Solvent Bar Micro-Extraction of Heavy Metals from Natural Water Samples Using 3-Hydroxy-2-Naphthoate-Based Ionic Liquids
Abstract
:1. Introduction
- (1)
- Based on earlier results [30], we evaluated the influence of organic additives, pH, extraction time, stirring rate and volume of ionic liquid on extraction efficacy and leaching. The parameters that achieved the most promising results were selected for subsequent experiments to:
- (2)
- Study the influence of different salinities on the simultaneous extraction of the four metals from synthetic samples.
- (3)
- Study the applicability for the extraction of the four metals from metal-spiked natural feed solutions, including drinking water, sea water, hypersaline water and a wastewater treatment plant effluent.
2. Results and Discussion
2.1. Influence of Physico-Chemical Properties on SBME
2.1.1. Organic Additives
2.1.2. Feed Solution pH
2.1.3. Time Dependence
2.1.4. Stirring Rate
2.1.5. Fiber Length
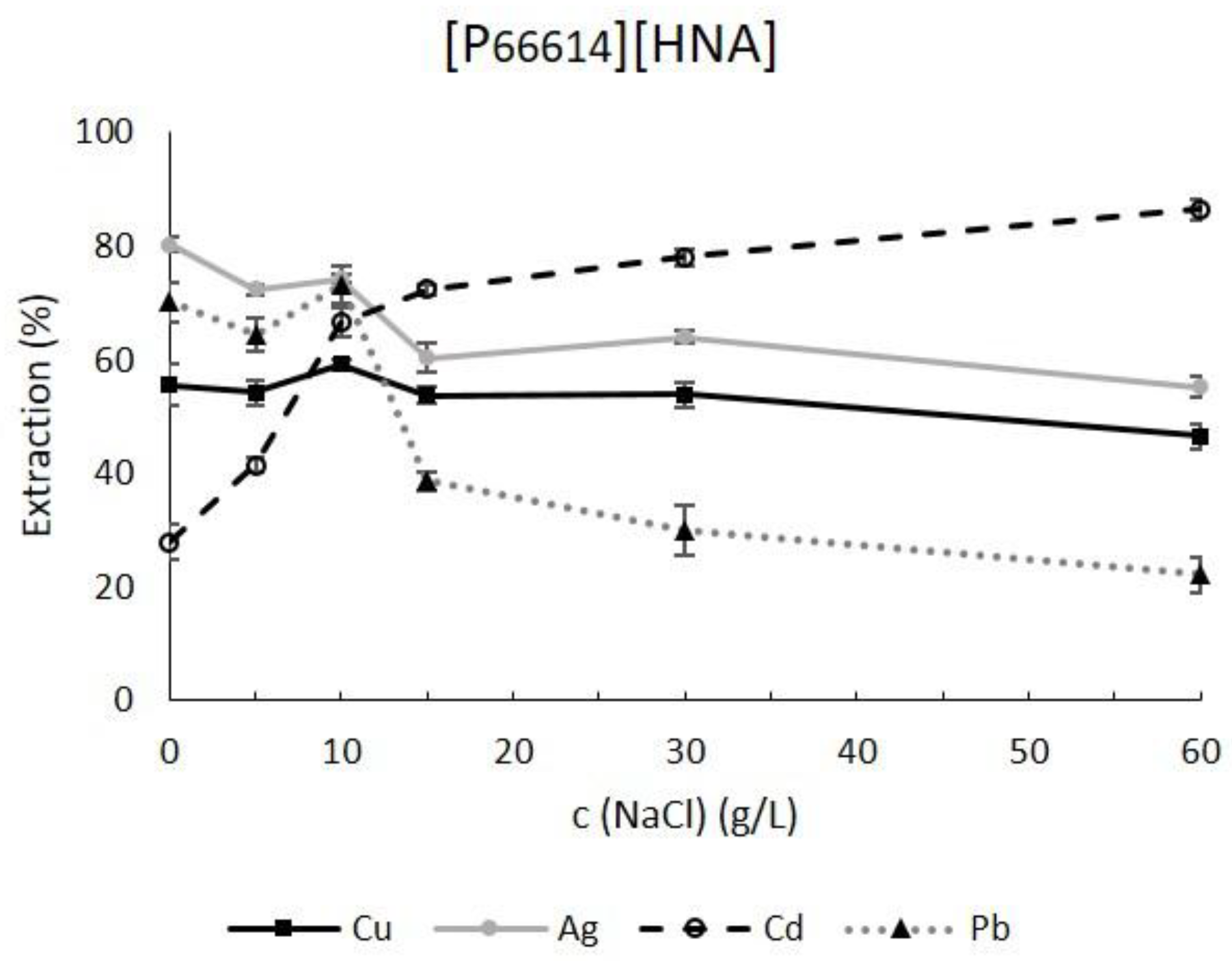
2.2. Synthetic Saline Samples
2.3. Applicability in Natural Water Samples
3. Materials and Methods
3.1. Solvents and Reagents
3.2. Instrumentation
3.3. Preparation of Solvent Bars (SB) and Setup of Extraction Experiments
3.4. Feed Solutions and Sample Conditions
3.4.1. Influence of Physico-Chemical Properties on SBME
Organic Additives
Feed Solution pH
Time Dependence
Stirring Rate
Fiber Length
3.4.2. Synthetic Saline Samples
3.4.3. Natural Water Samples
3.5. Quantification
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ahamed, M.; AlSalhi, M.S.; Siddiqui, M. Silver nanoparticle applications and human health. Clin. Chim. Acta 2010, 411, 1841–1848. [Google Scholar] [CrossRef] [PubMed]
- Plessl, C.; Jandrisits, P.; Krachler, R.; Keppler, B.K.; Jirsa, F. Heavy metals in the mallard Anas platyrhynchos from eastern Austria. Sci. Total Environ. 2017, 580, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Förstner, U.; Wittmann, G.T.W. Metal Pollution in the Aquatic Environment, 2nd ed.; Springer Science & Business Media: Heidelberg, Germany, 2012. [Google Scholar]
- Stojanovic, A.; Keppler, B.K. Ionic liquids as extracting agents for heavy metals. Sep. Sci. Technol. 2012, 47, 189–203. [Google Scholar] [CrossRef]
- Komjarova, I.; Blust, R. Comparison of liquid–liquid extraction, solid-phase extraction and co-precipitation preconcentration methods for the determination of cadmium, copper, nickel, lead and zinc in seawater. Anal. Chim. Acta 2006, 576, 221–228. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Trujillo-Rodríguez, M.J.; Pino, V.; Anderson, J.L. Non-conventional solvents in liquid phase microextraction and aqueous biphasic systems. J. Chromatogr. A 2017, 1500, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Dadfarnia, S.; Shabani, A.M.H. Recent development in liquid phase microextraction for determination of trace level concentration of metals—A review. Anal. Chim. Acta 2010, 658, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Rutkowska, M.; Dubalska, K.; Konieczka, P.; Namieśnik, J. Microextraction techniques used in the procedures for determining organomercury and organotin compounds in environmental samples. Molecules 2014, 19, 7581–7609. [Google Scholar] [CrossRef] [PubMed]
