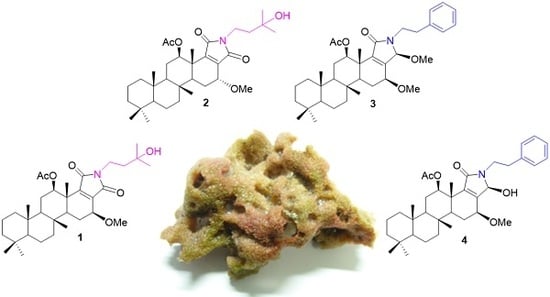
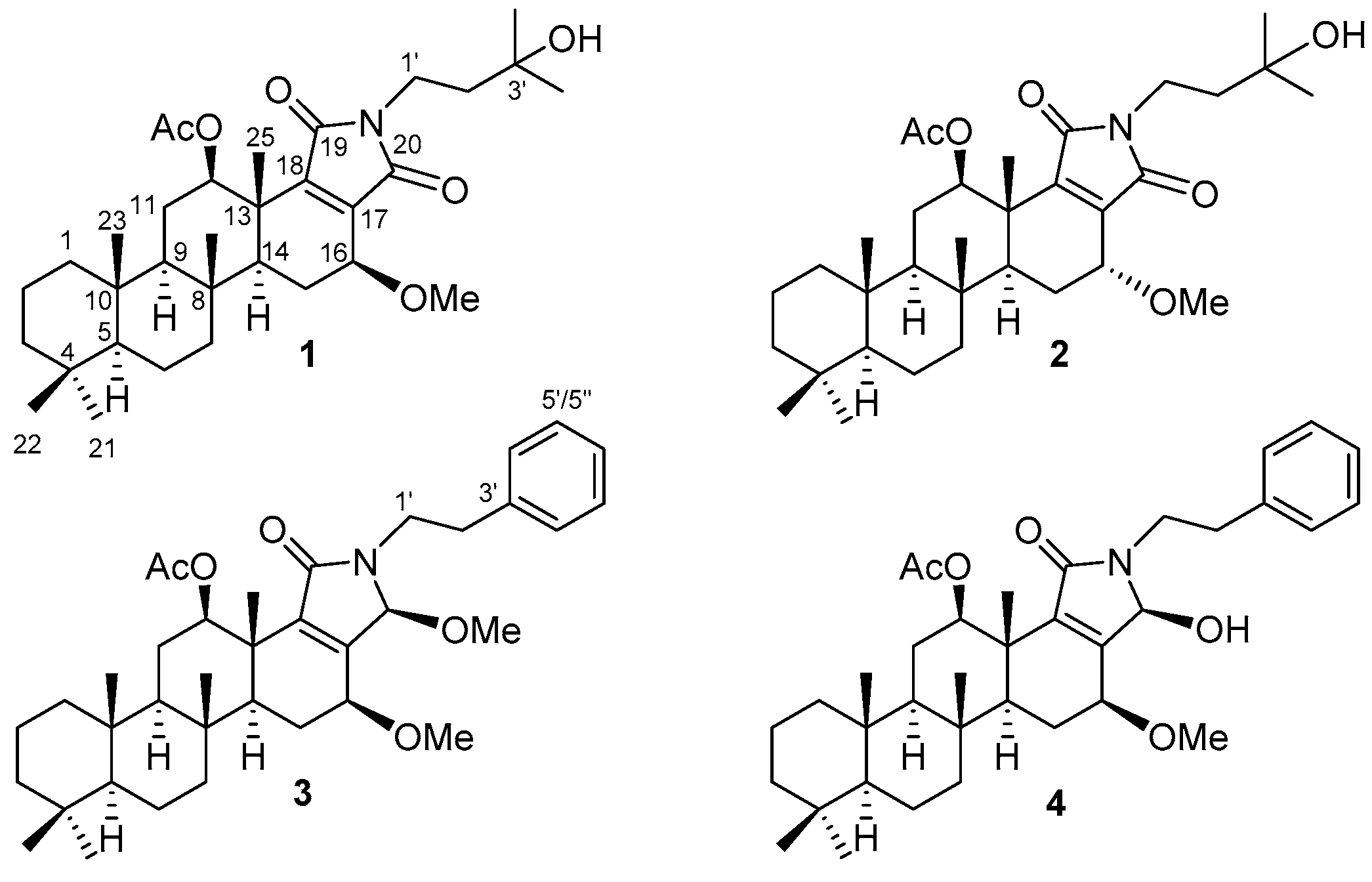
Scalalactams A–D, Scalarane Sesterterpenes with a γ-Lactam Moiety from a Korean Spongia Sp. Marine Sponge
Abstract
:1. Introduction
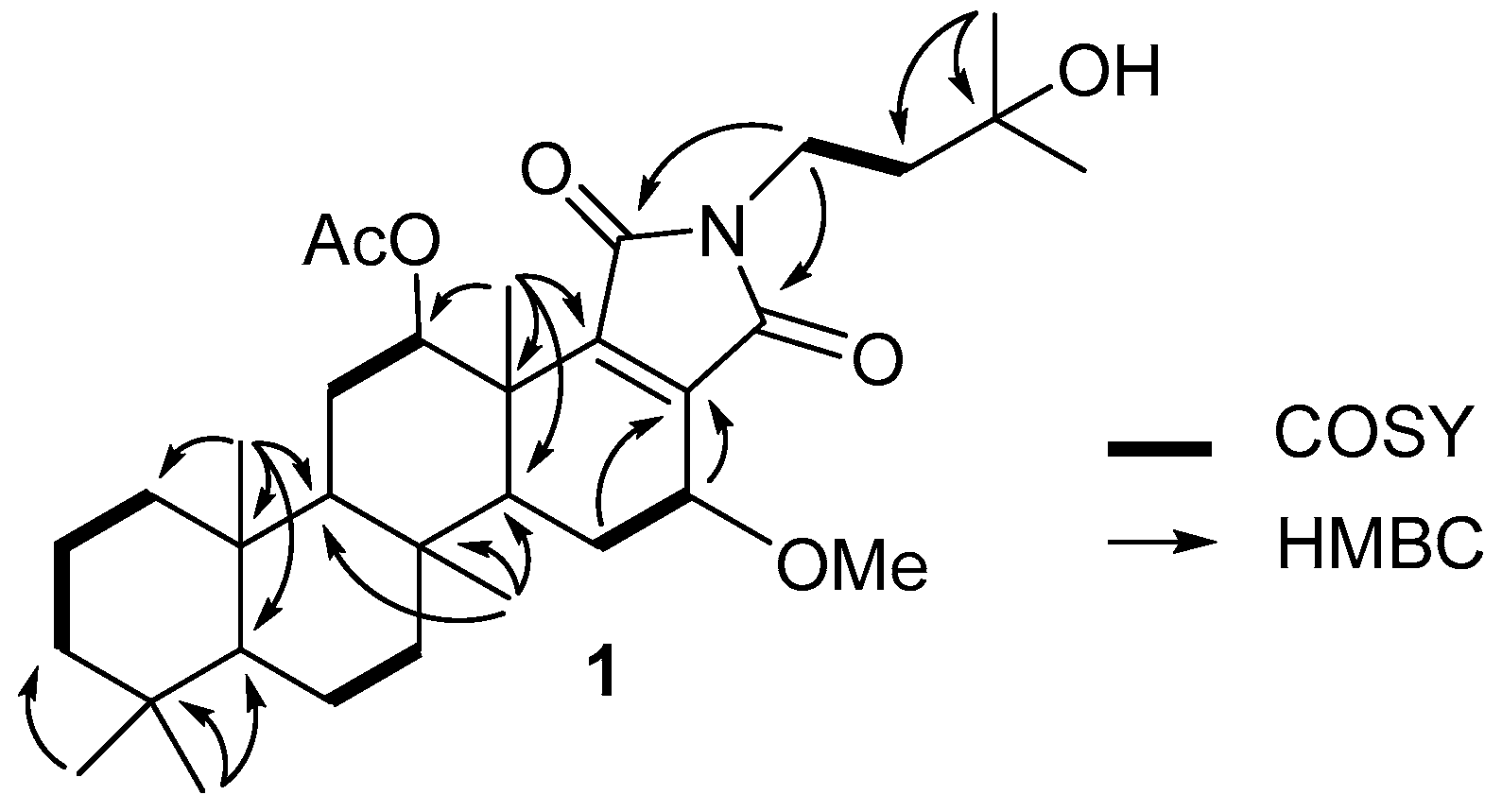
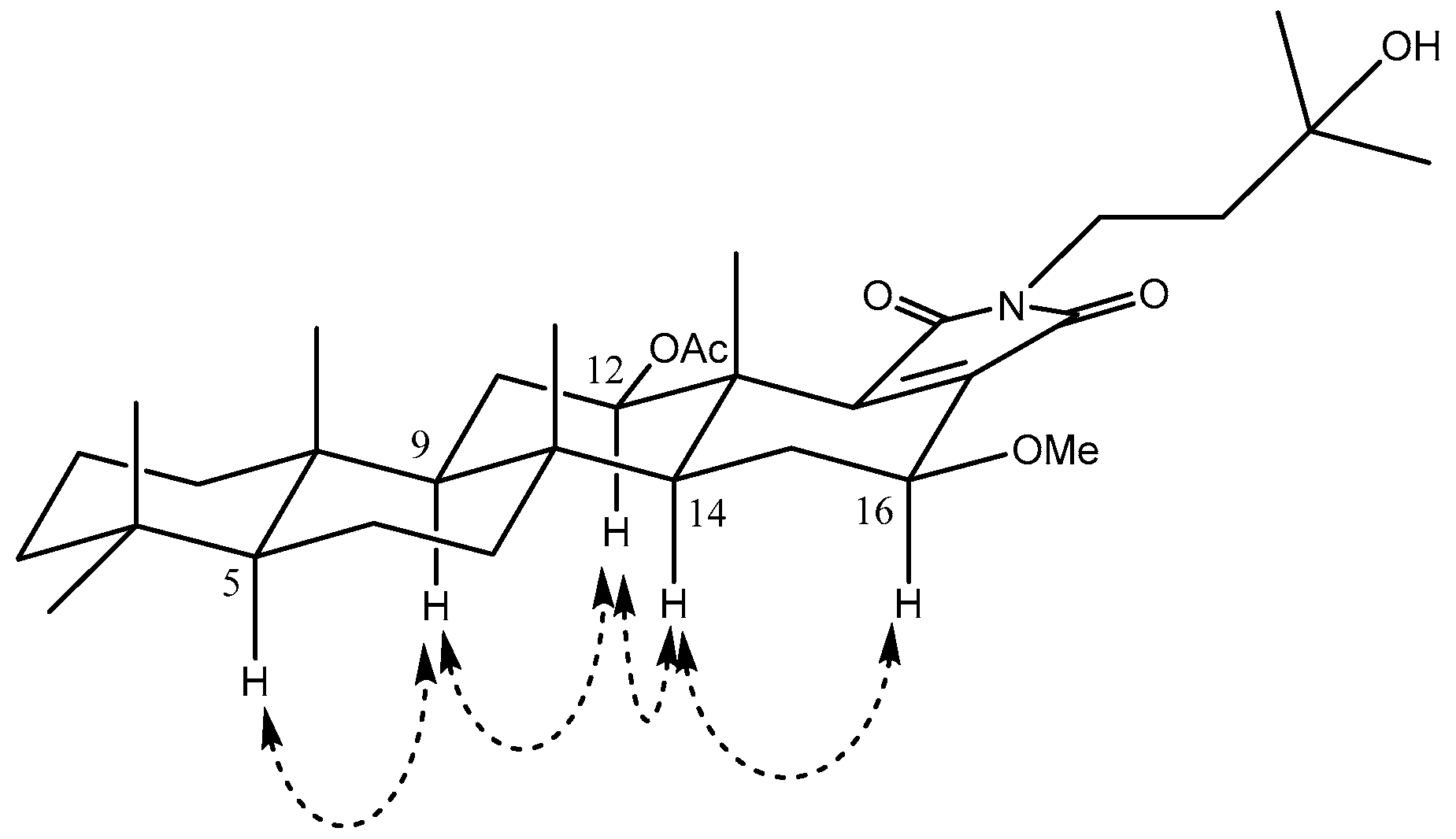
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Animal Material
3.3. Extraction and Isolation
3.4. Co-transfection Assay
3.5. Cytotoxicity Assay
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gonzalez, A.M. Scalarane sesterterpenoids. Curr. Bioact. Compd. 2010, 6, 178–206. [Google Scholar] [CrossRef]
- Fattorusso, E.; Magno, S.; Santacroce, C.; Sica, D. Scalarin, a new pentacyclic C-25 terpenoid from the sponge Cacospongia scalaris. Tetrahedron 1972, 28, 5993–5997. [Google Scholar] [CrossRef]
- Kulciţki, V.; Ungur, N.; Gavagnin, M.; Castelluccio, F.; Cimino, G. Ring B functionalization of scalarane sesterterpenes by radical relay halogenation. Tetrahedron 2007, 63, 7617–7623. [Google Scholar] [CrossRef]
- Cafieri, F.; De Napoli, L.; Fattorusso, E.; Santacroce, C.; Sica, D. Molliorin-A: A unique scalarin-like pyrroloterpene from the sponge Cacospongia mollior. Tetrahedron Lett. 1977, 18, 477–480. [Google Scholar] [CrossRef]
- Cafieri, F.; De Napoli, L.; Fattorusso, E.; Santacroce, C. Molliorin-B, a second scalarin-like pyrroloterpene from the sponge Cacospongia mollior. Experientia 1977, 33, 994–995. [Google Scholar] [CrossRef] [PubMed]
- Cafieri, F.; De Napoli, L.; Iengo, A.; Santacroce, C. Molliorin-c, a further pyrroloterpene present in the sponge Cacospongia mollior. Experientia 1978, 34, 300–301. [Google Scholar] [CrossRef]
