A Novel Synthetic Dihydroindeno[1,2-b] Indole Derivative (LS-2-3j) Reverses ABCB1- and ABCG2-Mediated Multidrug Resistance in Cancer Cells
Abstract
1. Introduction
2. Results
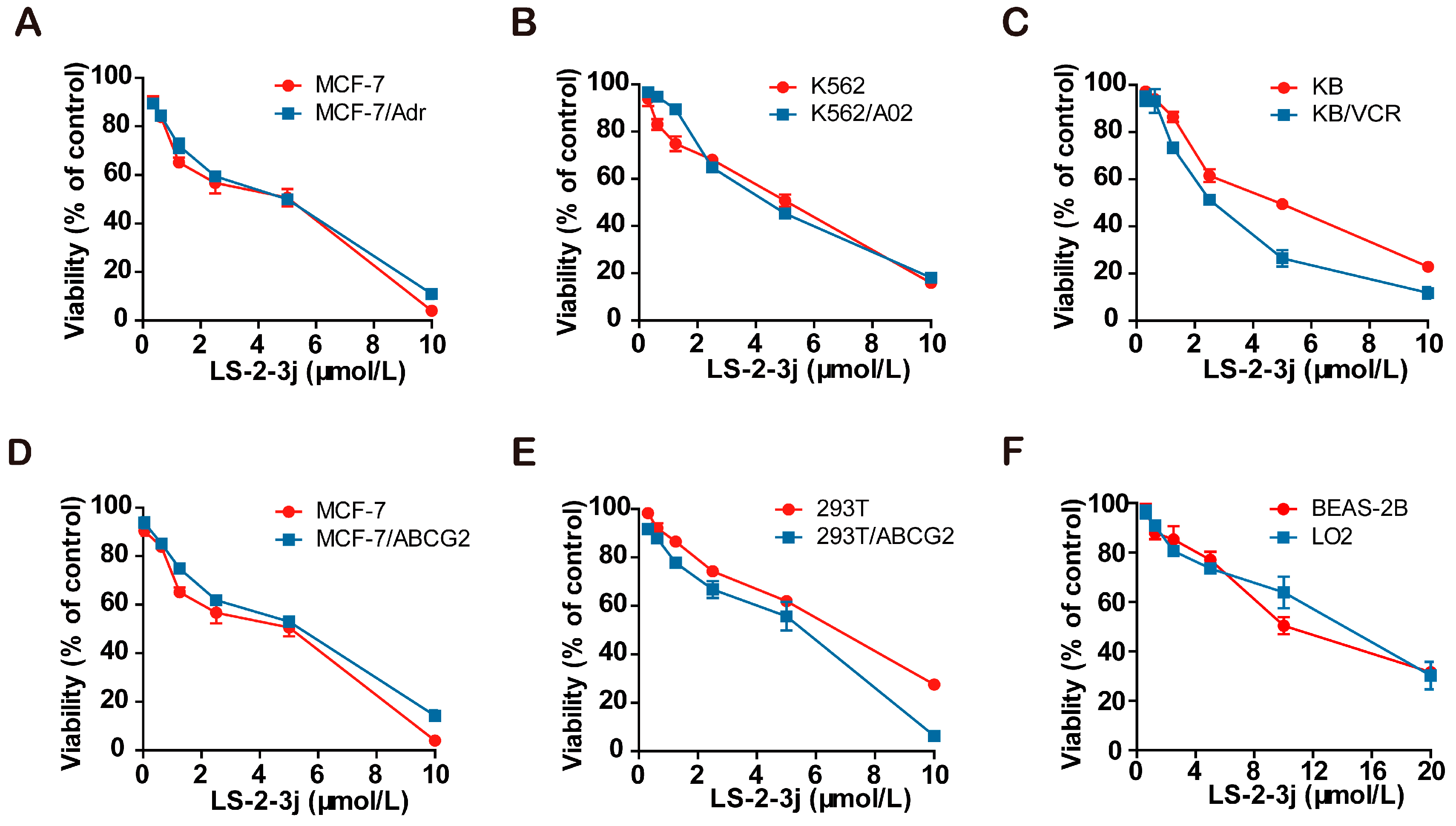
2.1. Cell Cytotoxity Assay
2.2. LS-2-3j Enhances the Sensitivity of Chemotherapeutic Agents
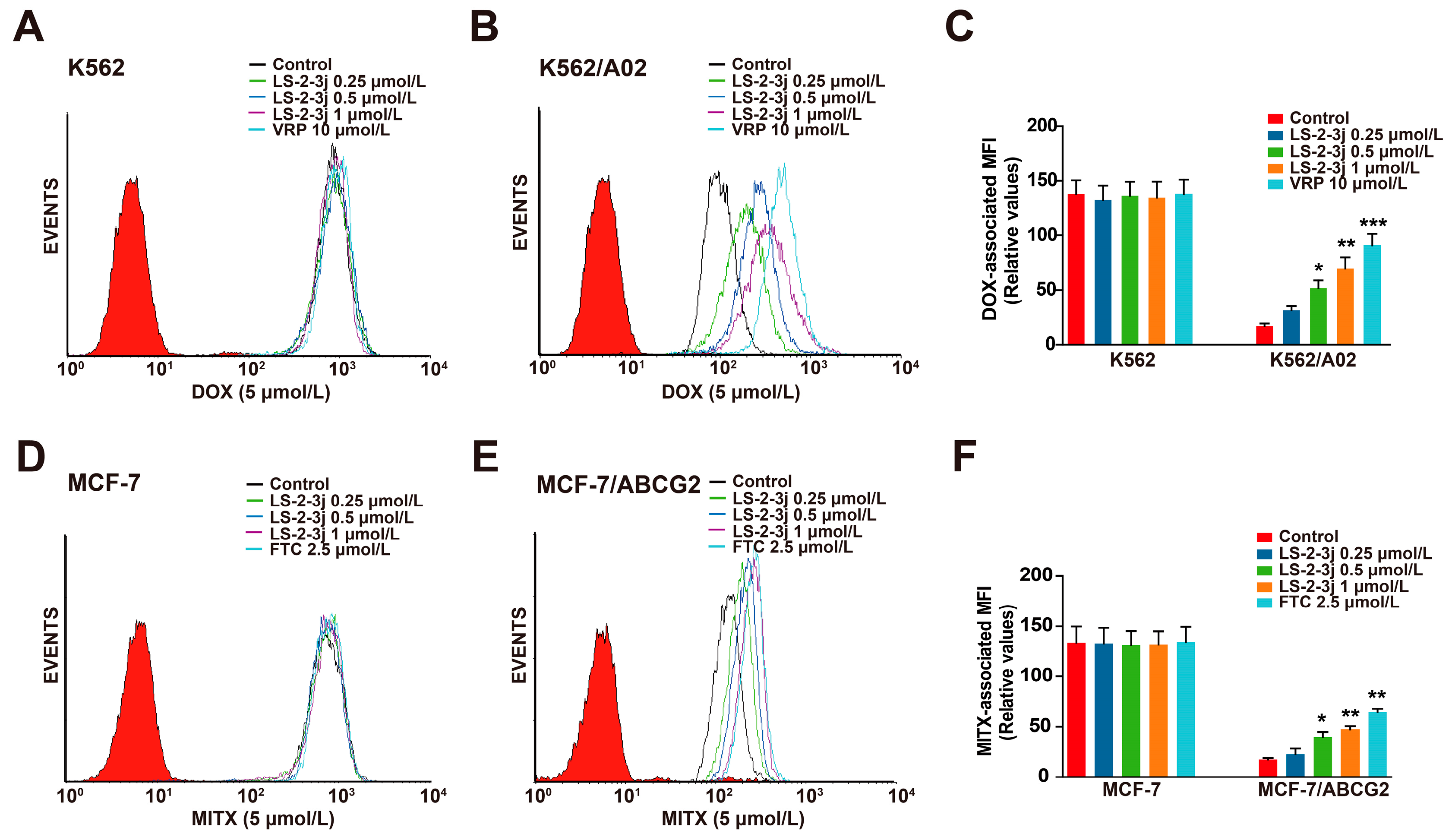
2.3. LS-2-3j Enhances the Accumulation of DOX and MITX
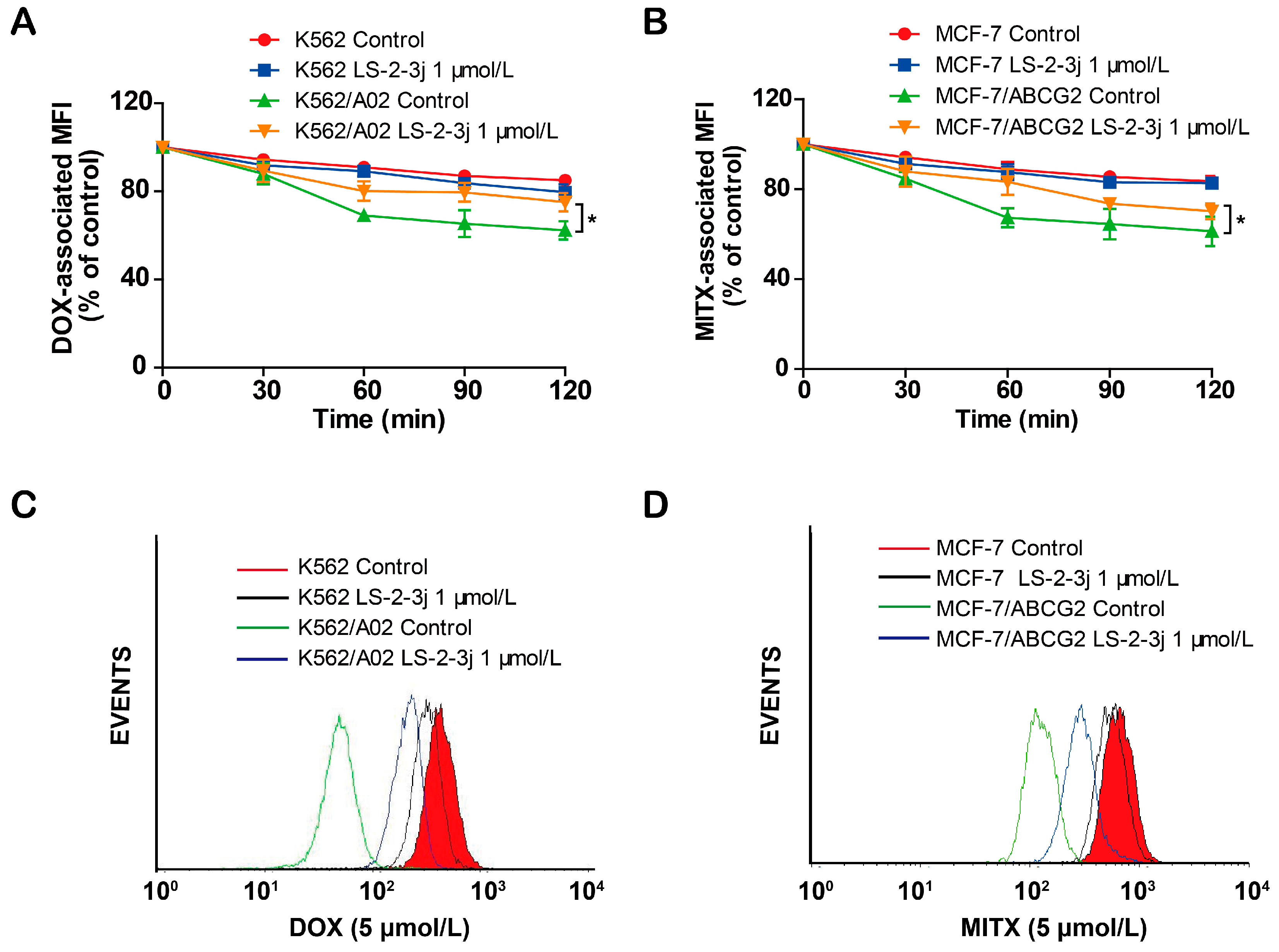
2.4. LS-2-3j Inhibits the Efflux of DOX and MITX
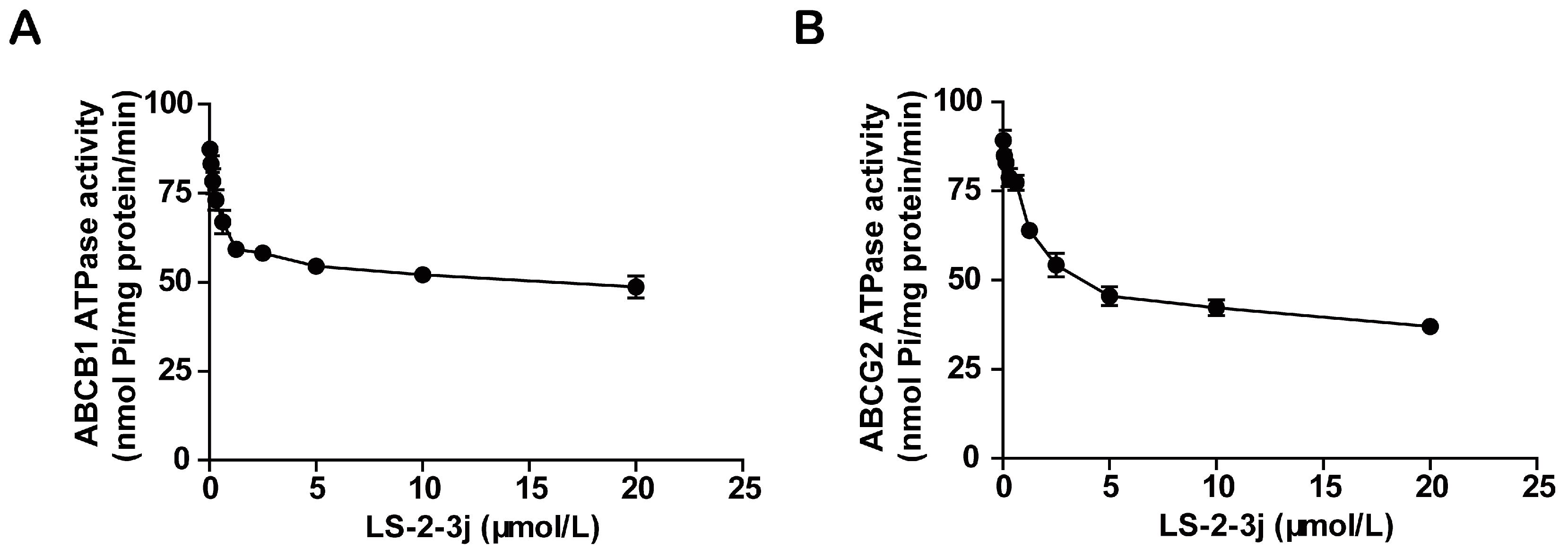
2.5. LS-2-3j Inhibits the ATPase Activity of ABCB1 and ABCG2
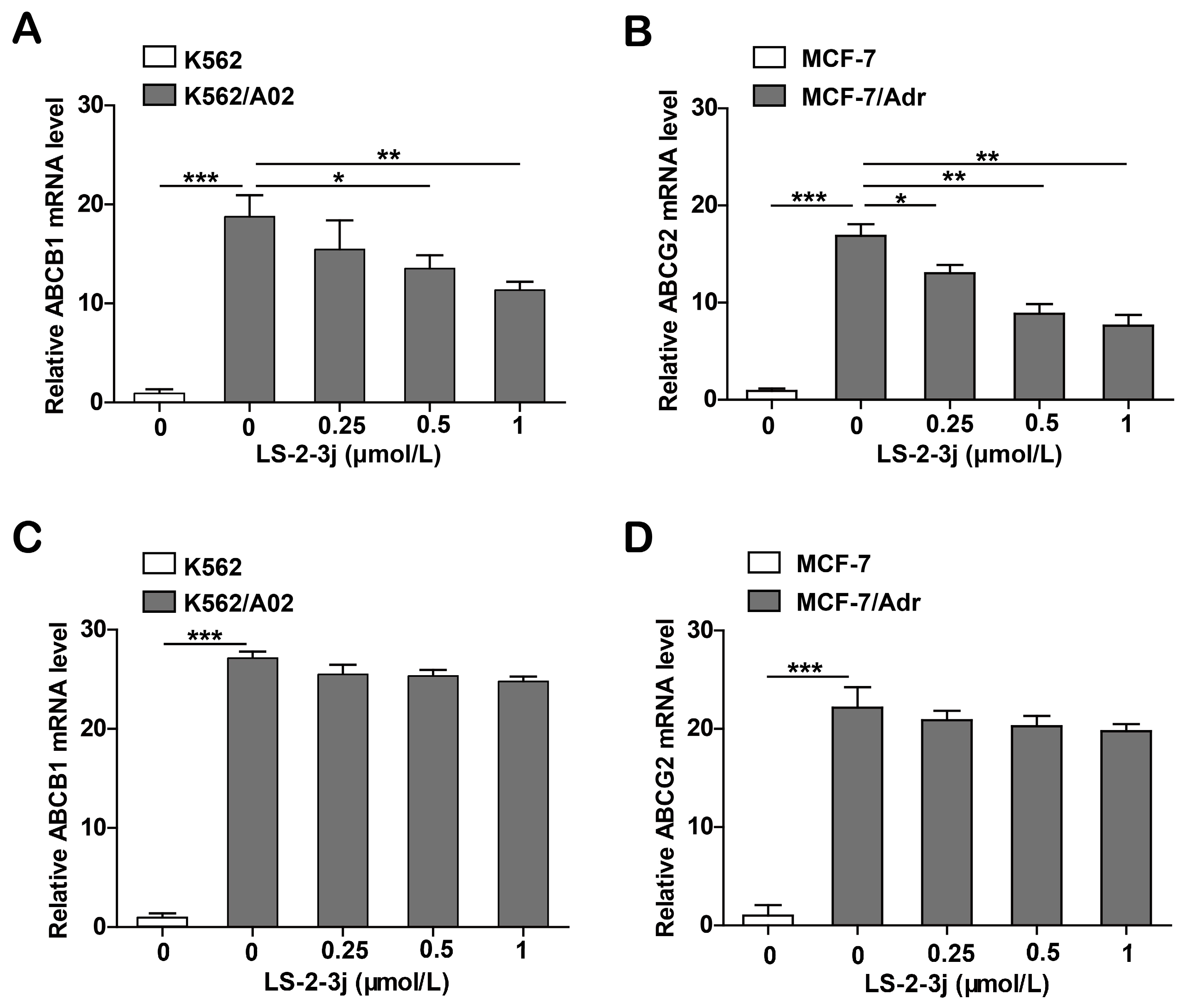
2.6. LS-2-3j Decreases the mRNA Expression Levels of ABCB1 and ABCG2
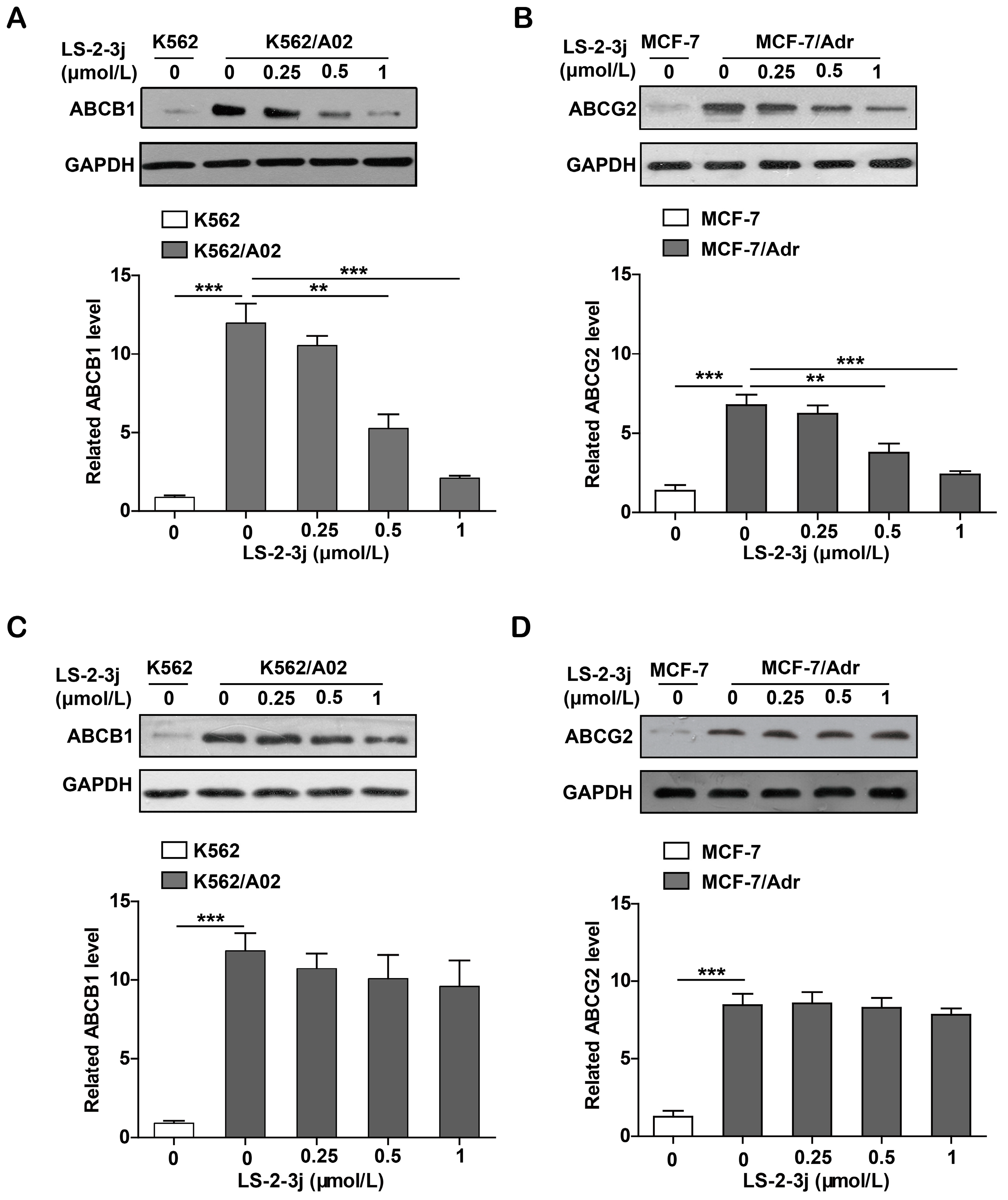
2.7. LS-2-3j Decreases the Protein Expression of ABCB1 and ABCG2
3. Discussion
4. Materials and Methods
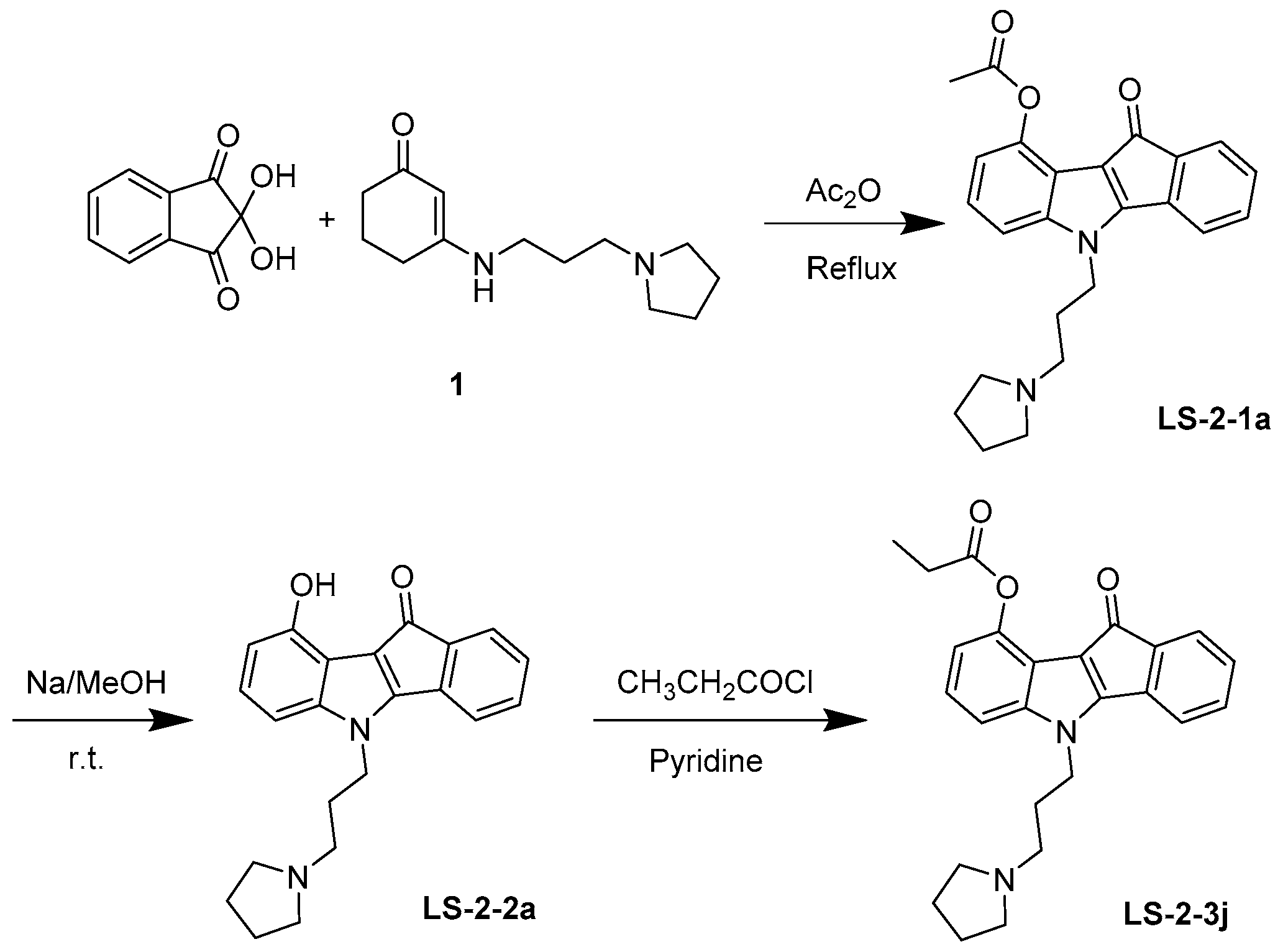
4.1. Synthesis and Analysis of LS-3-2j
4.2. Chemicals and Regents
4.3. Cell Lines and Cell Culture
4.4. MTT Assay
4.5. Drug Intracellular Accumulation Assay
4.6. Drug Efflux Assay
4.7. ATPase Activity Assay
4.8. RNA Extraction and Real-Time PCR
4.9. Western Blot Analysis
4.10. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Baker, E.K.; El-Osta, A. The rise of DNA methylation and the importance of chromatin on multidrug resistance in cancer. Exp. Cell Res. 2003, 290, 177–194. [Google Scholar] [CrossRef]
- Sodani, K.; Patel, A.; Kathawala, R.J.; Chen, Z.S. Multidrug resistance associated proteins in multidrug resistance. Chin. J. Cancer 2012, 31, 58–72. [Google Scholar] [CrossRef] [PubMed]
- Bentires-Alj, M.; Barbu, V.; Fillet, M.; Chariot, A.; Relic, B.; Jacobs, N.; Gielen, J.; Merville, M.-P.; Bours, V. NF-[kappa] B transcription factor induces drug resistance through MDR1 expression in cancer cells. Oncogene 2003, 22, 90. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.