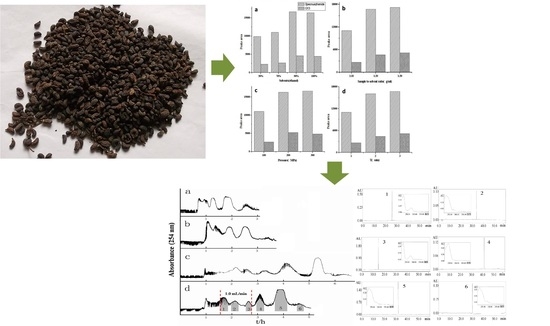
Preparative Separation of Phenylethanoid and Secoiridoid Glycosides from Ligustri Lucidi Fructus by High-Speed Counter-Current Chromatography Coupled with Ultrahigh Pressure Extraction
Abstract
:1. Introduction
2. Results
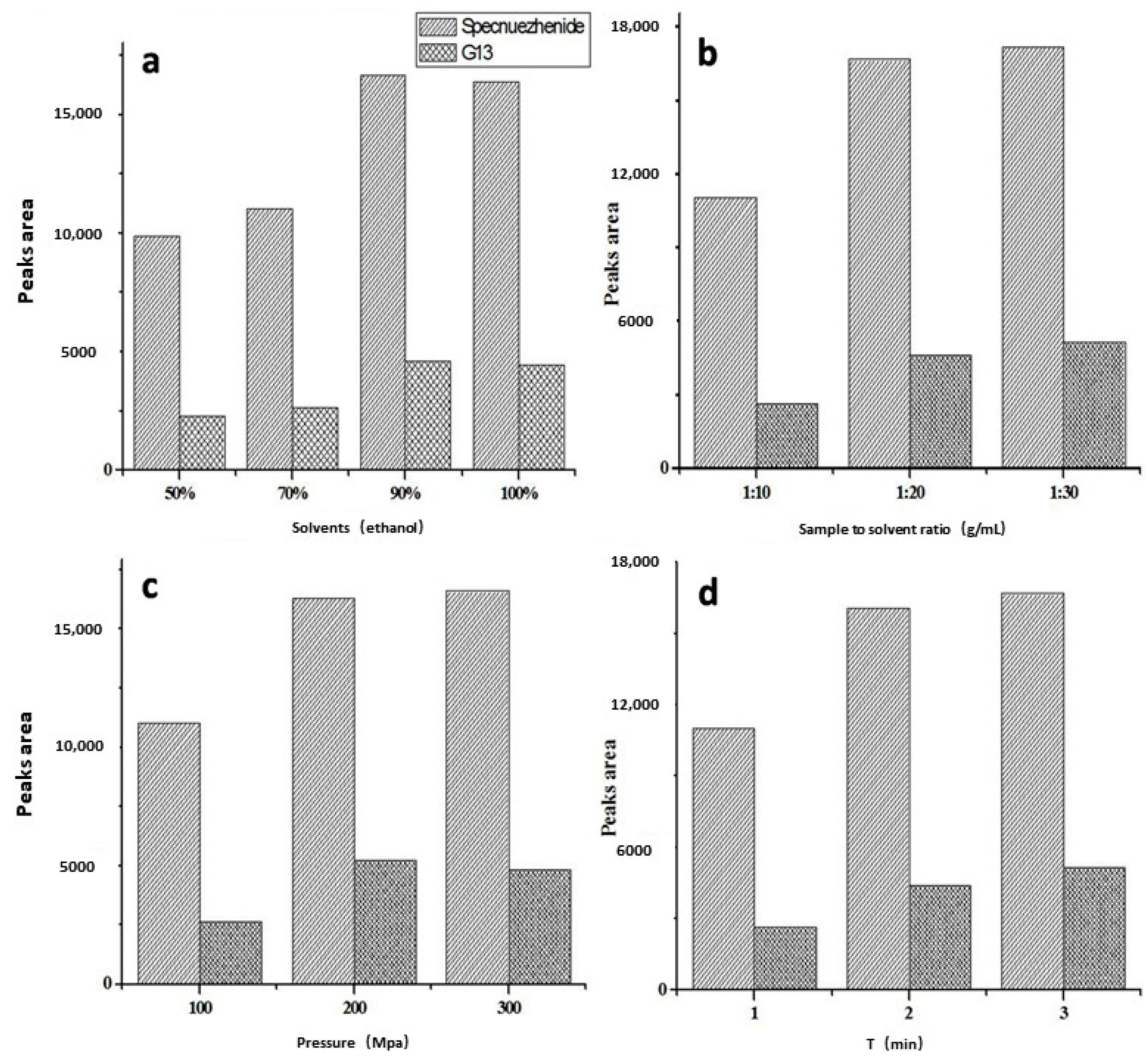
2.1. Ultrahigh Pressure Extraction Parameters
2.2. Optimization of the High-Speed Counter-Current Chromatography Conditions
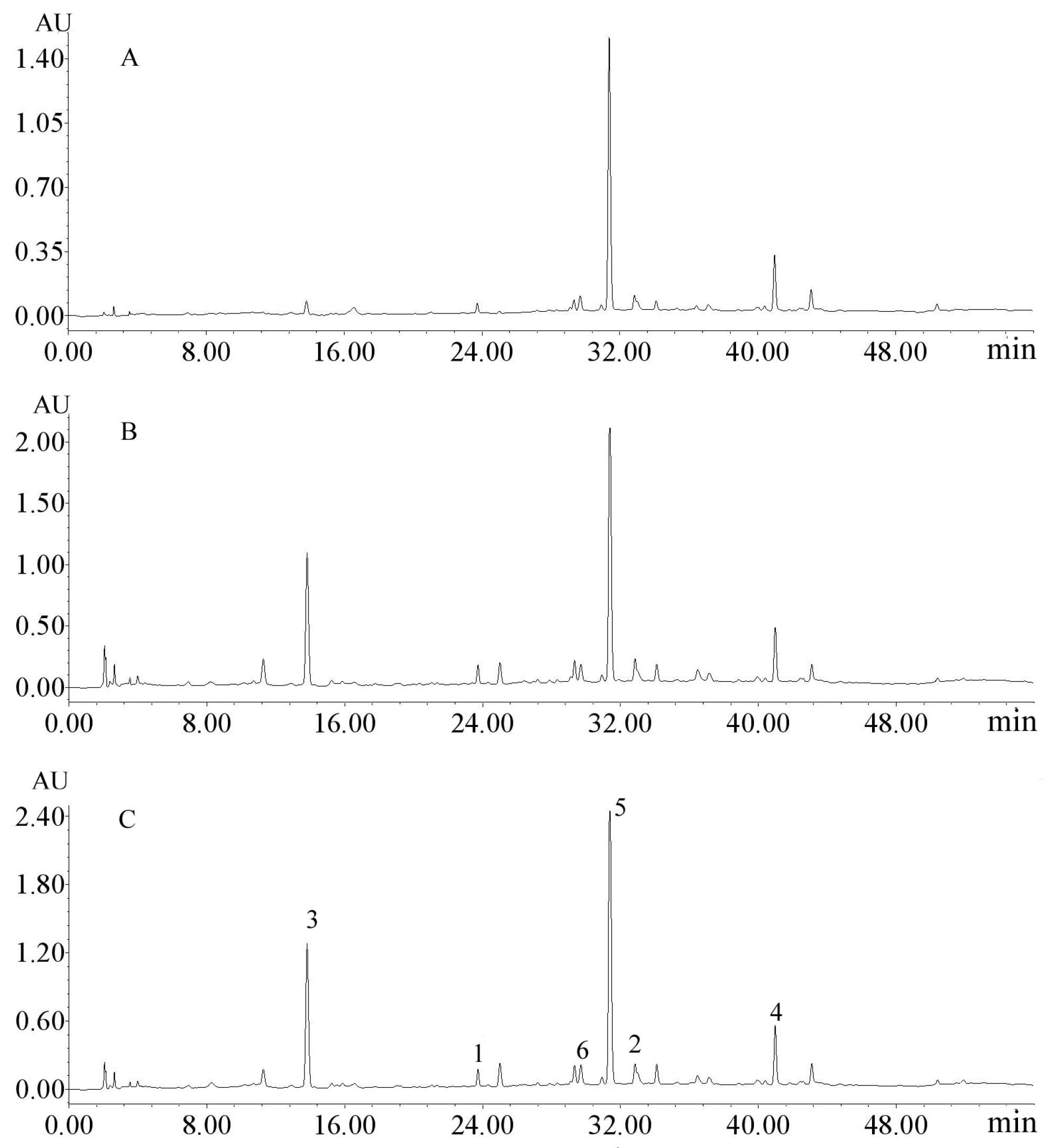
2.3. Purification of Phenylethanoid and Secoiridoid Glycosides by HSCCC
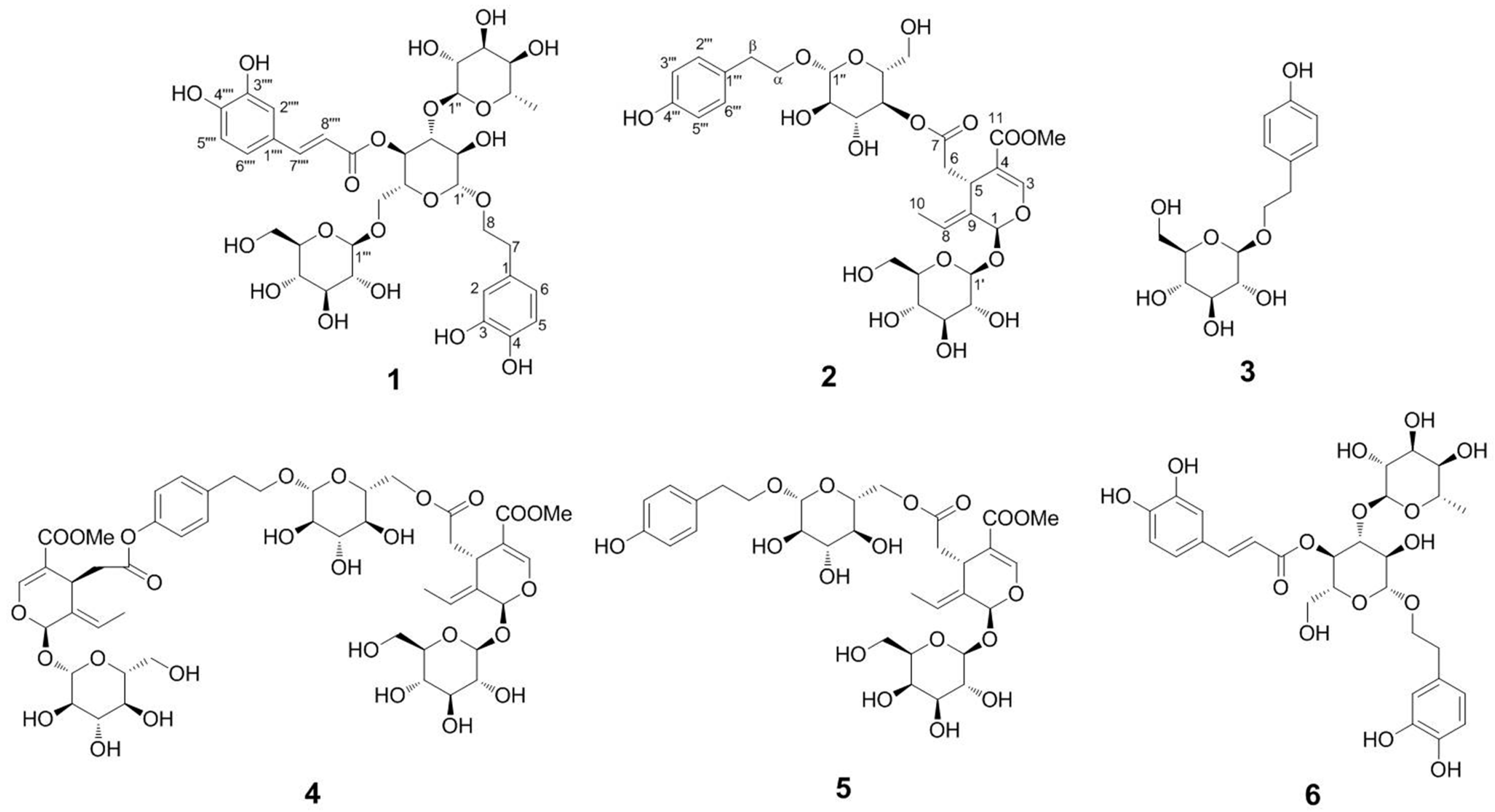
2.4. Structure Identification of the Isolated Compounds
3. Materials and Methods
3.1. Reagents and Materials
3.2. Apparatus
3.3. Ultrahigh Pressure Extraction
3.4. Traditional Extraction
3.5. HSCCC Separation Procedure
3.6. HPLC Analysis and Identification of the Fractions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hu, B.; Du, Q.; Deng, S.; An, H.M.; Pan, C.F.; Shen, K.P.; Xu, L.; Wei, M.M.; Wang, S.S. Ligustrum lucidum Ait. fruit extract induces apoptosis and cell senescence in human hepatocellular carcinoma cells through upregulation of p21. Oncol. Rep. 2014, 32, 1037–1042. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.L.; Fan, W.X. The effects of Nü-zhen-zi formula on chemotherapy induced alopecia. Acta. Univ. Med. Nanjing Nat. Sci. 2004, 24, 305–306. [Google Scholar]
