Effect of New Thiophene-Derived Aminophosphonic Derivatives on Growth of Terrestrial Plants. Part 2. Their Ecotoxicological Impact and Phytotoxicity Test Toward Herbicidal Application in Agriculture
Abstract
1. Introduction
2. Results
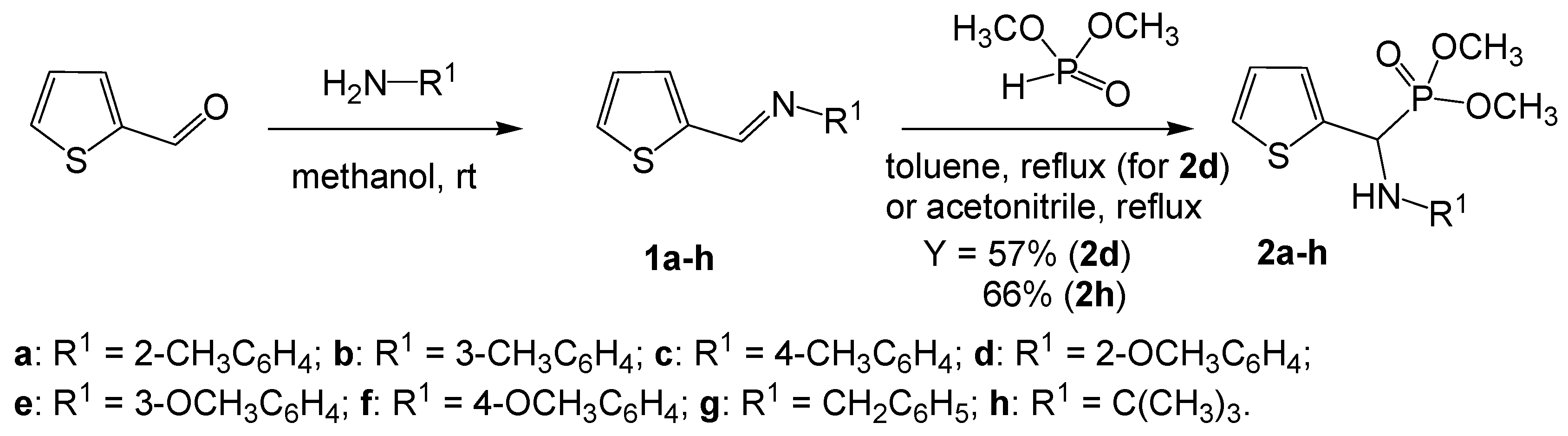
2.1. Synthesis of Aminophosphonates 2a–h
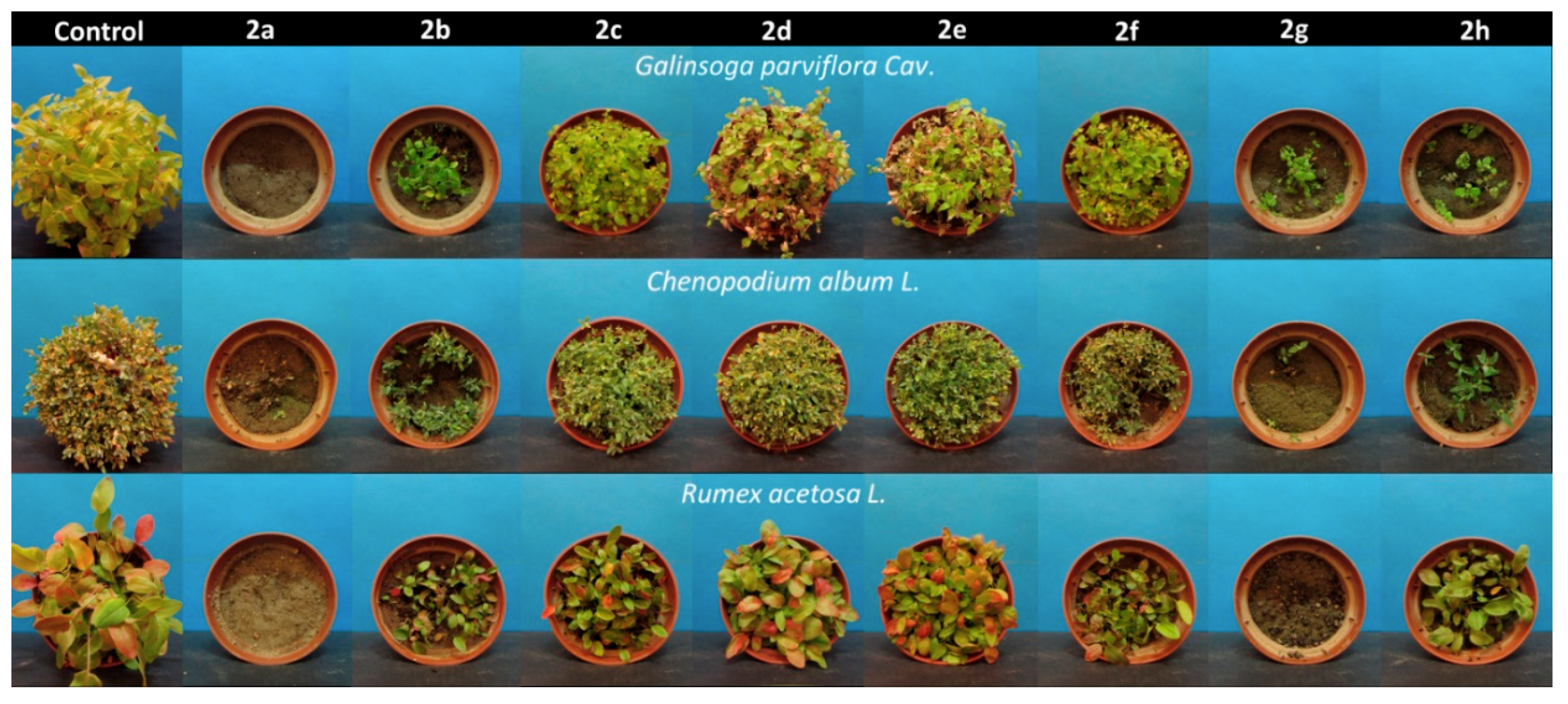
2.2. Phytotoxicity of Tested Aminophosphonates 2a–h
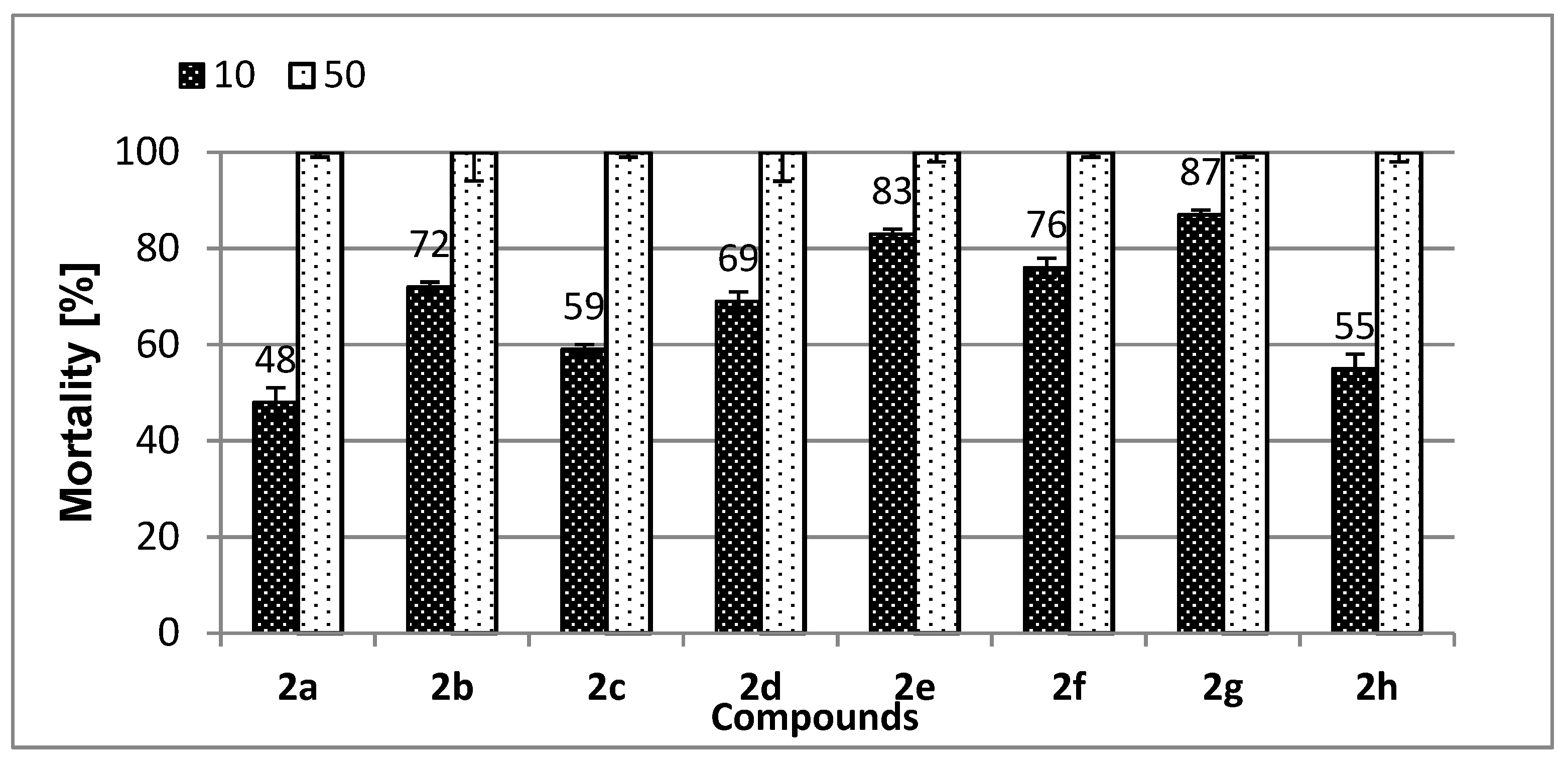
2.3. Evaluation of Ecotoxicity of Tested Compounds 2a–h
2.3.1. Microtox® Assay with Aliivibrio fischeri
2.3.2. OSTRACODTOXKIT™ Test with Heterocypris incongruens
3. Discussion
4. Materials and Methods
4.1. Chemistry
4.1.1. Preparation of Amino(2-thienyl)methylphosphonates 2a–c and 2e–h
4.1.2. Preparation of Dimethyl N-(2-methoxyphenyl)amino(2-thienyl)methylphosphonate (2d)
