Cytisine-Pterocarpan Derived Compounds: Biomimetic Synthesis and Apoptosis-Inducing Activity in Human Breast Cancer Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthsis of Compound 4 with 1 and 2
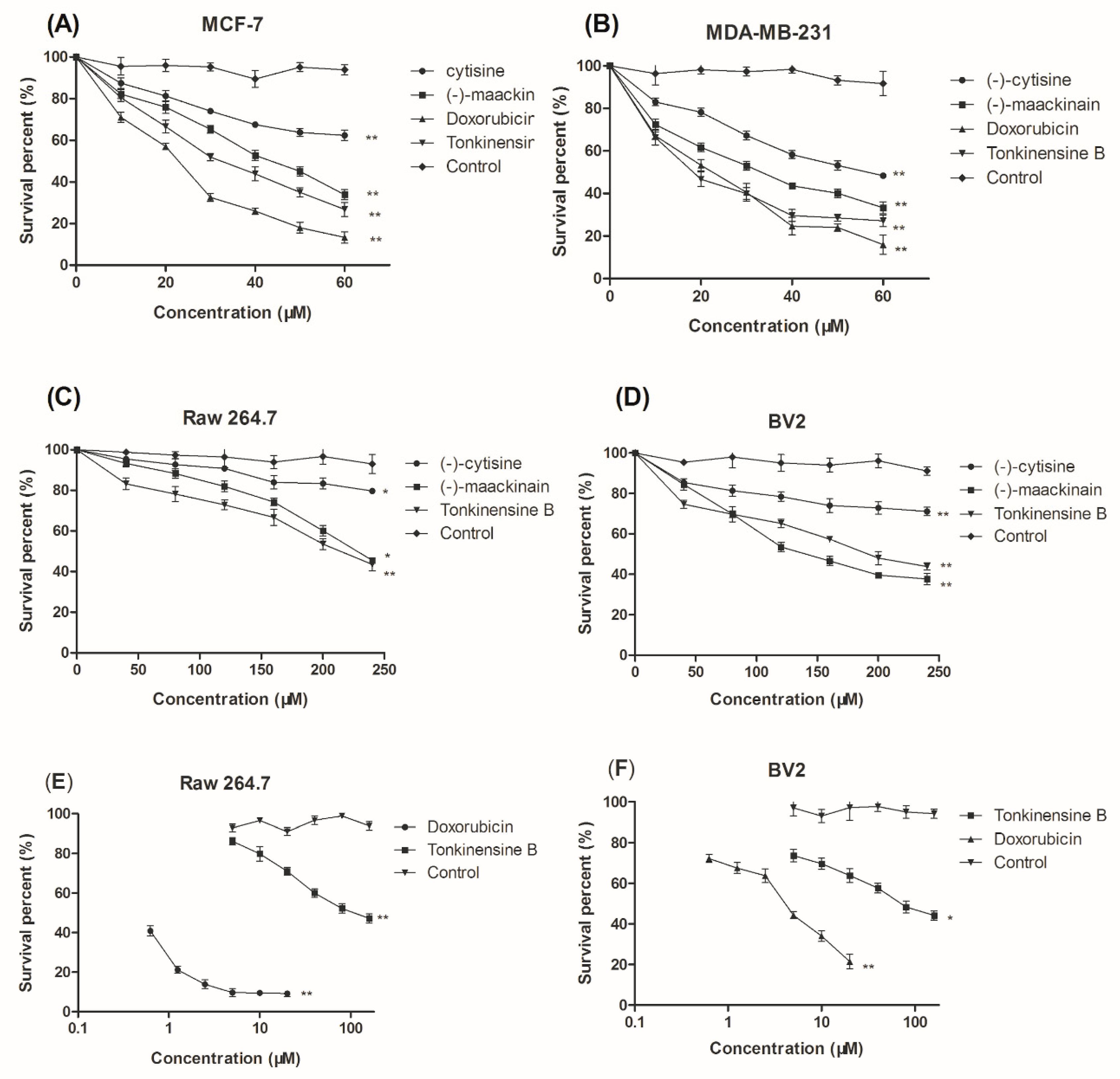
2.2. Cytotoxic Activity In Vitro against Select Cell Lines
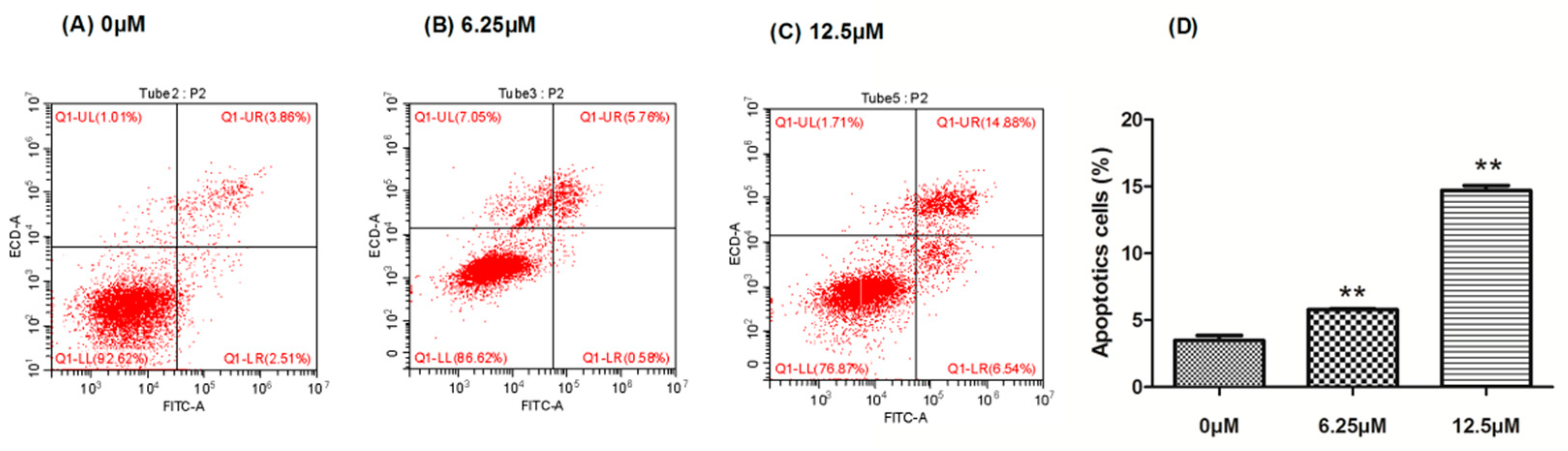
2.3. The Effect of Compound 4 on Apoptosis Induction in MDA-MB-231 Cell
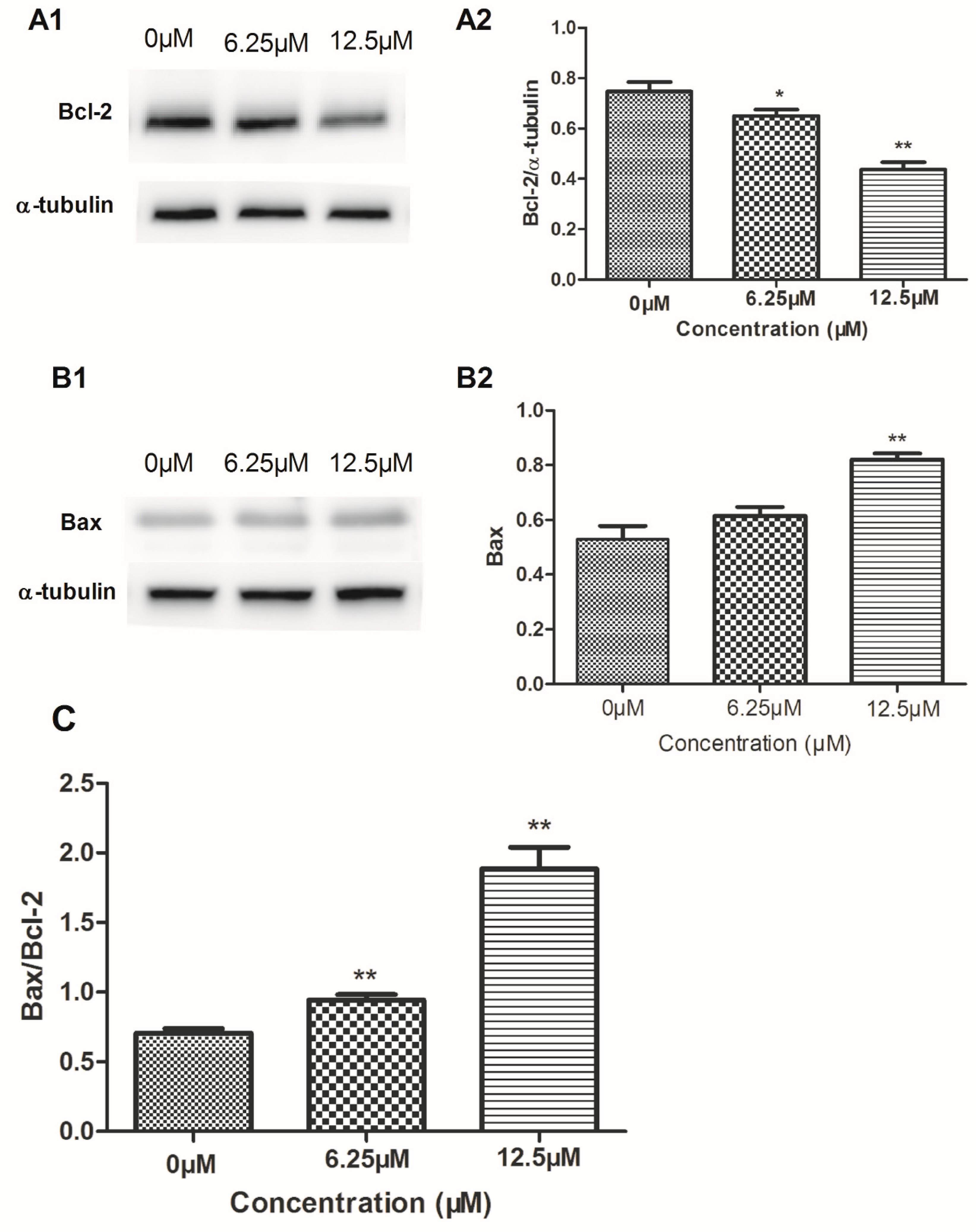
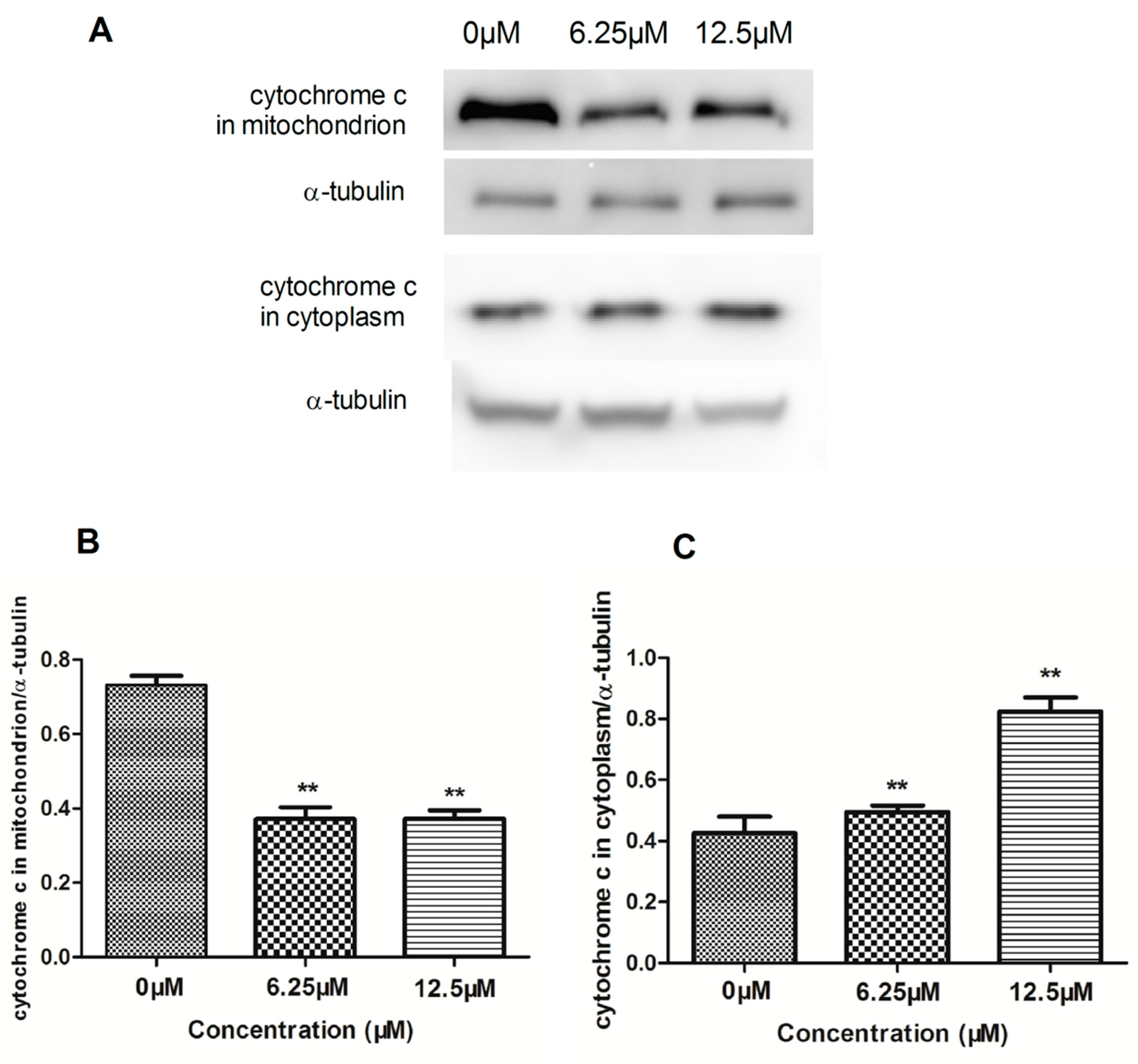
2.4. The Effect of Compound 4 on Mitochondrion-Mediated Apoptosis in MDA-MB-231 Cell
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Compound 4
3.3. Biological Studies
3.3.1. Cell Lines, Cell Culture
3.3.2. Cytotoxicity Assay In Vitro
3.3.3. MTT Assay
3.3.4. Annexin V-FITC/ Propidium Iodide Analysis
3.3.5. Western Blot Analysis
3.3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.; Ma, J.M.; Zou, Z.H.; Jemal, A. Cancer statistics. CA-Cancer J. Clinic. 2014, 64, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.Q.; Zheng, R.S.; Zuo, T.T. National cancer incidence and mortality in China, 2012. Chin. J. Cancer Res. 2016, 1, 1–11. [Google Scholar]
- Liu, D.X.; Guo, P.; Carthy, C.M.; Wang, B.R.; Tao, Y.; Augueste, D. Peptide density targets and impedes triple negative breast cancer metastasis. Nat. Commun. 2018, 9, 2612–2623. [Google Scholar] [CrossRef] [PubMed]