- Wu, X.F.; Han, S.Y.; Zhu, L.S.; Bai, J.; Liu, S.M. The effects of Ligustrum lucidum Ait. fruits extract on cyclophosphomidum induced immunosuppression. J. North China Coal Med. Coll. 2008, 10, 303–304. [Google Scholar]
- Fukuda, T.; Kitada, Y.; Chen, X.M.; Yang, L.; Miyase, T. Two new monoterpene glycosides from ku-ding-cha. Inhibitors of acyl-CoA: Cholesterol acyltransferase (ACAT). Chem. Pharm. Bull. 1996, 44, 2173–2176. [Google Scholar] [CrossRef] [PubMed]
- He, Z.D.; Ueda, S.; Akaji, M.; Fujita, T.; Inoue, K.; Yang, C.R. Monoterpenoid and phenylethanoid glycosides from Ligustrum pedunculare. Phytochemistry 1994, 36, 709–716. [Google Scholar] [CrossRef]
- He, Z.D.; Dong, H.; Xu, H.X.; Ye, W.C.; Sun, H.D.; But, P.P.H. Secoiridoid constituents from the fruits of Ligustrum lucidum. Phytochemistry 2001, 56, 327–330. [Google Scholar] [CrossRef]
- Gao, L.L.; Li, C.; Wang, Z.M.; Liu, X.Q.; You, Y.; Wei, H.; Guo, T. Ligustri Lucidi Fructus as a traditional Chinese medicine: A review of its phytochemistry and pharmacology. Nat. Prod. Res. 2015, 29, 493–510. [Google Scholar] [CrossRef] [PubMed]
- He, Z.D.; But, P.P.H.; Chan, T.W.D.; Dong, H.; Xu, H.X.; Lau, C.P.; Sun, H.D. Antioxidative glucosides from the fruits of Ligustrum lucidum. Chem. Pharm. Bull. 2001, 49, 780–784. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Yang, L.; Zhang, G.; Wang, F. Bioactivity-guided isolation of antiosteoporotic compounds from Ligustrum lucidum. Phytother. Res. 2013, 27, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Pharmacopoeia Commission of the Ministry of Public Health. Chinese Pharmacopoeia, Part I.; China Medical Science and Technology Press: Beijing, China, 2015; pp. 45–46. [Google Scholar]