4.2. Plant Growth Test of Aminophosphonates 2a–h
4.3. Microtox®® Toxicity Assay
4.4. Ostracod Test Kit
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Andersen, S.M.; Hertz, P.B.; Holst, T.; Bossi, R.; Jacobsen, C.S. Mineralisation studies of C14-labelled metsulfuron-methyl, tribenuron-methyl, chlorsulfuron and thifensulfuronmethyl in one Danish soil and groundwater sediment profile. Chemosphere 2001, 45, 775–782. [Google Scholar] [CrossRef]
- Polati, S.; Bottaro, M.; Frascarolo, P.; Gosetti, F.; Gianotti, V.; Gennaro, M.C. HPLC-UV and HPLC-MSn multiresidue determination of amidosulfuron, azimsulfuron, nicosulfuron, rimsulfuron, thifensulfuron methyl, tribenuron methyl and azoxystrobin in surface waters. Anal. Chim. Acta 2006, 579, 146–151. [Google Scholar] [CrossRef] [PubMed]
- EFSA (European Food Safety Authority). Conclusion on the peer review of the pesticide risk assessment of the active substance thifensulfuron-methyl. EFSA J. 2015, 13, 4201. [Google Scholar] [CrossRef]
- Rosenkrantz, R.Y.; Baun, A.; Kusk, O. Growth inhibition and recovery of Lemna gibba after pulse exposure to sulfonylurea herbicides. Ecotoxicol. Environ. Saf. 2013, 89, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Beckie, H.J.; Tardif, F.J. Herbicide cross resistance in weeds. Crop Prot. 2012, 35, 15–28. [Google Scholar] [CrossRef]
- 2,4-D and Dicamba-Resistant Crops and Their Implications for Susceptible Non-Target Crops. Available online: http://msue.anr.msu.edu/news/24_d_and_dicamba_resistant_crops_and_their_implications_for_susceptible_non (accessed on 28 November 2018).