- Kokosa, J.M. Advances in solvent-microextraction techniques. Trends Analyt. Chem. 2013, 43, 2–13. [Google Scholar] [CrossRef]
- Pinto, J.J.; Martín, M.; Herce-Sesa, B.; López-López, J.A.; Moreno, C. Solvent bar micro-extraction: Improving hollow fiber liquid phase micro-extraction applicability in the determination of Ni in seawater samples. Talanta 2015, 142, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Trujillo-Rodríguez, M.J.; Rocío-Bautista, P.; Pino, V.; Afonso, A.M. Ionic liquids in dispersive liquid-liquid microextraction. Trends Analyt. Chem. 2013, 51, 87–106. [Google Scholar] [CrossRef]
- Zhao, H.; Xia, S.; Ma, P. Use of ionic liquids as ‘green’ solvents for extractions. J. Chem. Technol. Biotechnol. 2005, 80, 1089–1096. [Google Scholar] [CrossRef]
- Visser, A.E.; Swatloski, R.P.; Reichert, W.M.; Mayton, R.; Sheff, S.; Wierzbicki, A.; Davis, J.H., Jr.; Rogers, R.D. Task-specific ionic liquids for the extraction of metal ions from aqueous solutions. Chem. Commun. 2001, 135–136. [Google Scholar] [CrossRef]
- Leyma, R.; Platzer, S.; Jirsa, F.; Kandioller, W.; Krachler, R.; Keppler, B.K. Novel thiosalicylate-based ionic liquids for heavy metal extractions. J. Hazardous Mater. 2016, 314, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Platzer, S.; Kar, M.; Leyma, R.; Chib, S.; Roller, A.; Jirsa, F.; Krachler, R.; MacFarlane, D.R.; Kandioller, W.; Keppler, B.K. Task-specific thioglycolate ionic liquids for heavy metal extraction: synthesis, extraction efficacies and recycling properties. J. Hazardous Mater. 2017, 324, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Platzer, S.; Sap, O.; Leyma, R.; Wallner, G.; Jirsa, F.; Kandioller, W.; Krachler, R.; Keppler, B.K. Extraction of natural radionuclides from aqueous solutions by novel maltolate-based task-specific ionic liquids. J. Radioanal. Nucl. Chem. 2014. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, J.; Li, H.; Yang, M.; Zou, D.; Deng, Y.; Liu, Y. Applying basic research on a dialkylphosphoric acid based task-specific ionic liquid for the solvent extraction and membrane separation of yttrium. Sep. Sci. Technol. 2018, 207, 179–186. [Google Scholar] [CrossRef]
- Freire, M.G.; Carvalho, P.J.; Gardas, R.L.; Marrucho, I.M.; Santos, L.M.; Coutinho, J.A. Mutual solubilities of water and the [C(n)mim][Tf(2)N] hydrophobic ionic liquids. J. Phys. Chem. B 2008, 112, 1604–1610. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Qin, L.; Mu, T.; Xue, Z.; Gao, G. Are ionic liquids chemically stable? Chem. Rev. 2017, 117, 7113–7131. [Google Scholar] [CrossRef] [PubMed]