- Xia, E.Q.; Yu, Y.Y.; Xu, X.R.; Deng, G.F.; Guo, Y.J.; Li, H.B. Ultrasound-assisted extraction of oleanolic acid and ursolic acid from Ligustrum lucidum Ait. Ultrason. Sonochem. 2012, 19, 772–776. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.C.; Zhao, X.X.; Wu, Q.C.; Fu, J.W.; Dai, Y.; Wong, M.S.; Yao, X.S. New secoiridoids from the fruits of Ligustrum lucidum. J. Asian Nat. Prod. Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.Q.; Zhao, H.W.; Wang, D.J.; Song, X.Y.; Zhao, L.; Wang, X. Extraction and purification of five terpenoids from olibanum by ultrahigh pressure technique and high-speed countercurrent chromatography. J. Sep. Sci. 2017, 40, 2732–2740. [Google Scholar] [CrossRef] [PubMed]
- Li, H.L.; Zhang, Y.Q.; Liu, Q.; Sun, C.L.; Li, J.; Yang, P.; Wang, X. Preparative separation of phenolic compounds from Chimonanthus praecox Flowers by high-speed counter-current chromatography using a stepwise elution mode. Molecules 2016, 21, 1016. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.; Ji, W.; Wei, Y.; Zhao, R.; Chen, Z.; Geng, Y.; Jing, F.; Wang, X. Separation and Purification of Fructo-Oligosaccharide by High-Speed Counter-Current Chromatography Coupled with Precolumn Derivatization. Molecules 2018, 23, 381. