Metabolomic Study to Determine the Mechanism Underlying the Effects of Sagittaria sagittifolia Polysaccharide on Isoniazid- and Rifampicin-Induced Hepatotoxicity in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Instruments and Reagents
2.2. Mouse Model and SSP Administration
2.3. Plasma Sample Preparation
2.4. UPLC-HRMS Analysis
2.5. Histological Analysis
2.6. Data Processing
3. Results
3.1. Protective Effects of SSP on Ultrastructural Liver Damage Induced by INH/RFP
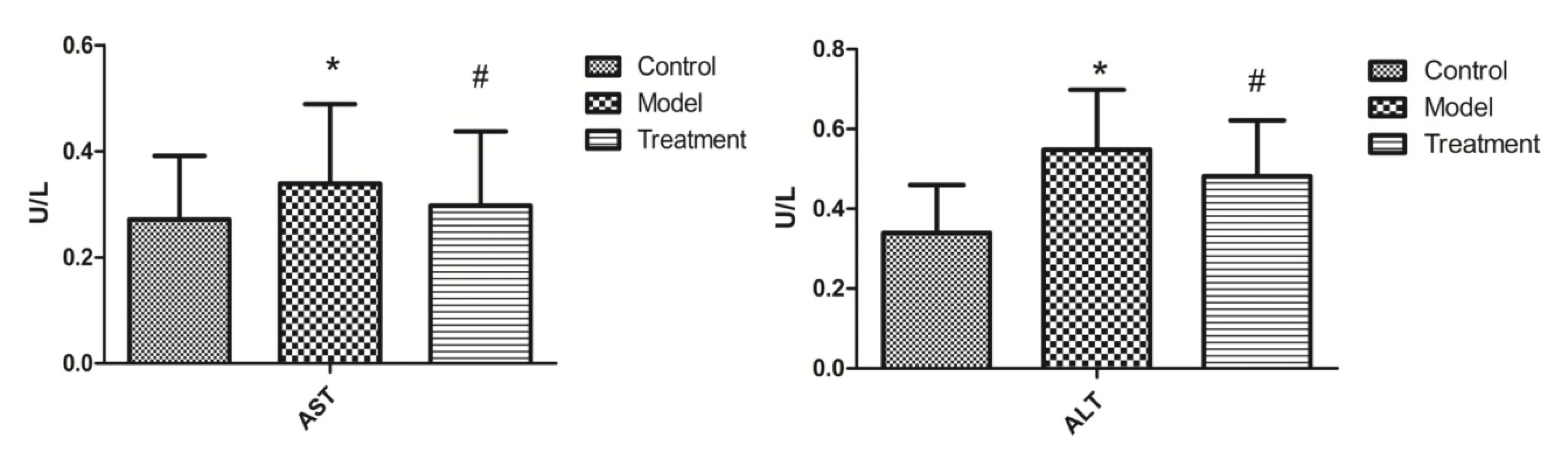
3.2. Effects of SSP on ALT and AST Content
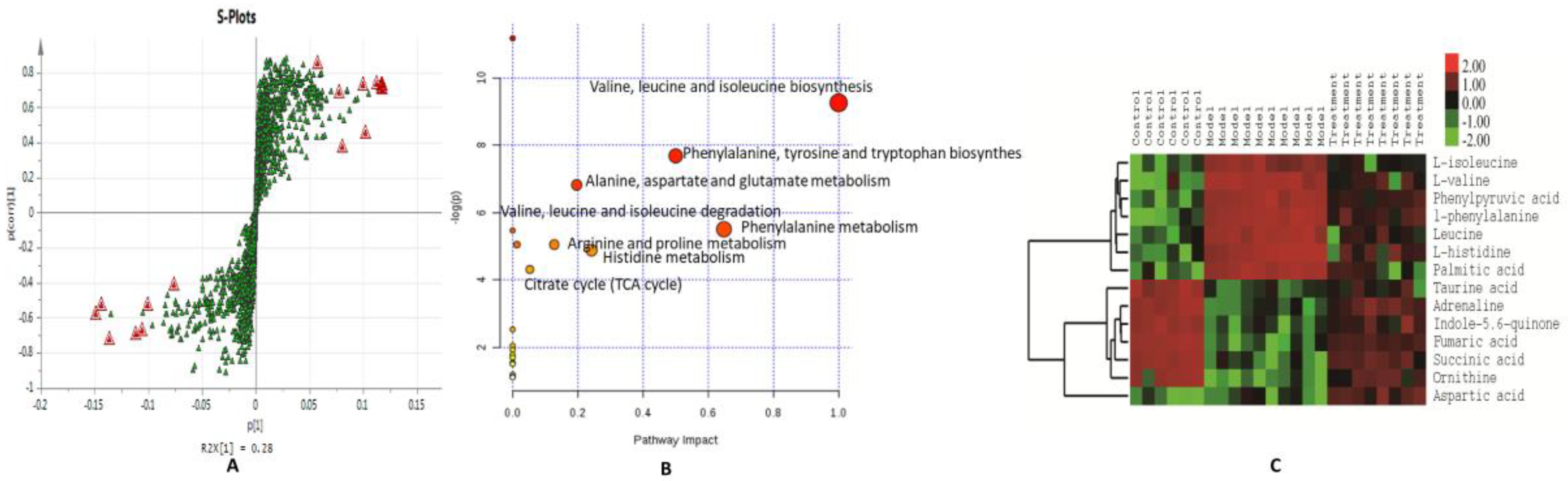
3.3. Normalization and Multivariate Statistical Analysis
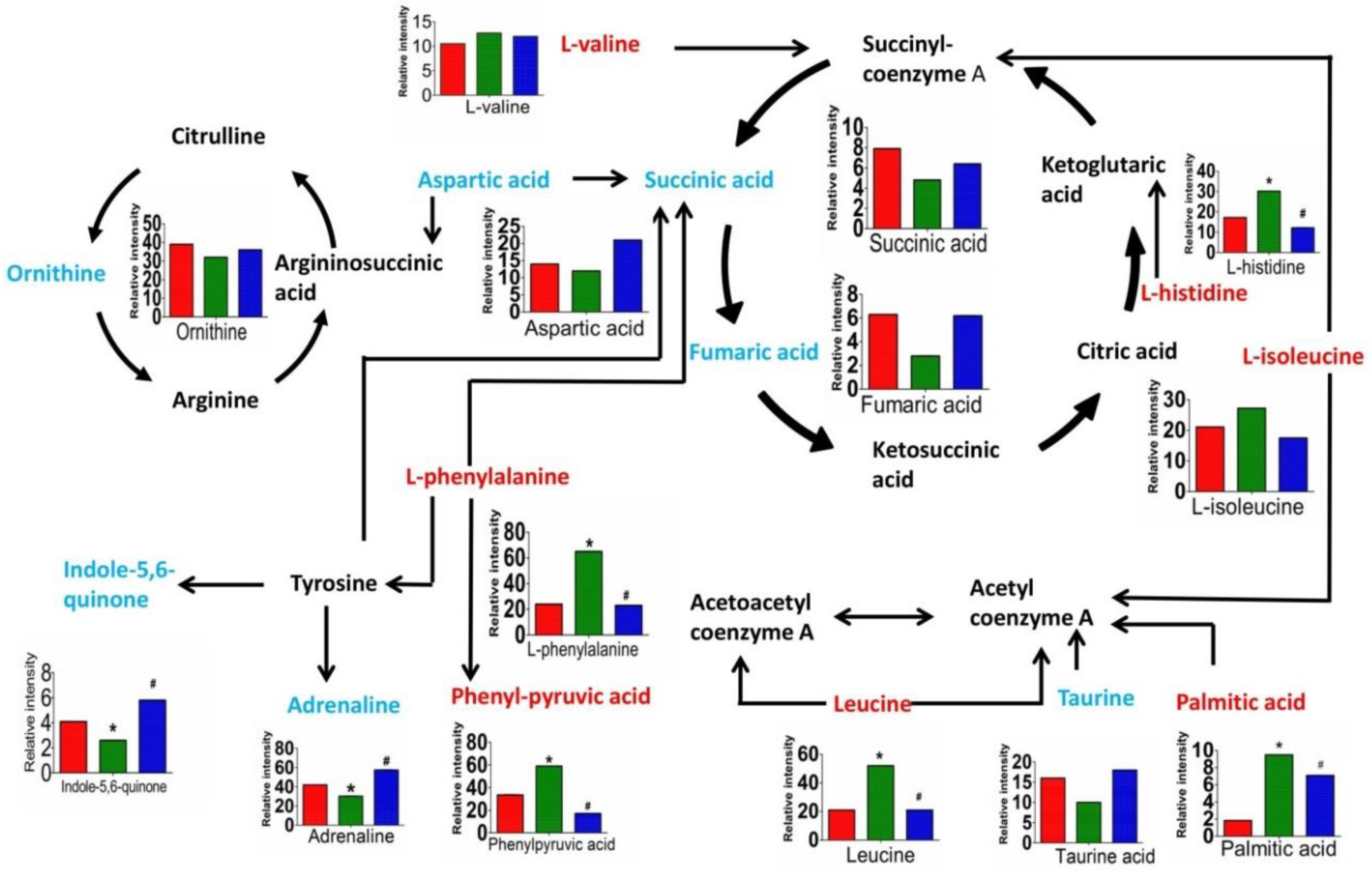
3.4. Discovery and Identification of Differential Metabolites
3.5. Metabolic Pathway Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Günther, G. Multidrug-resistant and extensively drug-resistant tuberculosis: A review of current concepts and future challenges. Clin. Med. 2014, 14, 279. [Google Scholar]
- Ramanathan, R.; Sivanesan, K. Evaluation of ameliorative ability of Silibinin metabolism against zidovudine and isoniazid-induced hepatotoxicity and hyperlipidaemia in rats: Role of Silibinin in Phase I and II drug metabolism. Chem. Biol. Interact. 2017, 273, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Adhvaryu, M.R.; Reddy, N.; Vakharia, B.C. Prevention of hepatotoxicity due to anti tuberculosis treatment: A novel integrative approach. World J. Gastroenterol. 2008, 14, 4753. [Google Scholar] [CrossRef] [PubMed]
- Harrington, T.; Manangan, L.; Jereb, J.; Navin, T.; Powell, K. Severe Isoniazid-Associated Liver Injuries Among Persons Being Treated for Latent Tuberculosis Infection—United States, 2004–2008. MMWR-Morb. Mortal. Wkly. 2010, 59, 224–229. [Google Scholar]
- Westphal, J.F.; Vetter, D.; Brogard, J.M. Hepatic side-effects of antibiotics. J. Antimicrob. Chemother. 1994, 33, 387–401. [Google Scholar] [CrossRef] [PubMed]
- Sarich, T.C.; Youssefi, M.; Zhou, T.; Adams, S.P.; Wall, R.A.; Wright, J.M. Role of hydrazine in the mechanism of isoniazid hepatotoxicity in rabbits. Arch Toxicol. 1996, 70, 835–840. [Google Scholar] [CrossRef] [PubMed]
