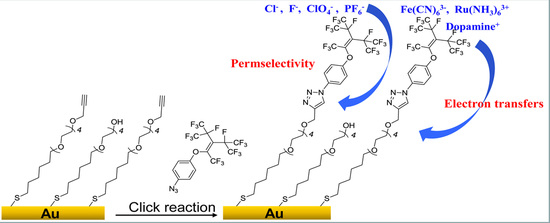
Electrochemistry Study of Permselectivity and Interfacial Electron Transfers of a Branch-Tailed Fluorosurfactant Self-Assembled Monolayer on Gold
Abstract
1. Introduction
2. Results
2.1. Characterization of the FS-SAM
2.2. Electrolyte Ion Permeability of the FS-SAM
2.3. Electrochemical Response of Hydrophilic Probes on the FS-SAM
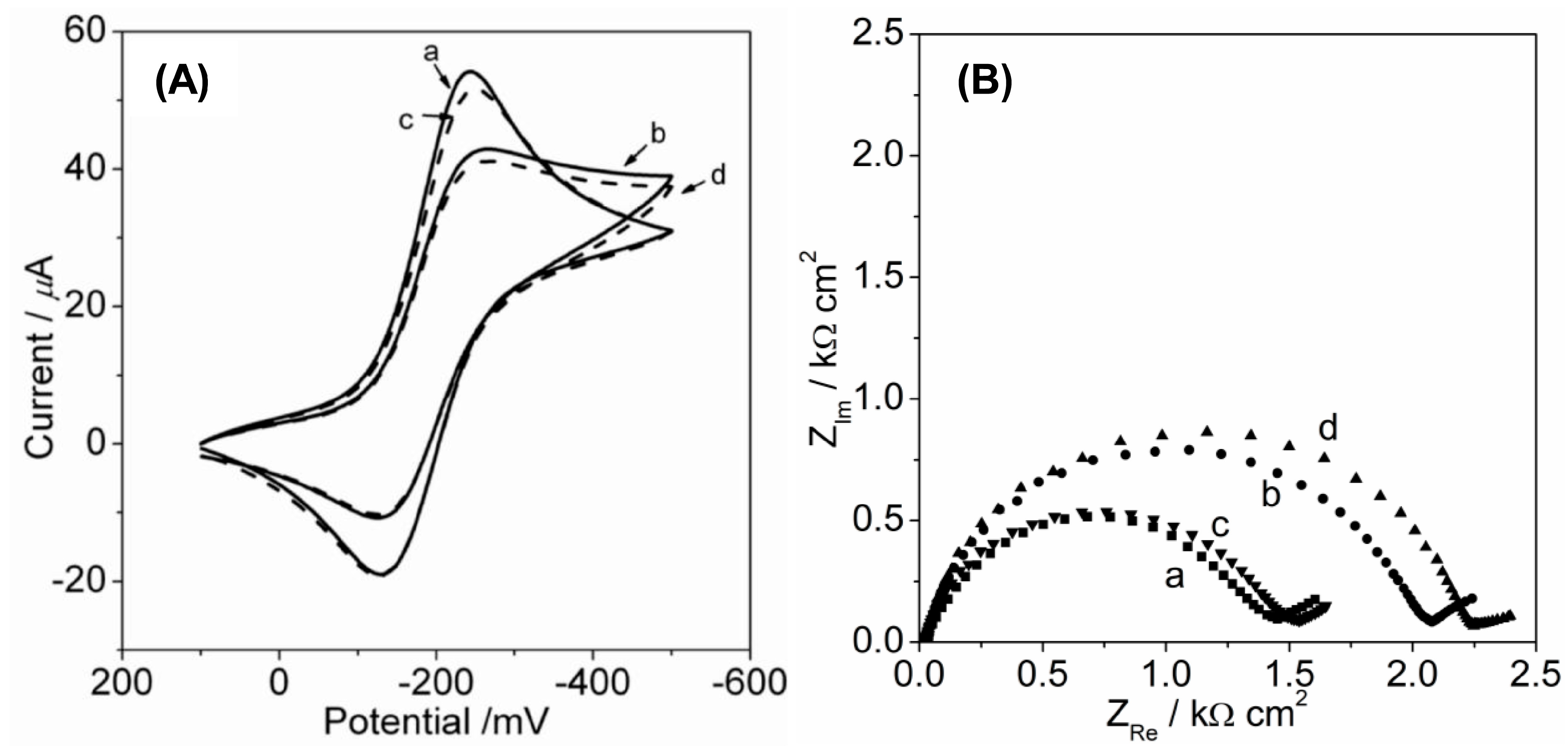
2.3.1. Fe(CN)63− Redox Reaction
2.3.2. Ru(NH3)63+ Redox Reaction
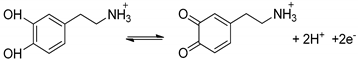
2.4. Electrochemical Response of Hydrophobic Dopamine+ on the FS-SAM
3. Materials and Methods
3.1. Materials
3.2. Fabrication of the FS-SAM
3.3. Surface Characterization
3.4. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zaggia, A.; Ameduri, B. Recent advances on synthesis of potentially non-bioaccumulable fluorinated surfactants. Curr. Opin. Colloid Interface Sci. 2012, 17, 188–195. [Google Scholar] [CrossRef]
- Buck, R.C.; Murphy, P.M.; Pabon, M. Chemistry, properties, and uses of commercial fluorinated surfactants. In Polyfluorinated Chemicals and Transformation Products; Knepper, T.P., Lange, F.T., Eds.; Springer: Heidelberg, Germany, 2012; Volume 17, pp. 1–24. [Google Scholar]
- Wang, L.; Zhang, L.; Lu, C. Applications in analytical chemistry using the attractive properties of non-ionic fluorosurfactants. TRAC-Trend Anal. Chem. 2014, 54, 45–55. [Google Scholar] [CrossRef]
- Brown, P.S.; Atkinson, O.D.L.A.; Badyal, J.P.S. Ultrafast oleophobic–hydrophilic switching surfaces for antifogging, self-cleaning, and oil–water separation. ACS Appl. Mater. Int. 2014, 6, 7504–7511. [Google Scholar] [CrossRef] [PubMed]
- Thebault, P.; de Givenchy, E.T.; Géribaldi, S.; Levy, R.; Vandenberghe, Y.; Guittard, F. Surface and antimicrobial properties of semi-fluorinated quaternary ammonium thiol surfactants potentially usable for self-assembled monolayers. J. Fluorine Chem. 2010, 131, 592–596. [Google Scholar] [CrossRef]
- Brown, P.S.; Bhushan, B. Mechanically durable, superoleophobic coatings prepared by layer-by-layer technique for anti-smudge and oil-water separation. Sci. Rep.-UK 2015, 5, 8701. [Google Scholar] [CrossRef] [PubMed]