- Vanlaeys, A.; Dubuisson, F.; Seralini, G.-E.; Travert, C. Formulants of glyphosate-based herbicides have more deleterious impact than glyphosate on TM4 Sertoli cells. Toxicol. In Vitro 2018, 52, 14–22. [Google Scholar] [CrossRef]
- Defarge, N.; Spiroux de Vendômois, J.; Séralini, G.E. Toxicity of formulants and heavy metals in glyphosate-based herbicides and other pesticides. Toxicol. Rep. 2018, 5, 156–163. [Google Scholar] [CrossRef]
- Mesnage, R.; Bernay, B.; Séralini, G.-E. Ethoxylated adjuvants of glyphosate-based herbicides are active principles of human cell toxicity. Toxicology 2013, 313, 122–128. [Google Scholar] [CrossRef]
- Filipe, O.M.S.; Santos, S.A.O.; Domingues, M.R.M.; Vidal, M.M.; Silvestre, A.J.D.; Neto, C.P.; Santos, E.B.H. Photodegradation of the fungicide thiram in aqueous solutions. Kinetic studies and identification of the photodegradation products by HPLC–MS/MS. Chemosphere 2013, 91, 993–1001. [Google Scholar] [CrossRef]
- Sanchirico, R.; Pinto, G.; Pollio, A.; Cordella, M.; Cozzani, V. Thermal degradation of Fenitrothion: Identification and eco-toxicity of decomposition products. J. Hazard. Mater. 2012, 199–200, 390–400. [Google Scholar] [CrossRef]
- Baghestani, M.A.; Zand, E.; Soufizadeh, S.; Jamali, M.; Mighany, F. Evaluation of sulfosulfuron for broadleaved and grass weed control in wheat (Triticum aestivum L.) in Iran. Crop Prot. 2007, 26, 1385–1389. [Google Scholar] [CrossRef]
- Lewkowski, J.; Malinowski, Z.; Matusiak, A.; Morawska, M.; Rogacz, D.; Rychter, P. The Effect of New Thiophene-Derived Aminophosphonic Derivatives on Growth of Terrestrial Plants: A Seedling Emergence and Growth Test. Molecules 2016, 21, 694. [Google Scholar] [CrossRef] [PubMed]
- Terrestrial plant test: Seedling emergence and seedling growth test. In OECD/OCDE Guidelines for the Testing of Chemicals. Section 2. Effects on Biotic Systems; Organization for Economic and Cooperation Development (OECD) Publishing: Paris, France, 2006; ISSN 20745761.
- Lewkowski, J.; Morawska, M.; Karpowicz, R.; Rychter, P.; Rogacz, D.; Lewicka, K.; Dobrzyński, P. Evaluation of Ecotoxicological Impact of New Pyrrole-derived Aminophosphonates Using Selected Bioassay Battery. Ecotoxicology 2017, 26, 914–929. [Google Scholar] [CrossRef] [PubMed]
- Lewkowski, J.; Karpowicz, R.; Morawska, M.; Rychter, P.; Rogacz, D.; Lewicka, K.; Dobrzyński, P. Synthesis and ecotoxicological impact of ferrocene-derived amino-phosphonates using a battery of bioassays. RSC Adv. 2017, 7, 38399–38409. [Google Scholar] [CrossRef]
- Lewkowski, J.; Morawska, M.; Karpowicz, R.; Rychter, P.; Rogacz, D.; Lewicka, K. Novel (5-nitrofurfuryl)-substituted esters of phosphonoglycine—Their synthesis and phyto- and ecotoxicological properties. Chemosphere 2017, 188, 618–632. [Google Scholar] [CrossRef]
- Alshallash, K.S. Germination of weed species (Avena fatua, Bromus catharticus, Chenopodium album and Phalaris minor) with implications for their dispersal and control. Ann. Agric. Sci. 2018, 63, 91–97. [Google Scholar] [CrossRef]
- Parsons, D.; Lane, P.; Hall, E.; Galloway, A.; Pham Van, B. Effective Weed Control for Talish and Hairy Canary Clover Seed Crops; Rural Industries Research and Development Corporation: Barton, Australia, 2013; p. 5. ISBN 9 978-1-74254-567-7. [Google Scholar]
- Hernando, M.D.; De Vettori, S.; Martinez Bueno, M.J.; Fernandez-Alba, A.R. Toxicity evaluation with Vibrio fischeri test of organic chemicals used in aquaculture. Chemosphere 2007, 68, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Technical Guidance Documents in Support of Directive 93/67/EEC on Risk Assessment of New Notified Substances and Regulation (EC) No. 1488/94 on Risk Assessment of Existing Substances (Parts I, II, III and IV). Available online: https://ec.europa.eu/jrc/en/publication/eur-scientific-and-technical-research-reports/technical-guidance-document-risk-assessment-part-1-part-2 (accessed on 28 November 2018).