- Bhadauria, S.; Singh, G.; Sinha, N.; Srivastava, S. Isoniazid induces oxidative stress, mitochondrial dysfunction and apoptosis in hep g2 cells. Cell Mol. Biol. (Noisy-le-Grand, France) 2007, 53, 102. [Google Scholar]
- Verma, A.K.; Yadav, A.; Dewangan, J.; Singh, S.V.; Mishra, M.; Singh, P.K. Isoniazid prevents nrf2 translocation by inhibiting erk1 phosphorylation and induces oxidative stress and apoptosis. Redox Biol. 2015, 6, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Boelsterli, U.A.; Lee, K.K. Mechanisms of isoniazid-induced idiosyncratic liver injury: Emerging role of mitochondrial stress. J. Gastroenterol. Hepatol. 2014, 29, 678–687. [Google Scholar] [CrossRef] [PubMed]
- Chao, W.; Rui, Q.; Zhang, Y.X. Naringenin protects against isoniazid- and rifampicininduced apoptosis in hepatic injury. World J. Gastroenterol. 2016, 22, 9775–9783. [Google Scholar]
- Gao, Y.; Zhong, M.L.; Zhong, J.L.; Zhang, K.F. Study of Dicliptera Chinensis Polysaccharide in Counteracting Liver Injury Induced by Antituberculosis Drugs. J. Guangzhou Univ. Tradit. Chin. Med. 2014, 6, 953–956. [Google Scholar]
- Shwe, H.; Huang, R.B.; He, Q.L.; Tang, X.J.; Huang, J.C. Protective effect of Yulangsan polysaccharide on the hepatocytes injury induced by anti-tubercular drug. West China J. Pharm. Sci. 2015, 30, 292–294. [Google Scholar]
- Ou, L.L.; Shui, P.X.; Zhu, Y.; Zhang, C. Study on Extraction of Polysaccharides and Antioxidant Activity of Sagittaria sagittifolia L. Polysaccharide. Agric. Sci. Technol. 2017, 18, 724–728. [Google Scholar]
- Li, B.; Wang, C.G.; Wang, J.; Li, G.M.; Ge, D.Y.; Xi, X.; Luo, W.Z.; Liao, Y.; Lin, Y. Water extract from Sagittaria Sagittifolia on Hepatic Injury Induced by Co-administration of Isoniazid and Rifampicin. Jilin J. Tradit. Chin. Med. 2016, 36, 818–821. [Google Scholar]
- Wang, J.; Luo, W.Z.; Li, B.; Lv, J.P.; Ke, X.H.; Ge, D.Y.; Dong, R.J.; Wang, C.G.; Han, Y.; Zhang, C.; et al. Sagittaria sagittifolia polysaccharide protects against isoniazid- and rifampicin-induced hepatic injury via activation of nuclear factor E2-related factor 2 signaling in mice. J. Ethnopharmacol. 2018, 227, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Fernie, A.R.; Trethewey, R.N.; Krotzky, A.J.; Willmitzer, L. Metabolite profiling: From diagnostics to systems biology. Nat. Rev. Mol. Cell. Biol. 2004, 5, 763–769. [Google Scholar] [CrossRef] [PubMed]
