Taxifolin Resensitizes Multidrug Resistance Cancer Cells via Uncompetitive Inhibition of P-Glycoprotein Function
Abstract
:1. Introduction
2. Results
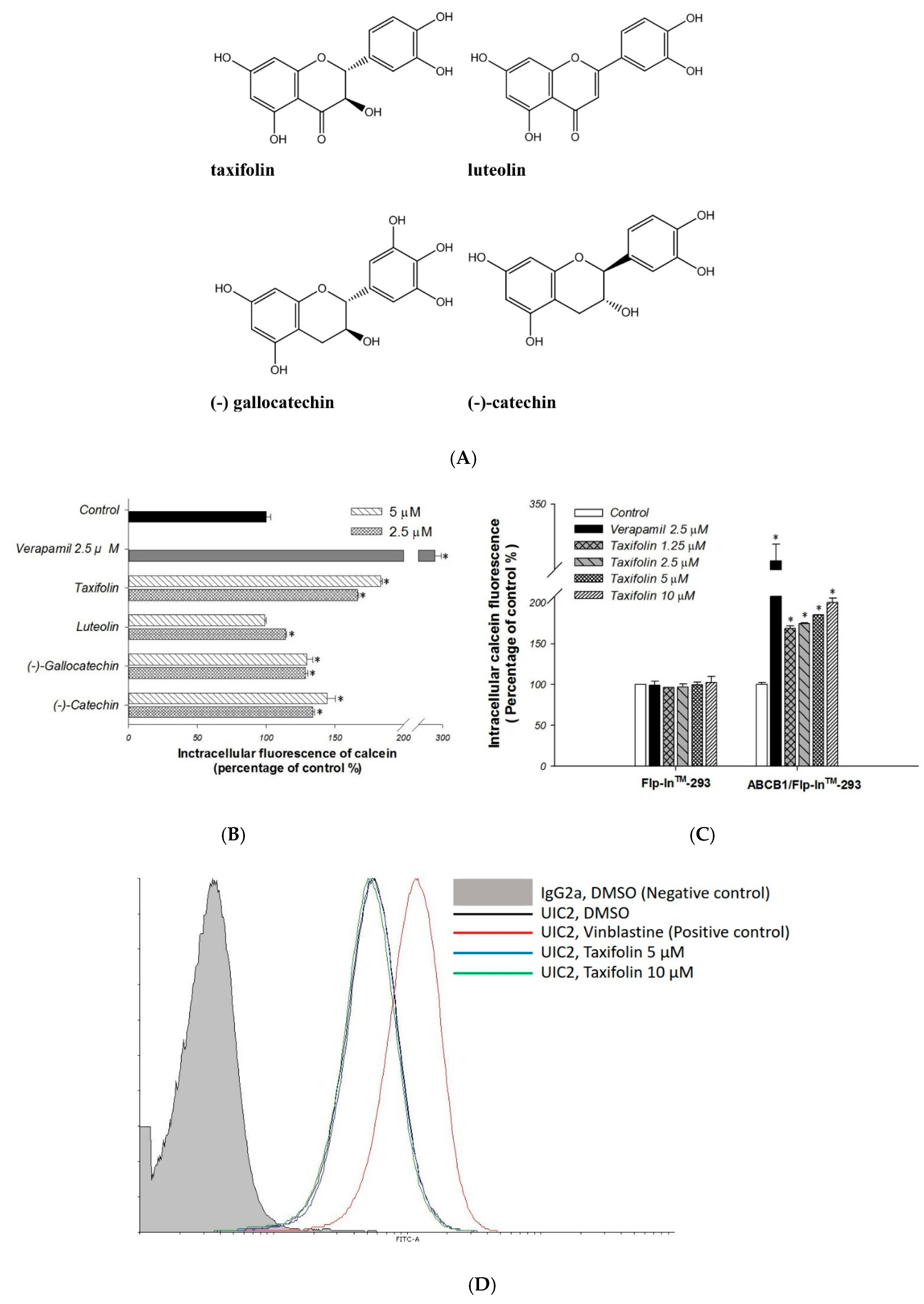
2.1. Primary Screen of Effects on P-gp Efflux Function
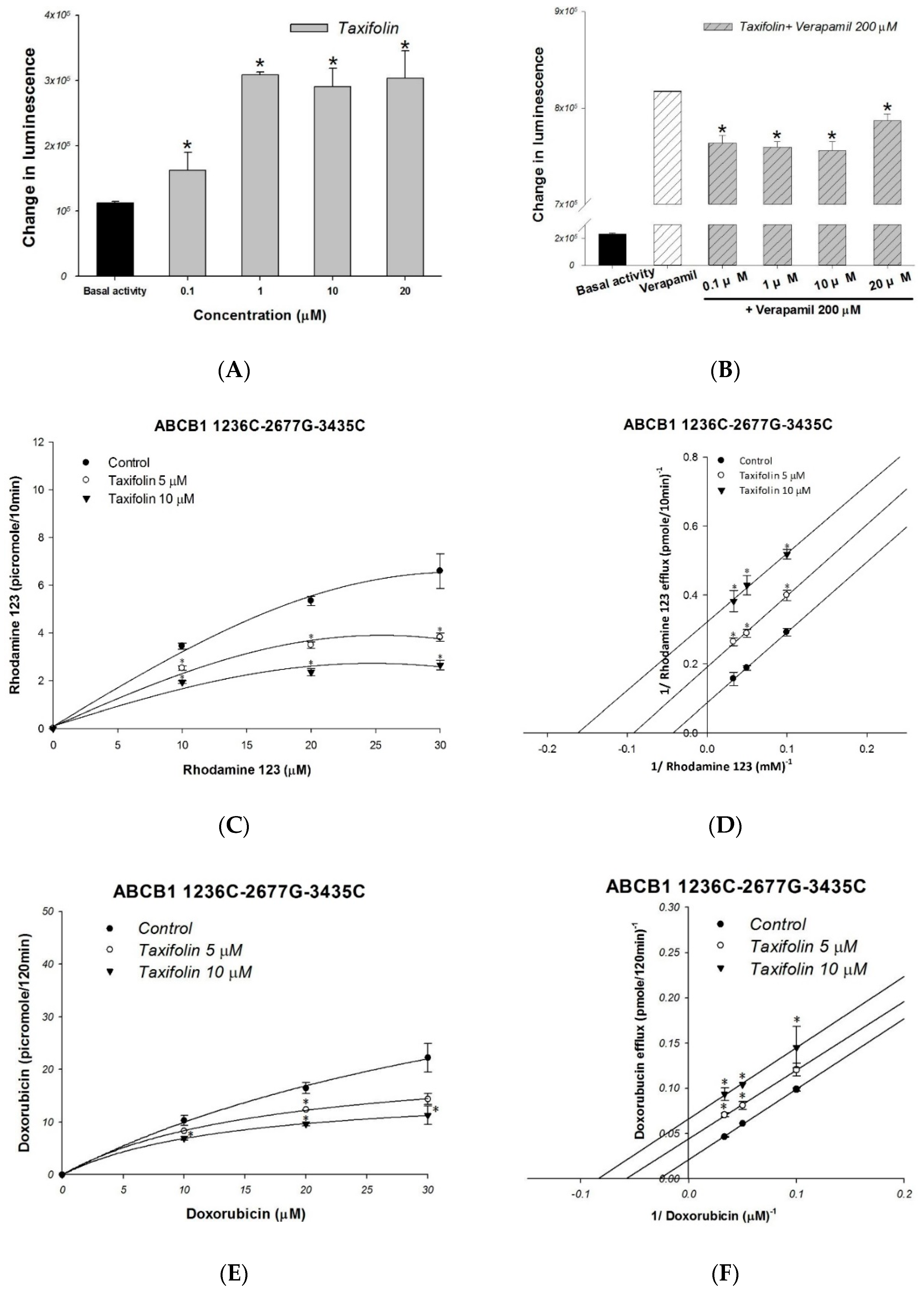
2.2. The Molecular and Kinetic Mechanism of Interaction between Taxifolin and Human P-gp
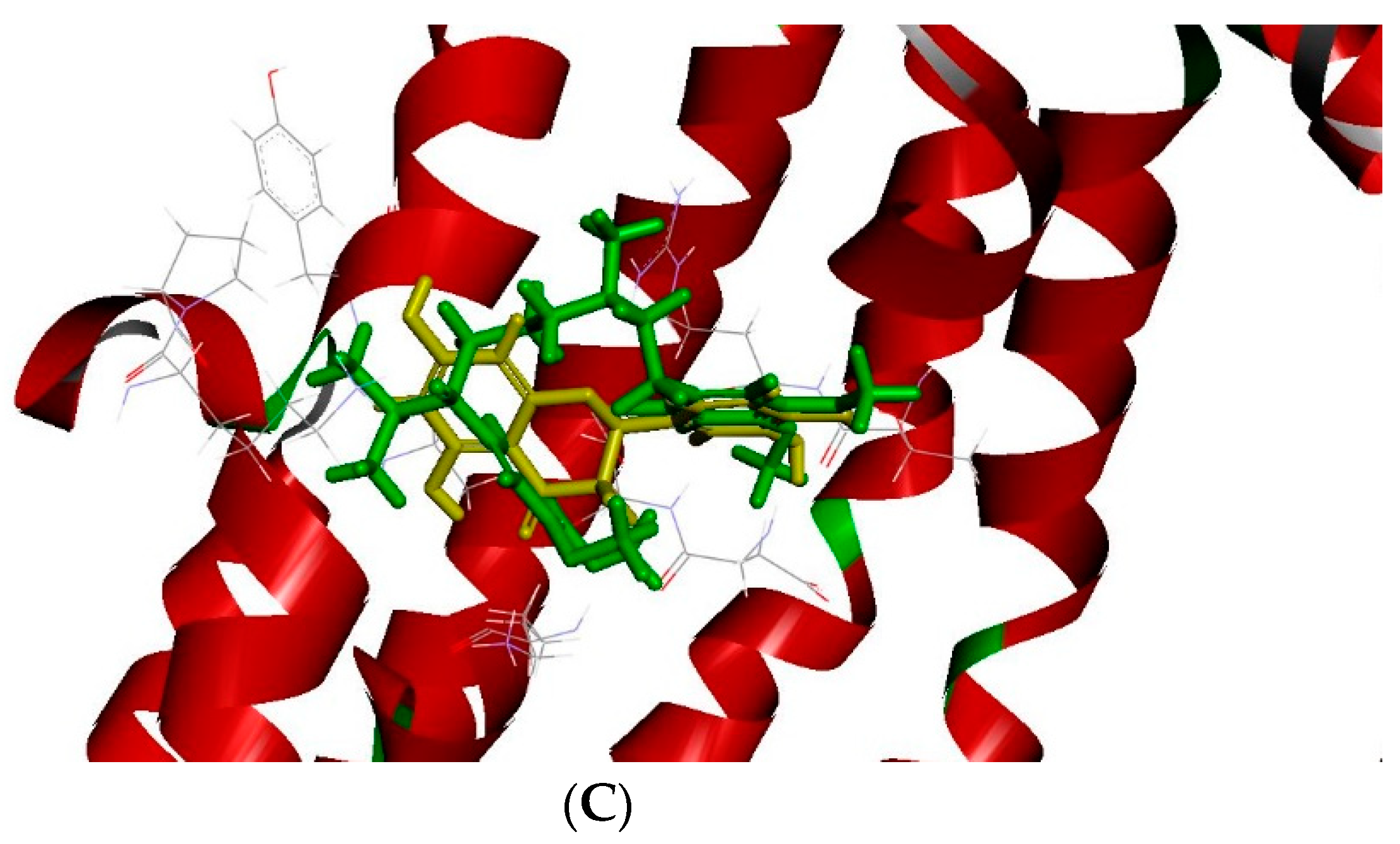
2.3. The Docking Model of Taxifolin on P-gp
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell lines and Culture Condition
4.3. Cell Line Establishment
4.4. Calcein-AM Accumulation Assay
4.5. Doxorubicin and Rhodamine123 Efflux Assay
4.6. MDR1 Shift Assay
4.7. P-gp ATPase Activity Assays
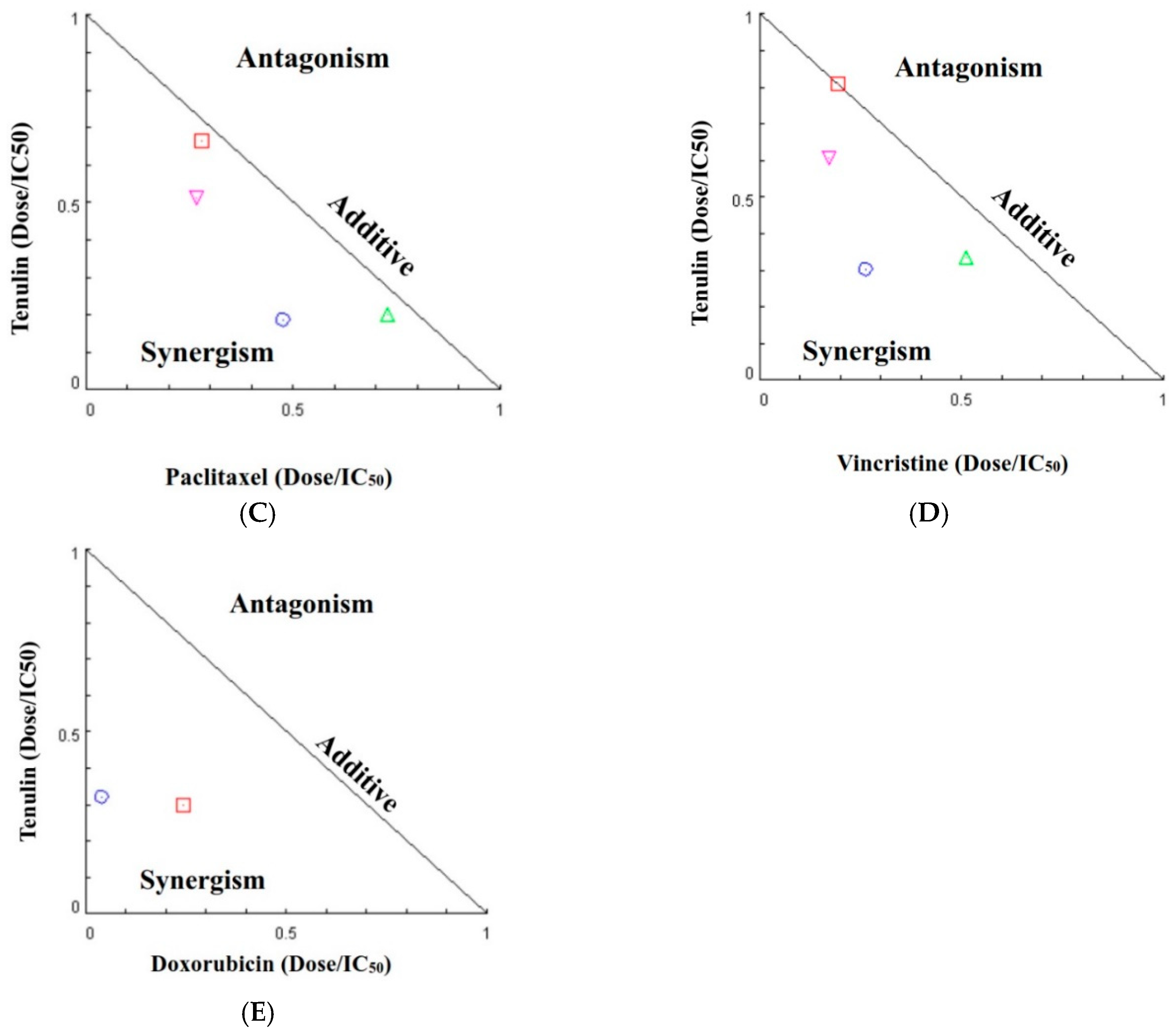
4.8. Cell Viability Assay and Drug Combination Assay
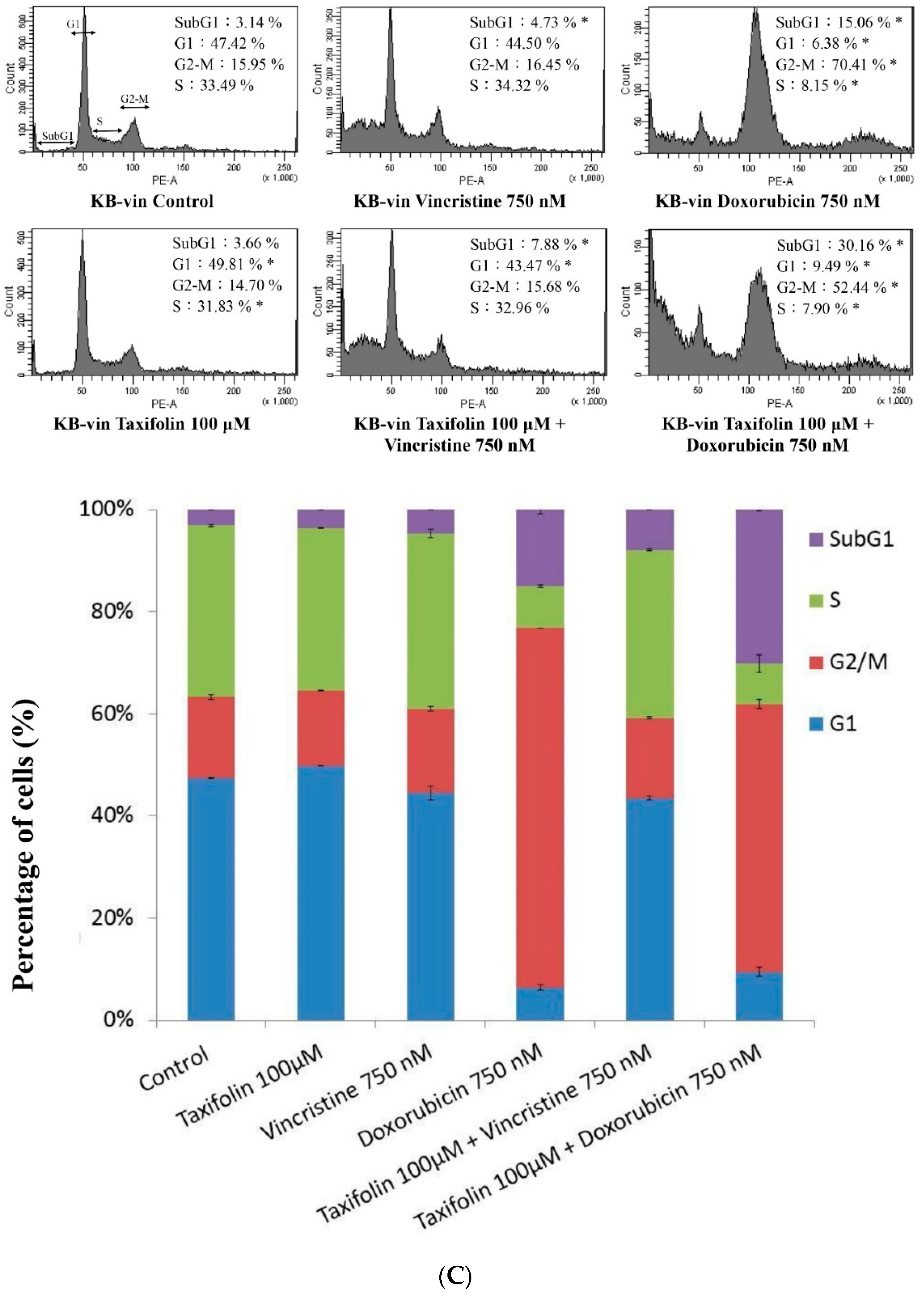
4.9. Cell-Cycle Analysis
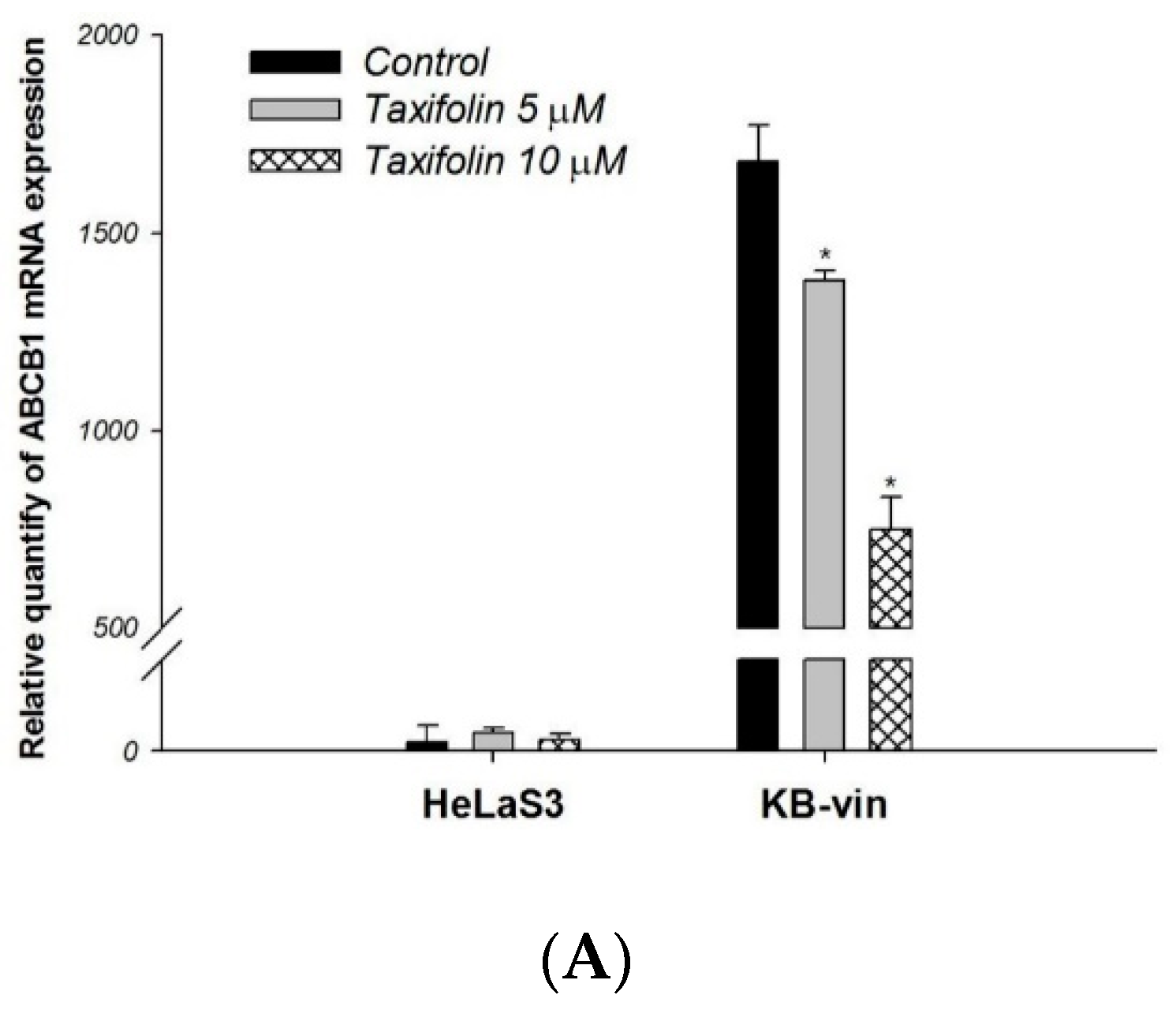
4.10. RNA Extraction and Real-Time Quantitative RT-PCR
4.11. Docking Simulation
4.12. Data and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| MDR | multidrug resistance |
| ABC | ATP-binding cassette |
| P-gp | P-glycoprotein |
References
- Gillet, J.P.; Gottesman, M.M. Mechanisms of multidrug resistance in cancer. Methods Mol. Biol. 2010, 596, 47–76. [Google Scholar] [CrossRef] [PubMed]
- Bugde, P.; Biswas, R.; Merien, F.; Lu, J.; Liu, D.X.; Chen, M.; Zhou, S.; Li, Y. The therapeutic potential of targeting ABC transporters to combat multi-drug resistance. Expert Opin. Ther. Targets 2017, 21, 511–530. [Google Scholar] [CrossRef] [PubMed]