- Cafieri, F.; De Napoli, L.; Iengo, A.; Santacroce, C. Minor pyrroloterpenoids from the marine sponge Cacospongia mollior. Experientia 1979, 35, 157–158. [Google Scholar] [CrossRef]
- Hernández-Guerrero, C.J.; Zubía, E.; Ortega, M.J.; Carballo, J.L. Sesterterpene metabolites from the sponge Hyatella intestinalis. Tetrahedron 2006, 62, 5392–5400. [Google Scholar] [CrossRef]
- Jeon, J.-E.; Bae, J.; Lee, K.J.; Oh, K.-B.; Shin, J. Scalarane sesterterpenes from the sponge Hyatella sp. J. Nat. Prod. 2011, 74, 847–851. [Google Scholar] [CrossRef]
- Festa, C.; Cassiano, C.; D’Auria, M.V.; Debitus, C.; Monti, M.C.; De Marino, S. Scalarane sesterterpenes from Thorectidae sponges as inhibitors of TDP-43 nuclear factor. Org. Biomol. Chem. 2014, 12, 8646–8655. [Google Scholar] [CrossRef]
- Elhady, S.S.; El-Halawany, A.M.; Alahdal, A.M.; Hassanean, H.A.; Ahmed, S.A. A new bioactive metabolite isolated from the Red Sea marine sponge Hyrtios erectus. Molecules 2016, 21, 82. [Google Scholar] [CrossRef] [PubMed]
- Walker, R. P.; Thompson, J.E.; Faulkner, D.J. Sesterterpenes from Spongia idia. J. Org. Chem. 1980, 45, 4976–4979. [Google Scholar] [CrossRef]
- Cimino, G.; De Rosa, S.; De Stefano, S.; Sodano, G. The chemical defense of four Mediterranean nudibranchs. Comp. Biochem. Physiol. B: Comp. 1982, 73, 471–474. [Google Scholar] [CrossRef]
- Thompson, J.E.; Walker, R.P.; Faulkner, D.J. Screening and bioassays for biologically-active substances from forty marine sponge species from San Diego, California, USA. Mar. Biol. 1985, 88, 11–21. [Google Scholar] [CrossRef]
- Rogers, S.D.; Paul, V.J. Chemical defenses of three Glossodoris nudibranchs and their dietary Hyrtios sponges. Mar. Ecol. Prog. Ser. 1991, 77, 221–232. [Google Scholar] [CrossRef]
