1. Introduction
Hydrogen is regarded as a fuel of the future due to its vast abundance and the possibility of its sustainable production. However, hydrogen does not occur in Nature in its elemental form and thus hydrogen production requires the use of various conversion technologies. Methods of hydrogen production include fossil fuel reforming, coal gasification, plasma arc decomposition of fossil fuels, water electrolysis, photocatalysis, photo-electrolysis or dark fermentation [
1]. Among the above-mentioned methods, dark fermentation is believed to be the most promising method of hydrogen production from renewable energy sources as the net energy ratio for this method is equal to 1.9 [
2,
3].
Production of hydrogen via dark fermentation from various biomass feedstocks is widely reported in the literature [
4,
5,
6,
7,
8,
9,
10]. Dark fermentation enables a low energy input production of hydrogen from renewable feedstocks [
11]. Various lignocellulosic materials may serve as substrates for hydrogen production, including cornstalk, rice straw, wheat straw, grass, switchgrass, sugarcane bagasse,
Miscantus, energy willow, and energy poplar leaves [
12,
13,
14,
15,
16,
17,
18]. However, efficient fermentative hydrogen production from the abovementioned substrates requires their pre-treatment and efficient saccharification.
The need for lignocellulosic biomass pre-treatment prior to dark fermentation results primarily from the composition of lignocellulose. The complex chemical structure of the raw materials (
Table 1) necessitates the initial pre-treatment step, usually by means of chemical hydrolysis. The purpose of the pre-treatment is to destroy the structure of cellulosic biomass plant cell walls and to make cellulose and hemicellulose more accessible to the subsequent enzymatic hydrolysis process. In general, the plant cell wall is a heterogeneous mix of polymers that constitutes a matrix in which above mentioned carbohydrates are encapsulated. The performance of enzymatic hydrolysis of cellulose and hemicellulose in the cell wall is highly dependent on the pre-treatment operations. In this perspective, one of the most effective pre-treatment method is the alkaline pre-treatment which enhances the rate of enzymatic hydrolysis and the yield of TRS as fermentable sugars, including mainly glucose and xylose, released to the hydrolysate [
10]. Pre-treatment of lignocelllulosic biomass may be realized by means of various methods including physical, chemical, physicochemical and other methods, depending on the type of substrate and the resulting final products [
19,
20,
21].
Efficient fermentation of biomass must be preceded by at least two steps, i.e., biomass pre-treatment and saccharification. Saccharification may be realized e.g., either by acid or enzymatic hydrolysis [
20]. Enzymatic hydrolysis is carried out by highly specific cellulase enzymes to yield reducing sugars, including glucose [
23]. The rate of enzymatic hydrolysis of lignocellulosic residues is limited by many factors such as the degree of polymerization of the raw material, the moisture content, hemicellulose and lignin content or porosity of the raw material [
24,
25]. Thus, the efficiency of the enzymatic hydrolysis is primarily dependent on the results of pre-treatment operations. The main goals of pre-treatment are: (i) to achieve a high degradation of sugars (including those derived from hemicellulose), (ii) to minimize the formation of inhibitors for subsequent fermentation step, (iii) to remove lignin derivatives and possibly recover value-added products from hydrolysates, (iv) to reduce energy consumption [
19,
26]. Pre-treatment of raw materials should also provide a reduction of the cellulose crystallinity [
27] and consequently an increase of the contact surface between raw material and enzymes [
28,
29]. Various approaches towards pre-treatment of lignocellulosic materials is presented in
Table 2.
Studies on the direct conversion of lignocellulosic materials to H
2 exist, however most microorganisms require pre-treated biomass residues as substrates for dark fermentation [
30,
31,
32,
33,
34,
35,
36]. The degree of pretreatment depends on the nature of the raw material and on the type of inoculated microorganism [
37]. Pre-treatment has great improvement potential when the monosugar-releasing efficiency from cellulose and hemicellulose is considered. However, the pre-treatment also strongly influences the total process cost by reducing enzyme loading, mixing power, waste treatment demands and power generation [
38]. The conversion of cellulose and hemicellulose is catalysed by cellulases and hemicellulases, respectively. Cellulases are usually a mixture of several enzymes. The three predominant ones are: endo-1,4-β-glucanase, which hydrolyzes the inner β-1,4-glycosidic bonds; exo-1,4-β-glucanase, which removes glucose or cellobiose from the free chain-ends; and β-glucosidase (cellobiase), which hydrolyzes cellobiose [
25,
39,
40,
41]. Cellulose hydrolysis starts with adsorption of cellulase enzymes onto the surface of cellulose. Afterwards, cellulose is biodegraded to the fermentable sugars and cellulase is desorbed from the biomass surface.
Cellulose microfibers are surrounded by hemicellulose polysaccharides. Therefore, auxiliary enzymes that attack hemicellulose are also used in the saccharification process. In the first step, endo-1,4-β-xylanase depolymerizes xylan into xylooligosaccharides. Further, xylanases, such as β-glucuronidase, α-arabinofuranosidase, and acetyl xylan esterase, cleave side chains and side groups of heteroxylan, while galactomannanase and glucomannanase hydrolyze glucomannan [
12,
47]. The optimal balanced combination of enzymes is needed to effectively modify the complex structure of lignocellulosic materials.
The main advantages of enzymatic vs. acid hydrolysis of lignocellulosic biomass are the elimination of corrosion problems and mitigation of fermentation inhibitor formation, such as hydroxymethylfurfural (HMF), furan, furfural, levulinic acid and formic acid [
48,
49]. The drawbacks of enzymatic hydrolysis include high cost of enzymes, relatively long reaction times and the necessity to separate the hydrolysis products from the enzymes [
50]. Enzyme immobilization and recycling are solutions to both the mentioned problems [
51]. Enzyme immobilization enables the easy separation of the enzymes after the hydrolysis as well as their possible reuse. The recycling of Eupergit C-immobilized β-glucosidase revealed that the stability of enzymes was maintained during five successive rounds of enzymatic hydrolysis [
50,
51]. Among a number of possible carriers used for enzyme immobilization, after examining the present state of art, we decided to use diatomaceous earth (DE) for this purpose. The choice of DE as a carrier was determined by its following properties. It is a material of natural origin, relatively cheap and with a high content of silica. What’s more, it has a high porosity, developed surface, low density, low conduction coefficient and is chemically inert. Moreover, the enzyme can be immobilized on the DE surface either by adsorption or by covalent binding to the functional groups present on the DE surface. What was particularly interesting to our research team was the possibility of modifying the structure of DE leading to give them magnetic properties. This allows for effective removal of the immobilized enzyme from the bioreactor after its use with the use of magnetic biosorption technology [
52,
53]. In our opinion, in future studies, the magnetic DE nanoparticles technology gives an interesting opportunity for developing novel chemically modified DE carriers for enzymes immobilization.