- Vosgueritchian, M.; Lipomi, D.J.; Bao, Z. Highly conductive and transparent PEDOT: PSS films with a fluorosurfactant for stretchable and flexible transparent electrodes. Adv. Funct. Mater. 2012, 22, 421–428. [Google Scholar] [CrossRef]
- Li, Q.; Liu, F.; Lu, C.; Lin, J.M. Aminothiols sensing based on fluorosurfactant-mediated triangular gold nanoparticle-catalyzed luminol chemiluminescence. J. Phys. Chem. C 2011, 115, 10964–10970. [Google Scholar] [CrossRef]
- Tang, Y.; Yan, J.; Zhu, F.; Sun, C.; Mao, B. Comparative electrochemical scanning tunneling microscopy study of nonionic fluorosurfactant zonyl FSN self-assembled monolayers on Au (111) and Au (100): A potential-induced structural transition. Langmuir 2011, 27, 943–947. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Tang, Y.; Sun, C.; Su, Y.; Mao, B. STM study on nonionic fluorosurfactant zonyl FSN self-assembly on Au (100):(3−111) molecular lattice, corrugations, and adsorbate-enhanced mobility. Langmuir 2010, 26, 3829–3834. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Xu, G. Applications and trends in electrochemiluminescence. Chem. Soc. Rev. 2010, 39, 3275–3304. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zheng, H.; Lu, C.; Zu, Y. Oxidation of l-cysteine at a fluorosurfactant-modified gold electrode: Lower overpotential and higher selectivity. Langmuir 2007, 23, 10816–10822. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zu, Y. Electrogenerated chemiluminescence of the tris (2, 2′-bipyridine) ruthenium (II)/tertiary amine systems: Effects of electrode surface hydrophobicity on the low-oxidation-potential emission. J. Phys. Chem. C 2009, 113, 21877–21882. [Google Scholar] [CrossRef]
- Arvand, M.; Farahpour, M.; Ardaki, M.S. Electrochemical characterization of in situ functionalized gold organosulfur self-assembled monolayer with conducting polymer and carbon nanotubes for determination of rutin. Talanta 2018, 176, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.J.; Li, J.; Cao, T.Y.; Li, J.; Xia, X.H. Chain-length dependent interfacial immunoreaction kinetics on self-assembled monolayers revealed by surface-enhanced infrared absorption spectroscopy. Talanta 2018, 176, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Astruc, D. The copper (I)-catalyzed alkyne-azide cycloaddition (CuAAC) “click” reaction and its applications. An overview. Coord. Chem. Rev. 2011, 255, 2933–2945. [Google Scholar] [CrossRef]