- Poole, C.F. Editorial on “liquid-phase microextraction with porous hollow fibers, a miniaturized and highly flexible format for liquid–liquid extraction” by s. Pedersen-Bjergaard and K.E. Rasmussen. J. Chromatogr. Coruña 2008, 1184, 131. [Google Scholar] [CrossRef] [PubMed]
- Kissoudi, M.; Samanidou, V. Recent advances in applications of ionic liquids in miniaturized microextraction techniques. Molecules 2018, 23, 1437. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Hu, L.; Lu, R.; Zhou, W.; Gao, H. Application of ionic liquids for liquid–liquid microextraction. Analyt. Methods 2013, 5, 5376–5385. [Google Scholar] [CrossRef]
- Pabby, A.K.; Sastre, A.M. State-of-the-art review on hollow fibre contactor technology and membrane-based extraction processes. J. Membr. Sci. 2013, 430, 263–303. [Google Scholar] [CrossRef]
- Herce-Sesa, B.; López-López, J.A.; Moreno, C. Ionic liquid solvent bar micro-extraction of CdCln(n-2)− species for ultra-trace Cd determination in seawater. Chemosphere 2017, 193, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Stanisz, E.; Werner, J.; Zgoła-Grześkowiak, A. Liquid-phase microextraction techniques based on ionic liquids for preconcentration and determination of metals. TrAC Trends Anal. Chem. 2014, 61, 54–66. [Google Scholar] [CrossRef]
- Jiang, X.; Lee, H.K. Solvent bar microextraction. Analyt. Chem. 2004, 76, 5591–5596. [Google Scholar] [CrossRef] [PubMed]
- Abulhassani, J.; Manzoori, J.L.; Amjadi, M. Hollow fiber based-liquid phase microextraction using ionic liquid solvent for preconcentration of lead and nickel from environmental and biological samples prior to determination by electrothermal atomic absorption spectrometry. J. Hazardous Mater. 2010, 176, 481–486. [Google Scholar] [CrossRef] [PubMed]
- López-López, J.A.; Herce-Sesa, B.; Moreno, C. Solvent bar micro-extraction with graphite atomic absorption spectrometry for the determination of silver in ocean water. Talanta 2016, 159, 117–121. [Google Scholar] [CrossRef] [PubMed]
- López-López, J.A.; Pirkwieser, P.; Leyma, R.; Kandioller, W.; Krachler, R.; Keppler, B.K.; Jirsa, F.; Moreno, C. Solvent bar micro-extraction for greener application of task specific ionic liquids in multi-elemental extraction. J. Clean. Prod. 2018, 201, 22–27. [Google Scholar] [CrossRef]
- Pirkwieser, P.; López-López, J.A.; Kandioller, W.; Keppler, B.K.; Moreno, C.; Jirsa, F. Novel 3-hydroxy-2-naphthoate-based task-specific ionic liquids for an efficient extraction of heavy metals. Front. Chem. 2018, 6, 172. [Google Scholar] [CrossRef] [PubMed]
- Sastry, N.V.; Valand, M.K. Viscosities and densities for heptane + 1-pentanol, +1-hexanol, +1-heptanol, +1-octanol, +1-decanol, and +1-dodecanol at 298.15 k and 308.15 k. J. Chem. Eng. Data 1996, 41, 1426–1428. [Google Scholar] [CrossRef]