Each step of biomass treatment affects its consecutive processing. The harsh pH conditions of chemical hydrolysis, for example, require further pH adjustment before the enzymatic hydrolysis and may also result in the formation of undesired products [
20,
54,
55,
56,
57]. Thus, the harsh conditions of acid pre-treatment may lead to the degradation of monomeric sugars into by-products such as 5-hydroxymethylfurfural or furfural, which may inhibit the fermentative hydrogen production [
10]. On the other hand, alkaline pre-treatment is mostly effective for low lignin content biomass [
19]. It is therefore reasonable to investigate other chemicals that may be of use for efficient biomass alkaline pre-treatment. Monoethanolamine (MEA), is an interesting example of a reagent for such a purpose. It maintains mild pH conditions during chemical hydrolysis and it is cost-effective if compared to hydrolysis with ammonia. MEA has been applied for the removal of lignin from woody biomass as a component of an ionic liquid [
58].
The aim of the present work is threefold: (i) to investigate the effectiveness and optimize the conditions of chemical hydrolysis of the terrestrial part of energetic poplar (EP) with MEA, (ii) to investigate the possibility of reusing MEA solution for further chemical hydrolysis, (iii) to investigate the possibility and resulting efficiency of recycling of the enzymes. The selection of the substrate (branches and trunk elements of energetic poplar) is justified by its popularity towards biofuels production, low soil and water requirements as well as relatively fast growth [
18,
59,
60].
2. Materials and Methods
2.1. Biomass Characterization
The energy poplar (EP) used in this study was obtained from a local producer (Wejherowo, Poland). The terrestrial part of the plant was used. The composition of EP was determined according to NREL procedures [
61,
62,
63,
64,
65].
Milled and minced biomass (garden shredder 425, Meec Tools, 729-162, Jula AB, Skara, Sweden and an Ultra Centrifugal Mill ZM 200 EP, RETCH, Verder Scientific, Dusseldorf, Germany) was sieved through a 0.75 mm screen. The material after the grinding was dried and stored at room temperature in sealed containers. Prior to the alkaline pre-treatment, the material was dried in a laboratory dryer at 105 °C for 4 h and then stored in a desiccator with NaOH flakes (drying agent).
2.2. Analytical Methods
Total solids, ash and extractives were determined according to the National Renewable Energy Laboratory (NREL) analytical procedures [
61,
62,
63,
64,
65]. The content of cellulose and hemicellulose was determined by HPLC with a Rezex Pb
2+ column (Phenomenex, California, CA, USA, 300 × 7.8 mm, 8 μm) and a refractometric detection (Knauer Smartline RID 2300, Berlin, Germany,). Water with a flow rate of 0.6 mL/min was used as the eluent. The EP composition was found to be 39.5% cellulose, 22.2% hemicellulose, 26.3% lignin, 0.1% ash, 3.5% water, 8.4% ethanol extractives, 6.4% water extractives.
The presence and the concentration of reducing monosugars and disugars (glucose, xylose, arabinose, mannose, galactose and cellobiose) were determined using HPLC with a Rezex Pb2+ column and a refractometric detector (1755 Refractive Index Monitor, Bio-Rad Model 1755). Mobile phase was water, flow: 0.6 mL/min, column temperature was set at t = 80 °C.
The volatile fatty acids and alcohols in the fermentation broth and fermentation inhibitors (furfural, 5-HMF, and levulinic acid) in the hydrolysates were determined using HPLC with a Shodex SH1011 (city, Japan) column and a refractometric detection (Knauer). Mobile phase was 5 mM H2SO4 solution, 0.6 mL/min flow, column temperature was set at t = 60 °C. Gaseous products of fermentation (H2 and CO2) were analyzed using a gas chromatograph (AutoSystem XL, Perkin-Elmer, Norwalk, CT, USA) equipped with a Porapak Q column (Sigma-Aldrich, Merck, Darmstadt, Germany), 100–120 mesh, 6.5 m × 1/8) and a thermal conductivity detector (TCD). Oven temperature was set at 60 °C. Turbochrom software was used for recording and processing of chromatograms. Gas samples were taken from the reactor during the lag, exponential and decline phases of culture growth.
