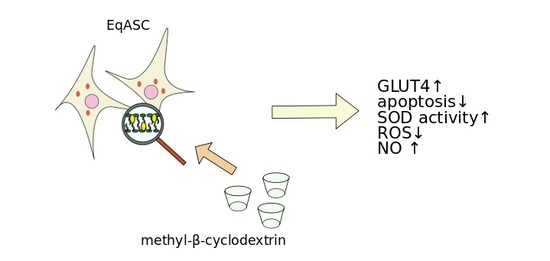
The Effect of Methyl-β-cyclodextrin on Apoptosis, Proliferative Activity, and Oxidative Stress in Adipose-Derived Mesenchymal Stromal Cells of Horses Suffering from Metabolic Syndrome (EMS)
Abstract
1. Introduction
2. Results
2.1. Proliferation of EqASCs
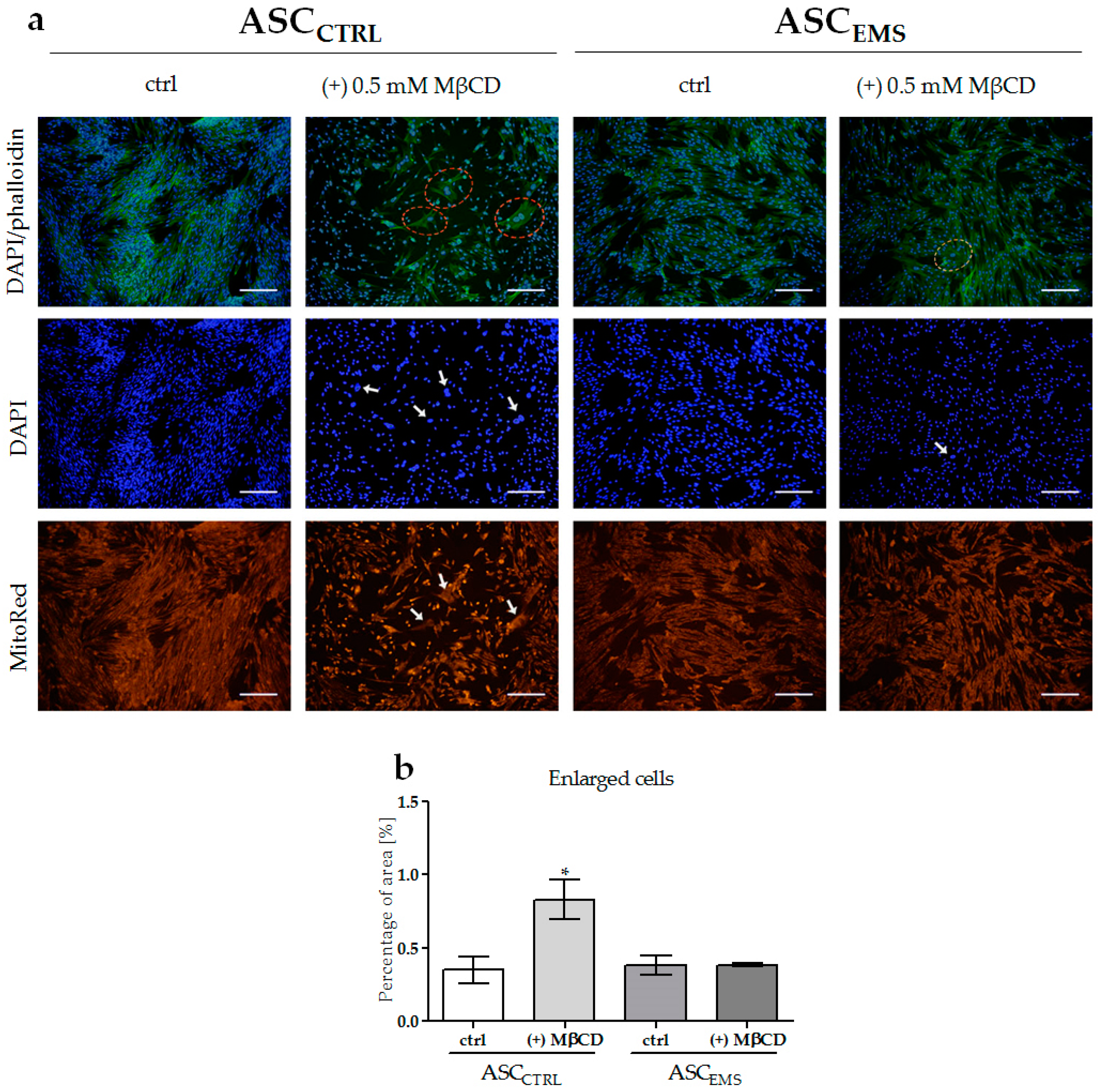
2.2. Morphology and Ultrastructure of EqASCs
2.3. Oxidative Stress Factors Analysis and the Impact of MβCD Treatment on Endoplasmic Reticulum Stress
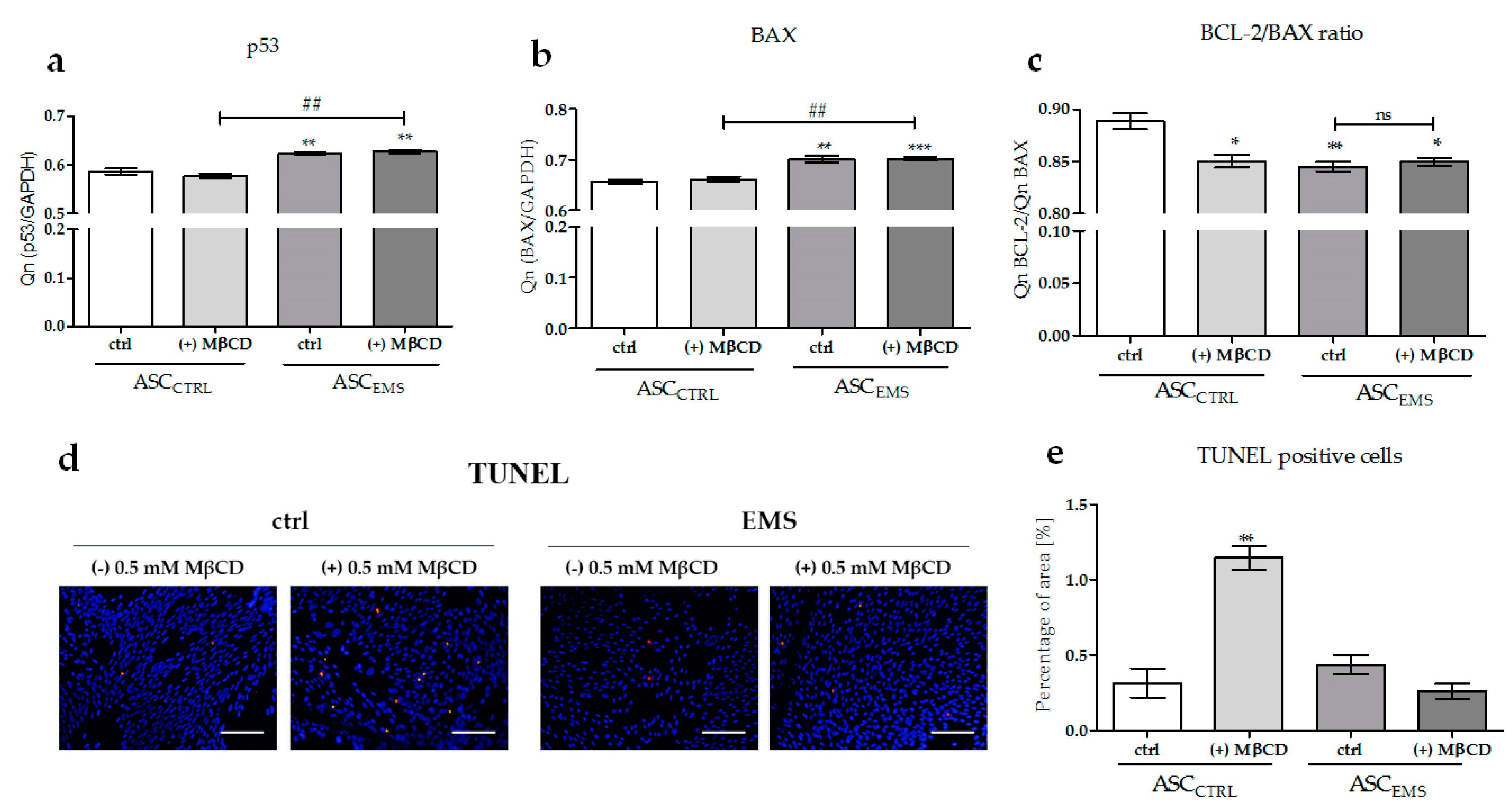
2.4. The Effects of MβCD on the Apoptotic Process
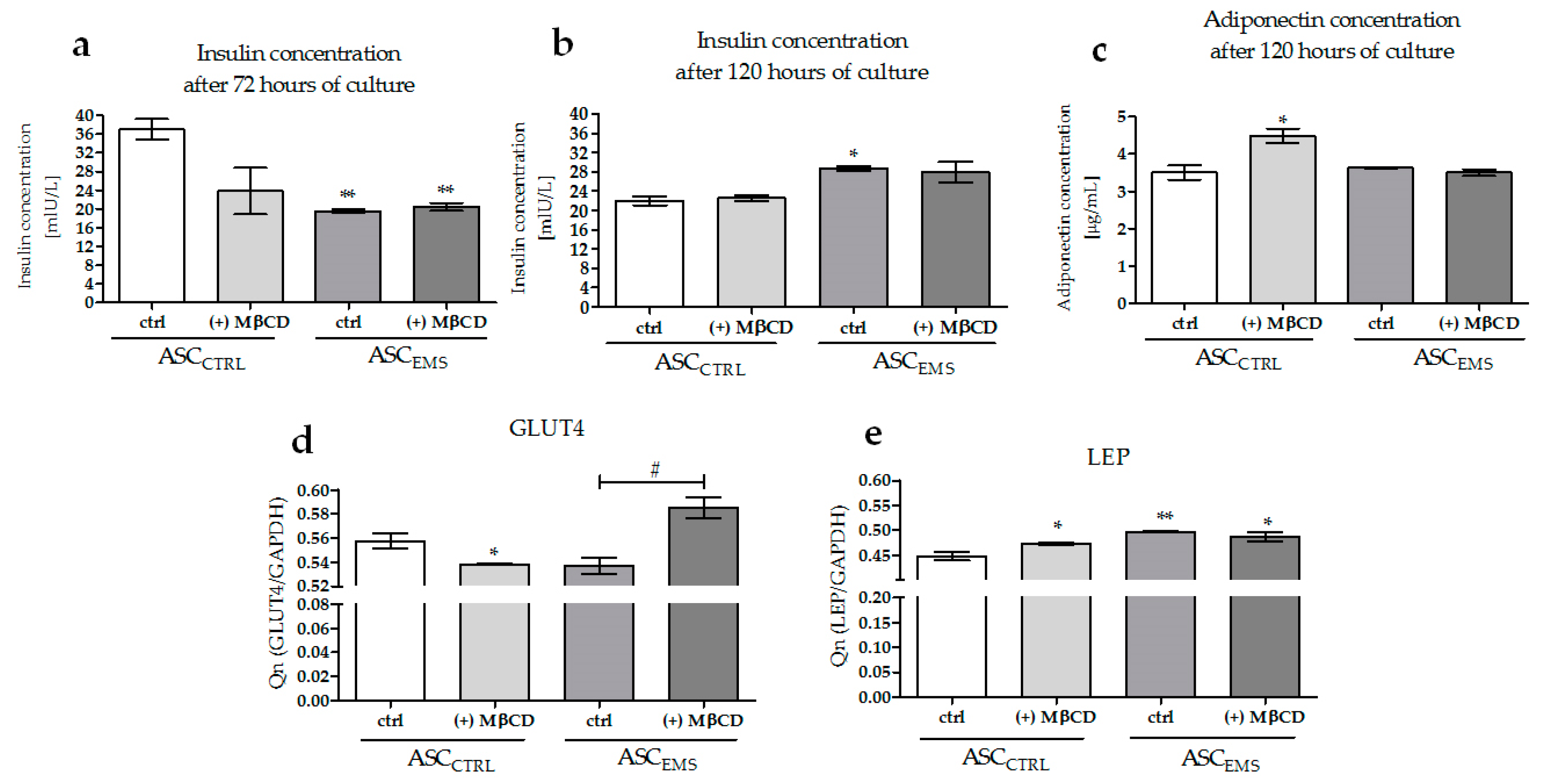
2.5. Analysis of the Expression Levels of LAMP-2, GLUT4, and LEP and of the Extracellular Secretion of Adiponectin and Insulin
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Cell Proliferation Assay
4.3. Evaluation of the Cell Morphology and Plasma Membrane Ultrastructure
4.4. TUNEL Staining
4.5. Measurement of the Oxidative Stress Factor
4.6. Analysis of Gene Expression
4.7. Enzyme-Linked Immunosorbent Assay (ELISA)
4.8. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Giles, S.L.; Rands, S.A.; Nicol, C.J.; Harris, P.A. Obesity prevalence and associated risk factors in outdoor living domestic horses and ponies. PeerJ 2014, 2, e299. [Google Scholar] [CrossRef] [PubMed]
- Ertelt, A.; Barton, A.-K.; Schmitz, R.R.; Gehlen, H. Metabolic syndrome: Is equine disease comparable to what we know in humans? Endocr. Connect. 2014, 3, R81–R93. [Google Scholar] [CrossRef] [PubMed]
- Marycz, K.; Kornicka, K.; Basinska, K.; Czyrek, A. Equine Metabolic Syndrome Affects Viability, Senescence, and Stress Factors of Equine Adipose-Derived Mesenchymal Stromal Stem Cells: New Insight into EqASCs Isolated from EMS Horses in the Context of Their Aging. Oxid. Med. Cell. Longev. 