Purification, Preliminary Structure and Antitumor Activity of Exopolysaccharide Produced by Streptococcus thermophilus CH9
Abstract
:1. Introduction
2. Results and Discussion
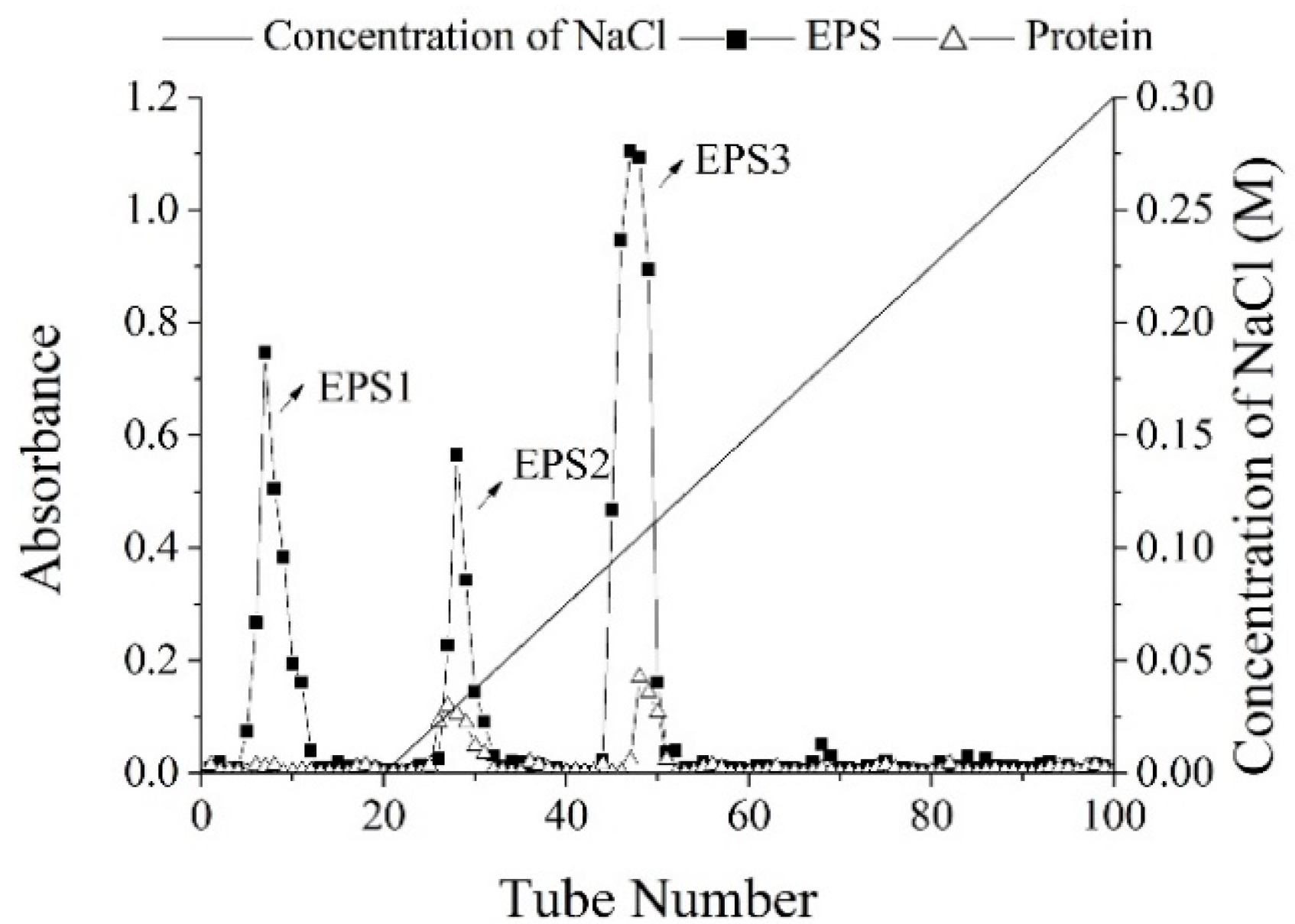
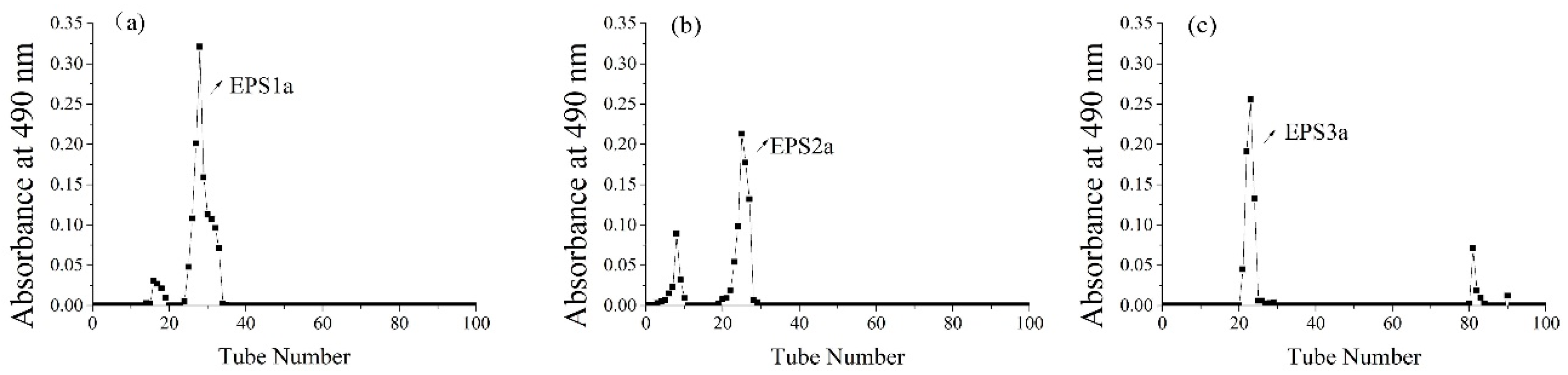
2.1. Isolation and Purification of EPS
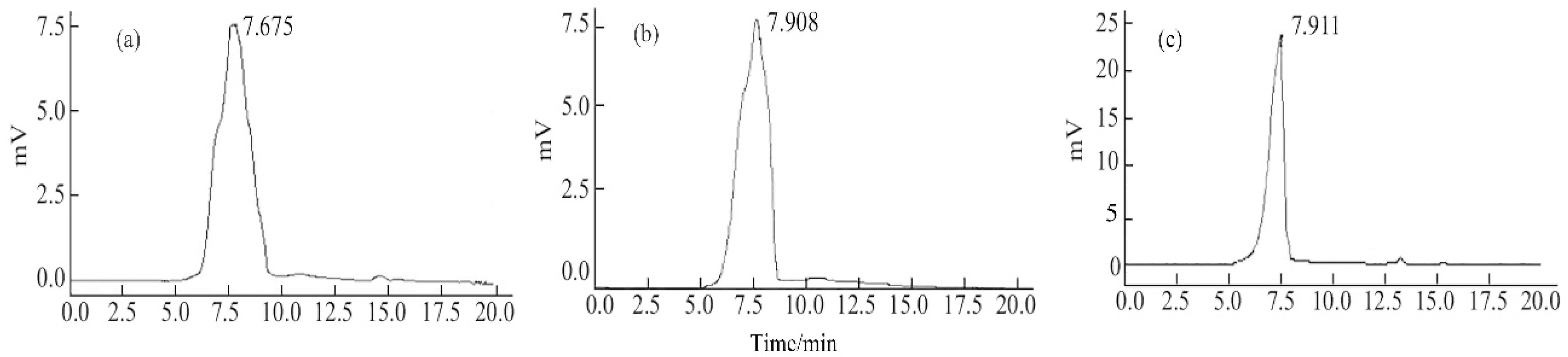
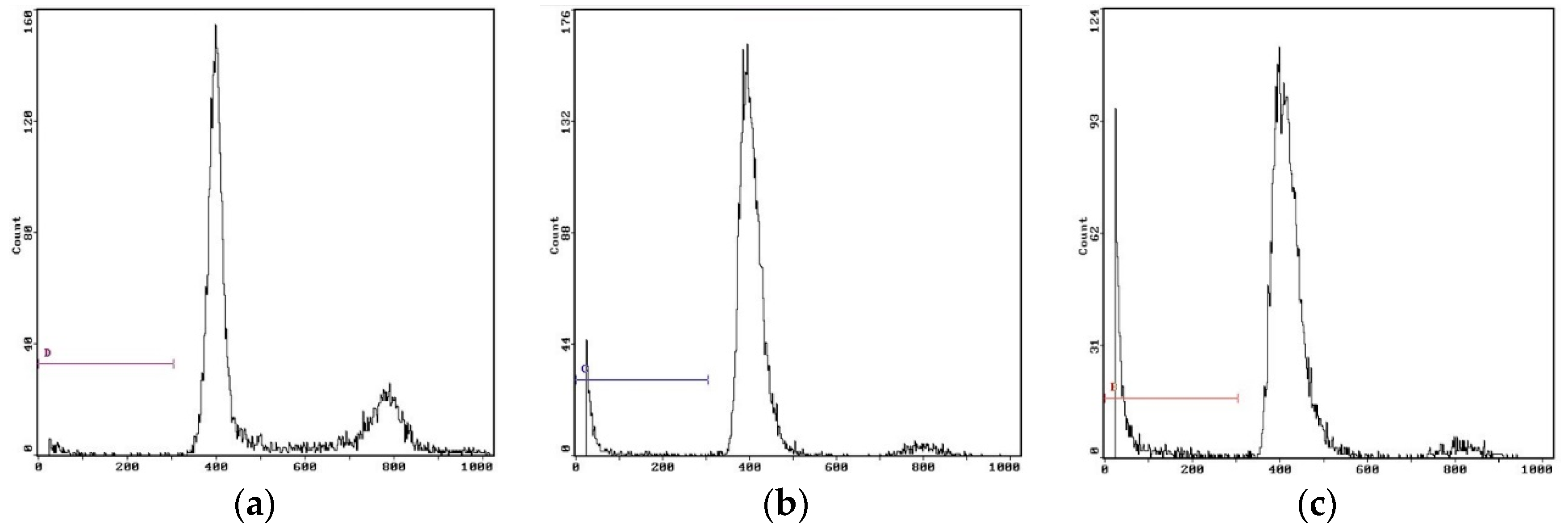
2.2. Molecular Weight
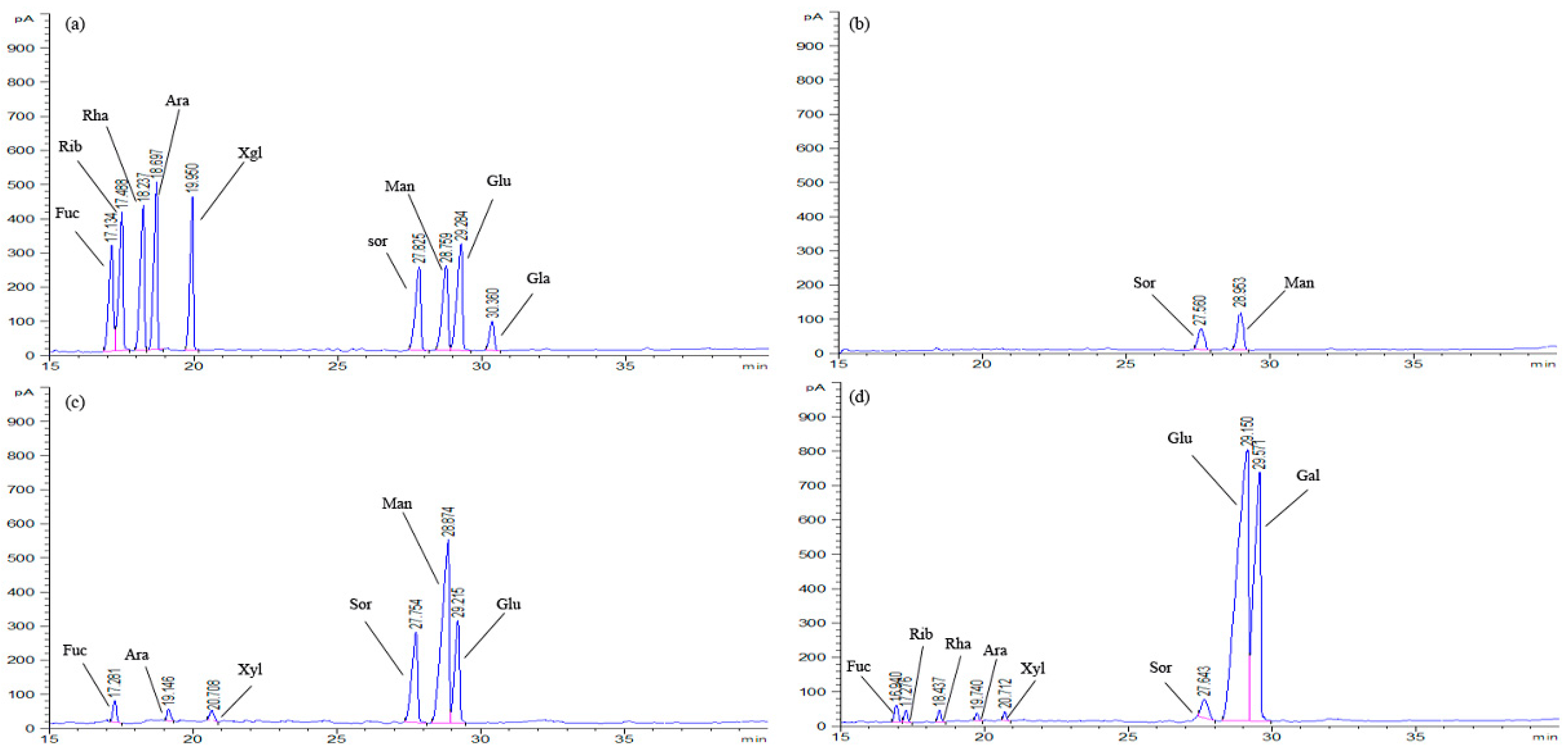
2.3. Monosaccharide Composition Analysis
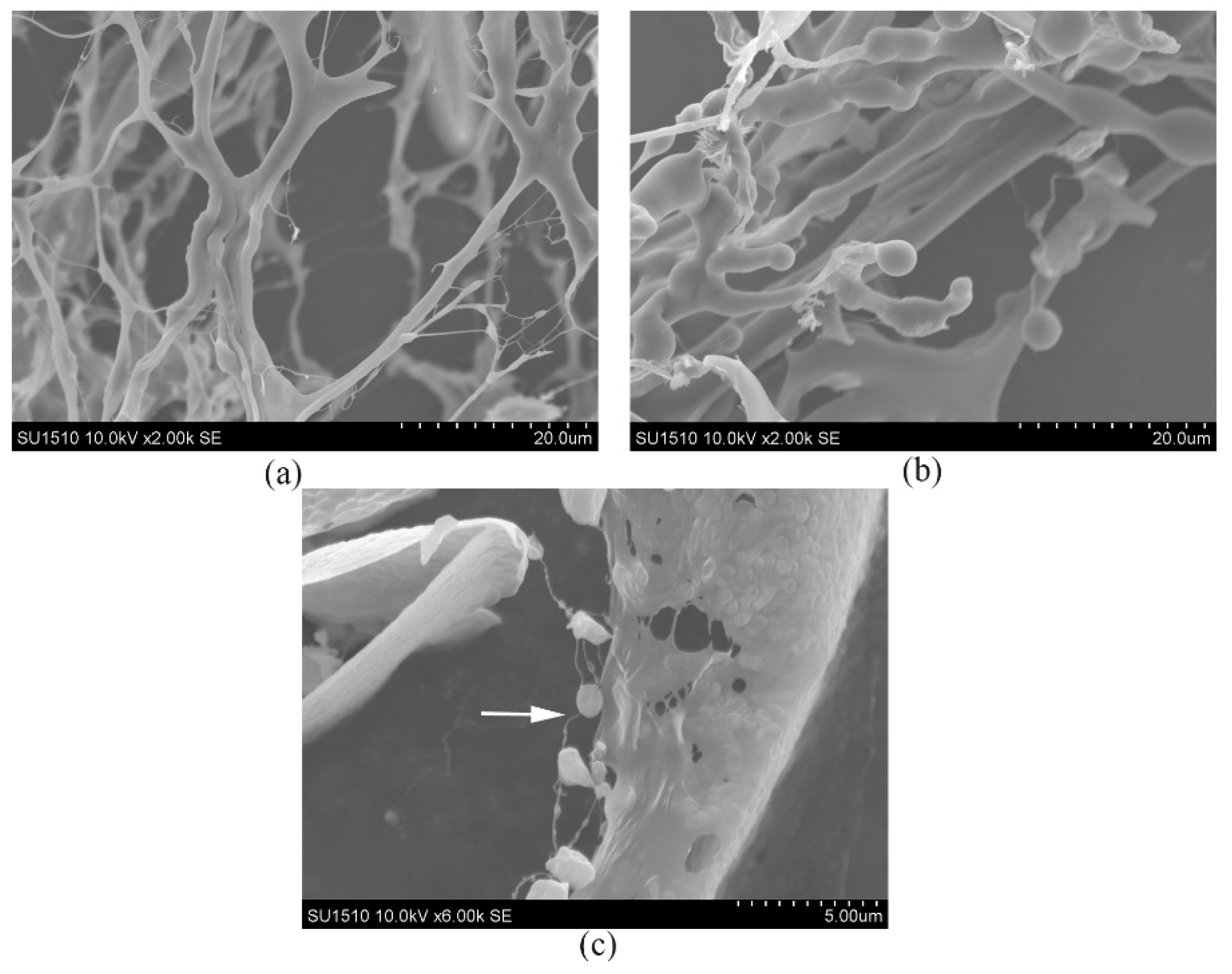
2.4. SEM Analysis
2.5. AFM Analysis
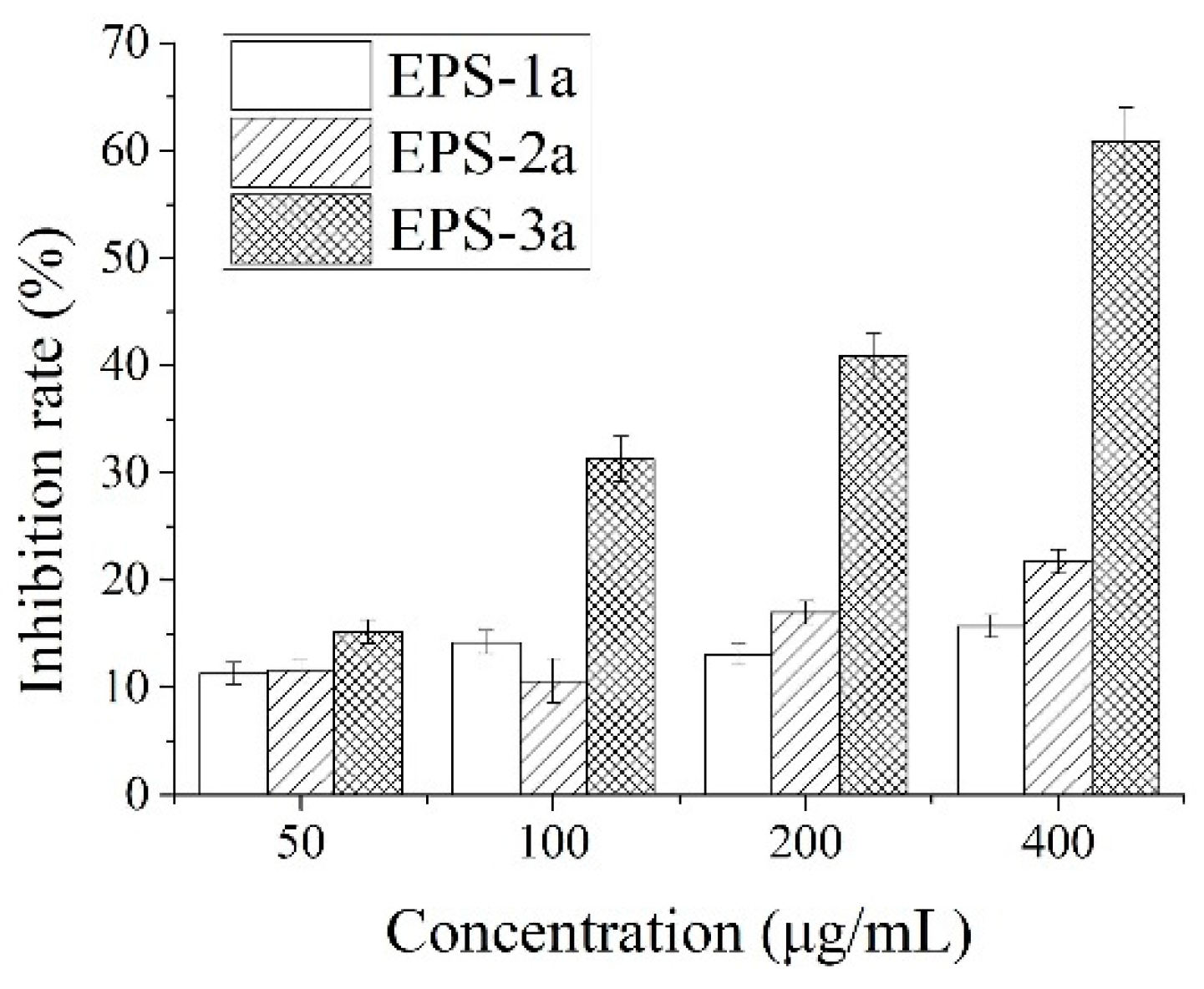
2.6. Antitumor Activity against HepG2 Cells In Vitro
2.6.1. Effect of EPS on HepG2 Proliferation
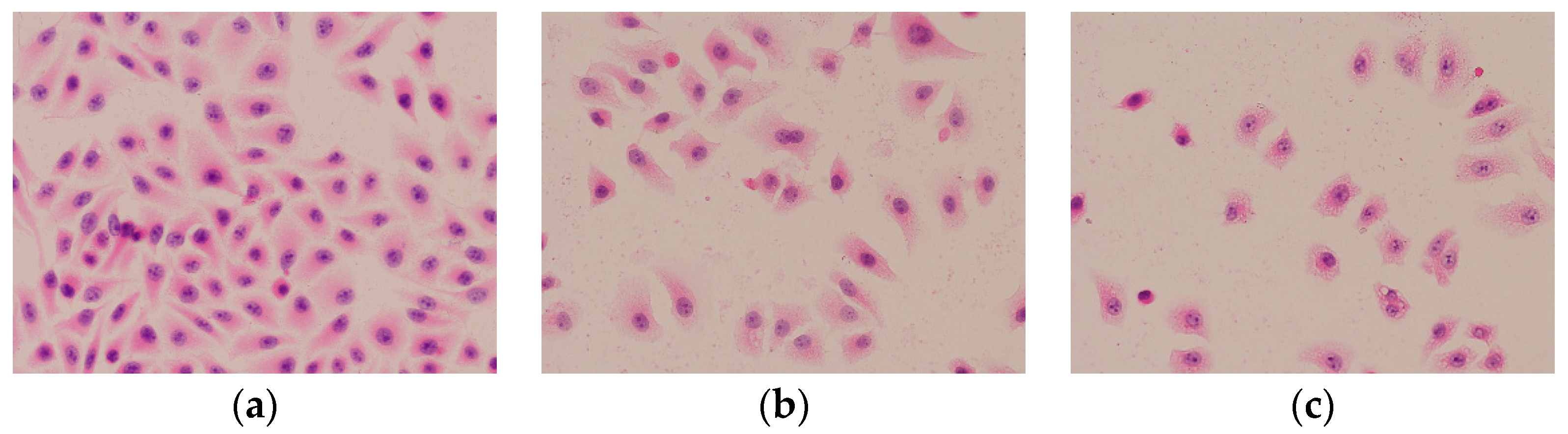
2.6.2. HE Staining
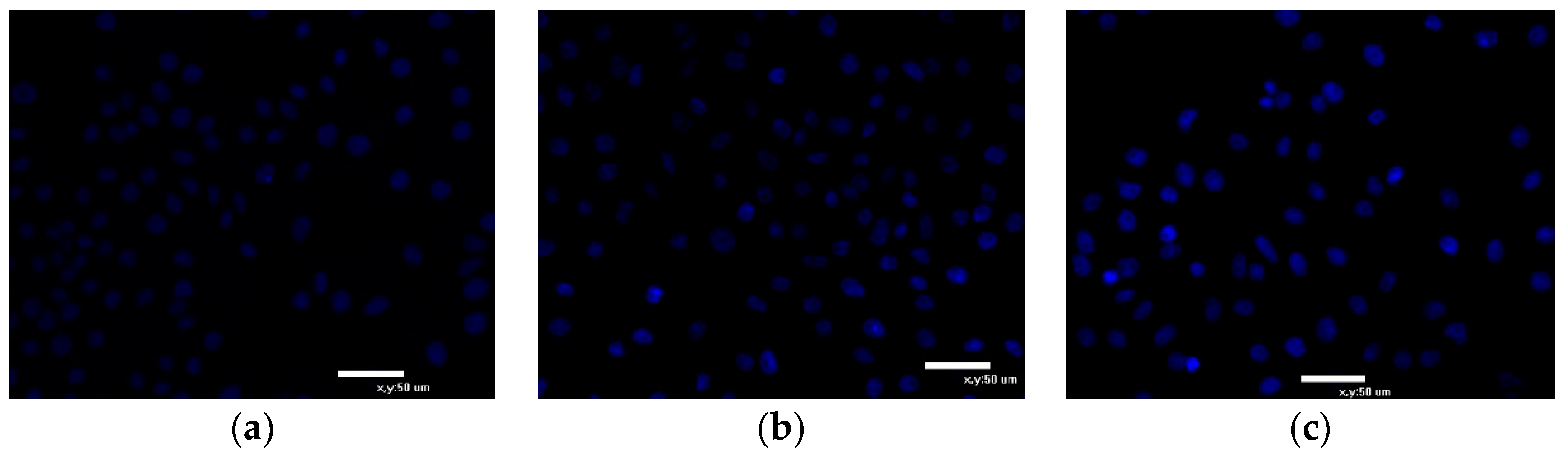
2.6.3. Hoechst 33342 Staining
2.6.4. Cell Cycle Analysis
3. Materials and Methods
3.1. Preparation and Purification of Polysaccharides
3.1.1. Strain and Growth Medium
3.1.2. Preparation of Crude EPS
3.1.3. Purification of Crude EPS