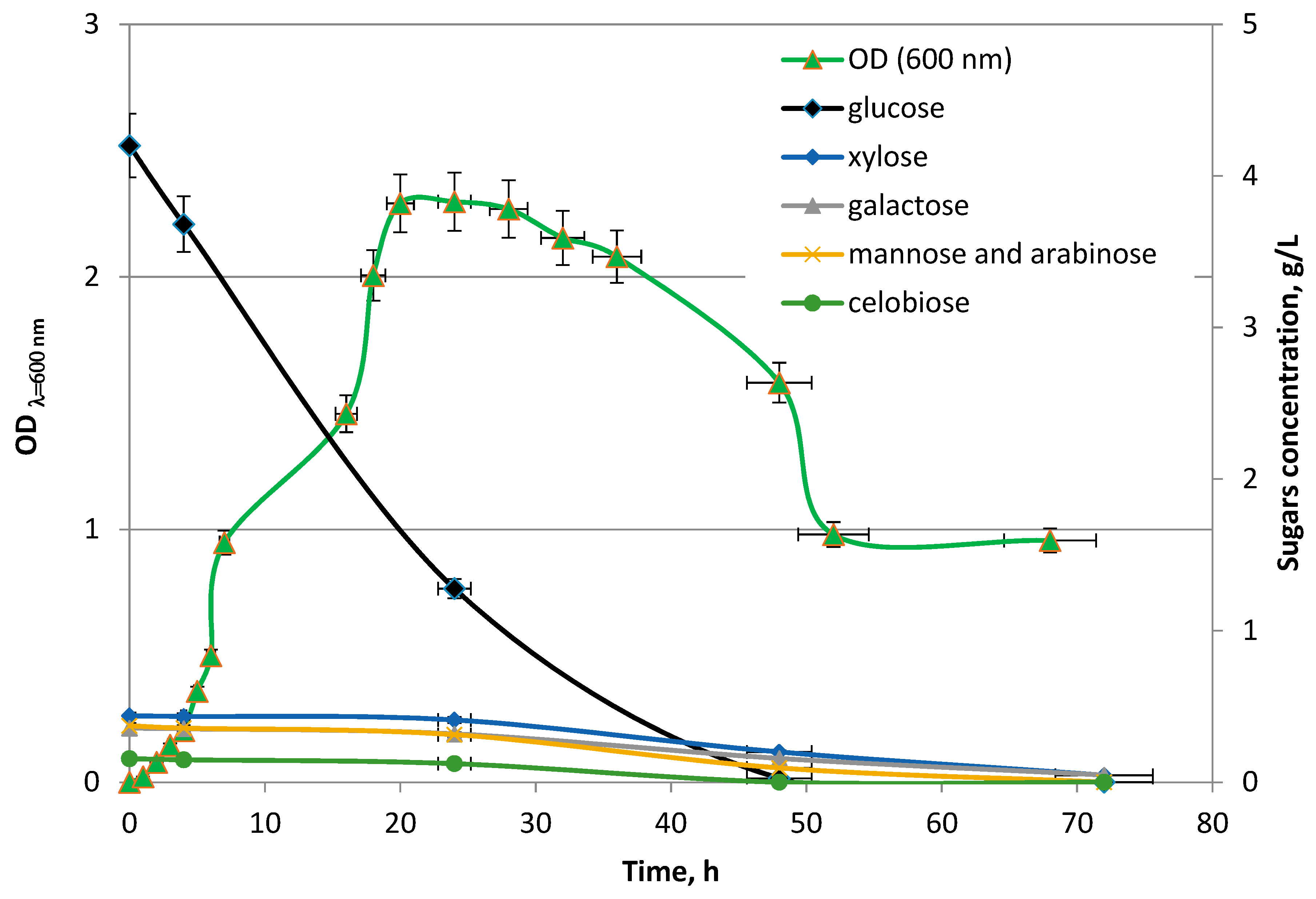
The pH was continuously monitored during the fermentation (Arduino Microcontroller data-logger, Continuous pH regulative system developed at Gdańsk University of Technology, Gdańsk, Poland). The growth of
E. aerogenes ATCC 13048 culture was monitored by measuring of OD
− at λ = 600 nm (optical density of culture). Concentration of total phenolic compounds was determined by UV-VIS. The calibration curve was prepared for vanillin [
66,
67,
68,
69,
70]. The absorbance measurement was made at λ = 280 nm. To obtain the sum of the phenolic compounds, the concentrations of furfural and 5-HMF (determined by HPLC) were subtracted from the concentrations determined by the UV-VIS method [
66,
67,
68,
69,
70].
2.3. Alkaline Pre-Treatment
Alkaline pre-treatment was carried with MEA solution. In this work, the influence of three variable process parameters (reaction time, MEA concentration, temperature) on the yield of reducing sugars obtained as a result of lignocellulosic biomass saccharification was examined by means of RSM (
Table 3).
During the alkaline pre-treatment, 20 mL of catalyst solution was used per each gram of lignocellulosic material. The reactions were carried out in 100 mL glass reactors. Details are listed in
Table 3. After the pre-treatment, the slurry was filtered through Buchner funnel to separate the solid fraction. The solid residue was washed three times by water and two times by acetone. In addition, the alkaline pre-treatment experiment was carried out with repeated use of the catalyst solution (threefold).
To this end, experiments were carried out using two approaches. The first consisted in the direct reuse of the catalyst solution for the pre-treatment of the new lignocellulosic feed (
Figure 1a.) while the second approach included an additional step of purifying the alkaline solution prior to reuse for further processing (
Figure 1b). For this purpose, activated carbon was used to remove chemical compounds from the catalyst solution.
2.4. Enzymatic Hydrolysis
After completion of the pre-treatment with MEA, the pre-treated EP samples were further processed using biochemical methods i.e., by enzymatic hydrolysis. In this study, high quality enzyme preparations for the hydrolysis of lignocellulosic residues were used. Enzyme immobilization on diatomite was applied, taking into account both the need for the removal of enzymes prior to fermentation and the possibility of immobilized enzyme separation by centrifugation [
55].
According to the manufacturer′s declaration, the supplementation of cellulolytic enzyme mixtures with an additional portion of β-glucosidase may increase the hydrolysis yield to monosaccharides, due to the fact, that cellobiose is an inhibitor of cellulolytic enzymes [
71]. Therefore, in this commercially available cellulolytic enzyme mixtures Viscozyme L (Novozymes Corp., Copenhagen, Denmark) supplemented with commercially available glucosidase (Sigma-Aldrich, Stockholm, Sweden) were used. The immobilized enzyme preparation consisted in placing 20 mL of enzymatic solution (Viscozyme L: glucosidase from
Aspergillus niger; 0.95:0.15
m/
m) in a 50 mL beaker with 2.5 g dry diatomite and stirring the solution for 1 h at low speed on a magnetic stirrer at room temperature. The diatomite with the immobilized enzyme was washed in a column with a small amount (about 10 mL) of McIlvaine’s buffer. The bed stabilized after 20 min. Next, the diluted diatomite was stored under a layer of buffer (about 5 mm).