- Alshallash, K.S. Effect of pendimethalin, triflufarin, and terbutryn on Lolium multiflorum growing with barley during pre-emergence stage. Ann. Agric. Sci. 2014, 59, 239–242. [Google Scholar] [CrossRef]
- Chikoye, D.; Abaidoo, R.; Fontem, L.A. Response of weeds and soil microorganisms to imazaquin and pendimethalin in cowpea and soybean. Crop Prot. 2014, 65, 168–172. [Google Scholar] [CrossRef]
- Solaimalai, A.; Ramesh, R.T.; Baskar, M. Pesticides and environment. In Environmental Contamination and Bioreclamation; Kumar, A., Ed.; APH Publishing Co.: New Dehli, India, 2004; pp. 345–382. [Google Scholar]
- Soltani, N.; Shropshire, C.; Sikkema, P.H. Response of spring planted barley (Hordeum vulgare L.), oats (Avena sativa L.) and wheat (Triticum aestivum L.) to mesotrione. Crop Prot. 2011, 30, 849–853. [Google Scholar] [CrossRef]
- Pannacci, E.; Covarelli, G. Efficacy of mesotrione used at reduced doses for post-emergence weed control in maize (Zea mays L.). Crop Prot. 2009, 28, 57–61. [Google Scholar] [CrossRef]
- Nurse, R.E.; Hamill, A.S.; Swanton, C.J.; Tardif, F.J.; Sikkema, P.H. Weed control and yield response to mesotrione in maize (Zea mays). Crop Prot. 2010, 29, 652–657. [Google Scholar] [CrossRef]
- Sihtmäe, M.; Blinova, I.; Künnis-Beres, K.; Kanarbik, L.; Heinlaan, M.; Kahru, A. Ecotoxicological effects of different glyphosate formulations. Appl. Soil Ecol. 2013, 72, 215–224. [Google Scholar] [CrossRef]
- Rogacz, D.; Lewkowski, J.; Cal, D.; Rychter, P. C-Aryl Substituted Derivatives of Glyphosate—Their Phyto- and Ecotoxicological Properties. (in preparation)
- Baylis, A.D. Why glyphosate is a global herbicide: Strengths, weaknesses and prospects. Pest Manag. Sci. 2000, 56, 299–308. [Google Scholar] [CrossRef]
- Batisson, I.; Sancelme, M.; Mallet, C.; Besse-Hoggan, P. Fate and environmental impact of the recently marketed herbicide Mesotrione: Coupling biological and chemical studies for a global overview. In Current Research, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology; Méndez-Vilas, A., Ed.; FORMATEX: Badajoz, Spain, 2010; Volume 1, pp. 287–294. [Google Scholar]
- Bonnet, J.-L.; Bonnemoy, F.; Dusser, M.; Bohatier, J. Toxicity assessment of the herbicides sulcotrione and mesotrione toward two reference environmental microorganisms: Tetrahymena pyriformis and Vibrio fischeri. Arch. Environ. Contam. Toxicol. 2008, 55, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Kyriakopoulou, K.; Anastasiadou, P.; Machera, K. Comparative Toxicities of Fungicide and Herbicide Formulations on Freshwater and Marine Species. Bull. Environ. Contam. Toxicol. 2009, 82, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Bražėnaitė, J.; Šakalienė, O. Availability and toxicity of pendimethalin to aquatic microorganisms. Biologija 2006, 3, 59–62. [Google Scholar]
- Bonnet, J.-L.; Bonnemoy, F.; Dusser, M.; Bohatier, J. Assessment of the potential toxicity of herbicides and their degradation products to nontarget cells using two microorganisms, the bacteria Vibrio fischeri and the ciliate Tetrahymena pyriformis. Environ. Toxicol. 2007, 22, 78–91. [Google Scholar] [CrossRef]
- Tsui, M.T.K.; Chu, L.M. Aquatic toxicity of glyphosate-based formulations: Comparison between different organisms and the effects of environmental factors. Chemosphere 2003, 52, 1189–1197. [Google Scholar] [CrossRef]
- IUPAC THE PPDB Pesticide Properties Database. Available online: https://sitem.herts.ac.uk/aeru/iupac/index.htm (accessed on 24 October 2018).