3.2. Preliminary Structure of EPS
3.2.1. Molecular Weight
3.2.2. Analysis of Monosaccharide Composition
3.2.3. Scanning Electron Microscopy (SEM) Analysis of EPS
3.2.4. Atomic Force Microscope (AFM) of EPS
3.3. Assay of Antitumor Activity against HepG2 Cell Proliferation In Vitro
3.3.1. Cell Lines and Culture, and MTT Assay
3.3.2. Cell Morphologic Examination (HE Staining and Hoechst 33342 Staining)
3.3.3. Flow Cytometry Analysis
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aslim, B.; Yüksekdag, Z.N.; Beyatli, Y.; Mercan, N. Exopolysaccharide production by Lactobacillus delbruckii subsp. bulgaricus and Streptococcus thermophilus strains under different growth conditions. World J. Microbiol. Biotechnol. 2005, 21, 673–677. [Google Scholar] [CrossRef]
- Ismail, B.; Nampoothiri, K.M. Production, purification and structural characterization of an exopolysaccharide produced by a probiotic Lactobacillus plantarum MTCC 9510. Arch. Microbiol. 2010, 192, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Pigeon, R.M.; Cuesta, E.P.; Gililliand, S.E. Binding of free bile acids by cells of yogurt starter culture bacteria. J. Dairy Sci. 2002, 85, 2705–2710. [Google Scholar] [CrossRef]
- Kitazawa, H.; Harata, T.; Uemura, J.; Saito, T.; Kaneko, T.; Itoh, T. Phosphate group requirement for mitogenic activation of lymphocytes by an extracellular phosphopolysaccharide from Lactobacillus delbrueckii ssp. bulgaricus. Int. J. Food Microbiol. 1998, 40, 169–175. [Google Scholar] [CrossRef]
- Chabot, S.; Yu, H.L.; Léséleuc, L.D.; Cloutier, D.; Calsteren, M.R.V.; Lessard, M.; Roy, D.; Lacroix, M.; Oth, D. Exopolysaccharides from Lactobacillus rhamnosus RW-9595M stimulate TNF, IL-6 and IL-12 in human and mouse cultured immunocompetent cells, and IFN-gamma mouse splenocytes. Le Lait 2001, 81, 683–697. [Google Scholar] [CrossRef]
- Welman, A.D.; Maddox, I.S. Exopolysaccharides from lactic acid bacteria: Perspectives and challenges. Trends Biotechnol. 2003, 21, 269–274. [Google Scholar] [CrossRef]