Milled and minced EP after alkaline pre-treatment (0.2 g) was added to the flasks and supplemented with a suspension of cellulolytic enzymes immobilized on diatomite to 10 mL. The reaction flasks were incubated in a shaker at 42 °C for 24 h. Subsequently, samples were taken, the enzyme containing bed was separated by centrifugation and filtration, and the contents of monosaccharides and cellobiose in the supernatant solution were analyzed. Control experiments were carried without the addition of EP. For the use of the enzymatic hydrolyzing preparations, the immobilized bed was shaken and then introduced to the solid residue of lignocellulosic biomass solution after alkaline pre-treatment. To recover the enzymes after enzymatic hydrolysis, diatomite was separated from EP residues and double washed using McIlvaine’s buffer, and then dried for 24 h in 42 °C.
2.5. Design of Experiments
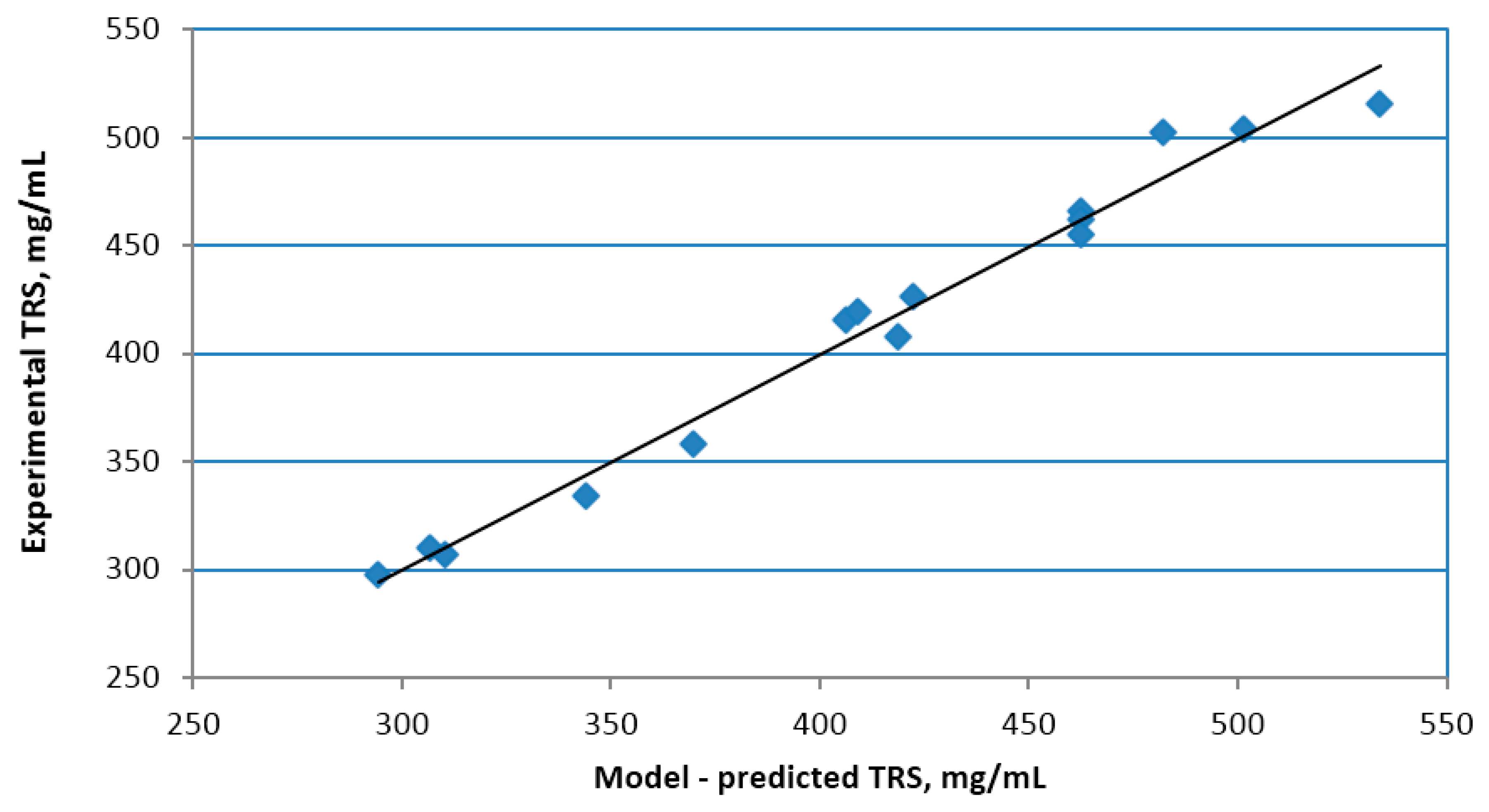
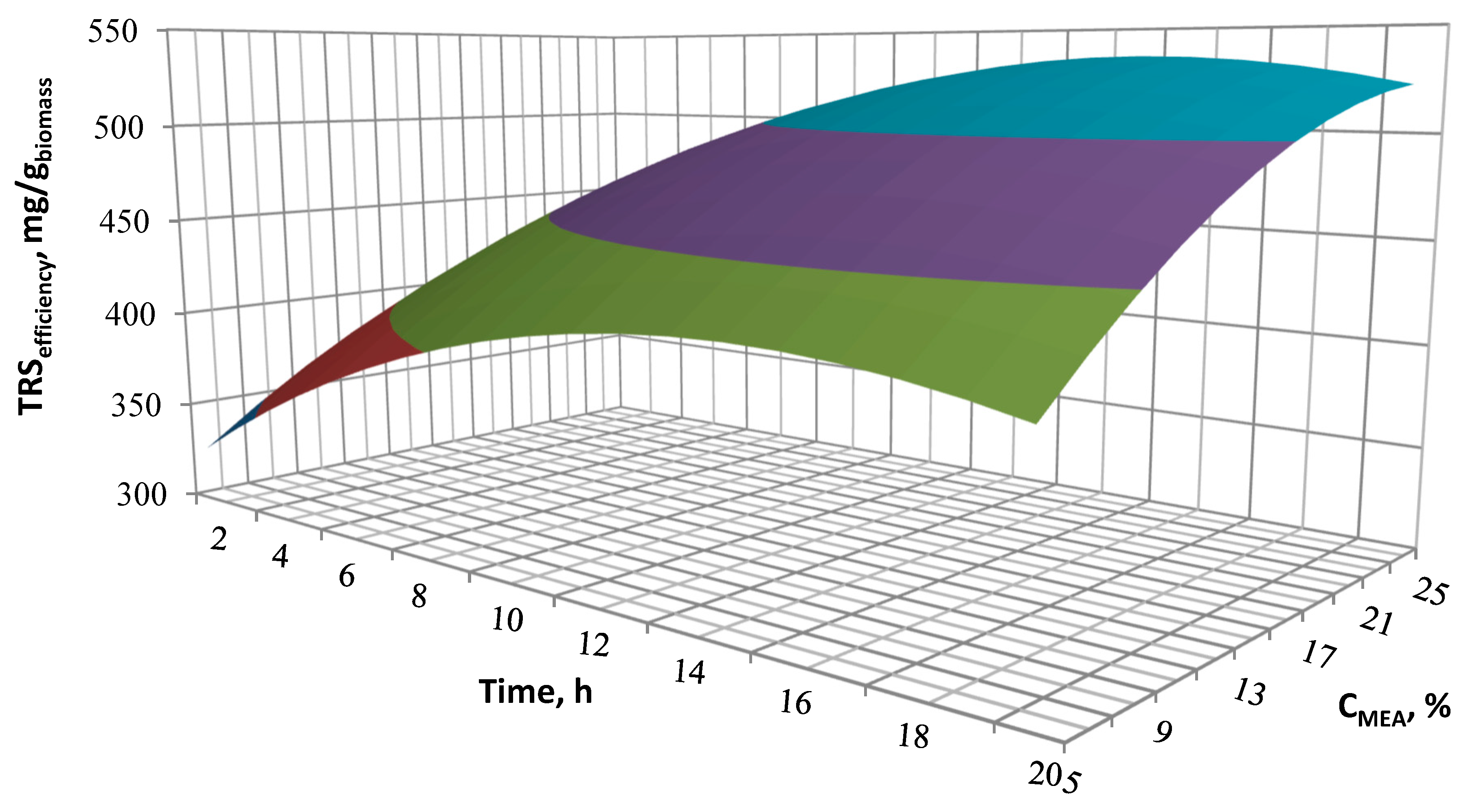
The response surface methodology (RSM) was used to determine the optimal alkaline pre-treatment conditions resulting in the highest total released sugar yield. Three variable parameters (temperature, MEA concentration, time) were selected to optimize the alkaline pre-treatment conditions. The research was carried out using the Box-Behnken design. The plan includes 15 experiments with different levels of three variables (