2016, 2016, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Marędziak, M.; Marycz, K.; Lewandowski, D.; Siudzińska, A.; Śmieszek, A. Static magnetic field enhances synthesis and secretion of membrane-derived microvesicles (MVs) rich in VEGF and BMP-2 in equine adipose-derived stromal cells (EqASCs)—A new approach in veterinary regenerative medicine. In Vitro Cell. Dev. Biol. Anim. 2015, 51, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, K. Mesenchymal Stem Cells and Metabolic Syndrome: Current Understanding and Potential Clinical Implications. Stem Cells Int. 2016, 2016, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Baraniak, P.R.; McDevitt, T.C. Stem cell paracrine actions and tissue regeneration. Regen. Med. 2010, 5, 121–143. [Google Scholar] [CrossRef] [PubMed]
- Basinska, K.; Marycz, K.; Śieszek, A.; Nicpoń, J. The production and distribution of IL-6 and TNF-a in subcutaneous adipose tissue and their correlation with serum concentrations in Welsh ponies with equine metabolic syndrome. J. Vet. Sci. 2015, 16, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Yang, X.; Han, Z.; Qu, F.; Shao, L.; Shi, Y. Mesenchymal stem cells: A new trend for cell therapy. Acta Pharmacol. Sin. 2013, 34, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Marycz, K.; Kornicka, K.; Marędziak, M.; Golonka, P.; Nicpoń, J. Equine metabolic syndrome impairs adipose stem cells osteogenic differentiation by predominance of autophagy over selective mitophagy. J. Cell. Mol. Med. 2016, 20, 2384–2404. [Google Scholar] [CrossRef] [PubMed]
- Marycz, K.; Kornicka, K.; Grzesiak, J.; Śmieszek, A.; Szłapka, J.; Szłapka, J. Macroautophagy and Selective Mitophagy Ameliorate Chondrogenic Differentiation Potential in Adipose Stem Cells of Equine Metabolic Syndrome: New Findings in the Field of Progenitor Cells Differentiation. Oxid. Med. Cell. Longev. 2016, 2016, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Gylling, H.; Hallikainen, M.; Pihlajamäki, J.; Simonen, P.; Kuusisto, J.; Laakso, M.; Miettinen, T.A. Insulin sensitivity regulates cholesterol metabolism to a greater extent than obesity: Lessons from the METSIM Study. J. Lipid Res. 2010, 51, 2422–2427. [Google Scholar] [CrossRef] [PubMed]