- Mack, N.; Mazzio, E.A.; Bauer, D.; Flores-Rozas, H.; Soliman, K. Stable shRNA silencing of lactate dehydrogenase A (LDHA) in human MDA-MB-231 breast cancer cells fails to alter lactic acid production, glycolytic activity, ATP or survival. Anticancer Res. 2017, 37, 1205–1212. [Google Scholar] [PubMed]
- Wurfel, F.; Erberb, R.; Huebnera, H.; Hein, A.; Lux, M.P.; Jud, S. TILGen: A program to investigate immune targets in breast cancer patients-first results on the influence of tumor-infiltrating lymphocytes. Breast Care. 2018, 13, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Sun, J.Z.; Yao, X.L.; Li, J.J.; Tu, Y.; Yao, F.; Sun, S.R. Knockdown of B7H6 inhibits tumor progression in triple-negative breast cancer. Oncol. Lett. 2018, 16, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.V.; Agarwal, S.; Singh, P.; Godbole, M.M.; Misra, K. Curcumin conjugates induce apoptosis via a mitochondrion dependent pathway in MCF-7 and MDA-MB-231 cell lines. Asian Pac. J. Cancer Prev. 2013, 14, 5797–5804. [Google Scholar] [CrossRef]
- Hengartner, M.O. The biochemistry of apoptosis. Nature 2000, 407, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Lill, R.; Mühlenhoff, U. Maturation of iron-sulfur proteins in eukaryotes: Mechanisms, connected processes, and diseases. Annu. Rev. Biochem. 2008, 77, 669–700. [Google Scholar] [CrossRef] [PubMed]