Table 2) used to determine the influence of each of the variable parameters and their mutual interactions on the TRS obtained from one gram of biomass. While using the Box-Behnken design, a high concentration of points in the optimal area is obtained. This allows for a correct selection of the most favorable process parameters. The authors wanted to avoid the situation where the corner points in the central composite design are very extreme, i.e., they are at the highest level of several factors. The R-studio [
72] software was used to determine and evaluate the coefficients of the regression model equation and their statistical significance. Box-Behnken designs are efficient designs for fitting second polynomials to response surfaces, because they use relatively small number of observations to estimate the parameters. Rotatability is a reasonable basis for the selection of a response surface design. The purpose is the optimisation when the location of the optimum is unkown, therefore it makes sense to use a design that provides equal precision of estimation in all directions. Experimental data was evaluated using three-dimensional diagrams showing the response surface area. RSM results are presented using MS Excel. In order to match the dependences of the parameter variables with the response value, the second order polynomial Equation (1) presented in the general form was applied:
YTRS (mg/gbiomass) is the expected value of TRS after two-stage alkaline pre-treatment and enzymatic hydrolysis, Xi (X1, X2, X3) describe values of independent variables of the alkaline pre-treatment process, catalyst concentration, temperature and time, respectively β0 is the intercept value, βi (β1, β2, β3) stand for linear coefficients, βij (β12, β23, β13) for coefficients of mutual interaction of parameters, βii (β11, β22, β33) for squared linear coefficients.
2.6. Dark Fermentation
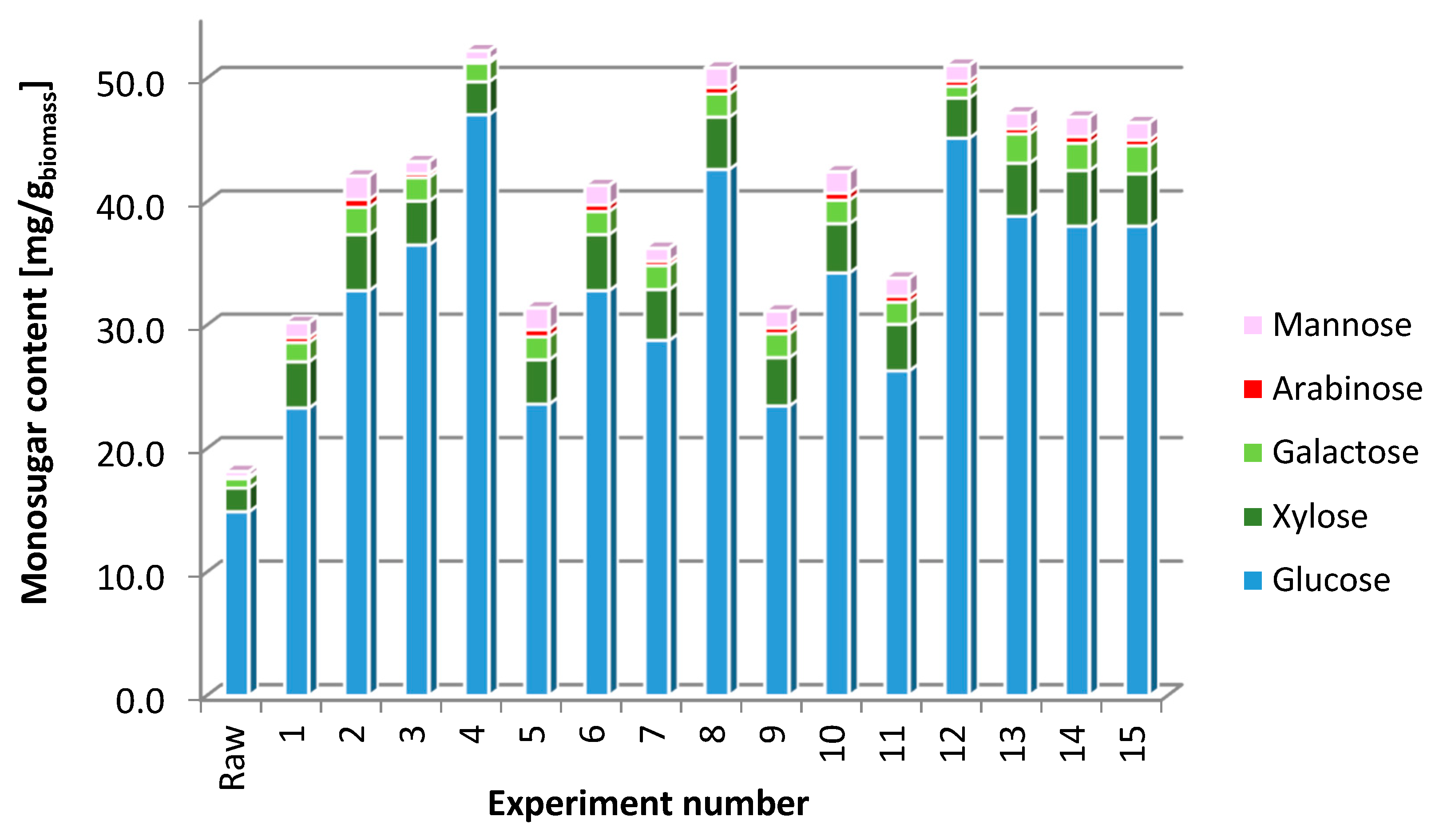
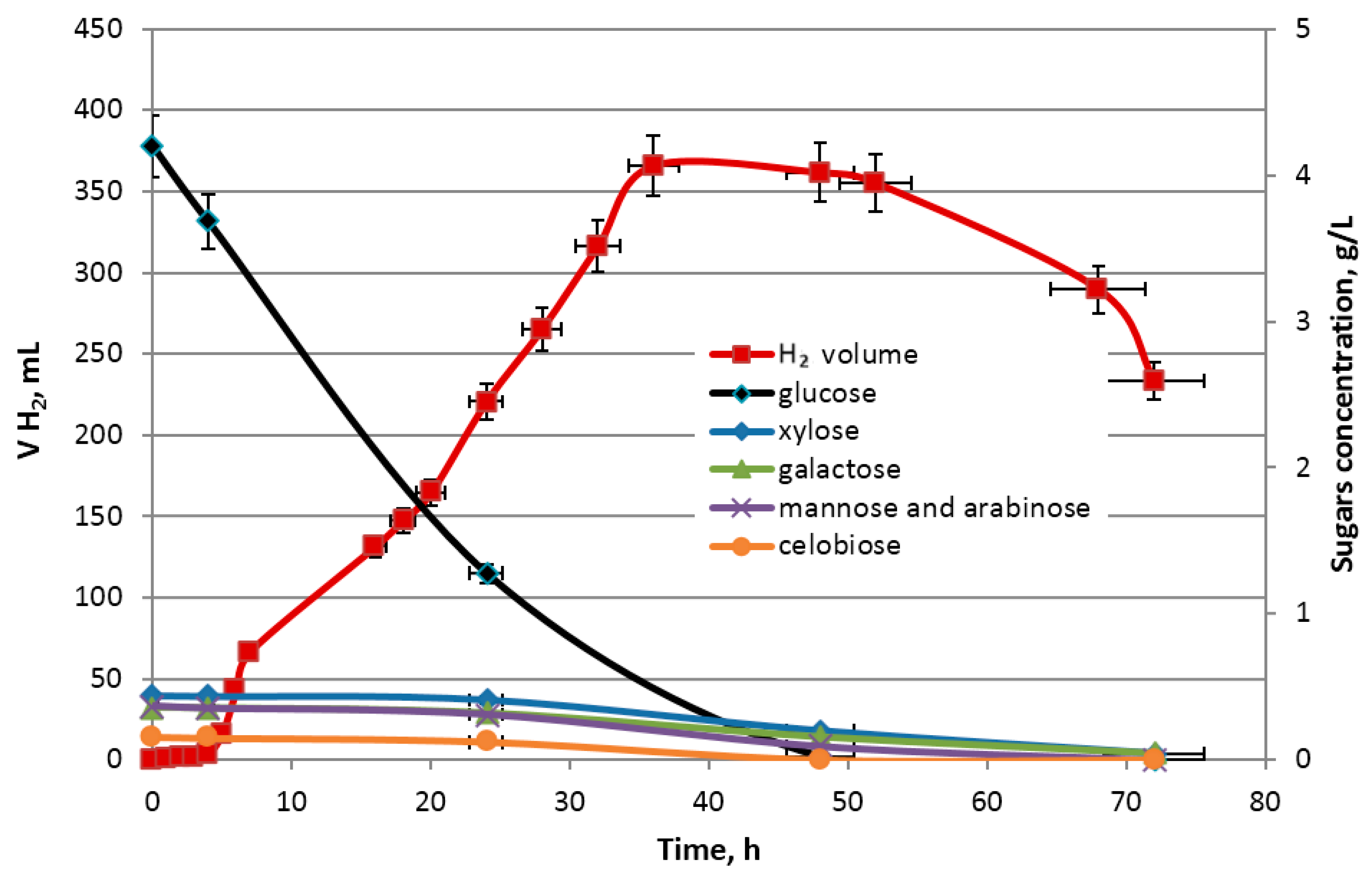
Enterobacter aerogenes ATCC 13048 (Selectrol TCS Biosciences Ltd., Buckingham, UK) was used as a model microorganism for hydrogen production in dark fermentation process. The dark fermentation was carried out in triplicate in sterile 1200 mL glass bioreactors with an initial working volume of 1 L fermentation broth and under regulated pH conditions. The initial fermentation broth was composed of an appropriate portion of a sterile minimal medium Buffered Peptone Water (Biomaxima, Gdańsk, Poland) and EP hydrolysate. The total sugar concentration (see
Table 4), utilized as a sole carbon source by
Enterobacter aerogenes ATCC 13048, in the initial fermentation broth was set as 5.5 g/L. The proportions of sugars in hydrolysates obtained during optimization are presented in the
Figure 2. For the purposes of fermentation, an optimal hydrolysate
Hopt was used. The sugar content in
Hopt is equal to 528.7 mg/g
biomass (including 462 mg/g
biomass glucose, 26,15 mg/g
biomass xylose, 14.97 mg/g
biomass galactose, 2,4 arabinose, 7,19 mg/g
biomass cellobiose). The initial pH of fermentation broth was adjusted to 7.00 with 1 M NaOH.