- Kim, Y.; Oh, S.; Kim, S.H. Released exopolysaccharide (r-EPS) produced from probiotic bacteria reduce biofilm formation of enterohemorrhagic Escherichia coli O157:H7. Biochem. Biophys. Res. Commun. 2009, 379, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.D.; Mei, X.M. Antioxidant activity of an exopolysaccharide purified from Lactococcus lactis subsp. lactis 12. Carbohydr. Polym. 2010, 80, 908–914. [Google Scholar] [CrossRef]
- Li, W.; Ji, J.; Chen, X.; Jiang, M.; Rui, X.; Dong, M. Structural elucidation and antioxidant activities of exopolysaccharides from Lactobacillus helveticus MB2-1. Carbohydr. Polym. 2014, 102, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, X.; Yang, Y.; Zhao, A.; Yang, Z. Characterization and bioactivities of an exopolysaccharide produced by Lactobacillus plantarum YW32. Int. J. Biol. Macromol. 2015, 74, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Surayot, U.; Wang, J.; Seesuriyachan, P.; Kuntiya, A.; Tabarsa, M.; Lee, Y.; Kim, J.; Park, W.; You, S. Exopolysaccharides from lactic acid bacteria: Structural analysis, molecular weight effect on immunomodulation. Int. J. Biol. Macromol. 