 1997, 13, 450–455. [Google Scholar] [CrossRef]
- Naue, J.; Horer, S.; Sanger, T.; Strobl, C.; Hatzer-Grubwieser, P.; Parson, W.; Lutz-Bonengel, S. Evidence for frequent and tissue-specific sequence heteroplasmy in human mitochondrial DNA. Mitochondrion 2015, 20, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Pakendorf, B.; Stoneking, M. Mitochondrial DNA and human evolution. Ann. Rev. Genom. Hum. Genet. 2005, 6, 165–183. [Google Scholar] [CrossRef] [PubMed]
- Gocke, C.D.; Benko, F.A.; Rogan, P.K. Transmission of mitochondrial DNA heteroplasmy in normal pedigrees. Hum. Genet. 1998, 102, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Holt, I.J.; Miller, D.H.; Harding, A.E. Genetic heterogeneity and mitochondrial DNA heteroplasmy in leber’s hereditary optic neuropathy. J. Med. Genet. 1989, 26, 739–743. [Google Scholar] [CrossRef] [PubMed]
- Monnat, R.J.; Maxwell, C.L.; Loeb, L.A. Nucleotide sequence preservation of human leukemic mitochondrial DNA. Cancer Res. 1985, 45, 1809–1814. [Google Scholar] [PubMed]
- Monnat, R.J.; Reay, D.T. Nucleotide sequence identity of mitochondrial DNA from different human tissues. Gene 1986, 43, 205–211. [Google Scholar] [CrossRef]
- Holt, I.J.; Harding, A.E.; Morganhughes, J.A. Deletions of muscle mitochondrial DNA in patients with mitochondrial myopathies. Nature 1988, 331, 717–719. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.; Vissing, J. Paternal inheritance of mitochondrial DNA. N. Engl. J. Med. 2002, 347, 576–580. [Google Scholar] [CrossRef] [PubMed]
- Kraytsberg, Y.; Schwartz, M.; Brown, T.A.; Ebralidse, K.; Kunz, W.S.; Clayton, D.A.; Vissing, J.; Khrapko, K. Recombination of human mitochondrial DNA. Science 2004, 304, 981. [Google Scholar] [CrossRef] [PubMed]
- Gaziev, A.I.; Abdullaev, S.; Podlutsky, A. Mitochondrial function and mitochondrial DNA maintenance with advancing age. Biogerontology 2014, 15, 417–438. [Google Scholar] [CrossRef] [PubMed]
- Alexeyev, M.; Ledoux, S.P.; Wilson, G.L. Mitochondrial DNA and aging. Clin. Sci. 2004, 107, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Elson, J.L.; Samuels, D.C.; Turnbull, D.M.; Chinnery, P.F. Random intracellular drift explains the clonal expansion of mitochondrial DNA mutations with age. Am. J. Hum. Genet. 2001, 68, 802–806. [Google Scholar] [CrossRef] [PubMed]