Next, each bioreactor was inoculated with 100 mL of pure bacterial cultures of Enterobacter aerogenes ATCC 13048 (inoculum OD600 = 2435) propagated in sterile Thioglycollate Broth Alternative (Biomaxima, Gdańsk, Poland) at 37 °C with an agitation (280 RPM). Before inoculation, anaerobic conditions for Enterobacter aerogenes growth in bioreactor were created by purging the reactors with sterile nitrogen gas for 60 min. Operational set-points were set at 37 °C and 320 RPM for temperature and agitation, respectively. The pH control system in bioreactor was connected with a peristaltic dosage pump dispensing a portion of 1 M NaOH when the pH drifted below the set-point value (7.00 ± 0.1). Experiments were carried for a process time of 72 h which corresponds to the late logarithmic death phase of Enterobacter aerogenes culture.
4. Conclusions
The object of this study was to explore the possibility of reusing both types of catalysts during energy willow hydrolysis. The authors found that in order for the technology to be profitable it was necessary to undertake attempts to return catalysts. During the experiments it turned out that the preferred directions of research may vary depending on the approach, mainly due to the very high potential of the obtained hydrolysates. In order to use the raw material efficiently, it is necessary to analyze the potential economics as well as energetic effects of the pre-treatment process. Consequently, for each biofuel production, optimization should be carried out, preferably in a two-step manner.
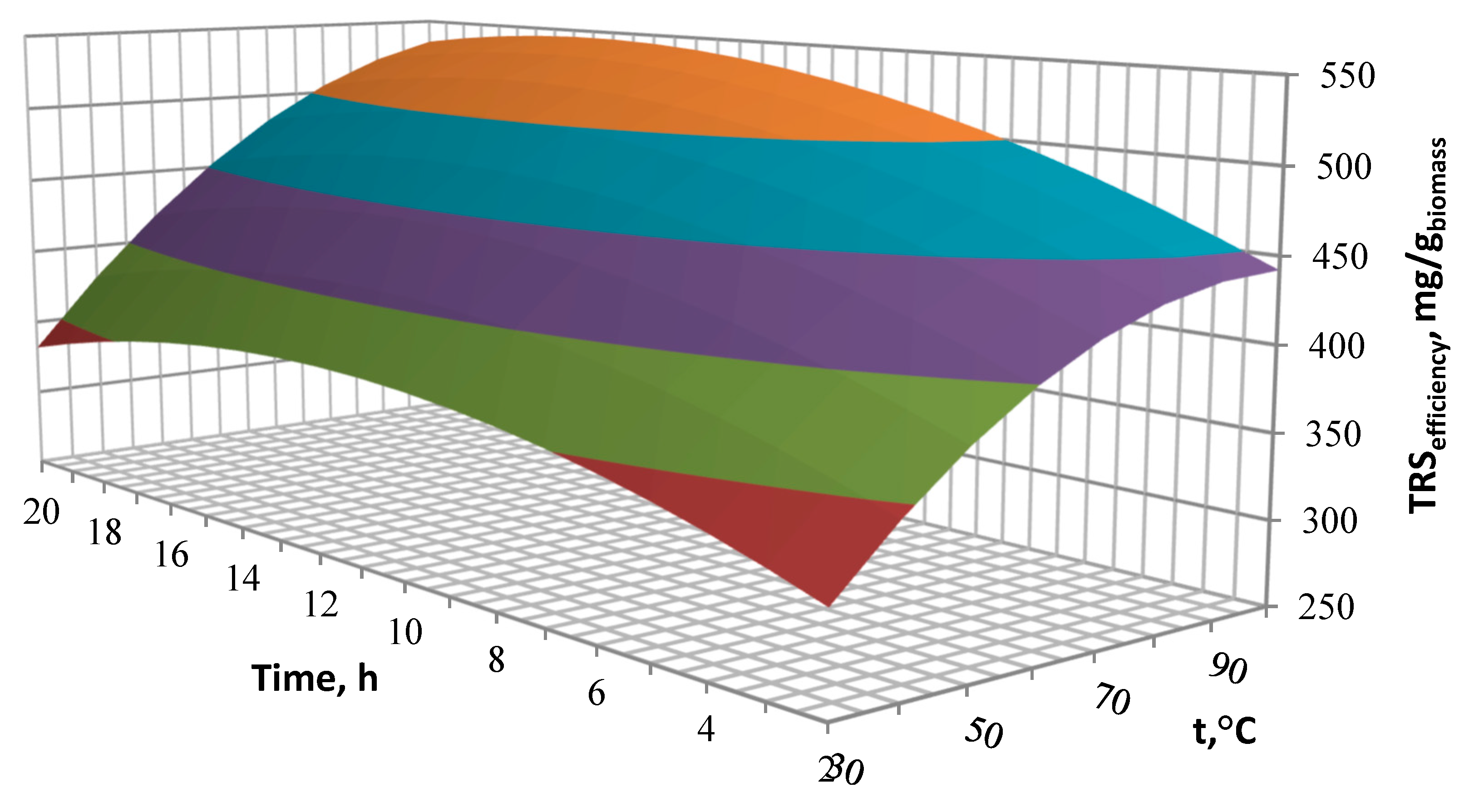
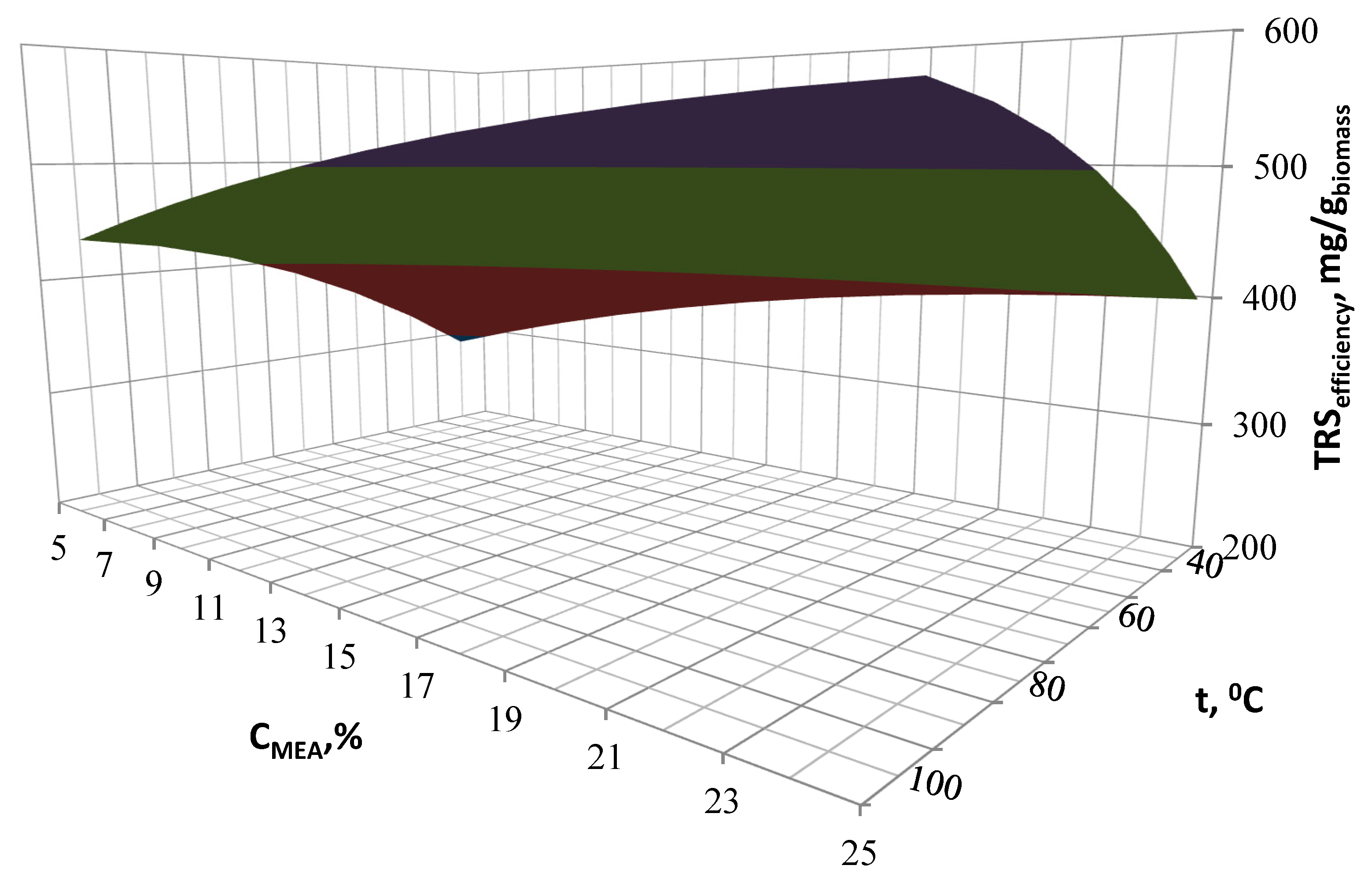
Biomass must undergo the saccharification process prior to its fermentation. The need of lignocellulosic biomass pre-treatment results primarily from its composition. The efficiency of the saccharification process depends on the degree of lignin removal obtained during the biomass pre-treatment. It was found that higher glucose concentration in the hydrolysates corresponds with a higher degree of lignin removal. Therefore, the pre-treatment has a great improvement potential when monosugars efficiency is considered. Both the increase in temperature and the catalyst concentration have an influence on the better efficiency of the cleavage of ester bonds linking lignin and hemicellulose. This affects the biomass structure and thus allows for a better