- Branzei, D.; Foiani, M. Regulation of DNA repair throughout the cell cycle. Nat. Rev. Mol. Cell Biol. 2008, 30, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.Y.; Chen, W.; Fan, J.T.; Song, R.; Wang, L.; Gu, Y.H.; Zheng, G.Z.; Shen, Y.; Wu, X.F.; Tan, N.H.; et al. Plant cyclopeptide RA-V kills huamna breast cancer cells by inducing mitochondria-mediated apoptosis through blocking PDK1-AKT interaction. Toxicol. Appl. Pharm. 2013, 267, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Stead, D.; O’brien, P. Total synthesis of the lupin alkaloid cytisine: Comparison of synthetic strategies and routes. Cheminform 2007, 38, 1885–1897. [Google Scholar] [CrossRef]
- Makshakov, G.; Dravolina, O.; Bespalov, A.; Kayukova, E.; Dorofeikova, M.; Zvartau, E. Characterisation of the effects of partial agonist of α4β2* nACh receptor cytisine in the two-choice serial reaction time task. Eur. Neuropsychopharm. 2013, 23, 41. [Google Scholar] [CrossRef]
- Rouden, J.; Lasne, M.C.; Blanchet, J.; Baudoux, J. (-)-cytisine and derivatives: Synthesis, reactivity, and applications. Chem. Rev. 2014, 114, 712–778. [Google Scholar] [CrossRef] [PubMed]
- Mizuguchi, H.; Nariai, Y.; Kato, S.; Nakano, T.; Kanayama, T.; Kashiwada, Y.; Nemoto, H. Maackiain is a novel antiallergic compound that suppresses transcriptional upregulation of the histamine H1 receptor and interleukin-4 genes. PR&P 2015, 3, 166–178. [Google Scholar]