- Alfarouk, K.O.; Stock, C.M.; Taylor, S.; Walsh, M.; Muddathir, A.K.; Verduzco, D.; Bashir, A.H.; Mohammed, O.Y.; Elhassan, G.O.; Harguindey, S.; et al. Resistance to cancer chemotherapy: Failure in drug response from ADME to P-gp. Cancer Cell Int. 2015, 15, 71. [Google Scholar] [CrossRef] [PubMed]
- Coley, H.M. Overcoming multidrug resistance in cancer: Clinical studies of P-glycoprotein inhibitors. Methods Mol. Biol. 2010, 596, 341–358. [Google Scholar] [CrossRef] [PubMed]
- Mohana, S.; Ganesan, M.; Agilan, B.; Karthikeyan, R.; Srithar, G.; Beaulah Mary, R.; Ananthakrishnan, D.; Velmurugan, D.; Rajendra Prasad, N.; Ambudkar, S.V. Screening dietary flavonoids for the reversal of P-glycoprotein-mediated multidrug resistance in cancer. Mol. BioSyst. 2016, 12, 2458–2470. [Google Scholar] [CrossRef] [PubMed]
- Batra, P.; Sharma, A.K. Anti-cancer potential of flavonoids: Recent trends and future perspectives. 3 Biotech 2013, 3, 439–459. [Google Scholar] [CrossRef] [PubMed]
- Limtrakul, P.; Khantamat, O.; Pintha, K. Inhibition of P-glycoprotein function and expression by kaempferol and quercetin. J. Chemother. 2005, 17, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Wang, H.; Hu, Z.; Huang, Y.; Yao, F.; Sun, S.; Wu, B. Quercetin inhibits proliferation and drug resistance in KB/VCR oral cancer cells and enhances its sensitivity to vincristine. Nutr. Cancer 