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.T.; Fan, G.R.; Hong, Z.Y.; Chai, Y.F.; Wu, Y.T. Large-scale isolation and purification of geniposide from the fruit of Gardenia jasminoides Ellis by high-speed counter-current chromatography. J. Chromatogr. A 2005, 1100, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Oguchi, H.; Takizawa, N. New phenylethanoid glycosides from Cistanche tubulosa (SCHRENK) Hook. F. I. Chem. Pharm. Bull. 1987, 35, 3309–3314. [Google Scholar] [CrossRef]
- Tolonen, A.; Gyorgy, Z.; Jalonen, J. LC/MS/MS identification of glycosides produced by biotransformation of cinnamyl alcohol in Rhodiola rosea compact callus aggregates. Biomed. Chromatogr. 2004, 18, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Fukuyama, Y.; Koshino, K.; Hasegawa, T.; Yamada, T.; Nakagawa, K. New Secoiridoid Glucosides from Ligustrum japonicum. Planta Med. 1987, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.F.; Cao, Y.Y.; Chen, H.S.; Dong, J.P. Isolation and identification of two new secoiridoids of water-soluble chemical constituents from the fruits of Ligustrum lucidum Ait. Acta Pharm. Sin. 1997, 32, 442–446. [Google Scholar]
Sample Availability: Samples of the compounds (1–6) are available from the authors. |

| Solvent System Ethyl Acetate:n-Butanol:Water | Peak No. | |||||
|---|---|---|---|---|---|---|
| 1(KD) | 2(KD) | 3(KD) | 4(KD) | 5(KD) | 6(KD) | |
| 1:0:1 | 0.11 | 0.10 | 0.13 | 0.05 | 0.23 | 0.06 |