- Ikonen, E. Mechanisms for Cellular Cholesterol Transport: Defects and Human Disease. Physiol. Rev. 2006, 86, 1237–1261. [Google Scholar] [CrossRef] [PubMed]
- Goluszko, P.; Nowicki, B. Membrane cholesterol: A crucial molecule affecting interactions of microbial pathogens with mammalian cells. Infect. Immun. 2005, 73, 7791–7796. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Arjonilla, M.; Schwarcz, M.; Swerdloff, R.S.; Wang, C. Obesity, low testosterone levels and erectile dysfunction. Int. J. Impot. Res. 2009, 21, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Grantham, J.P.; Henneberg, M. The Estrogen Hypothesis of Obesity. PLoS ONE 2014, 9, e99776. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Brown, M.S.; Anderson, D.D.; Goldstein, J.L.; Radhakrishnan, A. Three pools of plasma membrane cholesterol and their relation to cholesterol homeostasis. eLife 2014, 3. [Google Scholar] [CrossRef] [PubMed]
- Llanos, P.; Contreras-Ferrat, A.; Georgiev, T.; Osorio-Fuentealba, C.; Espinosa, A.; Hidalgo, J.; Hidalgo, C.; Jaimovich, E. The cholesterol-lowering agent methyl-β-cyclodextrin promotes glucose uptake via GLUT4 in adult muscle fibers and reduces insulin resistance in obese mice. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E294–E305. [Google Scholar] [CrossRef] [PubMed]
- Le Lay, S.; Krief, S.; Farnier, C.; Lefrère, I.; Le Liepvre, X.; Bazin, R.; Ferré, P.; Dugail, I. Cholesterol, a Cell Size-dependent Signal That Regulates Glucose Metabolism and Gene Expression in Adipocytes. J. Biol. Chem. 2001, 276, 16904–16910. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Chiang, S.