- Engler, T.A.; Latessa, K.O.; Iyengar, R.; Chai, W.Y.; Agrios, K. Stereoselective syntheses of substituted pterocarpans with anti-HIV activity, and 5-aza-/5-thia-pterocarpan and 2-aryl-2,3-dihydrobenzofuran analogues. Bioorgan. Med. Chem. 1996, 4, 1755–1769. [Google Scholar] [CrossRef]
- Li, X.N.; Lu, Z.Q.; Qin, S.; Yan, H.X.; Yang, M.; Guan, S.H.; Liu, X.; Hua, H.M.; Wu, L.J.; Guo, D.A. Tonkinensines A and B, two novel alkaloids from Sophora tonkinensis. Tetrahedron Letters. Tetrahedron Lett. 2008, 49, 3797–3801. [Google Scholar] [CrossRef]
- Pan, Q.M.; Zhang, G.J.; Huang, R.Z.; Pan, Y.M.; Wang, H.S.; Liang, D.J. J Cytisine-type alkaloids and flavonoids from the rhizomes of Sophora tonkinensis. Asian. Nat. Prod. Res. 2016, 18, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.P.; Wu, X.; Tan, M.; Gong, J.; Tan, W.; Bian, B.L.; Chen, M.W.J. Fighting fire with fire: Poisonous chinese herbal medicine for cancer therapy. Ethnopharmacol 2012, 140, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Reber, K.P.; Burdge, H.E. Total synthesis of pyrophen and campyrones A–C. J. Nat. Prod. 2018, 81, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Mandai, H.; Fujii, K.; Suga, S. Recent topics in enantioselective acyl transfer reactions with dialkylaminopyridine-based nucleophilic catalysts. Tetrahedron Lett. 2018, 59, 1787–1803. [Google Scholar] [CrossRef]