- Egorov, V.M.; Djigailo, D.I.; Momotenko, D.S.; Chernyshov, D.V.; Torocheshnikova, I.I.; Smirnova, S.V.; Pletnev, I.V. Task-specific ionic liquid trioctylmethylammonium salicylate as extraction solvent for transition metal ions. Talanta 2010, 80, 1177–1182. [Google Scholar] [CrossRef] [PubMed]
- Platzer, S.; Leyma, R.; Wolske, S.; Kandioller, W.; Heid, E.; Schröder, C.; Schagerl, M.; Krachler, R.; Jirsa, F.; Keppler, B.K. Thioglycolate-based task-specific ionic liquids: Metal extraction abilities vs acute algal toxicity. J. Hazard. Mater. 2017, 340, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Janssen, C.H.; Macías-Ruvalcaba, N.A.; Aguilar-Martínez, M.; Kobrak, M.N. Metal extraction to ionic liquids: the relationship between structure, mechanism and application. Int. Rev. Phys. Chem. 2015, 34, 591–622. [Google Scholar] [CrossRef]
- Dietz, M.L.; Dzielawa, J.A.; Laszak, I.; Young, B.A.; Jensen, M.P. Influence of solvent structural variations on the mechanism of facilitated ion transfer into room-temperature ionic liquids. Green Chem. 2003, 5, 682–685. [Google Scholar] [CrossRef]
- Diabate, P.; Dupont, L.; Boudesocque, S.; Mohamadou, A. Novel task specific ionic liquids to remove heavy metals from aqueous effluents. Metals 2018, 8, 412. [Google Scholar] [CrossRef]
- Barton, A.F. Alcohols with Water: Solubility Data Series; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Messadi, A.; Mohamadou, A.; Boudesocque, S.; Dupont, L.; Guillon, E. Task-specific ionic liquid with coordinating anion for heavy metal ion extraction: cation exchange versus ion-pair extraction. Sep. Purif. Technol. 2013, 107, 172–178. [Google Scholar] [CrossRef]
- Herrmann, R. Laura sigg und werner stumm: aquatische chemie. Eine einführung in die chemie wäßriger lösungen und in die chemie natürlicher Gewässer. Catena 1990, 17, 584. [Google Scholar] [CrossRef]
- Devi, N.B.; Nathsarma, K.C.; Chakravortty, V. Separation of divalent manganese and cobalt ions from sulphate solutions using sodium salts of d2ehpa, pc 88a and cyanex 272. Hydrometallurgy 2000, 54, 117–131. [Google Scholar] [CrossRef]
- Salgado, A. Recovery of zinc and manganese from spent alkaline batteries by liquid–liquid extraction with cyanex 272. J. Power Sources 2003, 115, 367–373. [Google Scholar] [CrossRef]
- Herce-Sesa, B.; López-López, J.A.; Pinto, J.J.; Moreno, C. Ionic liquid based solvent micro-extraction of Ag and Cd from saline and hyper-saline waters. Chem. Eng. J. 2017, 308, 649–655. [Google Scholar] [CrossRef]
- Bhatluri, K.K.; Manna, M.S.; Saha, P.; Ghoshal, A.K. Supported liquid membrane-based simultaneous separation of cadmium and lead from wastewater. J. Membr. Sci. 2014, 459, 256–263. [Google Scholar] [CrossRef]
- Fischer, L.; Falta, T.; Koellensperger, G.; Stojanovic, A.; Kogelnig, D.; Galanski, M.; Krachler, R.; Keppler, B.K.; Hann, S. Ionic liquids for extraction of metals and metal containing compounds from communal and industrial waste water. Water Res. 2011, 45, 4601–4614. [Google Scholar] [CrossRef] [PubMed]