- The Plant List. Available online: http://www.theplantlist.org (accessed on 25 July 2017).
- Liang, D.; Zhou, Q.; Gong, W.; Wang, Y.; Nie, Z.; He, H. Studies on the antioxidant and hepatoprotective activities of polysaccharides from talinum triangulare. J. Ethnopharmacol. 2011, 136, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Huang, J.; Lin, X.; Zhang, S.; Jiao, Y.; Liang, T. Hepatoprotective effects of yulangsan polysaccharide against isoniazid and rifampicin-induced liver injury in mice. J. Ethnopharmacol. 2014, 152, 201–206. [Google Scholar] [CrossRef] [PubMed]
- KEGG. Available online: http://www.genome.jp/kegg/ (accessed on 10 October 2017).
- Sun, Q.; Liao, Y.; Lin, Y.; Zhang, C.; Zhang, Y.P.; Zhang, Y. Textual Research on Wuyu, Arrowhead and Water Chestnut. World Chin. Med. 2013, 8, 81–84. [Google Scholar]
- Lu, Z.F.; Wu, X.N.; Wang, J.L.; Huang, F. Hepatoprotective effect of Sagittaria sagittifolia against cadimum-induced acute liver injury on rats. China Public Health 2002, 18, 388–389. [Google Scholar]
- Wu, X.N.; Wang, J.L. Effect of Sagittaria sagittifolia on antioxidant capacity in rats. China Public Health 1996, 15, 89. [Google Scholar]
- Liao, Y.; Sun, Q.; Peng, G.Y.; Li, G.M.; Ge, D.Y.; Lin, Y. Protective effect of Sagittaria Sagittifolia on liver damaged by isoniazid and rifampin in rats. J. Tradit. Chin. Med. 2012, 35, 466–469. [Google Scholar]
- Munro, H.N.; Femstrom, J.D.; Wurtman, R.J. Plasma Amino Acid Imbalance and Hepatic Coma. In Fortschritte in der Parenteralen Ernährung; Springer: Berlin/Heidelberg, Germany, 1977; pp. 103–112. [Google Scholar]
- Yan, W.L.; Sun, D.Y.; Lin, X.T.; Jiang, Y.B.; Sun, X. l-[1-13C] phenylalanine breath test results reflect the activity of phenylalanine hydroxylase in carbon tetrachloride acute injured rat liver. Life Sci. 2006, 78, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Mcallan, L.; Cotter, P.D.; Roche, H.M.; Korpela, R.; Nilaweera, K.N. Impact of leucine on energy balance. J. Physiol. Biochem. 