- Nekhaeva, E.; Bodyak, N.; Kraytsberg, Y.; McGrath, S.B.; Van Orsouw, N.J.; Pluzhnikov, A.; Wei, J.Y.; Vijg, J.; Khrapko, K. Clonally expanded mtdna point mutations are abundant in individual cells of human tissues. Proc. Natl. Acad. Sci. USA 2002, 99, 5521–5526. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Schroder, R.; Ni, S.; Madea, B.; Stoneking, M. Extensive tissue-related and allele-related mtdna heteroplasmy suggests positive selection for somatic mutations. Proc. Natl. Acad. Sci. USA 2015, 112, 2491–2496. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, K.; Kondookamoto, N. Mitochondria and autophagy: Critical interplay between the two homeostats. Biochim. Biophys. Acta 2012, 1820, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Krjutskov, K.; Koltsina, M.; Grand, K.; Vosa, U.; Sauk, M.; Tonisson, N.; Salumets, A. Tissue-specific mitochondrial heteroplasmy at position 16,093 within the same individual. Curr. Genet. 2014, 60, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Sekiguchi, K.; Sato, H.; Kasai, K. Mitochondrial DNA heteroplasmy among hairs from single individuals. J. Forensic Sci. 2004, 49, 986–991. [Google Scholar] [CrossRef] [PubMed]
- Cavelier, L.; Johannisson, A.; Gyllensten, U. Analysis of mtdna copy number and composition of single mitochondrial particles using flow cytometry and pcr. Exp. Cell Res. 2000, 259, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.W.; Taylor, G.A.; Durham, S.E.; Turnbull, D.M. The determination of complete human mitochondrial DNA sequences in single cells: Implications for the study of somatic mitochondrial DNA point mutations. Nucleic Acids Res. 2001, 29, E74. [Google Scholar] [CrossRef] [PubMed]
- Matthews, P.M.; Brown, R.M.; Morten, K.; Marchington, D.; Poulton, J.; Brown, G. Intracellular heteroplasmy for disease-associated point mutations in mtdna: Implications for disease expression and evidence for mitotic segregation of heteroplasmic units of mtdna. Hum. Genet. 1995, 96, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Lynn, S.; Borthwick, G.M.; Charnley, R.M.; Walker, M.; Turnbull, D.M. Heteroplasmic ratio of the a3243g mitochondrial DNA mutation in single pancreatic beta cells. Diabetologia 2003, 46, 296–299. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, S.; Feckler, C.; Lehr, S.; Wallbrecht, K.; Wolgast, H.; Müller-Wieland, D.; Kotzka, J. A critical comparison between two classical and a kit-based method for mitochondria isolation. Proteomics 2009, 9, 3209–3214. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Vizarra, E.; Ferrín, G.; Pérez-Martos, A.; Fernández-Silva, P.; Zeviani, M.; Enríquez, J.A. Isolation of mitochondria for biogenetical studies: An update. Mitochondrion 2010, 10, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Tobler, H.; Gut, C. Mitochondrial DNA from 4-cell stages of ascaris lumbricoides. J. Cell Sci. 1974, 16, 593–601. [Google Scholar] [PubMed]