- EPA United States Environmental Protection Agency. Available online: https://www3.epa.gov/pesticides/endanger/litstatus/effects/pendimeth/analysis.pdf (accessed on 24 October 2018).
- Thurston County Washington Public Health & Social Services. Available online: https://www.co.thurston.wa.us/health/ehipm/pdf_terr/terrestrial%20actives/pendimethalin.pdf (accessed on 24 October 2018).
- Vighi, M.; Matthies, M.; Solomon, K.R. Critical assessment of pendimethalin in terms of persistence, bioaccumulation, toxicity, and potential for long-range transport. J. Toxicol. Environ. Health B 2017, 20, 1–21. [Google Scholar] [CrossRef]
- Costa, A.G.F.; Sofiatti, V.; Maciel, C.D.G.; Lira, A.J.S.; Cordeiro, A.F., Jr.; Silva, R.L.M. Weed Management With Herbicides Applied in Pre and Postemergence on Castor Crop. Planta Daninha 2015, 33, 551–559. [Google Scholar] [CrossRef]
- Lewkowski, J.; Rodriguez Moya, M.; Chmielak, M.; Rogacz, D.; Lewicka, K.; Rychter, P. Synthesis, spectral characterization of several novel pyrene-derived aminophosphonates and their ecotoxicological evaluation using Heterocypris incongruens and Vibrio fischeri tests. Molecules 2016, 21, 936. [Google Scholar] [CrossRef] [PubMed]
- Doe, K.; Scroggins, R.; Mcleay, D.; Wohlgeschaffen, G. Solid-phase test for sediment toxicity using the luminescent bacterium Vibrio fischeri. In Small-Scale Freshwater Toxicity Investigations; Blaise, C., Férard, J.F., Eds.; Springer: Dordrecht, Netherlands, 2005; Volume 1, pp. 107–136. [Google Scholar]
- Martínez-Sanchez, M.J.; Pérez-Sirvent, C.; García-Lorenzo, M.L.; Martínez-López, S.; Bech, J.; García-Tenorio, R.; Bolívar, J.P. Use of bioassays for the assessment of areas affected by phosphate industry wastes. J. Geochem. Explor. 2014, 147, 130–138. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 2a–h are available upon request from the authors. |







| Compound Concentration [mg/kg s.d.w.] | Inhibition Biomarkers [%] Shoot Height | |||||||
|---|---|---|---|---|---|---|---|---|
| Galinsoga parviflora Cav. | ||||||||
| 2a | 2b | 2c | 2d | 2e | 2f | 2g | 2h | |
| Control | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 100 | 16.34 ± 2.21 | 11.32 ± 1.43 | 6.34 ± 0.56 | 8.21 ± 1.78 | 9.15 ± 1.09 | 7.83 ± 1.31 | 23.42 ± 0.75 | 21.54 ± 1.03 |
| 400 | 62.45 ± 3.32 | 29.24 ± 2.11 | 17.03 ± 1.54 | 14.15 ± 1.53 | 15.76 ± 1.67 | 13.31 ± 2.21 | 68.23 ± 1.09 | 65.22 ± 1.77 |
| 1000 | 100.00 ± 0.00 | 81.63 ± 4.21 | 67.54 ± 2.26 | 28.35 ± 2.09 | 29.93 ± 2.43 | 42.87 ± 3.92 | 95.12 ± 1.62 | 92.34 ± 2.68 |
| Chenopodium album L. | ||||||||
| 2a | 2b | 2c | 2d | 2e | 2f | 2g | 2h | |
| Control | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 100 | 19.32 ± 0.03 | 14.32 ± 1.12 | 11.21 ± 1.21 | 9.16 ± 1.87 | 8.31 ± 1.03 | 14.31 ± 0.37 | 16.76 ± 1.56 | 15.23 ± 1.34 |
| 400 | 37.97 ± 1.21 | 28.43 ± 0.43 | 29.43 ± 0.21 | 17.09 ± 1.09 | 11.67 ± 2.26 | 19.98 ± 1.73 | 39.23 ± 2.84 | 35.24 ± 1.93 |
| 1000 | 100.00 ± 0.00 | 79.21 ± 2.09 | 41.28 ± 0.54 | 29.66 ± 1.56 | 27.01 ± 2.87 | 36.13 ± 1.55 | 96.53 ± 1.03 | 93.41 ± 2.41 |