2015, 67, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Jodoin, J.; Demeule, M.; Beliveau, R. Inhibition of the multidrug resistance P-glycoprotein activity by green tea polyphenols. Biochim. Biophys. Acta 2002, 1542, 149–159. [Google Scholar] [CrossRef]
- Zhang, Z.R.; Al Zaharna, M.; Wong, M.M.; Chiu, S.K.; Cheung, H.Y. Taxifolin enhances andrographolide-induced mitotic arrest and apoptosis in human prostate cancer cells via spindle assembly checkpoint activation. PLoS ONE 2013, 8, 54577. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.T.; Moon, J.H.; Choi, J.Y.; Park, S.J.; Kim, S.J.; Saadeldin, I.M.; Lee, B.C. Effect of antioxidant flavonoids (quercetin and taxifolin) on in vitro maturation of porcine oocytes. Asian-Australasian J. Anim. Sci. 2016, 29, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Alzaharna, M.; Alqouqa, I.; Cheung, H.Y. Taxifolin synergizes Andrographolide-induced cell death by attenuation of autophagy and augmentation of caspase dependent and independent cell death in HeLa cells. PLoS ONE 2017, 12, 0171325. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, S.; Nabekura, T.; Kamiyama, S. Inhibition of P-glycoprotein function by tea catechins in KB-C2 cells. J. Pharm. Pharmacol. 2004, 56, 1001–1005. [Google Scholar] [CrossRef] [PubMed]
- Esser, L.; Zhou, F.; Pluchino, K.M.; Shiloach, J.; Ma, J.; Tang, W.K.; Gutierrez, C.; Zhang, A.; Shukla, S.; Madigan, J.P.; et al. Structures of the multidrug transporter P-glycoprotein reveal asymmetric ATP binding and the mechanism of polyspecificity. J. Biol. Chem. 2017, 292, 446–461. [Google Scholar] [CrossRef] [PubMed]