- Ouaar, F.; Negadi, A.; Bahadur, I.; Phadagi, R.; Feddal-Benabed, F.; Negadi, L. Volumetric, acoustic and transport properties of mixtures containing dimethyl sulfoxide and some amines or alkanolamines: Measurement and correlation. J. Chem. Thermodyn. 2018, 121, 187–198. [Google Scholar] [CrossRef]
- Bondarenko, S.P.; Frasinyuk, M.S.; Vinogradova, V.I.; Khilya, V.P. Synthesis of flavonoid derivatives of cytisine. 1. Aminomethylation of 7-hydroxy-3-arylcoumarins. Chem. Nat. Compd. 2010, 46, 771–773. [Google Scholar] [CrossRef]
- Bondarenko, S.P.; Frasinyuk, M.S.; Vinogradova, V.I.; Khilya, V.P. Synthesis of cytisine derivatives of flavonoids. 2. Aminomethylation of 7-hydroxyisoflavones. Chem. Nat. Compd. 2011, 47, 604–607. [Google Scholar] [CrossRef]
- Kosheleva, N.V.; Chernyak, E.I.; Morozov, S.V.; Vinogradova, V.I.; Sagdullaev, S.S.; Abdullaev, N.D.; Grigorev, I.A. Synthesis of the first dihydroquercetin-cytisine conjugates. Chem. Nat. Compd. 2014, 50, 443–445. [Google Scholar] [CrossRef]
- Ma, Y.; He, Y.; Yin, T.J.; Chen, H.Q.; Gao, S.; Hu, M. Metabolism of phenolic compounds in LPS-stimulated Raw264.7 cells can impact their anti-inflammatory efficacy: Indication of hesperetin. J. Agr. Food Chem. 2018, 66, 6042–6052. [Google Scholar] [CrossRef] [PubMed]