- Avila, C.; Paul, V.J. Chemical ecology of the nudibranch Glossodoris pallida: Is the location of diet-derived metabolites important for defense? Mar. Ecol. Prog. Ser. 1997, 150, 171–180. [Google Scholar] [CrossRef]
- Sera, Y.; Adachi, K.; Shizuri, Y. A new epidioxy sterol as an antifouling substance from a palauan marine sponge, Lendenfeldia chondrodes. J. Nat. Prod. 1999, 62, 152–154. [Google Scholar] [CrossRef]
- Cimino, G.; Fontana, A.; Giménez, F.; Marin, A.; Mollo, E.; Trivellone, E.; Zubía, E. Biotransformation of a dietary sesterterpenoid in the Mediterranean nudibranch Hypselodoris orsini. Experientia 1993, 49, 582–586. [Google Scholar] [CrossRef]
- Miyaoka, H.; Nishijima, S.; Mitome, H.; Yamada, Y. Three new scalarane sesterterpenoids from the Okinawan sponge Hyrtios erectus. J. Nat. Prod. 2000, 63, 1369–1372. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Miura, S.; van Soest, R.W.M.; Ohta, T. Three new cytotoxic sesterterpenes from a marine sponge Spongia sp. J. Nat. Prod. 2003, 66, 438–440. [Google Scholar] [CrossRef]
- Hahn, D.; Won, D.H.; Mun, B.; Kim, H.; Han, C.; Wang, W.; Chun, T.; Park, S.; Yoon, D.; Choi, H.; et al. Cytotoxic scalarane sesterterpenes from a Korean marine sponge Psammocinia sp. Bioorg. Med. Chem. Lett. 2013, 23, 2336–2339. [Google Scholar] [CrossRef] [PubMed]
- Aoki, S.; Higuchi, K.; Isozumi, N.; Matsui, K.; Miyamoto, Y.; Itoh, N.; Tanaka, K.; Kobayashi, M. Differentiation in chronic myelogenous leukemia cell K562 by spongean sesterterpene. Biochem. Biophys. Res. Commun. 2001, 282, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Liu, Y.; Zhou, Y.-D.; Nagle, D.G. Cytotoxic metabolites from an Indonesian sponge Lendenfeldia sp. J. Nat. Prod. 2007, 70, 1824–1826. [Google Scholar] [CrossRef] [PubMed]