-Q.; Furukawa, T.; Aoki, S.; Nakajima, T.; Sumizawa, T.; Haraguchi, M.; Chen, Z.-S.; Kobayashi, M.; Akiyama, S.-I. Glutathione-dependent binding of a photoaffinity analog of agosterol A to the C-terminal half of human multidrug resistance protein. J. Biol. Chem. 2001, 276, 23197–23206. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Cai, X.; Fan, D. Roles of epithelial-mesenchymal transition in cancer drug resistance. Curr. Cancer Drug Targets 2013, 13, 915–929. [Google Scholar] [CrossRef] [PubMed]
- Gottesman, M.M.; Fojo, T.; Bates, S.E. Multidrug resistance in cancer: Role of ATP-dependent transporters. Nat. Rev. Cancer 2002, 2, 48. [Google Scholar] [CrossRef] [PubMed]
- Vasiliou, V.; Vasiliou, K.; Nebert, D.W. Human ATP-binding cassette (ABC) transporter family. Hum. Genomics 2009, 3, 281. [Google Scholar] [CrossRef] [PubMed]
- Borst, P.; Elferink, R.O. Mammalian ABC transporters in health and disease. Annu. Rev. Biochem. 2002, 71, 537–592. [Google Scholar] [CrossRef]
- Fojo, T.; Coley, H.M. The role of efflux pumps in drug-resistant metastatic breast cancer: New insights and treatment strategies. Clin. Breast Cancer 2007, 7, 749–756. [Google Scholar] [CrossRef]
- Han, J.Y.; Lim, H.S.; Yoo, Y.K.; Shin, E.S.; Park, Y.H.; Lee, S.Y.; Lee, J.E.; Lee, D.H.; Kim, H.T.; Lee, J.S. Associations of ABCB1, ABCC2, and ABCG2 polymorphisms with irinotecan-pharmacokinetics and clinical outcome in patients with advanced non-small cell lung cancer. Cancer 2007, 110, 138–147. [Google Scholar] [CrossRef]
- Matsuo, K.; Eno, M.L.; Ahn, E.H.; Shahzad, M.M.K.; Im, D.D.; Rosenshein, N.B.; Sood, A.K. Multidrug resistance gene (MDR-1) and risk of brain metastasis in epithelial ovarian, fallopian tube, and peritoneal cancer. Am. J. Clin. Oncol. 2011, 34, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Russell, A.; Beesley, J.; Chen, X.Q.; Healey, S.; Henderson, M.; Wong, M.; Emmanuel, C.; Galletta, L.; Johnatty, S.E.; et al. Paclitaxel sensitivity in relation to ABCB1 expression, efflux and single nucleotide polymorphisms in ovarian cancer. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef]