2013, 69, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.H.; Bae, S.H.; Kim, H.L.; Park, N.R.; Choi, E.S.; Jung, E.S. Branched-chain amino acids ameliorate fibrosis and suppress tumor growth in a rat model of hepatocellular carcinoma with liver cirrhosis. PLoS ONE 2013, 8, e77899. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhao, S.; Yan, W.; Xia, Y.; Chen, X.; Wang, W. Branched chain amino acids cause liver injury in obese/diabetic mice by promoting adipocyte lipolysis and inhibiting hepatic autophagy. Ebiomedicine 2016, 13, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Blonde-Cynober, F.; Aussel, C.; Cynober, L. Abnormalities in branched-chain amino acid metabolism in cirrhosis: Influence of hormonal and nutritional factors and directions for future research. Clin. Nutr. 1999, 18, 5–13. [Google Scholar] [CrossRef]
- Kerai, M.D.; Waterfield, C.J.; Kenyon, S.H.; Asker, D.S.; Timbrell, J.A. The effect of taurine depletion by beta-alanine treatment on the susceptibility to ethanol-induced hepatic dysfunction in rats. Alcohol Alcohol. 2011, 36, 29. [Google Scholar] [CrossRef]
- Timbrell, J.A.; Seabra, V.; Waterfield, C.J. The in vivo and in vitro protective properties of taurine. Gen. Pharmacol. 1995, 26, 453–462. [Google Scholar] [CrossRef]
- Jensen, M.D.; Cardin, S.; Edgerton, D.; Cherrington, A. Splanchnic free fatty acid kinetics. Am. J. Physiol. Endocrinol. Metab. 2003, 284, E1140. [Google Scholar] [CrossRef] [PubMed]
- Angulo, P.; Lindor, K.D. Non-alcoholic fatty liver disease. J. Gastroen. Hepatol. 2002, 17, S186–S190. [Google Scholar] [CrossRef]
- Pessayre, D.; Berson, A.; Fromenty, B.; Mansouri, A. Mitochondria in steatohepatitis. Semin. Liver Dis. 2001, 21, 57–69. [Google Scholar]
- Moravcová, A.; Červinková, Z.; Kučera, O.; Mezera, V.; Rychtrmoc, D.; Lotková, H. The effect of oleic and palmitic acid on induction of steatosis and cytotoxicity on rat hepatocytes in primary culture. Physiol. Res. 2015, 5, S627. [Google Scholar]