- Block, M.L.; Zecca, L.J.; Hong, S. Microglia-mediated neurotoxicity: Uncovering the molecular mechanisms. Neuroscience 2007, 8, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.S.; Bowman, L.; Magaye, R.; Leonard, S.S.; Castranova, V.; Ding, M. Apoptosis induced by tungsten carbide-cobalt nanoparticles in JB6 cells involves ROS generation through both extrinsic and intrinsic apoptosis pathways. Int. J. Oncol. 2013, 42, 1349–1359. [Google Scholar] [CrossRef] [PubMed]
- Lou, L.L.; Yao, G.D.; Wang, J.; Zhao, W.Y.; Wang, X.B.; Huang, X.X.; Song, S.J. Enantiomeric neolignans from Picrasma quassioides exhibit distinctive cytotoxicity on hepatic carcinoma cells through ROS generation and apoptosis induction. Bioorg. Med. Chem. Lett. 2018, 28, 1263–1268. [Google Scholar] [CrossRef] [PubMed]
- Ghobrial, I.M.; Witzig, T.E.; Adjei, A.A. Targeting apoptosis pathways in cancer therapy. CA-Cancer J. Clin. 2005, 55, 178–194. [Google Scholar] [CrossRef] [PubMed]
- Li-Weber, M. Targeting apoptosis pathways in cancer by Chinese medicine. Cancer Lett. 2013, 332, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y. A structure view of mitochondria-mediated apoptosis. Nat. Struct. Biol. 2001, 8, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Indran, I.R.; Tufo, G.; Pervaiz, S.; Brenner, C. Recent advances in apoptosis, mitochondria and drug resistance in cancer cells. BBA-Bioenerg. 