- Borska, S.; Sopel, M.; Chmielewska, M.; Zabel, M.; Dziegiel, P. Quercetin as a potential modulator of P-glycoprotein expression and function in cells of human pancreatic carcinoma line resistant to daunorubicin. Molecules 2010, 15, 857–870. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Singh, A.; Mishra, A. Dual inhibition of chaperoning process by taxifolin: Molecular dynamics simulation study. J. Mol. Graphics Modell. 2012, 37, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Akinmoladun, A.C.; Oladejo, C.O.; Josiah, S.S.; Famusiwa, C.D.; Ojo, O.B.; Olaleye, M.T. Catechin, quercetin and taxifolin improve redox and biochemical imbalances in rotenone-induced hepatocellular dysfunction: Relevance for therapy in pesticide-induced liver toxicity? Pathophysiology 2018. [Google Scholar] [CrossRef] [PubMed]
- Van Zanden, J.J.; Geraets, L.; Wortelboer, H.M.; van Bladeren, P.J.; Rietjens, I.M.; Cnubben, N.H. Structural requirements for the flavonoid-mediated modulation of glutathione S-transferase P1-1 and GS-X pump activity in MCF7 breast cancer cells. Biochem. Pharmacol. 2004, 67, 1607–1617. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.D.; Meng, M.X.; Gao, L.B.; Liu, T.; Xu, Q.; Zeng, S. Permeation of astilbin and taxifolin in Caco-2 cell and their effects on the P-gp. Int. J. Pharm. 