- Sarkadi, B.; Homolya, L.; Szakács, G.; Váradi, A. Human multidrug resistance ABCB and ABCG transporters: Participation in a chemoimmunity defense system. Physiol. Rev. 2006, 86, 1179–1236. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.S.; Davidson, G.S.; Martin, S.B.; Andries, E.; Potter, J.; Harvey, R.; Ar, K.; Xu, Y.; Kopecky, K.J.; Ankerst, D.P. Gene expression profiling of adult acute myeloid leukemia identifies novel biologic clusters for risk classification and outcome prediction. Blood 2006, 108, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Chen, Y.F.; To, K.K.W.; Wang, F.; Li, D.L.; Chen, L.K.; Fu, L.W. Alectinib (CH5424802) antagonizes ABCB1- and ABCG2-mediated multidrug resistance in vitro, in vivo and ex vivo. Exp. Mol. Med. 2017, 49. [Google Scholar] [CrossRef]
- Kühnle, M.; Egger, M.; Müller, C.; Mahringer, A.; Bernhardt, G.; Fricker, G.; König, B.; Buschauer, A. Potent and selective inhibitors of breast cancer resistance protein (ABCG2) derived from the p-glycoprotein (ABCB1) modulator tariquidar. J. Med. Chem. 2009, 52, 1190–1197. [Google Scholar] [CrossRef] [PubMed]
- Weidner, L.D.; Zoghbi, S.S.; Lu, S.; Shukla, S.; Ambudkar, S.V.; Pike, V.W.; Mulder, J.; Gottesman, M.M.; Innis, R.B.; Hall, M.D. The inhibitor Ko143 is not specific for ABCG2. J. Pharm. Exp. Ther. 2015, 354, 384–393. [Google Scholar] [CrossRef]
- Rothenberg, M.; Ling, V. Multidrug resistance: Molecular biology clinical relevance. J. Natl. Cancer Inst. 1989, 81, 907–910. [Google Scholar] [CrossRef]
- Fojo, T.; Bates, S. Strategies for reversing drug resistance. Oncogene 2003, 22, 7512. [Google Scholar] [CrossRef]