- Sims, N.R.; Anderson, M.F. Isolation of mitochondria from rat brain using percoll density gradient centrifugation. Nat. Protoc. 2008, 3, 1228–1239. [Google Scholar] [CrossRef] [PubMed]
- Nishiguchi, M.K.; Doukakis, P.; Egan, M.; Kizirian, D.; Phillips, A.; Prendini, L.; Rosenbaum, H.C.; Torres, E.; Wyner, Y.; DeSalle, R. DNA isolation procedures. In Techniques in Molecular Systematics and Evolution; Springer: New York, NY, USA, 2002; pp. 249–287. [Google Scholar]
- Briggs, A.W.; Good, J.M.; Green, R.E.; Krause, J.; Maricic, T.; Stenzel, U.; Lalueza-Fox, C.; Rudan, P.; Brajković, D.; Kućan, Ž.; et al. Targeted retrieval and analysis of five neandertal mtdna genomes. Science 2009, 325, 318. [Google Scholar] [CrossRef] [PubMed]
- Maricic, T.; Whitten, M.; Paabo, S. Multiplexed DNA sequence capture of mitochondrial genomes using pcr products. PLoS ONE 2010, 5, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, X.; Xie, L.; Tan, M.; Li, Z.; Su, X.; Zhang, H.; Misof, B.; Kjer, K.M.; Tang, M. Mitochondrial capture enriches mito-DNA 100 fold, enabling pcr-free mitogenomics biodiversity analysis. Mol. Ecol. Resour. 2016, 16, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Lippold, S.; Xu, H.; Ko, A.; Li, M.; Renaud, G.; Butthof, A.; Schröder, R.; Stoneking, M. Human paternal and maternal demographic histories: Insights from high-resolution y chromosome and mtdna sequences. Investig. Genet. 2014, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Templeton, J.E.L.; Brotherton, P.; Llamas, B.; Soubrier, J.; Haak, W.; Cooper, A.; Austin, J.J. DNA capture and next-generation sequencing can recover whole mitochondrial genomes from highly degraded samples for human identification. Investig. Genet. 2013, 4, 26. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Cai, Q.; Samuels, D.C.; Ye, F.; Long, J.; Li, C.I.; Winther, J.F.; Tawn, E.J.; Stovall, M.; Lahteenmaki, P.; et al. The use of next generation sequencing technology to study the effect of radiation therapy on mitochondrial DNA mutation. Mutat. Res. 2012, 744, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Cui, H.; Wong, L.J. Comprehensive one-step molecular analyses of mitochondrial genome by massively parallel sequencing. Clin. Chem. 2012, 58, 1322–1331. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Li, F.; Chen, D.; Wang, G.; Truong, C.K.; Enns, G.M.; Graham, B.; Milone, M.; Landsverk, M.L.; Wang, J.; et al. Comprehensive next-generation sequence analyses of the entire mitochondrial genome reveal new insights into the molecular diagnosis of mitochondrial DNA disorders. Genet. Med. 2013, 15, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Aird, D.; Ross, M.G.; Chen, W.S.; Danielsson, M.; Fennell, T.; Russ, C.; Jaffe, D.B.; Nusbaum, C.; Gnirke, A. Analyzing