2009, 378, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ambudkar, S.V.; Dey, S.; Hrycyna, C.A.; Ramachandra, M.; Pastan, I.; Gottesman, M.M. Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Ann. Rev. Pharmacol. Toxicol. 1999, 39, 361–398. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.J.; Ferreira, M.J.; dos Santos, D.J. Molecular docking characterizes substrate-binding sites and efflux modulation mechanisms within P-glycoprotein. J. Chem. Inf. Model. 2013, 53, 1747–1760. [Google Scholar] [CrossRef] [PubMed]
- Scambia, G.; Ranelletti, F.O.; Panici, P.B.; De Vincenzo, R.; Bonanno, G.; Ferrandina, G.; Piantelli, M.; Bussa, S.; Rumi, C.; Cianfriglia, M.; et al. Quercetin potentiates the effect of adriamycin in a multidrug-resistant MCF-7 human breast-cancer cell line: P-glycoprotein as a possible target. Cancer Chemother. Pharmacol. 1994, 34, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Van Zanden, J.J.; Wortelboer, H.M.; Bijlsma, S.; Punt, A.; Usta, M.; Bladeren, P.J.; Rietjens, I.M.; Cnubben, N.H. Quantitative structure activity relationship studies on the flavonoid mediated inhibition of multidrug resistance proteins 1 and 2. Biochem. Pharmacol. 2005, 69, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Schutte, M.E.; Freidig, A.P.; van de Sandt, J.J.; Alink, G.M.; Rietjens, I.M.; Groten, J.P. An in vitro and in silico study on the flavonoid-mediated modulation of the transport of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) through Caco-2 monolayers. Toxicol. Appl. Pharmacol. 