- Bergquist, P.R.; Cambie, R.C.; Kernan, M.R. Scalarane sesterterpenes from Collospongia auris, a new thorectid sponge. Biochem. Syst. Ecol. 1990, 18, 349–357. [Google Scholar] [CrossRef]
- Bowden, B.F.; Coll, J.C.; Li, H.; Cambie, R.C.; Kernan, M.R.; Bergquist, P.R. New cytotoxic scalarane sesterterpenes from the Dictyoceratid sponge Strepsichordaia lendenfeldi. J. Nat. Prod. 1992, 55, 1234–1240. [Google Scholar] [CrossRef] [PubMed]
- Kamel, H.N.; Kim, Y.B.; Rimoldi, J.M.; Fronczek, F.R.; Ferreira, D.; Slattery, M. Scalarane sesterterpenoids: Semisynthesis and biological activity. J. Nat. Prod. 2009, 72, 1492–1496. [Google Scholar] [CrossRef] [PubMed]
- Crews, P.; Bescansa, P.; Bakus, G.J. A non-peroxide norsesterterpene from a marine sponge Hyrtios erecta. Experientia 1985, 41, 690–691. [Google Scholar] [CrossRef] [PubMed]
- Crews, P.; Bescansa, P. Sesterterpenes from a common marine sponge, Hyrtios erecta. J. Nat. Prod. 1986, 49, 1041–1052. [Google Scholar] [CrossRef]
- Marshall, L.A.; Winkler, J.D.; Griswold, D.E.; Bolognese, B.; Roshak, A.; Sung, C.M.; Webb, E.F.; Jacobs, R. Effects of scalaradial, a type II phospholipase A2 inhibitor, on human neutrophil arachidonic acid mobilization and lipid mediator formation. J. Pharmacol. Exp. Ther. 1994, 268, 709–717. [Google Scholar]
- Nakagawa, M.; Hamamoto, Y.; Ishihama, M.; Hamasaki, S.; Endo, M. Pharmacologically active homosesterterpenes from Palauan sponges. Tetrahedron Lett. 1987, 28, 431–434. [Google Scholar] [CrossRef]
- Doi, Y.; Shigemori, H.; Ishibashi, M.; Mizobe, F.; Kawashima, A.; Nakaike, S.; Kobayashi, J. New sesterterpenes with nerve growth factor synthesis-stimulating activity from the Okinawan marine sponge Hyrtios sp. Chem. Pharm. Bull. 1993, 41, 2190–2191. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.-J.; Ko, H.; Shin, M.; Ham, J.; Chin, J.; Kim, Y.; Kim, H.; Shin, K.; Choi, H.; Kang, H. Farnesoid X-activated receptor antagonists from a marine sponge Spongia sp. Bioorg. Med. Chem. Lett. 2006, 16, 5398–5402. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.-J.; Ko, H.; Ju, M.K.; Hwang, H.; Chin, J.; Ham, J.; Lee, B.; Lee, J.; Won, D.H.; Choi, H.; et al. Scalarane sesterterpenes from a marine sponge of the genus Spongia and their FXR antagonistic activity. J. Nat. Prod. 2007, 70, 1691–1695. [Google Scholar] [CrossRef] [PubMed]
- Mahido, C.; Prawat, H.; Sangpetsiripan, S.; Ruchirawat, S. Bioactive scalaranes from the Thai sponge Hyrtios gumminae. J. Nat. Prod. 2009, 72, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Mehta, G.; Singh, V. Hybrid systems through natural product leads: An approach towards new molecular entities. Chem. Soc. Rev. 2002, 31, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Panda, G. A new example of a steroid–amino acid hybrid: Construction of constrained nine membered D-ring steroids. Org. Biomol. Chem. 2007, 5, 360–366. [Google Scholar] [PubMed]
- Banerjee, A.; Sergienko, E.; Vasile, S.; Gupta, V.; Vuori, K.; Wipf, P. Triple hybrids of steroids, spiroketals, and oligopeptides as new biomolecular chimeras. Org. Lett. 2009, 11, 65–68. [Google Scholar] [CrossRef]
- El Sayed, K.A.; Mayer, A.M.S.; Kelly, M.; Hamann, M.T. The biocatalytic transformation of furan to amide in the bioactive marine natural product palinurin. J. Org. Chem. 1999, 64, 9258–9260. [Google Scholar] [CrossRef]
Sample Availability: Not available. |
| 1 | 2 | |||||
|---|---|---|---|---|---|---|
| No. | δC, m a | δH, m a, J (Hz) | 1H-1H COSY | HMBC (10 Hz) | δC, m a | δH, m a, J (Hz) |