- Tsuruo, T.; Iida, H.; Tsukagoshi, S.; Sakurai, Y. Overcoming of vincristine resistance in P388 leukemia in vivo and in vitro through enhanced cytotoxicity of vincristine and vinblastine by verapamil. Cancer Res. 1981, 41, 1967–1972. [Google Scholar]
- Qadir, M.; O’Loughlin, K.L.; Fricke, S.M.; Williamson, N.A.; Greco, W.R.; Minderman, H.; Baer, M.R. Cyclosporin A is a broad-spectrum multidrug resistance modulator. Clin. Cancer Res. 2005, 11, 2320–2326. [Google Scholar] [CrossRef] [PubMed]
- Arai, T.; Miyoshi, Y.; Kim, S.; Akazawa, K.; Maruyama, N.; Taguchi, T.; Tamaki, Y.; Noguchi, S. Association of GSTP1 expression with resistance to docetaxel and paclitaxel in human breast cancers. Eur. J. Surg. Oncol. 2008, 34, 734–738. [Google Scholar] [CrossRef] [PubMed]
- Perez, E.A. Impact, mechanisms, and novel chemotherapy strategies for overcoming resistance to anthracyclines and taxanes in metastatic breast cancer. Breast Cancer Res. Treat. 2009, 114, 195. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.-L.; Liu, Y. Reversing multidrug resistance in Caco-2 by silencing MDR1, MRP1, MRP2, and BCL-2/BCL-xL using liposomal antisense oligonucleotides. PLoS ONE 2014, 9, e90180. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Mai, W.X.; Zhang, H.; Xue, M.; Xia, T.; Lin, S.; Wang, X.; Zhao, Y.; Ji, Z.; Zink, J.I. Codelivery of an optimal drug/siRNA combination using mesoporous silica nanoparticles to overcome drug resistance in breast cancer in vitro and in vivo. ACS Nano 2013, 7, 994–1005. [Google Scholar] [CrossRef] [PubMed]
- Bugde, P.; Biswas, R.; Merien, F.; Lu, J.; Liu, D.-X.; Chen, M.; Zhou, S.; Li, Y. The therapeutic potential of targeting ABC transporters to combat multi-drug resistance. Expert. Opin. Ther. Targets 2017, 21, 511–530. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.; Unadkat, J.D. Role of the breast cancer resistance protein (BCRP/ABCG2) in drug transport—An update. AAPS J. 2015, 17, 65–82. [Google Scholar] [CrossRef]