and minimizing pcr amplification bias in illumina sequencing libraries. Genome Biol. 2011, 12, R18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cui, X.; Schmitt, K.; Hubert, R.; Navidi, W.; Arnheim, N. Whole genome amplification from a single cell: Implications for genetic analysis. Proc. Natl. Acad. Sci. USA 1992, 89, 5847–5851. [Google Scholar] [CrossRef] [PubMed]
- Zong, C.; Lu, S.; Chapman, A.R.; Xie, X.S. Genome-wide detection of single-nucleotide and copy-number variations of a single human cell. Science 2012, 338, 1622–1626. [Google Scholar] [CrossRef] [PubMed]
- Tate, C.M.; Nunez, A.N.; Goldstein, C.A.; Gomes, I.; Robertson, J.; Kavlick, M.F.; Budowle, B. Evaluation of circular DNA substrates for whole genome amplification prior to forensic analysis. Forensic Sci. Int. Genet. 2012, 6, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Nara, A.; Harihara, S.; Iwadate, K.; Uemura, K. Sequence analysis for hv i region of mitochondrial DNA using wga (whole genome amplification) method. Leg. Med. 2009, 11, S115–S118. [Google Scholar] [CrossRef] [PubMed]
- Maragh, S.; Jakupciak, J.P.; Wagner, P.D.; Rom, W.N.; Sidransky, D.; Srivastava, S.; Oconnell, C.D. Multiple strand displacement amplification of mitochondrial DNA from clinical samples. BMC Med. Genet. 2008, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wolff, J.N.; Shearman, D.C.; Brooks, R.C.; Ballard, J.W.O. Selective enrichment and sequencing of whole mitochondrial genomes in the presence of nuclear encoded mitochondrial pseudogenes (numts). PLoS ONE 2012, 7, e37142. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.; Balbi, V.; Balducci, E.; Pirazzini, C.; Rosini, F.; Tavano, F.; Achilli, A.; Siviero, P.; Minicuci, N.; Bellavista, E. Evidence for sub-haplogroup h5 of mitochondrial DNA as a risk factor for late onset alzheimer’s disease. PLoS ONE 2010, 5, e12037. [Google Scholar] [CrossRef] [PubMed]
- Lasken, R.S.; Stockwell, T.B. Mechanism of chimera formation during the multiple displacement amplification reaction. BMC Biotechnol. 2007, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Samuels, D.C.; Han, L.; Li, J.; Quanghu, S.; Clark, T.A.; Shyr, Y.; Guo, Y. Finding the lost treasures in exome sequencing data. Trends Genet. 2013, 29, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Parr, R.L.; Maki, J.; Reguly, B.; Dakubo, G.D.; Aguirre, A.; Wittock, R.; Robinson, K.; Jakupciak, J.P.; Thayer, R.E. The pseudo-mitochondrial genome influences mistakes in heteroplasmy interpretation. BMC Genom. 2006, 7, 185. [Google Scholar] [CrossRef] [PubMed]