2006, 217, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Rotter, S.; Ladurner, A.; Heiss, E.H.; Oberlies, N.H.; Dirsch, V.M.; Atanasov, A.G. Silymarin constituents enhance ABCA1 Expression in THP-1 macrophages. Molecules 2016, 21, 55. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.C.; Chiou, M.H.; Teng, Y.N.; Hsieh, Y.W.; Huang, C.L.; Lane, H.Y. Functional impact of ABCB1 variants on interactions between P-glycoprotein and methadone. PLoS ONE 2013, 8, 59419. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.N.; Sheu, M.J.; Hsieh, Y.W.; Wang, R.Y.; Chiang, Y.C.; Hung, C.C. β-carotene reverses multidrug resistant cancer cells by selectively modulating human P-glycoprotein function. Phytomedicine 2016, 23, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.-N.; Chang, C.-S.; Lee, T.-E.; Hung, C.-C. Cordycepin re-sensitizes multidrug resistance cancer cells to chemotherapeutic agents through modulating P-glycoprotein expression and ATPase function. J. Funct. Foods 2016, 26, 681–690. [Google Scholar] [CrossRef]
- Sheu, M.J.; Teng, Y.N.; Chen, Y.Y.; Hung, C.C. The functional influences of common ABCB1 genetic variants on the inhibition of P-glycoprotein by Antrodia cinnamomea extracts. PLoS ONE 2014, 9, 89622. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-T.; Wang, C.C.N.; Wang, J.-Y.; Lee, T.-E.; Cheng, Y.-Y.; Morris-Natschke, S.L.; Lee, K.-H.; Hung, C.-C. Tenulin and isotenulin inhibit P-glycoprotein function and overcome multidrug resistance in cancer cells. Phytomedicine 2019, 53, 252–262. [Google Scholar] [CrossRef]