- Mao, Q. Role of the breast cancer resistance protein (ABCG2) in drug transport. AAPS J. 2005, 7, 118–133. [Google Scholar] [CrossRef]
- Shen, H.; Xu, W.; Luo, W.; Zhou, L.; Yong, W.; Chen, F.; Wu, C.; Chen, Q.; Han, X. Upregulation of mdr1 gene is related to activation of the MAPK/ERK signal transduction pathway and YB-1 nuclear translocation in B-cell lymphoma. Exp. Hematol. 2011, 39, 558–569. [Google Scholar] [CrossRef]
- Sui, H.; Zhou, S.; Wang, Y.; Liu, X.; Zhou, L.; Yin, P.; Fan, Z.; Li, Q. COX-2 contributes to p-glycoprotein-mediated multidrug resistance via phosphorylation of c-Jun at Ser63/73 in colorectal cancer. Carcinogenesis 2011, 32, 667–675. [Google Scholar] [CrossRef]
- Imai, Y.; Ohmori, K.; Yasuda, S.I.; Wada, M.; Suzuki, T.; Fukuda, K.; Ueda, Y. Breast cancer resistance protein/ABCG2 is differentially regulated downstream of extracellular signal-regulated kinase. Cancer Sci. 2010, 100, 1118–1127. [Google Scholar] [CrossRef]
- Zhu, M.M.; Tong, J.L.; Xu, Q.; Nie, F.; Xu, X.T.; Xiao, S.D.; Ran, Z.H. Increased JNK1 signaling pathway is responsible for ABCG2-mediated multidrug resistance in human colon cancer. PLoS ONE 2012, 7, e41763. [Google Scholar] [CrossRef] [PubMed]
- Tomiyasu, H.; Watanabe, M.; Sugita, K.; Gotokoshino, Y.; Fujino, Y.; Ohno, K.; Sugano, S.; Tsujimoto, H. Regulations of ABCB1 and ABCG2 expression through MAPK pathways in acute lymphoblastic leukemia cell lines. Anticancer Res. 2013, 33, 5317. [Google Scholar]
- Liu, B.; Zhao, L.; Ma, H.; Zhang, W.; Jin, Y. Knockdown of MRP4 by lentivirus-mediated siRNA improves sensitivity to adriamycin in adriamycin-resistant acute myeloid leukemia cells. Chin. Sci. Bull. 2012, 57, 90–97. [Google Scholar] [CrossRef]