- Tu, Q.; Zheng, R.; Li, J.; Hu, L.; Chang, Y.; Li, L.; Li, M.; Wang, R.; Huang, D.; Wu, M.; et al. Palmitic acid induces autophagy in hepatocytes via jnk2 activation. Acta Pharmacol. Sin. 2014, 35, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Slack, A.J.; Auzinger, G.; Willars, C.; Dew, T.; Musto, R.; Corsilli, D.; Sherwood, R.; Wendon, J.A.; Bernal, W. Ammonia clearance with haemofiltration in adults with liver disease. Liver Int. 2013, 34, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.J.; Sun, R.; Liu, X.R.; Yan, J.Y.; Gao, X.J.; Jia, B. Hyperammonemia-induced hepatic injury in rats: Characterization of a new animal model. Chin. J. Hepatol. 2013, 21, 467. [Google Scholar]
- Dai, N.; Zou, Y.; Zhu, L.; Wang, H.F.; Dai, M.G. Antioxidant properties of proanthocyanidins attenuate carbon tetrachloride (CCL4)–induced steatosis and liver injury in rats via cyp2e1 regulation. J. Med. Food 2014, 17, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Shih, T.Y.; Young, T.H.; Lee, H.S.; Hsieh, C.B.; Hu, O.Y. Protective effects of kaempferol on isoniazid- and rifampicin-induced hepatotoxicity. Aaps J. 2013, 15, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Taha, R.; Seidman, E.; Mailhot, G.; Boudreau, F.; Gendron, F.P.; Beaulieu, J.F. Oxidative stress and mitochondrial functions in the intestinal Caco-2/15 cell line. PLoS ONE 2010, 5, e11817. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds used in the study are available from the authors. |

| Total Score (Points) | ||||
|---|---|---|---|---|
| Groups | n | 1 | 2 | 3 |
| Control * | 6 | 0 | 0 | 0 |
| Model | 10 | 10 | 0 | 0 |
| Treatment * | 8 | 0 | 0 | 0 |
| Time | M/Z | Adduct | Formula | Model Group | VIP | Compound Name | Related Pathway | |
|---|---|---|---|---|---|---|---|---|
| Trend | p-Value | |||||||
| 12.25 | 118.0857 | M + H | C5H11NO2 | ↑ | 1.728 × 10−3 | 1.2453 | l-valine | Amino acid metabolism |
| 2.21 | 126.0217 | M + H | C2H7NO3S | ↓ | 1.23 × 10−2 | 1.3751 | Taurine acid | |
| 0.89 | 132.1016 | M + H | C6H13NO2 | ↑ | 1.737 × 10−2 | 1.4323 | l-isoleucine | |
| 2.02 | 131.0708 | M − H | C6H12O3 | ↑ | 2.23 × 10−3 | 1.8421 | Leucine | |
| 9.08 | 146.0244 | M − H | C8H5NO2 | ↓ | 1.32 × 10−3 | 2.0118 | Indole-5,6-quinone | |
| 0.58 | 165.0545 | M + H | C9H8O3 | ↑ | 1.4712 × 10−4 | 1.4421 | Phenylpyruvic acid | |
| 14.14 | 184.0965 | M + H | C9H13NO3 | ↓ | 1.08 × 10−4 | 1.2125 | Adrenaline | |