- Chou, T.C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |



| Nonlinear Kinetic Parameters | |||
|---|---|---|---|
| Vm (pmol/mg protein/10 min) | Km (μM) | ||
| Nonlinear regression | |||
| Rhodamine 123 only | 12.22 ± 1.95 | 25.30 ± 4.99 | |
| +taxifolin 5 μM | 5.27 ± 0.24 * | 10.93 ± 1.20 * | |
| +taxifolin 10 μM | 3.20 ± 0.37 * | 6.56 ± 1.53 * | |
| Ki from Lineweaver−Burk (μM) | 3.65 ± 0.15 | ||
| efflux IC50 (μM) | 4.45 ± 0.22 | ||
| Vm (pmol/mg protein/120 min) | Km (μM) | ||
| Nonlinear regression | |||
| Doxorubicin only | 48.38 ± 4.98 | 37.56 ± 4.89 | |
| +taxifolin 5 μM | 23.28 ± 2.63 * | 18.06 ± 3.23 * | |
| +taxifolin 10 μM | 16.57 ± 3.71 * | 13.72 ± 4.73 * | |
| Ki from Lineweaver−Burk (μM) | 5.19 ± 0.56 | ||
| efflux IC50 (μM) | 4.50 ± 0.31 | ||
| Chemotherapeutic Agent (nM) | Taxifolin (μM) | Fa a | CI b | Pharmacological Effect |
|---|---|---|---|---|
| Paclitaxel | ||||
| 1000 | 80 | 0.27 | 0.93 | Additive |
| 100 | 0.14 | 0.66 | Synergism | |
| 100 | 80 | 0.81 | 0.78 | Moderate synergism |
| 100 | 0.82 | 0.95 | Additive | |
| Vincristine | ||||
| 1000 | 80 | 0.58 | 0.85 | Moderate synergism |
| 100 | 0.37 | 0.56 | Synergism | |
| 100 | 80 | 0.87 | 0.78 | Moderate synergism |
| 100 | 0.89 | 1.00 | Additive | |
| Doxorubicin | ||||
| 1000 | 80 | 0.51 | 0.55 | Synergism |