- Wang, T.; Shannon, C. Electrochemical sensors based on molecularly imprinted polymers grafted onto gold electrodes using click chemistry. Anal. Chim. Acta 2011, 708, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Hayat, A.; Sassolas, A.; Marty, J.L.; Radi, A.E. Highly sensitive ochratoxin A impedimetric aptasensor based on the immobilization of azido-aptamer onto electrografted binary film via click chemistry. Talanta 2013, 103, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Gehan, H.; Fillaud, L.; Felidj, N.; Aubard, J.; Lang, P.; Chehimi, M.M.; Mangeney, C. A general approach combining diazonium salts and click chemistries for gold surface functionalization by nanoparticle assemblies. Langmuir 2009, 26, 3975–3980. [Google Scholar] [CrossRef] [PubMed]
- Ballav, N.; Terfort, A.; Zharnikov, M. Mixing of nonsubstituted and partly fluorinated alkanethiols in a binary self-assembled monolayer. J. Phys. Chem. C 2009, 113, 3697–3706. [Google Scholar] [CrossRef]
- Golub, M.A.; Lopata, E.S.; Finney, L.S. X-ray photoelectron spectroscopy study of the effect of hydrocarbon contamination on poly (tetrafluoroethylene) exposed to a nitrogen plasma. Langmuir 1993, 9, 2240–2242. [Google Scholar] [CrossRef]
- Gouget-Laemmel, A.C.; Yang, J.; Lodhi, M.A.; Siriwardena, A.; Aureau, D.; Boukherroub, R.; Szunerits, S. Functionalization of azide-terminated silicon surfaces with glycans using click chemistry: XPS and FTIR study. J. Phys. Chem. C 2012, 117, 368–375. [Google Scholar] [CrossRef]
- Li, S.; Yang, D.; Tu, H.; Deng, H.; Du, D.; Zhang, A. Protein adsorption and cell adhesion controlled by the surface chemistry of binary perfluoroalkyl/oligo (ethylene glycol) self-assembled monolayers. J. Colloid Interface Sci. 2013, 402, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, H.; Xiao, Y.; Ng, C.H.; Oh, T.S.; Tan, T.T.Y.; Ng, S.C. Preparation of cyclodextrin chiral stationary phases by organic soluble catalytic ‘click’ chemistry. Nat. Protoc. 2011, 6, 935. [Google Scholar] [CrossRef] [PubMed]
- Cassie, A.B.D. Discussions of the Faraday Society. Faraday Soc. 1948, 3, 11–16. [Google Scholar] [CrossRef]