| Rumex acetosa L. | ||||||||
| 2a | 2b | 2c | 2d | 2e | 2f | 2g | 2h | |
| Control | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 100 | 36.44 ± 0.21 | 33.65 ± 1.42 | 28.23 ± 1.43 | 19.32 ± 1.45 | 19.53 ± 2.02 | 25.21 ± 1.03 | 26.34 ± 1.35 | 27.54 ± 1.15 |
| 400 | 60.34 ± 1.54 | 58.85 ± 1.09 | 38.74 ± 2.12 | 28.64 ± 1.23 | 28.34 ± 2.21 | 33.72 ± 2.21 | 63.23 ± 1.23 | 63.28 ± 1.09 |
| 1000 | 100.00 ± 0.00 | 93.09 ± 1.73 | 56.96 ± 2.32 | 47.37 ± 1.34 | 48.32 ± 2.12 | 52.87 ± 1.56 | 100.00 ± 0.00 | 83.74 ± 3.21 |
| Weeds | Compounds | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | 2a | 2b | 2c | 2d | 2e | 2f | 2g | 2h | |
| Gallant soldier (Galinsoga parviflora Cav.) | - | 1 | 6 | 7 | 9 | 9 | 8 | 3 | 4 |
| White goosefoot (Chenopodium album L.) | - | 1 | 6 | 8 | 9 | 9 | 8 | 3 | 4 |
| Common sorrel (Rumex acetosa L.) | - | 1 | 4 | 7 | 8 | 8 | 8 | 1 | 5 |
| Compound | EC50 (Lower Limit, Upper Limit [mg/L]) | EC50 (Lower Limit, Upper Limit [mg/kg s.d.w.]) | Coefficient of Determination (R2) |
|---|---|---|---|
| 2a | 1345 (1169; 1546) | 1775.4 (1543.1; 2040.7) | 0.888 |
| 2b | 399.4 (334.1; 477.5) | 527.2 (441.0; 630.3) | 0.962 |
| 2c | 651.5 (542.1; 783.1) | 859.9 (715.8; 1033.7) | 0.947 |
| 2d | 420 (317.0; 556.5) | 554.4 (418.4; 734.6) | 0.905 |
| 2e | 308.4 (211.7; 449.4) | 407.1 (279.4; 593.2) | 0.839 |
| 2f | 349.9 (328,2; 373,2) | 461.9 (433.2; 492.6) | 0.994 |
| 2g | 222.6 (174.1; 283.7) | 293.8 (229.8; 374.5) | 0.927 |
| 2h | 747.5 (665.5; 839.6) | 986.7 (878.5; 1108.3) | 0.984 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rogacz, D.; Lewkowski, J.; Malinowski, Z.; Matusiak, A.; Morawska, M.; Rychter, P. Effect of New Thiophene-Derived Aminophosphonic Derivatives on Growth of Terrestrial Plants. Part 2. Their Ecotoxicological Impact and Phytotoxicity Test Toward Herbicidal Application in Agriculture. Molecules 2018, 23, 3173. https://doi.org/10.3390/molecules23123173
Rogacz D, Lewkowski J, Malinowski Z, Matusiak A, Morawska M, Rychter P. Effect of New Thiophene-Derived Aminophosphonic Derivatives on Growth of Terrestrial Plants. Part 2. Their Ecotoxicological Impact and Phytotoxicity Test Toward Herbicidal Application in Agriculture. Molecules. 2018; 23(12):3173. https://doi.org/10.3390/molecules23123173
Chicago/Turabian StyleRogacz, Diana, Jarosław Lewkowski, Zbigniew Malinowski, Agnieszka Matusiak, Marta Morawska, and Piotr Rychter. 2018. "Effect of New Thiophene-Derived Aminophosphonic Derivatives on Growth of Terrestrial Plants. Part 2. Their Ecotoxicological Impact and Phytotoxicity Test Toward Herbicidal Application in Agriculture" Molecules 23, no. 12: 3173. https://doi.org/10.3390/molecules23123173
APA StyleRogacz, D., Lewkowski, J., Malinowski, Z., Matusiak, A., Morawska, M., & Rychter, P. (2018). Effect of New Thiophene-Derived Aminophosphonic Derivatives on Growth of Terrestrial Plants. Part 2. Their Ecotoxicological Impact and Phytotoxicity Test Toward Herbicidal Application in Agriculture. Molecules, 23(12), 3173. https://doi.org/10.3390/molecules23123173