2011, 1807, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Czabotar, P.E.; Lessene, G.; Strasser, A.; Adams, J.M. Control of apoptosis by the Bcl-2 protein family: Implications for physiology and therapy. Nat. Rev. Mol. Cell Bio. 2014, 15, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Youle, R.J.; Strasser, A. The BCL-2 protein family: Opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 2009, 8, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Cory, S.; Huang, D.C.; Adams, J.M. The Bcl-2 family: Roles in cell survival and oncogenesis. Oncogene 2003, 22, 8590–8607. [Google Scholar] [CrossRef] [PubMed]
- Oltvai, Z.N.; Milliman, C.L.; Korsmeyer, S.J. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 1993, 74, 609–619. [Google Scholar] [CrossRef]
- Riedl, S.J.; Salvesen, G.S. The apoptosome: Signalling platform of cell death. Nat. Rev. Mol. Cell Biol. 2007, 8, 405–413. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 3 and 4 are available from the authors. |
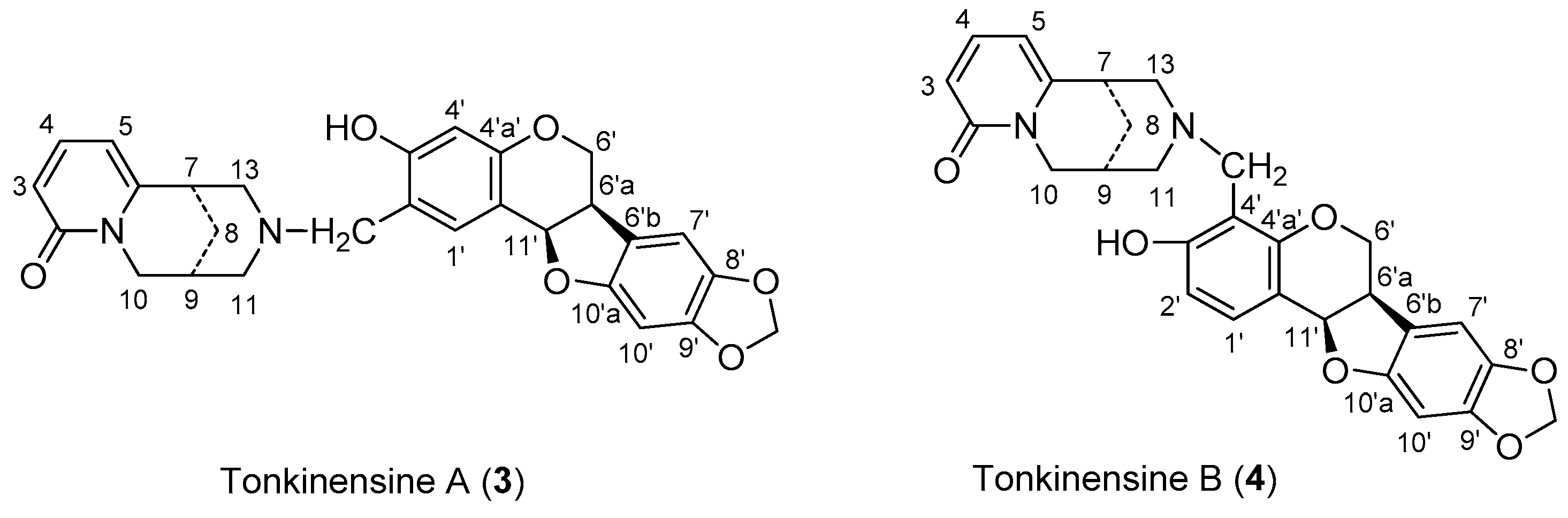
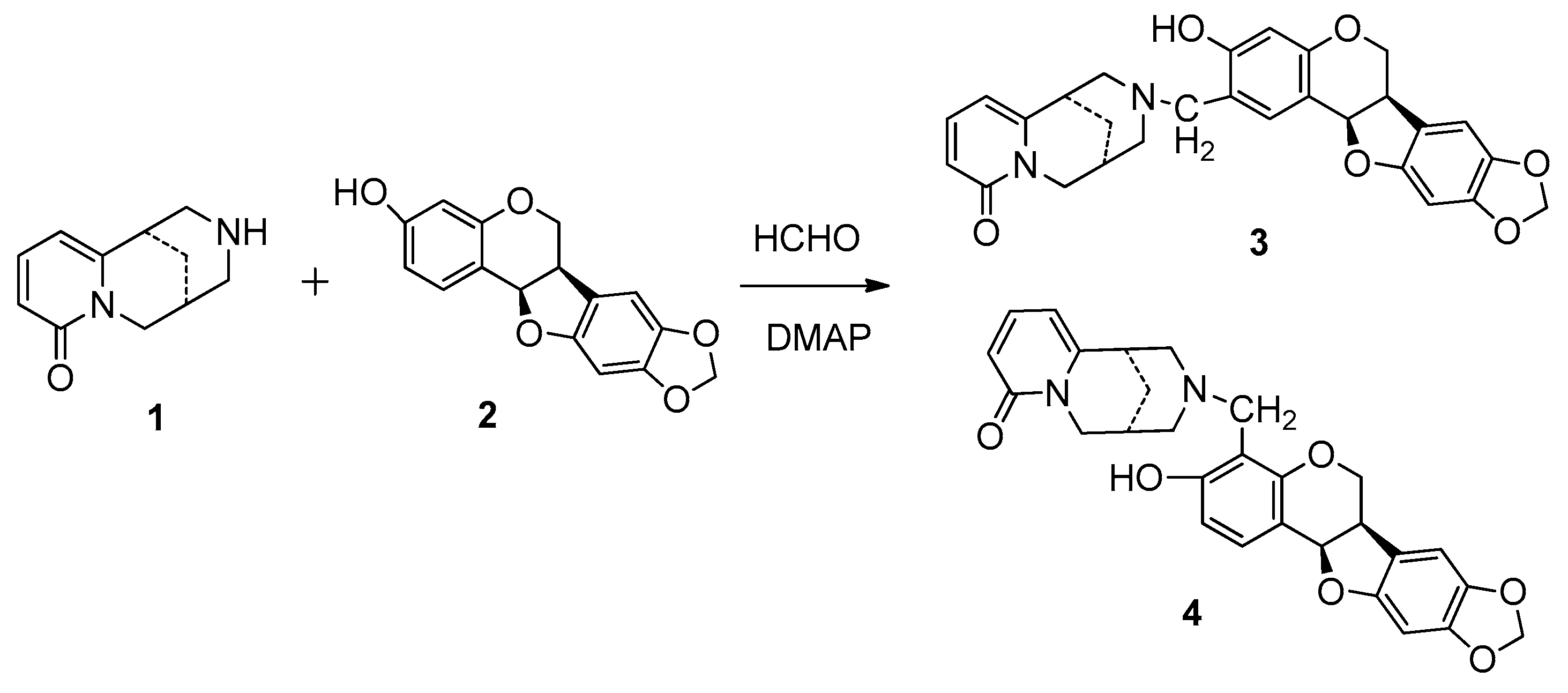
| Entry | Solvent | Ratio of 1/2 | Isolation Yield of 3 (%) | Isolation Yield of 4 (%) |
|---|---|---|---|---|
| 1 | 2-Propanol | 1.5:1 | 8 | 52 |
| 2 | 2-Propanol | 2.0:1 | 9 | 54 |
| 3 | 1,4-dioxane | 1.5:1 | 10 | 60 |
| 4 | 1,4-dioxane | 2.0:1 | 13 | 61 |
| 5 | Ethanol | 1.5:1 | 7 | 51 |
| 6 | Ethanol | 2.0:1 | 8 | 53 |
| Compound | IC50 (μM) | |||
|---|---|---|---|---|
| MCF-7 | MDA-MB-231 | RAW 264.7 | BV2 | |
| 1 | 101.7 ± 2.0 | 58.2 ± 0.98 | ||