| 100 | 0.41 | 0.36 | Synergism |
| PubChem CID | Chemical Names | -CDOCKER Energy (kcal/mole) |
|---|---|---|
| 712316 | (–)-Taxifolin | 32.12 |
| 2520 | Verapamil | 15.7402 |
| 5280343 | Quercetin | 28.62 |
| 9064 | Catechin | 28.16 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.-J.; Chung, Y.-L.; Li, C.-Y.; Chang, Y.-T.; Wang, C.C.N.; Lee, H.-Y.; Lin, H.-Y.; Hung, C.-C. Taxifolin Resensitizes Multidrug Resistance Cancer Cells via Uncompetitive Inhibition of P-Glycoprotein Function. Molecules 2018, 23, 3055. https://doi.org/10.3390/molecules23123055
Chen H-J, Chung Y-L, Li C-Y, Chang Y-T, Wang CCN, Lee H-Y, Lin H-Y, Hung C-C. Taxifolin Resensitizes Multidrug Resistance Cancer Cells via Uncompetitive Inhibition of P-Glycoprotein Function. Molecules. 2018; 23(12):3055. https://doi.org/10.3390/molecules23123055
Chicago/Turabian StyleChen, Hsiu-Ju, Yun-Lung Chung, Chia-Ying Li, Ying-Tzu Chang, Charles C. N. Wang, Hsiang-Yen Lee, Hui-Yi Lin, and Chin-Chuan Hung. 2018. "Taxifolin Resensitizes Multidrug Resistance Cancer Cells via Uncompetitive Inhibition of P-Glycoprotein Function" Molecules 23, no. 12: 3055. https://doi.org/10.3390/molecules23123055
APA StyleChen, H.-J., Chung, Y.-L., Li, C.-Y., Chang, Y.-T., Wang, C. C. N., Lee, H.-Y., Lin, H.-Y., & Hung, C.-C. (2018). Taxifolin Resensitizes Multidrug Resistance Cancer Cells via Uncompetitive Inhibition of P-Glycoprotein Function. Molecules, 23(12), 3055. https://doi.org/10.3390/molecules23123055