- Winter, S.S.; Ricci, J.; Luo, L.; Lovato, D.M.; Khawaja, H.M.; Serna-Gallegos, T.; Debassige, N.; Larson, R.S. ATP Binding Cassette C1 (ABCC1/MRP1)-mediated drug efflux contributes to disease progression in T-lineage acute lymphoblastic leukemia. Health 2013, 5, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Nagaraju, A.; Pranav, G.; Kathawala, R.J.; Atish, P.; Wurpel, J.N.D.; Zhe-Sheng, C. Tyrosine kinase inhibitors as reversal agents for ABC transporter mediated drug resistance. Molecules 2014, 19, 13848–13877. [Google Scholar] [CrossRef]
- Xu, Y.; Ohms, S.J.; Li, Z.; Wang, Q.; Gong, G.; Hu, Y.; Mao, Z.; Shannon, M.F.; Fan, J.Y. Changes in the expression of miR-381 and miR-495 are inversely associated with the expression of the MDR1 gene and development of multi-drug resistance. PLoS ONE 2013, 8, e82062. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
| Treatment | IC50 ± SD (μmol/L) (Reversal Fold, RF) | |||
| MCF-7 | MCF-7/Adr | |||
| Doxorubicin (DOX) | 0.298 ± 0.008 | (1.000) | 3.506 ± 0.637 | (1.000) |
| + LS-2-3j 0.25 μmol/L | 0.313 ± 0.019 | (0.953) | 0.827 ± 0.128 * | (4.241) |
| + LS-2-3j 0.5 μmol/L | 0.303 ± 0.012 | (0.986) | 0.471 ± 0.072 ** | (7.438) |
| + LS-2-3j 1 μmol/L | 0.300 ± 0.005 | (0.995) | 0.353 ± 0.031 ** | (9.937) |
| + VRP 10 μmol/L | 0.317 ± 0.017 | (0.941) | 0.274 ± 0.057 ** | (12.812) |
| Cisplatin | 7.004 ± 0.662 | (1.000) | 4.363 ± 0.189 | (1.000) |
| + LS-2-3j 1 μmol/L | 7.316 ± 0.913 | (0.957) | 4.581 ± 0.234 | (0.952) |
| Treatment | IC50 ± SD (μmol/L) (Reversal Fold, RF) | |||
| K562 | K562/A02 | |||
| DOX | 0.118 ± 0.001 | (1.000) | 13.317 ± 0.840 | (1.000) |
| + LS-2-3j 0.25 μmol/L | 0.118 ± 0.010 | (0.997) | 4.947 ± 0.580 * | (2.692) |
| + LS-2-3j 0.5 μmol/L | 0.127 ± 0.009 | (0.925) | 3.182 ± 0.162 * | (4.185) |
| + LS-2-3j 1 μmol/L | 0.126 ± 0.010 | (0.934) | 1.676 ± 0.269 ** | (7.944) |
| + VRP 10 μmol/L | 0.122 ± 0.007 | (0.962) | 1.180 ± 0.160 ** | (11.282) |