- Israelachvili, J.N.; Gee, M.L. Contact angles on chemically heterogeneous surfaces. Langmuir 1989, 5, 288–289. [Google Scholar] [CrossRef]
- Xing, Y.F.; Li, S.F.Y.; Lau, A.K.H.; O’Shea, S.J. Electrochemical impedance spectroscopy study of mixed thiol monolayers on gold. J. Electroanal. Chem. 2005, 583, 124–132. [Google Scholar] [CrossRef]
- Cheng, Q.; Brajter-Toth, A. Permselectivity and high sensitivity at ultrathin monolayers. Effect of film hydrophobicity. Anal. Chem. 1995, 67, 2767–2775. [Google Scholar] [CrossRef]
- Huddleston, J.G.; Visser, A.E.; Reichert, W.M.; Willauer, H.D.; Broker, G.A.; Rogers, R.D. Characterization and comparison of hydrophilic and hydrophobic room temperature ionic liquids incorporating the imidazolium cation. Green Chem. 2001, 3, 156–164. [Google Scholar] [CrossRef]
- Valente, N.I.; Muteto, P.V.; Farinha, A.S.; Tomé, A.C.; Oliveira, J.A.; Gomes, M.T.S. An acoustic wave sensor for the hydrophilic fluoride. Sens. Actuators B 2011, 157, 594–599. [Google Scholar] [CrossRef]
- Xiong, Z.; Zhao, D.; Harper, W.F. Sorption and desorption of perchlorate with various classes of ion exchangers: A comparative study. Ind. Eng. Chem. Res. 2007, 46, 9213–9222. [Google Scholar] [CrossRef]
- Shao, Q.; Jiang, S. Molecular understanding and design of zwitterionic materials. Adv. Mater. 2015, 27, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Porter, M.D.; Bright, T.B.; Allara, D.L.; Chidsey, C.E.D. Spontaneously organized molecular assemblies. 4. Structural characterization of n-alkyl thiol monolayers on gold by optical ellipsometry, infrared Spectroscopy, and electrochemistry. J. Am. Chem. Soc. 1987, 109, 3559–3568. [Google Scholar] [CrossRef]
- Ortiz, B.; Saby, C.; Champagne, G.Y.; Bélanger, D. Electrochemical modification of a carbon electrode using aromatic diazonium salts. 2. Electrochemistry of 4-nitrophenyl modified glassy carbon electrodes in aqueous media. J. Electroanal. Chem. 1998, 455, 75–81. [Google Scholar] [CrossRef]
- Dicke, C.; Hähner, G. Interaction between a hydrophobic probe and tri(ethylene glycol)-containing self-assembled monolayers on gold studied with force spectroscopy in aqueous electrolyte solution. J. Phys. Chem. B 2002, 106, 4450–4456. [Google Scholar] [CrossRef]