2014, 68, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Mende, S.; Peter, M.; Bartels, K.; Rohm, H.; Jaros, D. Addition of purified exopolysaccharide isolates from S. thermophilus, to milk and their impact on the rheology of acid gels. Food Hydrocoll. 2013, 32, 178–185. [Google Scholar] [CrossRef]
- Tieking, M.; Gänzle, M.G. Exopolysaccharides from cereal-associated lactobacilli. Trends Food Sci. Technol. 2005, 16, 79–84. [Google Scholar] [CrossRef]
- Izawa, N.; Hanamizu, T.; Sone, T.; Chiba, K. Effects of fermentation conditions and soybean peptide supplementation on hyaluronic acid production by Streptococcus thermophilus strain YIT 2084 in milk. J. Biosci. Bioeng. 2010, 109, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Chen, Y.; Ren, S.; Han, Y.; Cheng, H. Studies on the chemical structure and antitumor activity of an exopolysaccharide from Rhizobium sp. N613. Carbohydr. Res. 2010, 345, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Zeng, Y.; Yang, M.; Chen, S.; He, L.; Ao, X.; Zou, L.; Liu, S. Production, purification and structural study of an exopolysaccharide from Lactobacillus plantarum BC-25. Carbohydr. Polym. 2016, 144, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Vijayendra, S.V.N.; Palanivel, G.; Mahadevamma, S.; Tharanathan, R.N. Physico-chemical characterization of an exopolysaccharide produced by a non-ropy strain of Leuconostoc sp. CFR 2181 isolated from dahi, an Indian traditional lactic fermented milk product. Carbohydr. Polym. 2008, 72, 300–307. [Google Scholar] [CrossRef]
- Marshall, V.M.; Cowie, E.N.; Moreton, R.S. Analysis and production of two exopolysaccharides from Lactococcus lactis subsp. lactis LC330. J. Dairy Res. 1995, 62, 621–628. [Google Scholar] [CrossRef]
- Sheykhnazari, S.; Tabarsa, T.; Ashori, A.; Shakeri, A.; Golalipour, M. Bacterial synthesized cellulose nanofibers; Effects of growth times and culture mediums on the structural characteristics. Carbohydr. Polym. 2011, 86, 1187–1191. [Google Scholar] [CrossRef]