| Cisplatin | 2.747 ± 0.227 | (1.000) | 3.768 ± 0.124 | (1.000) |
| + LS-2-3j 1 μmol/L | 2.223 ± 0.124 | (1.236) | 3.577 ± 0.314 | (1.053) |
| KB | KB/VCR | |||
| Vincristine (VCR) | 0.010 ± 0.001 | (1.000) | 1.126 ± 0.134 | (1.000) |
| + LS-2-3j 0.25 μmol/L | 0.010 ± 0.010 | (1.030) | 0.589 ± 0.114 * | (1.913) |
| + LS-2-3j 0.5 μmol/L | 0.011 ± 0.012 | (0.955) | 0.291 ± 0.045 * | (3.874) |
| + LS-2-3j 1 μmol/L | 0.011 ± 0.030 | (0.939) | 0.150 ± 0.022 ** | (7.531) |
| + VRP 10 μmol/L | 0.011 ± 0.001 | (0.935) | 0.115 ± 0.018 ** | (9.808) |
| Cisplatin | 0.705 ± 0.263 | (1.000) | 1.183 ± 0.140 | (1.000) |
| + LS-2-3j 1 μmol/L | 0.703 ± 0.147 | (1.002) | 1.213 ± 0.118 | (0.975) |
| Treatment | IC50 ± SD (μmol/L) (RF) | |||
|---|---|---|---|---|
| MCF-7 | MCF-7/ABCG2 | |||
| Mitoxantrone (MITX) | 0.425 ± 0.014 | (1.000) | 3.550 ± 0.054 | (1.000) |
| + LS-2-3j 0.25 μmol/L | 0.434 ± 0.035 | (0.980) | 1.639 ± 0.177 * | (2.236) |
| + LS-2-3j 0.5 μmol/L | 0.462 ± 0.033 | (0.921) | 0.755 ± 0.021 * | (4.770) |
| + LS-2-3j 1 μmol/L | 0.436 ± 0.012 | (0.975) | 0.487 ± 0.050 ** | (7.479) |
| + Fumitremorgin C (FTC) 2.5 μmol/L | 0.444 ± 0.017 | (0.958) | 0.372 ± 0.023 ** | (8.793) |
| Cisplatin | 7.004 ± 0.662 | (1.000) | 5.478 ± 0.882 | (1.000) |
| + LS-2-3j 1 μmol/L | 7.316 ± 0.913 | (0.957) | 5.248 ± 0.142 | (1.044) |
| MCF-7 | MCF-7/Adr | |||
| MITX | 0.425 ± 0.014 | (1.000) | 39.242 ± 0.035 | (1.000) |
| + LS-2-3j 0.25 μmol/L | 0.434 ± 0.035 | (0.980) | 30.141 ± 0.264 | (1.302) |
| + LS-2-3j 0.5 μmol/L | 0.462 ± 0.033 | (0.921) | 13.772 ± 0.210 * | (2.849) |
| + LS-2-3j 1 μmol/L | 0.436 ± 0.012 | (0.975) | 8.253 ± 0.805 ** | (4.755) |
| + FTC 2.5 μmol/L | 0.444 ± 0.017 | (0.958) | 4.891 ± 0.337 ** | (8.023) |
| Cisplatin | 7.004 ± 0.662 | (1.000) | 8.995 ± 0.364 | (1.000) |
| + LS-2-3j 1 μmol/L | 7.316 ± 0.913 | (0.957) | 8.784 ± 0.963 | (1.024) |
