An Example of a Novel Efficient Plant Extraction Technique: Electromagnetic Induction Heating
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Plant Material
3.2. Chemistry
3.3. Extraction Procedures
3.4. HPLC conditions and Method Validation
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bimakr, M.; Rahman, R.A.; Taip, F.S.; Ganjloo, A.; Salleh, L.M.; Selamat, J.; Hamid, A.; Zaidul, I.S.M. Comparison of different extraction methods for the extraction of major bioactive flavonoid compounds from spearmint (Mentha spicata L.) leaves. Food Bioprod. Process 2011, 89, 67–72. [Google Scholar] [CrossRef]
- Pan, G.; Yu, G.; Zhu, C.; Qiao, J. Optimization of ultrasound-assisted extraction (UAE) of flavonoids compounds (FC) from hawthorn seed (HS). Ultrason. Sonochem. 2012, 19, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Taddeo, V.A.; Epifano, F.; Fiorito, S.; Genovese, S. Comparison of different extraction methods and HPLC quantification of prenylated and unprenylated phenylpropanoids in raw Italian propolis. J. Pharm. Biomed. Anal. 2016, 129, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Xue, D.; Farid, M.M. Pulsed electric field extraction of valuable compounds from white button mushroom (Agaricus bisporus). Innov. Food Sci. Emerg. Technol. 2015, 29, 178–186. [Google Scholar] [CrossRef]
- Babovic, N.; Djilas, S.; Jadranin, M.; Vajs, V.; Ivanovic, J.; Petrovic, S.; Zizovic, I. Supercritical carbon dioxide extraction of antioxidant f ractions from selected Lamiaceae herbs and their antioxidant capacity. Innov. Food Sci. Emerg. Technol. 2010, 11, 98–107. [Google Scholar] [CrossRef]
- Viganò, J.; Zaboti Brumer, I.; de Campos Braga, P.A.; Kelly da Silva, J.; Marostica Junior, M.R.; Reyes Reyes, F.G.; Martinez, J. Pressurized liquids extraction as an alternative process to readily obtain bioactive compounds from passion fruit rinds. Food Bioprod. Process 2016, 100A, 382–390. [Google Scholar]
- Kremer, D.; Kosalec, I.; Locatelli, M.; Epifano, F.; Genovese, S.; Carlucci, G.; Zovko Koncic, M. Anthraquinone profiles, antioxidant and antimicrobial properties of Frangula rupestris (Scop.) Schur and Frangula alnus Mill. Bark. Food Chem. 2012, 131, 1174–1180. [Google Scholar] [CrossRef]
- Taddeo, V.A.; Genovese, S.; de Medina, P.; Palmisano, R.; Epifano, F.; Fiorito, S. Quantification of biologically active O-prenylated and unprenylated phenylpropanoids in dill (Anethum graveolens), anise (Pimpinella anisum), and wild celery (Angelica archangelica). J. Pharm. Biomed. Anal. 2017, 134, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Meng, X.; Guo, X.; Lan, Y.; Zhang, S. Thermal analysis during partial carbonizing process of rhubarb, moutan, and burnet. PLoS ONE 2017, 12, e0173946. [Google Scholar] [CrossRef] [PubMed]