-H.; Saltiel, A.R. Insulin signaling and the regulation of glucose transport. Mol. Med. 2004, 10, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Antonescu, C.N.; Foti, M.; Sauvonnet, N.; Klip, A. Ready, set, internalize: Mechanisms and regulation of GLUT4 endocytosis. Biosci. Rep. 2009, 29, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, M.; Thorn, H.; Parpal, S.; Strålfors, P.; Gustavsson, J. Insulin induces translocation of glucose transporter GLUT4 to plasma membrane caveolae in adipocytes. FASEB J. 2001, 16, 249–251. [Google Scholar] [CrossRef] [PubMed]
- Barnes, K.; Ingram, J.C.; Bennett, M.D.M.; Stewart, G.W.; Baldwin, S.A. Methyl-beta-cyclodextrin stimulates glucose uptake in Clone 9 cells: A possible role for lipid rafts. Biochem. J. 2004, 378, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Hallikainen, M.; Toppinen, L.; Mykkänen, H.; Agren, J.J.; Laaksonen, D.E.; Miettinen, T.A.; Niskanen, L.; Poutanen, K.S.; Gylling, H. Interaction between cholesterol and glucose metabolism during dietary carbohydrate modification in subjects with the metabolic syndrome. Am. J. Clin. Nutr. 2006, 84, 1385–1392. [Google Scholar] [PubMed]
- Zidovetzki, R.; Levitan, I. Use of cyclodextrins to manipulate plasma membrane cholesterol content: Evidence, misconceptions and control strategies. Biochim. Biophys. Acta Biomembr. 2007, 1768, 1311–1324. [Google Scholar] [CrossRef] [PubMed]
- Christian, A.E.; Haynes, M.P.; Phillips, M.C.; Rothblat, G.H. Use of cyclodextrins for manipulating cellular cholesterol content. J. Lipid Res. 1997, 38, 2264–2272. [Google Scholar] [PubMed]
- Hao, M.; Head, W.S.; Gunawardana, S.C.; Hasty, A.H.; Piston, D.W. Direct effect of cholesterol on insulin secretion: A novel mechanism for pancreatic beta-cell dysfunction. Diabetes 2007, 56, 2328–2338. [Google Scholar] [CrossRef] [PubMed]
- Habegger, K.M.; Hoffman, N.J.; Ridenour, C.M.; Brozinick, J.T.; Elmendorf, J.S. AMPK Enhances Insulin-Stimulated GLUT4 Regulation via Lowering Membrane Cholesterol. Endocrinology 2012, 153, 2130–2141. [Google Scholar] [CrossRef] [PubMed]