access of enzymes to the digestion of cellulose. It is determined that the optimal parameters for twostep hydrolysis are as follows: MEA concentration 21%, temperature 90 °C and reaction time of 14 h. A total content of reducing sugars obtained in optimal hydrolysate is 528.7 mg/gbiomass. During the first step of pre-treatment, a wide matrix of value-added products may be obtained with high purity, as they are most commonly formed as a result of the transformation of lignin and hemicellulose derivatives. The changes occurring at this stage can and should definitely be an object of further research to make the process economically viable.
It is concluded that MEA reuse is possible, while the possibility of enzyme recovery must be further examined and developed, as it is a major part of the costs incurred in the multi-step biomass processing. As the approach concerning enzymatic hydrolysis increases the hydrolysis cost, a two–stage pre-treatment procedure is not economically profitable. On this premise, it is of no practical significance to study the optimization of technological conditions without taking into account the possibility of acquisition and purification of lignin derivatives. The enzymatic hydrolysis should be carried in a possibly simple matrix—after lignin and hemicelulose derivatives separation, as the presence of some chemical compounds may cause an inhibitory effect on the enzymes. Finally, the specific products, namely saccharides of cellulose and hemicellulose must be easily separated from other hydrolysis products in order to be used in bioconversion processes, as the presence of HMF or levulinic acid may inhibit the dark fermentation process.
The average amount of hydrogen generated during fermentation carried by
Enterobacter aerogenes ATCC 13048 in this study was equal to 0.6 mol H
2/mol TRS (22.99 mL H
2/g EP). The proposed approach of optimization of alkaline delignification, enzymatic saccharification, followed by dark fermentation allows to produce satisfying yields of hydrogen in a laboratory scale. The resulting amount of hydrogen is similar to the average results obtained in comparable studies described in the literature for hydrogen production by means of dark fermentation with the use of such complex media such as diary manure, rice winery wastewater, organic waste containing sucrose or xylose [
92,
98,
99,
100,
101,
102,
103].