- Parfait, B.; Rustin, P.; Munnich, A.; Rotig, A. Coamplification of nuclear pseudogenes and assessment of heteroplasmy of mitochondrial DNA mutations. Biochem. Biophys. Res. Commun. 1998, 247, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Picardi, E.; Pesole, G. Mitochondrial genomes gleaned from human whole-exome sequencing. Nat. Methods 2012, 9, 523–524. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Samuels, D.C.; Lehmann, B.; Stricker, T.; Pietenpol, J.; Shyr, Y.; Guo, Y. Mitochondria sequence mapping strategies and practicability of mitochondria variant detection from exome and rna sequencing data. Brief. Bioinform. 2016, 17, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Osborne, A.; Reis, A.H.; Bach, L.; Wangh, L.J. Single-molecule late-pcr analysis of human mitochondrial genomic sequence variations. PLoS ONE 2009, 4. [Google Scholar] [CrossRef] [PubMed]
- Paegel, B.M.; Emrich, C.A.; Wedemayer, G.J.; Scherer, J.R.; Mathies, R.A. High throughput DNA sequencing with a microfabricated 96-lane capillary array electrophoresis bioprocessor. Proc. Natl. Acad. Sci. USA 2002, 99, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Blazej, R.G.; Paegel, B.M.; Mathies, R.A. Polymorphism ratio sequencing: A new approach for single nucleotide polymorphism discovery and genotyping. Genome Res. 2003, 13, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Naue, J.; Sanger, T.; Schmidt, U.; Klein, R.; Lutz-Bonengel, S. Factors affecting the detection and quantification of mitochondrial point heteroplasmy using sanger sequencing and snapshot minisequencing. Int. J. Leg. Med. 2011, 125, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Sigurdsson, S.; Hedman, M.; Sistonen, P.; Sajantila, A.; Syvanen, A.C. A microarray system for genotyping 150 single nucleotide polymorphisms in the coding region of human mitochondrial DNA. Genomics 2006, 87, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Maitra, A.; Cohen, Y.; Gillespie, S.E.D.; Mambo, E.; Fukushima, N.; Hoque, M.O.; Shah, N.; Goggins, M.; Califano, J.A.; Sidransky, D. The human mitochip: A high-throughput sequencing microarray for mitochondrial mutation detection. Genome Res. 2004, 14, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.M.; Perin, J.C.; Schurr, T.G.; Dulik, M.C.; Zhadanov, S.I.; Baur, J.A.; King, M.P.; Place, E.; Clarke, C.; Grauer, M.; et al. Mitochondrial genome sequence analysis: A custom bioinformatics pipeline substantially improves affymetrix mitochip v2.0 call rate and accuracy. BMC Bioinform. 2011, 12, 402. [Google Scholar] [CrossRef] [PubMed]