- Portilho, D.M.; Soares, C.P.; Morrot, A.; Thiago, L.S.; Butler-Browne, G.; Savino, W.; Costa, M.L.; Mermelstein, C. Cholesterol depletion by methyl-β-cyclodextrin enhances cell proliferation and increases the number of desmin-positive cells in myoblast cultures. Eur. J. Pharmacol. 2012, 694, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.-A.; Chin, B.R.; Rhee, D.H.; Choi, H.-G.; Chang, H.-W.; Kim, J.-H.; Baek, S.-H. Methyl-β-cyclodextrin inhibits cell growth and cell cycle arrest via a prostaglandin E(2) independent pathway. Exp. Mol. Med. 2004, 36, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Hailstones, D.; Sleer, L.S.; Parton, R.G.; Stanley, K.K. Regulation of caveolin and caveolae by cholesterol in MDCK cells. J. Lipid Res. 1998, 39, 369–379. [Google Scholar] [PubMed]
- Mahavadi, S.; Nalli, A.; Kumar, D.; Bhattacharya, S.; Zhou, R.; Grider, J.; Murthy, K. Increased expression of caveolin-1 is associated with upregulation of the RhoA/Rho kinase pathway and smooth muscle contraction in diabetes (1110.11). FASEB J. 2014, 28. [Google Scholar] [CrossRef]
- Garofalo, T.; Matarrese, P.; Manganelli, V.; Marconi, M.; Tinari, A.; Gambardella, L.; Faggioni, A.; Misasi, R.; Sorice, M.; Malorni, W. Evidence for the involvement of lipid rafts localized at the ER-mitochondria associated membranes in autophagosome formation. Autophagy 2016, 12, 917–935. [Google Scholar] [CrossRef] [PubMed]
- Kameyama, K.; Motoyama, K.; Tanaka, N.; Yamashita, Y.; Higashi, T.; Arima, H. Induction of mitophagy-mediated antitumor activity with folate-appended methyl-β-cyclodextrin. Int. J. Nanomed. 2017, 12, 3433–3446. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Carroll, B.; Buganim, Y.; Maetzel, D.; Ng, A.H.M.; Cassady, J.P.; Cohen, M.A.; Chakraborty, S.; Wang, H.; Spooner, E.; et al. Impaired Autophagy in the Lipid-Storage Disorder Niemann-Pick Type C1 Disease. Cell Rep. 2013, 5, 1302–1315. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Czech, M.P. The GLUT4 Glucose Transporter. Cell Metab. 2007, 5, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Al-Makdissy, N.; Younsi, M.; Pierre, S.; Ziegler, O.; Donner, M. Sphingomyelin/cholesterol ratio: An important determinant of glucose transport mediated by GLUT-1 in 3T3-L1 preadipocytes. Cell. Signal. 2003, 15, 1019–1030. [Google Scholar] [CrossRef]