- Dicke, C.; Hähner, G. pH-Dependent force spectroscopy of tri(ethylene glycol)- and methyl-terminated self-assembled monolayers adsorbed on gold. J. Am. Chem. Soc. 2002, 124, 12619–12625. [Google Scholar] [CrossRef] [PubMed]
- Petrov, E.G.; May, V. A unified description of superexchange and sequential donor—Acceptor electron transfer mediated by a molecular bridge. J. Phys. Chem. A 2001, 105, 10176–10186. [Google Scholar] [CrossRef]
- Kim, J.; Bard, A.J. Electrodeposition of single nanometer-size Pt nanoparticles at a tunneling ultramicroelectrode and determination of fast heterogeneous kinetics for Ru(NH3)63+ reduction. J. Am. Chem. Soc. 2016, 138, 975–979. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Fryling, M.A.; McCreery, R.L. Electron transfer kinetics at modified carbon electrode surfaces: The role of specific surface sites. Anal. Chem. 1995, 67, 3115–3122. [Google Scholar] [CrossRef]
- Fleischmann, M.; Graves, P.R.; Robinson, J. The raman spectroscopy of the ferricyanide/ferrocyanide system at gold, β-palladium hydride and platinum electrodes. J. Electroanal. Chem. Interfacial Electrochem. 1985, 182, 87–98. [Google Scholar] [CrossRef]
- Malem, F.; Mandler, D. Self-assembled monolayers in electroanalytical chemistry: Application of. omega.-mercapto carboxylic acid monolayers for the electrochemical detection of dopamine in the presence of a high concentration of ascorbic acid. Anal. Chem. 1993, 65, 37–41. [Google Scholar] [CrossRef]
- Yang, L.; Wei, W.; Xia, J.; Tao, H.; Yang, P. Electrochemical studies of derivatized thiol self-assembled monolayers on gold electrode in the presence of surfactants. Anal. Sci. 2005, 21, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Finklea, H.O.; Snider, D.A.; Fedyk, J.; Sabatani, E.; Gafni, Y.; Rubinstein, I. Characterization of octadecanethiol-coated gold electrodes as microarray electrodes by cyclic voltammetry and ac impedance spectroscopy. Langmuir 1993, 9, 3660–3667. [Google Scholar] [CrossRef]
- Ganesh, V.; Pal, S.K.; Kumar, S.; Lakshminarayanan, V. Self-assembled monolayers (SAMs) of alkoxycyanobiphenyl thiols on gold-A study of electron transfer reaction using cyclic voltammetry and electrochemical impedance spectroscopy. J. Colloid Interface Sci. 2006, 296, 195–203. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |



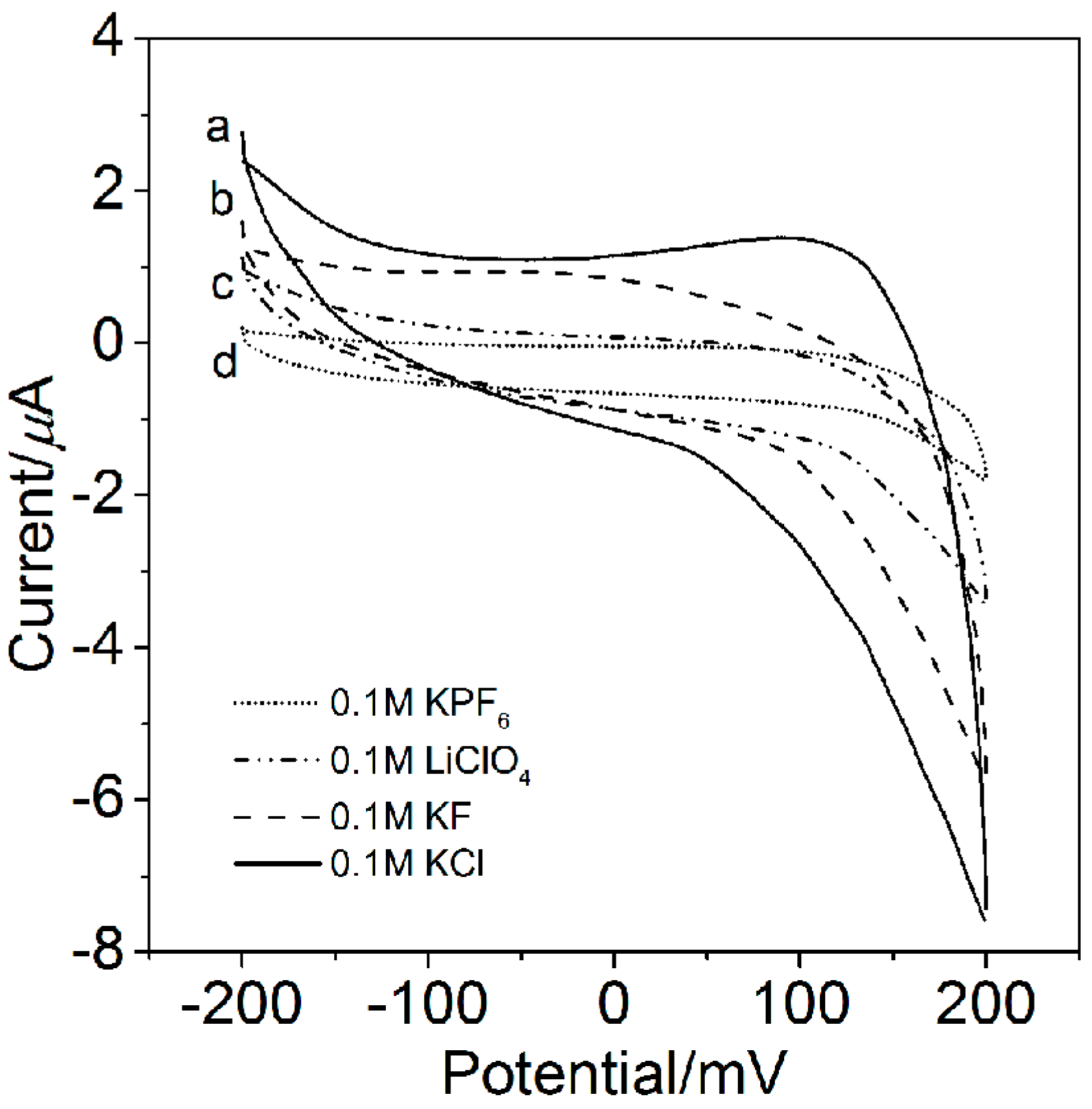



| Electrolyte | 0.1 M KCl | 0.1 M KF | 0.1 M LiClO4 | 0.1 M KPF6 |
|---|---|---|---|---|
| C (μF/cm2) | 0.73 ± 0.03 | 0.55 ± 0.04 | 0.25 ± 0.01 | 0.22 ± 0.06 |
| Probe | Electrode | Electrolyte | ΔEp/mV | Rct/Ω cm2 | k0/cm·s−1 |
|---|---|---|---|---|---|
| Fe(CN)63− | Binary OEG-SAM | 0.1M KCl | 391 | 6359 | 0.42 |
| 0.1 M KPF6 | 602 | 9374 | 0.28 | ||
| FS-SAM | 0.1M KCl | — | 15681 | 0.17 | |
| 0.1 M KPF6 | — | 58139 | 0.05 |
| Probe | Electrode | Electrolyte | ΔEp/mV | Rct/Ω cm2 | k0/cm·s−1 |
|---|---|---|---|---|---|
| Ru(NH3)63+ | Binary OEG-SAM | 0.1 M KCl | 114 | 1497 | 1.78 |
| 0.1 M KPF6 | 131 | 2064 | 1.29 | ||
| FS-SAM | 0.1 M KCl | 126 | 1524 | 1.75 | |
| 0.1 M KPF6 | 138 | 2196 | 1.22 |
| Electrode | Electrolyte | Ep,a/mV |
|---|---|---|
| Bare Au | 0.1 M KCl | 174 |
| Binary OEG-SAM | 0.1 M KCl | 347 |
| 0.1 KPF6 | 539 | |
| FS-SAM | 0.1 M KCl | — |
| 0.1 KPF6 | — |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Luo, Q.; Zhang, Z.; Shen, G.; Wu, H.; Chen, A.; Liu, X.; Li, M.; Zhang, A. Electrochemistry Study of Permselectivity and Interfacial Electron Transfers of a Branch-Tailed Fluorosurfactant Self-Assembled Monolayer on Gold. Molecules 2018, 23, 2998. https://doi.org/10.3390/molecules23112998
Li S, Luo Q, Zhang Z, Shen G, Wu H, Chen A, Liu X, Li M, Zhang A. Electrochemistry Study of Permselectivity and Interfacial Electron Transfers of a Branch-Tailed Fluorosurfactant Self-Assembled Monolayer on Gold. Molecules. 2018; 23(11):2998. https://doi.org/10.3390/molecules23112998
Chicago/Turabian StyleLi, Shanshan, Qingying Luo, Zhiqing Zhang, Guanghui Shen, Hejun Wu, Anjun Chen, Xingyan Liu, Meiliang Li, and Aidong Zhang. 2018. "Electrochemistry Study of Permselectivity and Interfacial Electron Transfers of a Branch-Tailed Fluorosurfactant Self-Assembled Monolayer on Gold" Molecules 23, no. 11: 2998. https://doi.org/10.3390/molecules23112998
APA StyleLi, S., Luo, Q., Zhang, Z., Shen, G., Wu, H., Chen, A., Liu, X., Li, M., & Zhang, A. (2018). Electrochemistry Study of Permselectivity and Interfacial Electron Transfers of a Branch-Tailed Fluorosurfactant Self-Assembled Monolayer on Gold. Molecules, 23(11), 2998. https://doi.org/10.3390/molecules23112998