| 4:1:5 | 0.56 | 0.49 | 1.39 | 0.65 | 0.46 | 0.34 |
| 2:1:3 | 0.58 | 0.50 | 1.38 | 0.78 | 0.55 | 0.46 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, F.; Chen, L.; Liu, Q.; Wang, X.; Li, J.; Yu, J. Preparative Separation of Phenylethanoid and Secoiridoid Glycosides from Ligustri Lucidi Fructus by High-Speed Counter-Current Chromatography Coupled with Ultrahigh Pressure Extraction. Molecules 2018, 23, 3353. https://doi.org/10.3390/molecules23123353
He F, Chen L, Liu Q, Wang X, Li J, Yu J. Preparative Separation of Phenylethanoid and Secoiridoid Glycosides from Ligustri Lucidi Fructus by High-Speed Counter-Current Chromatography Coupled with Ultrahigh Pressure Extraction. Molecules. 2018; 23(12):3353. https://doi.org/10.3390/molecules23123353
Chicago/Turabian StyleHe, Fengwei, Li Chen, Qian Liu, Xiao Wang, Jia Li, and Jinqian Yu. 2018. "Preparative Separation of Phenylethanoid and Secoiridoid Glycosides from Ligustri Lucidi Fructus by High-Speed Counter-Current Chromatography Coupled with Ultrahigh Pressure Extraction" Molecules 23, no. 12: 3353. https://doi.org/10.3390/molecules23123353
APA StyleHe, F., Chen, L., Liu, Q., Wang, X., Li, J., & Yu, J. (2018). Preparative Separation of Phenylethanoid and Secoiridoid Glycosides from Ligustri Lucidi Fructus by High-Speed Counter-Current Chromatography Coupled with Ultrahigh Pressure Extraction. Molecules, 23(12), 3353. https://doi.org/10.3390/molecules23123353