| 1 | 39.3, t | 0.83 c; 1.61 c | 2, 5, 9, 10 | 39.3, t | 0.83, m; 1.61, m | |
| 2 | 18.2, t | 1.40, m; 1.58, m | 18.3, t | 1.40, m; 1.59, m | ||
| 3 | 41.9, t | 1.11, m; 1.35, m | 3 | 41.9, t | 1.13, m; 1.35, m | |
| 4 | 33.1, s | 2 | 21, 22 | 33.1, s | ||
| 5 | 56.5, d | 0.81c | 56.5, d | 0.78, dd, J = 12.3, 1.5 | ||
| 6 | 18.5, t | 1.38, m; 1.57, m | 18.5, t | 1.38, m; 1.58, m | ||
| 7 | 42.1, t | 0.98, m; 1.77, m | 41.5, t | 0.94, m; 1.84, m | ||
| 8 | 39.1, s | 39.1, s | ||||
| 9 | 58.0, d | 1.05, dd, J = 13.1, 2.3 | 11 | 5, 7, 8, 11, 12, 14, 23, 24 | 58.0, d | 0.94, dd, J = 12.1, 2.3 |
| 10 | 37.1, s | 37.1, s | ||||
| 11 | 23.8, t | 1.56, m; 1.77, m | 9, 12 | 9, 12, 10, 13 | 24.4, t | 1.55, m; 1.74, m |
| 12 | 75.4, d | 4.96, dd, J = 11.3, 4.8 | 11 | 75.4, d | 4.88, dd, J = 11.1, 4.6 | |
| 13 | 43.5, s | 42.8, s | ||||
| 14 | 49.8, d | 1.61, m | 15 | 7, 9, 12, 15, 16, 18, 24, 25 | 54.7, d | 1.12, m |
| 15 | 22.2, t | 1.48, m; 2.08, br d, J = 14.0 | 14, 16 | 24.4, t | 1.75, d, J = 12.8; 2.28, dd, J = 12.8, 7.1 | |
| 16 | 68.1, d | 4.04, br d, J = 4.5 | 15 | 74.1, d | 4.12, dd, J = 9.5, 7.1 | |
| 17 | 138.8, s | 140.8, s | ||||
| 18 | 150.9, s | 150.5, s | ||||
| 19 | 169.1 b | 169.4b | ||||
| 20 | 169.1 b | 169.4b | ||||
| 21 | 33.2, q | 0.84, s | 3, 4, 5, 22 | 33.2, q | 0.84, s | |
| 22 | 21.8, q | 0.80, s | 3, 4, 5, 21 | 21.4, q | 0.80, s | |
| 23 | 16.3, q | 0.83, s | 1, 5, 9, 10 | 16.3, q | 0.82, s | |
| 24 | 16.9, q | 0.91, s | 7, 8, 9, 14 | 18.2, q | 0.92, s | |
| 25 | 16.1, q | 1.21, s | 12, 13, 14, 18 | 17.0, q | 1.32, s | |
| 12-OAc | 171.8, s | 171.8, s | ||||
| 22.0, q | 2.18, s | 22.2, q | 2.17, s | |||
| 16-OMe | 58.7, q | 3.44, s | 59.1, q | 3.55, s | ||