- Rudrappa, B.G.; Aski, B.S.; Kashinath, R.T. Effect of Membrane Cholesterol on Glucose Uptake in Diabetic Erythrocytes. Glob. J. Med. Res. 2012, 12, 18–22. [Google Scholar]
- Merry, T.L.; McConell, G.K. Skeletal muscle glucose uptake during exercise: A focus on reactive oxygen species and nitric oxide signaling. IUBMB Life 2009, 61, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Yashiro, A.; Nakashima, Y.; Nanri, H.; Ikeda, M.; Kuroiwa, A. Plasma nitrite/nitrate level is inversely correlated with plasma low-density lipoprotein cholesterol level. Clin. Cardiol. 1997, 20, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Gheibi, S.; Bakhtiarzadeh, F.; Jeddi, S.; Farrokhfall, K.; Zardooz, H.; Ghasemi, A. Nitrite increases glucose-stimulated insulin secretion and islet insulin content in obese type 2 diabetic male rats. Nitric Oxide 2017, 64, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Nakatani, K.; Morioka, K.; Urakawa, H.; Maruyama, N.; Kitagawa, N.; Katsuki, A.; Araki-Sasaki, R.; Hori, Y.; Gabazza, E.C.; et al. Nitric oxide stimulates glucose transport through insulin-independent GLUT4 translocation in 3T3-L1 adipocytes. Eur. J. Endocrinol. 2003, 149, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Cavener, D.R.; Gupta, S.; McGrath, B.C. PERK in beta cell biology and insulin biogenesis. Trends Endocrinol. Metab. 2010, 21, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Ballabio, A.; Gieselmann, V. Lysosomal disorders: From storage to cellular damage. Biochim. Biophys. Acta Mol. Cell Res. 2009, 1793, 684–696. [Google Scholar] [CrossRef] [PubMed]
- Doubling Time Online Computing with 2 Points. Available online: http://www.doubling-time.com/compute.php (accessed on 9 October 2017).