- Vasta, V.; Ng, S.B.; Turner, E.H.; Shendure, J.; Hahn, S.H. Next generation sequence analysis for mitochondrial disorders. Genome Med. 2009, 1, 100. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.T.; Hsiao, W.W.; Tsao, T.T.; Chang, C.M.; Liu, Y.W.; Fan, C.C.; Lin, H.; Chang, H.H.; Yeh, T.J.; Chen, J.C.; et al. Quantitative assessment of mitochondrial DNA copies from whole genome sequencing. BMC Genom. 2012, 13, S5. [Google Scholar]
- Chen, T.; He, J.; Shen, L.; Fang, H.; Nie, H.; Jin, T.; Wei, X.; Xin, Y.; Jiang, Y.; Li, H.; et al. The mitochondrial DNA 4,977-bp deletion and its implication in copy number alteration in colorectal cancer. BMC Med. Genet. 2011, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.K.; Wong, L.J. Detection and quantification of heteroplasmic mutant mitochondrial DNA by real-time amplification refractory mutation system quantitative pcr analysis: A single-step approach. Clin. Chem. 2004, 50, 996–1001. [Google Scholar] [CrossRef] [PubMed]
- Kraytsberg, Y.; Khrapko, K. Single-molecule pcr: An artifact-free pcr approach for the analysis of somatic mutations. Expert Rev. Mol. Diagn. 2014, 5, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Kraytsberg, Y.; Nicholas, A.; Caro, P.; Khrapko, K. Single molecule pcr in mtdna mutational analysis: Genuine mutations vs. Damage bypass-derived artifacts. Methods 2008, 46, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Dube, S.; Qin, J.; Ramakrishnan, R. Mathematical analysis of copy number variation in a DNA sample using digital pcr on a nanofluidic device. PLoS ONE 2008, 3, e2876. [Google Scholar] [CrossRef] [PubMed]
- Belmonte, F.R.; Martin, J.L.; Frescura, K.; Damas, J.; Pereira, F.; Tarnopolsky, M.A.; Kaufman, B.A. Digital pcr methods improve detection sensitivity and measurement precision of low abundance mtdna deletions. Sci. Rep. 2016, 6, 25186. [Google Scholar] [CrossRef] [PubMed]
- Beer, N.R.; Hindson, B.J.; Wheeler, E.K.; Hall, S.B.; Rose, K.A.; Kennedy, I.M.; Colston, B.W. On-chip, real-time, single-copy polymerase chain reaction in picoliter droplets. Anal. Chem. 2007, 79, 8471–8475. [Google Scholar] [CrossRef] [PubMed]
- Leng, X.; Zhang, W.; Wang, C.; Cui, L.; Yang, C.J. Agarose droplet microfluidics for highly parallel and efficient single molecule emulsion pcr. Lab Chip 2013, 10, 2841. [Google Scholar] [CrossRef] [PubMed]
- Dressman, D.; Yan, H.; Traverso, G.; Kinzler, K.W.; Vogelstein, B. Transforming single DNA molecules into fluorescent magnetic particles for detection and enumeration of genetic variations. Proc. Natl. Acad. Sci. USA 2003, 100, 8817–8822. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wu, J.; Dressman, D.C.; Iacobuzio-Donahue, C.; Markowitz, S.D.; Velculescu, V.E.; Diaz, L.A., Jr.; Kinzler, K.W.; Vogelstein, B.; Papadopoulos, N. Heteroplasmic mitochondrial DNA mutations in normal and tumour cells. Nature 2010, 464, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.W.; Balaj, L.; Liau, L.M.; Samuels, M.L.; Kotsopoulos, S.K.; Maguire, C.A.; Loguidice, L.; Soto, H.; Garrett, M.; Lin, D.Z. Beaming and droplet digital pcr analysis of mutant idh1 mrna in glioma patient serum and cerebrospinal fluid extracellular vesicles. Mol. Ther. Nucleic Acids 2013, 2, e109. [Google Scholar] [CrossRef] [PubMed]
- Casoli, T.; Spazzafumo, L.; Stefano, G.D.; Conti, F. Role of diffuse low-level heteroplasmy of mitochondrial DNA in alzheimer’s disease neurodegeneration. Front. Aging Neurosci. 2015, 7, 142. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Schonberg, A.; Schaefer, M.; Schroeder, R.; Nasidze, I.; Stoneking, M. Detecting heteroplasmy from high-throughput sequencing of complete human mitochondrial DNA genomes. Am. J. Hum. Genet. 2010, 87, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Ahn, E.H.; Hirohata, K.; Kohrn, B.F.; Fox, E.J.; Chang, C.; Loeb, L.A. Detection of ultra-rare mitochondrial mutations in breast stem cells by duplex sequencing. PLoS ONE 2015, 10, e0136216. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Huang, T. Characterization of mitochondrial DNA heteroplasmy using a parallel sequencing system. Biotechniques 2010, 48, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Leary, R.J.; Kinde, I.; Diehl, F.; Schmidt, K.; Clouser, C.; Duncan, C.; Antipova, A.; Lee, C.; McKernan, K.; De La Vega, F.M.; et al. Development of personalized tumor biomarkers using massively parallel sequencing. Sci. Transl. Med. 2010, 2, 20ra14. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, M.W.; Kennedy, S.R.; Salk, J.J.; Fox, E.J.; Hiatt, J.B.; Loeb, L.A. Detection of ultra-rare mutations by next-generation sequencing. Proc. Natl. Acad. Sci. USA 2012, 109, 14508–14513. [Google Scholar] [CrossRef] [PubMed]
- Stoler, N.; Arbeithuber, B.; Guiblet, W.; Makova, K.D.; Nekrutenko, A. Streamlined analysis of duplex sequencing data with du novo. Genome Biol. 2016, 17, 180. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, R.; Faustin, B.; Rocher, C.; Malgat, M.; Mazat, J.-P.; Letellier, T. Mitochondrial threshold effects. Biochem. J. 2003, 370, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Ye, K.; Lu, J.; Ma, F.; Keinan, A.; Gu, Z. Extensive pathogenicity of mitochondrial heteroplasmy in healthy human individuals. Proc. Natl. Acad. Sci. USA 2014, 111, 10654–10659. [Google Scholar] [CrossRef] [PubMed]
- Sondheimer, N.; Glatz, C.E.; Tirone, J.E.; Deardorff, M.A.; Krieger, A.M.; Hakonarson, H. Neutral mitochondrial heteroplasmy and the influence of aging. Hum. Mol. Genet. 2011, 20, 1653–1659. [Google Scholar] [CrossRef] [PubMed]