| 1′ | 33.5, t | 3.57, t, J = 7.1 | 33.5, t | 3.56, t, J = 6.8 | ||
| 2′ | 43.0, t | 1.68, t, J = 7.1 | 41.0, t | 1.68, q, J = 6.8 | ||
| 3′ | 69.9, s | 69.9, s | ||||
| 4′ | 28.9, q | 1.24, s | 28.8, q | 1.24, s | ||
| 5′ | 28.9, q | 1.23, s | 28.8, q | 1.22, s | ||
| 3 | 4 | |||
|---|---|---|---|---|
| No. | δC, m a | δH, m a, J (Hz) | δC, m a | δH, m a, J (Hz) |
| 1 | 40.0, t | 0.84, m; 1.61, m | 40.0, t | 0.83, m; 1.63, dt, J = 12.5, 1.5 |
| 2 | 18.4, t | 1.41, m; 1.59, m | 18.6, t | 1.41, m; 1.59, m |
| 3 | 41.9, t | 1.11, m; 1.36, m | 42.2, t | 1.17, m; 1.35, m |
| 4 | 33.4, s | 33.3, s | ||
| 5 | 56.5, d | 0.84 b | 56.5, d | 0.83 b |
| 6 | 18.6, t | 1.38, m; 1.56, m | 18.5, t | 1.38, m; 1.53, m |
| 7 | 41.3, t | 0.98, m; 1.79, dt, J = 12.3, 1.5 | 41.5, t | 0.94, m; 1.79, dt, J = 12.3, 1.5 |
| 8 | 37.0, s | 36.8, s | ||
| 9 | 57.6, d | 1.03, d, J = 12.3 | 57.8, d | 1.01, br d, J = 12.1 |
| 10 | 37.2, s | 39.8, s | ||
| 11 | 25.0, t | 1.55, m; 1.77, m | 24.9, t | 1.55, m; 1.75 b |
| 12 | 75.5, d | 4.97, dd, J = 11.1, 4.6 | 75.6, d | 4.95, dd, J = 11.1, 4.7 |
| 13 | 42.6, s | 42.0, s | ||
| 14 | 50.0, d | 1.47, m | 50.2, d | 1.43, m |
| 15 | 21.8, t | 1.54, m; 2.08, d, J = 13.5 | 22.1, t | 1.42, m; 2.06, d, J = 13.5 |
| 16 | 70.0, d | 3.75 b | 69.6, d | 3.92, d, J = 3.4 |
| 17 | 148.0, s | 150.2, s | ||
| 18 | 146.1, s | 144.0, s | ||
| 19 | 168.2, s | 167.5, s | ||
| 20 | 85.0, d | 5.10, s | 80.4, d | 5.05, d, J = 10.3 |
| 21 | 33.2, q | 0.84, s | 33.2, q | 0.82, s |
| 22 | 21.8, q | 0.80, s | 21.8, q | 0.79, s |
| 23 | 16.3, q | 0.82, s | 16.6, q | 0.82, s |
| 24 | 17.0, q | 0.91, s | 17.4, t | 0.91, s |
| 25 | 16.2, q | 1.22, s | 15.9, q | 1.20, s |
| 12-OAc | 172.0, s | 171.8, s | ||
| 21.1, q | 2.18, s | 21.4, q | 2.17, s | |