| 293T | 293T/ABCG2 | |||
| MITX | 0.187 ± 0.013 | (1.000) | 4.253 ± 0.200 | (1.000) |
| + LS-2-3j 0.25 μmol/L | 0.200 ± 0.005 | (0.934) | 1.739 ± 0.056 * | (2.446) |
| + LS-2-3j 0.5 μmol/L | 0.194 ± 0.004 | (0.963) | 1.205 ± 0.065 * | (3.530) |
| + LS-2-3j 1 μmol/L | 0.183 ± 0.008 | (1.020) | 0.707 ± 0.051 ** | (6.015) |
| + FTC 2.5 μmol/L | 0.189 ± 0.019 | (0.985) | 0.466 ± 0.016 ** | (9.120) |
| Cisplatin | 1.983 ± 0.132 | (1.000) | 1.411 ± 0.197 | (1.000) |
| + LS-2-3j 1 μmol/L | 1.970 ± 0.184 | (1.006) | 1.551 ± 0.189 | (0.910) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, C.; Liu, F.; Qi, J.; Ma, J.; Lin, S.; Zhang, C.; Zhang, Q.; Zhang, H.; Lu, R.; Li, X. A Novel Synthetic Dihydroindeno[1,2-b] Indole Derivative (LS-2-3j) Reverses ABCB1- and ABCG2-Mediated Multidrug Resistance in Cancer Cells. Molecules 2018, 23, 3264. https://doi.org/10.3390/molecules23123264
Guo C, Liu F, Qi J, Ma J, Lin S, Zhang C, Zhang Q, Zhang H, Lu R, Li X. A Novel Synthetic Dihydroindeno[1,2-b] Indole Derivative (LS-2-3j) Reverses ABCB1- and ABCG2-Mediated Multidrug Resistance in Cancer Cells. Molecules. 2018; 23(12):3264. https://doi.org/10.3390/molecules23123264
Chicago/Turabian StyleGuo, Chao, Fangyuan Liu, Jie Qi, Jiahui Ma, Shiqi Lin, Caiyun Zhang, Qian Zhang, Hangyu Zhang, Rong Lu, and Xia Li. 2018. "A Novel Synthetic Dihydroindeno[1,2-b] Indole Derivative (LS-2-3j) Reverses ABCB1- and ABCG2-Mediated Multidrug Resistance in Cancer Cells" Molecules 23, no. 12: 3264. https://doi.org/10.3390/molecules23123264
APA StyleGuo, C., Liu, F., Qi, J., Ma, J., Lin, S., Zhang, C., Zhang, Q., Zhang, H., Lu, R., & Li, X. (2018). A Novel Synthetic Dihydroindeno[1,2-b] Indole Derivative (LS-2-3j) Reverses ABCB1- and ABCG2-Mediated Multidrug Resistance in Cancer Cells. Molecules, 23(12), 3264. https://doi.org/10.3390/molecules23123264