- Levin, L.; Mishmar, D. A genetic view of the mitochondrial role in ageing: Killing us softly. In Longevity Genes; Springer: New York, NY, USA, 2015; pp. 89–106. [Google Scholar]
- DiMauro, S.; Mancuso, M. Mitochondrial diseases: Therapeutic approaches. Biosci. Rep. 2007, 27, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Kolesnikova, O.A.; Entelis, N.S.; Jacquin-Becker, C.; Goltzene, F.; Chrzanowska-Lightowlers, Z.M.; Lightowlers, R.N.; Martin, R.P.; Tarassov, I. Nuclear DNA-encoded trnas targeted into mitochondria can rescue a mitochondrial DNA mutation associated with the merrf syndrome in cultured human cells. Hum. Mol. Genet. 2004, 13, 2519–2534. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Hájek, P.; Chomyn, A.; Chan, E.; Seo, B.B.; Matsuno-Yagi, A.; Yagi, T.; Attardi, G. Lack of complex i activity in human cells carrying a mutation in mtdna-encoded nd4 subunit is corrected by thesaccharomyces cerevisiae nadh-quinone oxidoreductase (ndi1) gene. J. Biol. Chem. 2001, 276, 38808–38813. [Google Scholar] [CrossRef] [PubMed]
- Craven, L.; Tuppen, H.A.; Greggains, G.D.; Harbottle, S.J.; Murphy, J.L.; Cree, L.M.; Murdoch, A.P.; Chinnery, P.F.; Taylor, R.W.; Lightowlers, R.N. Pronuclear transfer in human embryos to prevent transmission of mitochondrial DNA disease. Nature 2010, 465, 82–85. [Google Scholar] [CrossRef] [PubMed]
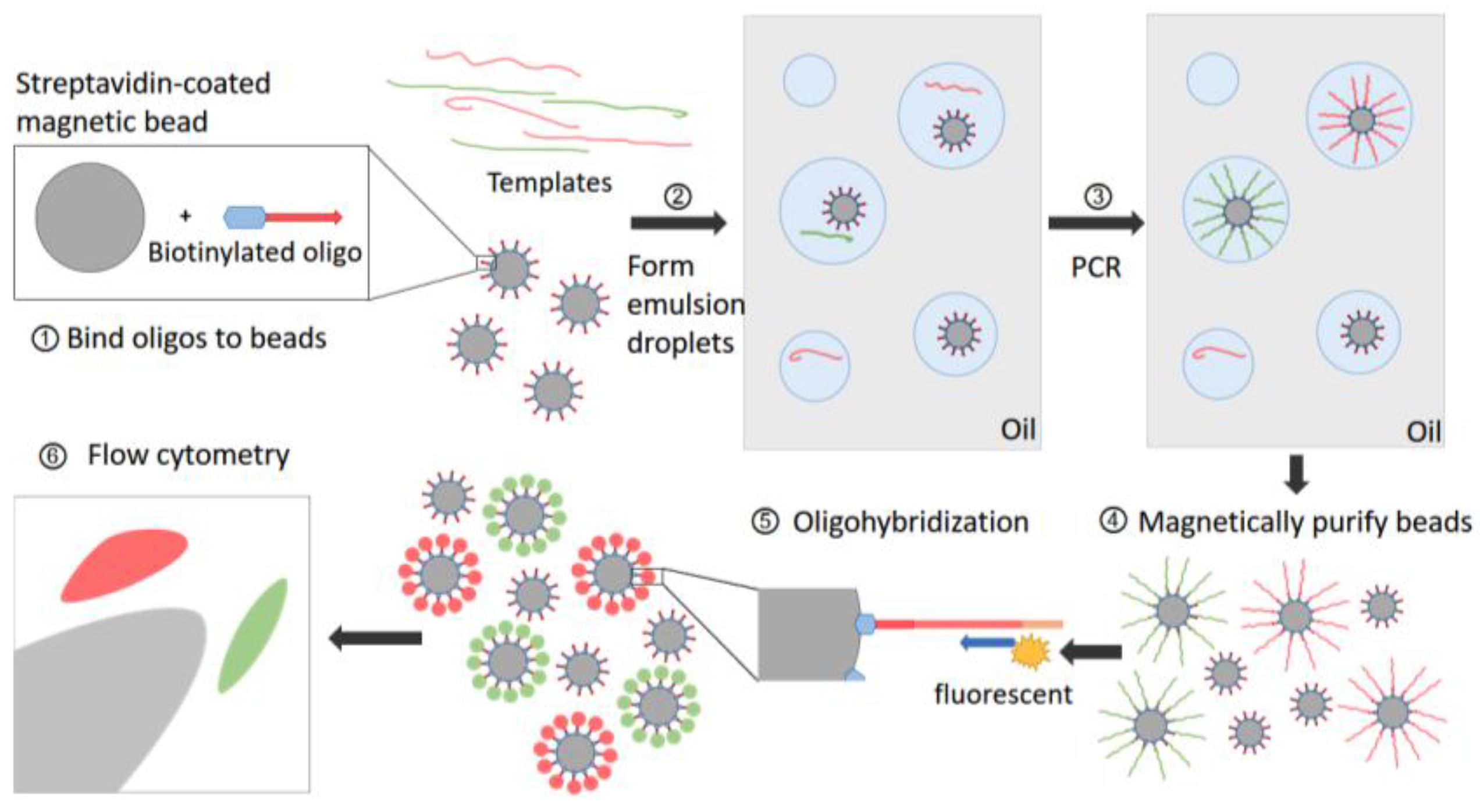
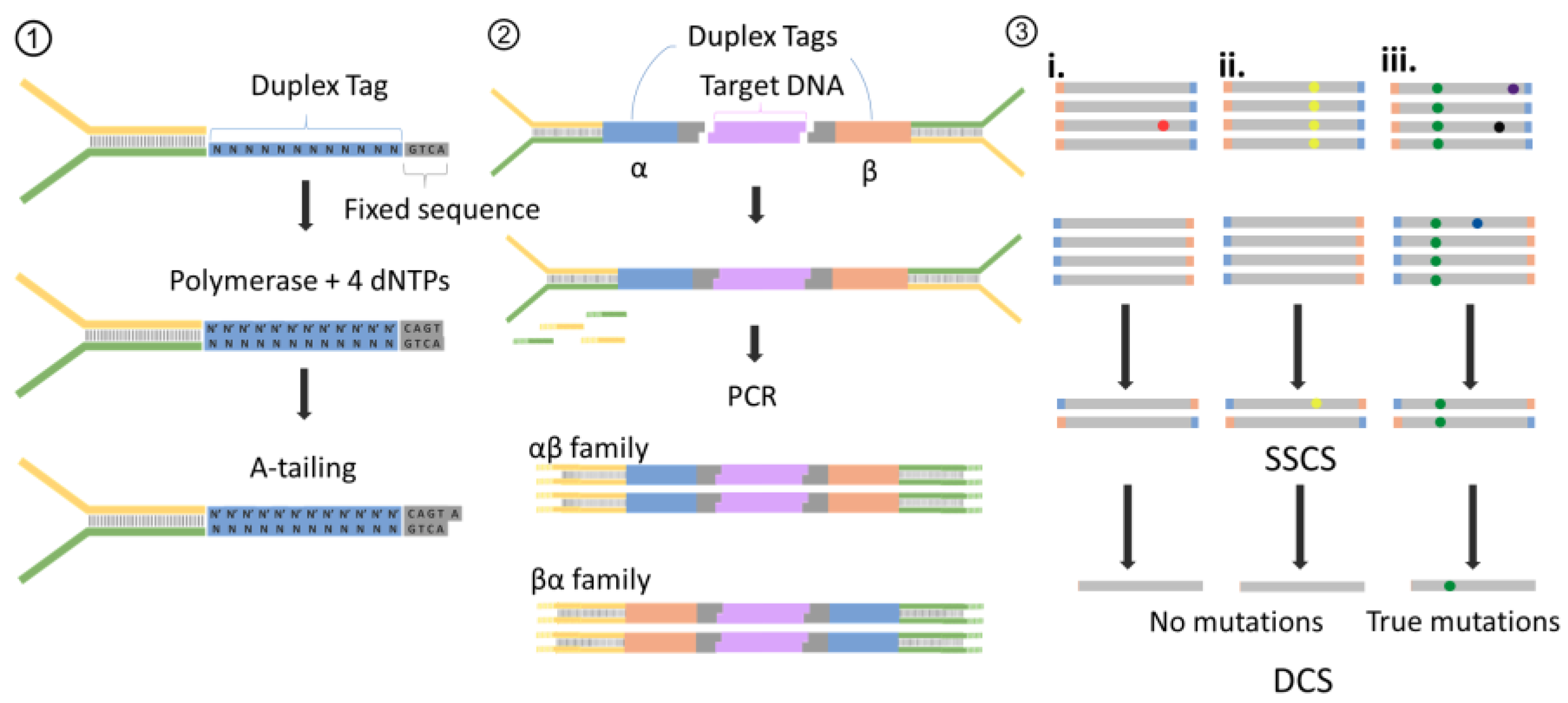
| Methods | Detection Limit | Variations Type 2 | |
|---|---|---|---|
| SNPs | Indels | ||
| Sanger sequencing | ~15% | Y | Y |
| Polymorphism ratio sequencing | ~5% | Y | Y |
| ARMS-qPCR | ~0.1% | Y (recorded) | N |
| Single-molecule PCR | ~7% [53] | Y | Y |
| Digital PCR | ~0.01% 1 | Y (recorded) | Y (recorded) |
| High-Throughput Sequencing | ~0.1% | Y | Y |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, M.; Tu, J.; Lu, Z. Recent Advances in Detecting Mitochondrial DNA Heteroplasmic Variations. Molecules 2018, 23, 323. https://doi.org/10.3390/molecules23020323
Duan M, Tu J, Lu Z. Recent Advances in Detecting Mitochondrial DNA Heteroplasmic Variations. Molecules. 2018; 23(2):323. https://doi.org/10.3390/molecules23020323
Chicago/Turabian StyleDuan, Mengqin, Jing Tu, and Zuhong Lu. 2018. "Recent Advances in Detecting Mitochondrial DNA Heteroplasmic Variations" Molecules 23, no. 2: 323. https://doi.org/10.3390/molecules23020323
APA StyleDuan, M., Tu, J., & Lu, Z. (2018). Recent Advances in Detecting Mitochondrial DNA Heteroplasmic Variations. Molecules, 23(2), 323. https://doi.org/10.3390/molecules23020323