| 16-OMe | 57.5, q | 3.35, s | 57.3, q | 3.34, s |
| 20-OMe | 49.1, q | 2.90 | ||
| 1′ | 41.6, t | 3.20, q, J = 8.0; 3.75 b | 41.9, t | 3.48, q, J = 8.0; 3.65, q, J = 8.0 |
| 2′ | 34.9, t | 2.83, m | 34.9, t | 2.85, m |
| 3′ | 139.0, s | 139.6, s | ||
| 4′, 4′′ | 128.7, d | 7.19, d, J = 7.3 | 128.7, d | 7.21, d, J = 7.9, |
| 5′, 5′′ | 128.5, d | 7.28, dd, J = 7.9, 7.3 | 128.5, d | 7.29, dd, J = 7.9, 7.9 |
| 6′ | 126.0, d | 7.21, d, J = 7.9 | 126.0, d | 7.19, d, J = 7.9 |
| 20-OH | 1.49, d, J = 10.3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, I.; Lee, J.; Lee, J.; Hahn, D.; Chin, J.; Won, D.H.; Ko, J.; Choi, H.; Hong, A.; Nam, S.-J.; et al. Scalalactams A–D, Scalarane Sesterterpenes with a γ-Lactam Moiety from a Korean Spongia Sp. Marine Sponge. Molecules 2018, 23, 3187. https://doi.org/10.3390/molecules23123187
Yang I, Lee J, Lee J, Hahn D, Chin J, Won DH, Ko J, Choi H, Hong A, Nam S-J, et al. Scalalactams A–D, Scalarane Sesterterpenes with a γ-Lactam Moiety from a Korean Spongia Sp. Marine Sponge. Molecules. 2018; 23(12):3187. https://doi.org/10.3390/molecules23123187
Chicago/Turabian StyleYang, Inho, Jusung Lee, Jihye Lee, Dongyup Hahn, Jungwook Chin, Dong Hwan Won, Jaeyoung Ko, Hyukjae Choi, Ahreum Hong, Sang-Jip Nam, and et al. 2018. "Scalalactams A–D, Scalarane Sesterterpenes with a γ-Lactam Moiety from a Korean Spongia Sp. Marine Sponge" Molecules 23, no. 12: 3187. https://doi.org/10.3390/molecules23123187
APA StyleYang, I., Lee, J., Lee, J., Hahn, D., Chin, J., Won, D. H., Ko, J., Choi, H., Hong, A., Nam, S.-J., & Kang, H. (2018). Scalalactams A–D, Scalarane Sesterterpenes with a γ-Lactam Moiety from a Korean Spongia Sp. Marine Sponge. Molecules, 23(12), 3187. https://doi.org/10.3390/molecules23123187