| 2.18 | 164.0711 | M − H | C9H11NO2 | ↑ | 1.5342 × 10−2 | 1.2421 | l-phenylalanine | |
| 1.6 | 156.0764 | M + H | C6H9N3O2 | ↑ | 1.241 × 10−4 | 1.2232 | l-histidine | |
| 2.66 | 134.0447 | M + H | C4H7NO4 | ↓ | 1.854 × 10−3 | 1.2524 | Aspartic acid | Ornithine cycle |
| 2.66 | 131.0823 | M − H | C5H12N2O2 | ↓ | 0.92 × 10−3 | 1.7635 | Ornithine | |
| 2.05 | 115.0033 | M − H | C4H4O4 | ↓ | 0.84 × 10−3 | 1.9302 | Fumaric acid | Tricarboxylic acid cycle |
| 1.05 | 117.0189 | M − H | C4H6O4 | ↓ | 0.76 × 10−2 | 1.4263 | Succinic acid | |
| 3.8 | 255.232 | M − H | C16H32O2 | ↑ | 1.22 × 10−3 | 1.4345 | Palmitic acid | Fatty acid metabolism |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ke, X.-H.; Wang, C.-G.; Luo, W.-Z.; Wang, J.; Li, B.; Lv, J.-P.; Dong, R.-J.; Ge, D.-Y.; Han, Y.; Yang, Y.-J.; et al. Metabolomic Study to Determine the Mechanism Underlying the Effects of Sagittaria sagittifolia Polysaccharide on Isoniazid- and Rifampicin-Induced Hepatotoxicity in Mice. Molecules 2018, 23, 3087. https://doi.org/10.3390/molecules23123087
Ke X-H, Wang C-G, Luo W-Z, Wang J, Li B, Lv J-P, Dong R-J, Ge D-Y, Han Y, Yang Y-J, et al. Metabolomic Study to Determine the Mechanism Underlying the Effects of Sagittaria sagittifolia Polysaccharide on Isoniazid- and Rifampicin-Induced Hepatotoxicity in Mice. Molecules. 2018; 23(12):3087. https://doi.org/10.3390/molecules23123087
Chicago/Turabian StyleKe, Xiu-Hui, Chun-Guo Wang, Wei-Zao Luo, Jing Wang, Bing Li, Jun-Ping Lv, Rui-Juan Dong, Dong-Yu Ge, Yue Han, Ya-Jie Yang, and et al. 2018. "Metabolomic Study to Determine the Mechanism Underlying the Effects of Sagittaria sagittifolia Polysaccharide on Isoniazid- and Rifampicin-Induced Hepatotoxicity in Mice" Molecules 23, no. 12: 3087. https://doi.org/10.3390/molecules23123087
APA StyleKe, X.-H., Wang, C.-G., Luo, W.-Z., Wang, J., Li, B., Lv, J.-P., Dong, R.-J., Ge, D.-Y., Han, Y., Yang, Y.-J., Tu-Erxun, R.-Y., Liu, H.-S., Wang, Y.-C., & Liao, Y. (2018). Metabolomic Study to Determine the Mechanism Underlying the Effects of Sagittaria sagittifolia Polysaccharide on Isoniazid- and Rifampicin-Induced Hepatotoxicity in Mice. Molecules, 23(12), 3087. https://doi.org/10.3390/molecules23123087