- Chomczynski, P.; Sacchi, N. Single-Step Method of RNA Isolation by Acid Guanidinium Thiocyanate–Phenol–Chloroform Extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds and methyl-β-cyclodextrin are available from the authors. |



| Gene | Sequence 5′-3′ | Amplicon Length (bp) | Accession No. |
|---|---|---|---|
| GAPDH | F: GATGCCCCAATGTTTGTGA R: AAGCAGGGATGATGTTCTGG | 250 | XM_014866500.1 |
| p53 | F: TACTCCCCTGCCCTCAACAA R: AGGAATCAGGGCCTTGAGGA | 252 | U37120.1 |
| BAX | F: GCCAGCAAATTGGTGCTCAA R: AGCAGTCACTTCCATGGCTC | 260 | XM_014830923.1 |
| BCL-2 | F: TTCTTTGAGTTCGGTGGGGT R: GGGCCGTACAGTTCCACAA | 164 | XM_014843802.1 |
| LAMP2 | F: GCACCCCTGGGAAGTTCTTA R: ATCCAGCGAACACTCTTGGG | 147 | XM_014831347.1 |
| PERK | F: GTGACTGCAATGGACCAGGA R: TCACGTGCTCACGAGGATATT | 283 | XM_014852775.1 |
| LEP | F: CACACGCAGTCAGTCTCCTC R: CGGAGGTTCTCCAGGTCAT | 176 | XM_014854289.1 |
| GLUT4 | F: TGGGCTCTCCGTGGCCATCTT R: GCTGCTGGCTGAGCTGCAGCA | 656 | XM_014861501.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szydlarska, J.; Weiss, C.; Marycz, K. The Effect of Methyl-β-cyclodextrin on Apoptosis, Proliferative Activity, and Oxidative Stress in Adipose-Derived Mesenchymal Stromal Cells of Horses Suffering from Metabolic Syndrome (EMS). Molecules 2018, 23, 287. https://doi.org/10.3390/molecules23020287
Szydlarska J, Weiss C, Marycz K. The Effect of Methyl-β-cyclodextrin on Apoptosis, Proliferative Activity, and Oxidative Stress in Adipose-Derived Mesenchymal Stromal Cells of Horses Suffering from Metabolic Syndrome (EMS). Molecules. 2018; 23(2):287. https://doi.org/10.3390/molecules23020287
Chicago/Turabian StyleSzydlarska, Joanna, Christine Weiss, and Krzysztof Marycz. 2018. "The Effect of Methyl-β-cyclodextrin on Apoptosis, Proliferative Activity, and Oxidative Stress in Adipose-Derived Mesenchymal Stromal Cells of Horses Suffering from Metabolic Syndrome (EMS)" Molecules 23, no. 2: 287. https://doi.org/10.3390/molecules23020287
APA StyleSzydlarska, J., Weiss, C., & Marycz, K. (2018). The Effect of Methyl-β-cyclodextrin on Apoptosis, Proliferative Activity, and Oxidative Stress in Adipose-Derived Mesenchymal Stromal Cells of Horses Suffering from Metabolic Syndrome (EMS). Molecules, 23(2), 287. https://doi.org/10.3390/molecules23020287