| 2 | 42.37 ± 0.87 | 31.2 ± 0.62 | 252.4 ± 4.8 | 147.4 ± 3.1 |
| 4 | 31.4 ± 0.63 | 19.2 ± 0.38 | 242.1 ± 4.3 | 206.7 ± 3.6 |
| Doxorubicin | 23.7 ± 0.76 | 21.4 ± 0.79 | 0.7 ± 0.07 | 7.9 ± 0.09 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, T.-T.; Sun, X.-R.; Liu, R.-H.; Hua, L.-X.; Cheng, D.-P.; Mao, B.; Li, X.-N. Cytisine-Pterocarpan Derived Compounds: Biomimetic Synthesis and Apoptosis-Inducing Activity in Human Breast Cancer Cells. Molecules 2018, 23, 3059. https://doi.org/10.3390/molecules23123059
Peng T-T, Sun X-R, Liu R-H, Hua L-X, Cheng D-P, Mao B, Li X-N. Cytisine-Pterocarpan Derived Compounds: Biomimetic Synthesis and Apoptosis-Inducing Activity in Human Breast Cancer Cells. Molecules. 2018; 23(12):3059. https://doi.org/10.3390/molecules23123059
Chicago/Turabian StylePeng, Ting-Ting, Xuan-Rong Sun, Ren-Hao Liu, Lu-Xia Hua, Dong-Ping Cheng, Bin Mao, and Xing-Nuo Li. 2018. "Cytisine-Pterocarpan Derived Compounds: Biomimetic Synthesis and Apoptosis-Inducing Activity in Human Breast Cancer Cells" Molecules 23, no. 12: 3059. https://doi.org/10.3390/molecules23123059
APA StylePeng, T.-T., Sun, X.-R., Liu, R.-H., Hua, L.-X., Cheng, D.-P., Mao, B., & Li, X.-N. (2018). Cytisine-Pterocarpan Derived Compounds: Biomimetic Synthesis and Apoptosis-Inducing Activity in Human Breast Cancer Cells. Molecules, 23(12), 3059. https://doi.org/10.3390/molecules23123059