- Byrne, R.H.; Kump, L.R.; Cantrell, K.J. The influence of temperature and ph on trace metal speciation in seawater. Mar. Chem. 1988, 25, 163–181. [Google Scholar] [CrossRef]
- Kerndorff, H.; Schnitzer, M. Sorption of metals on humic acid. Geochim. Cosmochim. Acta 1980, 44, 1701–1708. [Google Scholar] [CrossRef]
- Liu, A.; Gonzalez, R.D. Modeling adsorption of copper(ii), cadmium(ii) and lead(ii) on purified humic acid. Langmuir 2000, 16, 3902–3909. [Google Scholar] [CrossRef]
- Gunsolus, I.L.; Mousavi, M.P.; Hussein, K.; Bühlmann, P.; Haynes, C.L. Effects of humic and fulvic acids on silver nanoparticle stability, dissolution, and toxicity. Environ. Sci. Technol. 2015, 49, 8078–8086. [Google Scholar] [CrossRef] [PubMed]
- López-López, J.A.; Herce-Sesa, B.; Moreno, C. Three-phase solvent bar micro-extraction as an approach to silver ultra-traces speciation in estuarine water samples. Talanta 2015, 132, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Consorcio de Aguas de la Zona Gaditana. Available online: https://www.cazg.es/cicloagua.cfm?apartado=estaciones&op=ca&opcflash=5 (accessed on 18 October 2017).
Sample Availability: Samples of the compounds [P66614][HNA], [P1888][HNA] and [N1888][HNA] are available from the authors. |



| Sample | pH | NaCl (g L−1) | Conductivity (mS cm−1) | DOC (mg L−1) |
|---|---|---|---|---|
| Drinking water | 7.88 | 0.04 | 0.56 | 0.6 |
| WWTP effluent | 7.86 | 0.59 | 1.82 | 12.2 |
| Sea water | 8.06 | 36.4 | 42.9 | 2.2 |
| Hypersaline water | 8.17 | 55.9 | 55.9 | 9.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pirkwieser, P.; López-López, J.A.; Kandioller, W.; Keppler, B.K.; Moreno, C.; Jirsa, F. Solvent Bar Micro-Extraction of Heavy Metals from Natural Water Samples Using 3-Hydroxy-2-Naphthoate-Based Ionic Liquids. Molecules 2018, 23, 3011. https://doi.org/10.3390/molecules23113011
Pirkwieser P, López-López JA, Kandioller W, Keppler BK, Moreno C, Jirsa F. Solvent Bar Micro-Extraction of Heavy Metals from Natural Water Samples Using 3-Hydroxy-2-Naphthoate-Based Ionic Liquids. Molecules. 2018; 23(11):3011. https://doi.org/10.3390/molecules23113011
Chicago/Turabian StylePirkwieser, Philip, José A. López-López, Wolfgang Kandioller, Bernhard K. Keppler, Carlos Moreno, and Franz Jirsa. 2018. "Solvent Bar Micro-Extraction of Heavy Metals from Natural Water Samples Using 3-Hydroxy-2-Naphthoate-Based Ionic Liquids" Molecules 23, no. 11: 3011. https://doi.org/10.3390/molecules23113011
APA StylePirkwieser, P., López-López, J. A., Kandioller, W., Keppler, B. K., Moreno, C., & Jirsa, F. (2018). Solvent Bar Micro-Extraction of Heavy Metals from Natural Water Samples Using 3-Hydroxy-2-Naphthoate-Based Ionic Liquids. Molecules, 23(11), 3011. https://doi.org/10.3390/molecules23113011