- Wianowska, D. Hydrolytical instability of hydroxyanthraquinone glycosides in pressurized liquid extraction. Anal. Bioanal. Chem. 2014, 406, 3219–3227. [Google Scholar] [CrossRef] [PubMed]
- Zoumbia, Y.; Moulai-Mostefa, N. A new approach for pectin extraction: electromagnetic induction heating. Arab. J. Chem. 2017, 10, 480–487. [Google Scholar] [CrossRef]
- Megateli, S.; Krea, M. Enhancement of total phenolic and flavonoids extraction from Rosmarinus officinalis L. using electromagnetic induction heating (EMIH) process. Physiol. Mol. Biol. Plants 2018, 4, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Fiorito, S.; Epifano, F.; Palmisano, R.; Genovese, S.; Taddeo, V.A. A reinvestigation of the phytochemical composition of the edible herb Amaranthus retroflexus L. J. Pharm. Biomed. Anal. 2017, 143, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Armbruster, D.A.; Pry, T. Limit of blank, limit of detection, limit of quantitation. Clin. Biochem. Rev. 2008, 29, 49–52. [Google Scholar]
Sample Availability: Samples of plant extracts are available from the authors. |

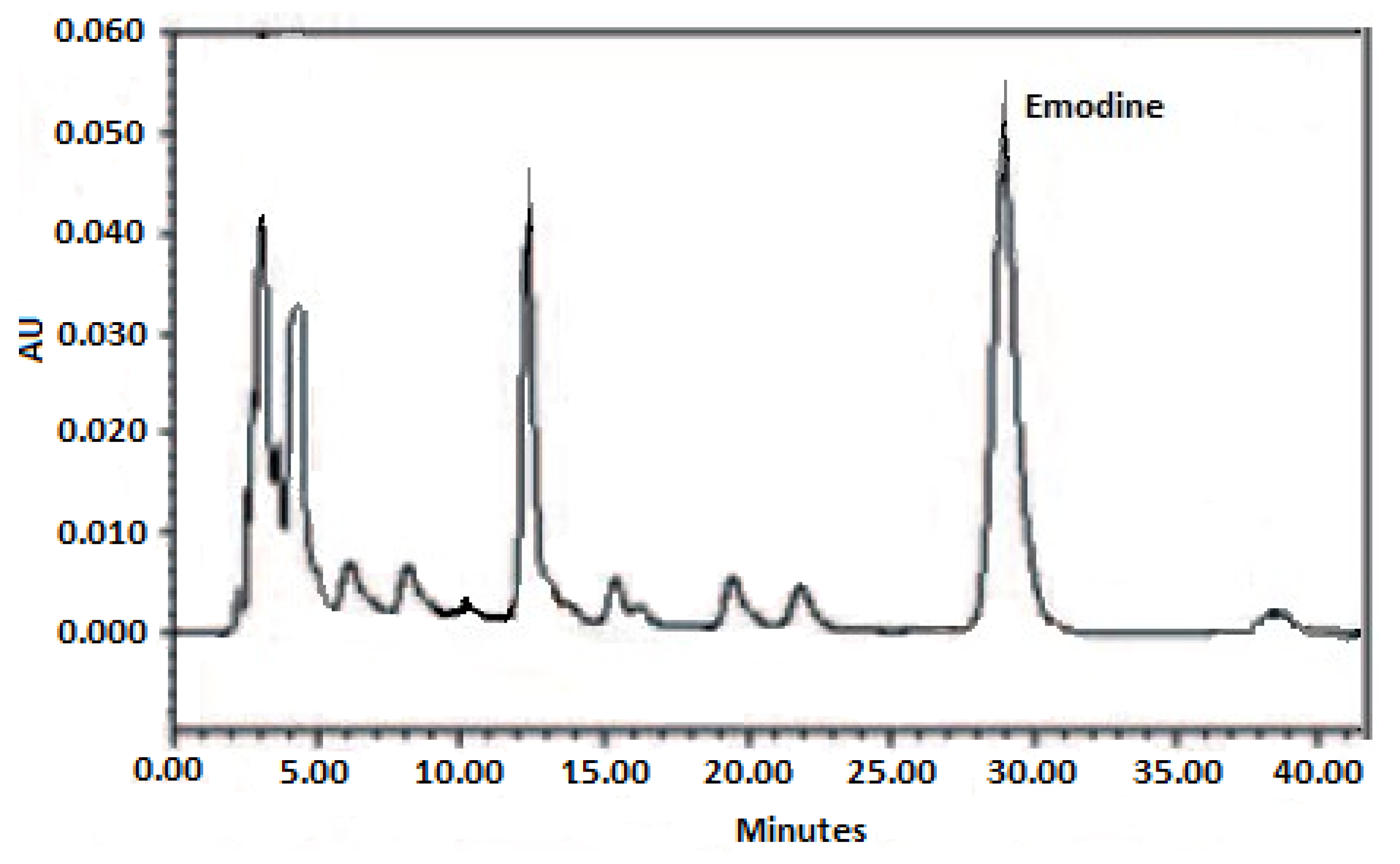
| Extraction Method * | Emodin Content (mg/g of Dry Weight) |
|---|---|
| A | 2.81 ± 0.05 |
| B | 3.46 ± 0.07 |
| C | 1.98 ± 0.12 |
| D | 2.01 ± 0.12 |
| E | 9.98 ± 0.12 |
| Extraction Method * | % Free Emodin Recorded |
|---|---|
| A | 0.44 ± 0.03 |
| B | 6.12 ± 0.06 |
| C | 1.44 ± 0.03 |
| D | 2.37 ± 0.04 |
| E | 0.88 ± 0.05 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Epifano, F.; Preziuso, F.; Taddeo, V.A.; Fiorito, S.; Genovese, S. An Example of a Novel Efficient Plant Extraction Technique: Electromagnetic Induction Heating. Molecules 2018, 23, 3048. https://doi.org/10.3390/molecules23113048
Epifano F, Preziuso F, Taddeo VA, Fiorito S, Genovese S. An Example of a Novel Efficient Plant Extraction Technique: Electromagnetic Induction Heating. Molecules. 2018; 23(11):3048. https://doi.org/10.3390/molecules23113048
Chicago/Turabian StyleEpifano, Francesco, Francesca Preziuso, Vito Alessandro Taddeo, Serena Fiorito, and Salvatore Genovese. 2018. "An Example of a Novel Efficient Plant Extraction Technique: Electromagnetic Induction Heating" Molecules 23, no. 11: 3048. https://doi.org/10.3390/molecules23113048
APA StyleEpifano, F., Preziuso, F., Taddeo, V. A., Fiorito, S., & Genovese, S. (2018). An Example of a Novel Efficient Plant Extraction Technique: Electromagnetic Induction Heating. Molecules, 23(11), 3048. https://doi.org/10.3390/molecules23113048