- Ghasemlou, M.; Khodaiyan, F.; Oromiehie, A.; Yarmand, M.S. Development and characterisation of a new biodegradable edible film made from kefiran, an exopolysaccharide obtained from kefir grains. Food Chem. 2011, 127, 1496–1502. [Google Scholar] [CrossRef]
- Zhang, Q.; Marszalek, P.E. Identification of sugar isomers by single-molecule force spectroscopy. J. Am. Chem. Soc. 2006, 128, 5596–5597. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.M.; Peng, X.; Lee, K.L.D.; Tang, C.O.; Cheung, C.K.; Wu, J.Y. Structural characterisation and immunomodulatory property of an acidic polysaccharide from mycelial culture of Cordyceps sinensis fungus Cs-HK1. Food Chem. 2011, 125, 637–643. [Google Scholar] [CrossRef]
- Tao, F.; Biao, G.Z.; Yu, J.Z.; Ning, Z.H. Isolation and characterization of an acidic polysaccharide from Mesona Blumes gum. Carbohydr. Polym. 2008, 71, 159–169. [Google Scholar] [CrossRef]
- Jin, Y.; Zhang, L.; Zhang, M.; Chen, L.; Cheung, P.C.K.; Oi, V.E.C.; Lin, Y. Antitumor activities of heteropolysaccharides of Poria cocos mycelia from different strains and culture media. Carbohydr. Res. 2003, 338, 1517–1521. [Google Scholar] [CrossRef]
- Zaidman, B.Z.; Yassin, M.; Mahajna, J.; Wasser, S.P. Medicinal mushroom modulators of molecular targets as cancer therapeutics. Appl. Microbiol. Biotechnol. 2005, 67, 453–468. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J.F.R.; Wyllie, A.H.; Currie, A.R. Apoptosis: A basic biological phenomenon with wideranging implications in tissue kinetics. Br. J. Cancer 1972, 26, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Kiechle, F.L.; Zhang, X. Apoptosis: Biochemical aspects and clinical implications. Clin. Chim. Acta 2002, 326, 27–45. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Monchy, S. Texture analysis of fluorescence lifetime images of AT- and GC-rich regions in nuclei. J. Histochem. Cytochem. 2001, 49, 1443–1451. [Google Scholar]
- Zhou, G.Z.; Shi, Y.Y.; Cui, L.S.; Li, A.; Wang, Q.Q.; Liu, M. Oxymatrine induces A549 human non-small lung cancer cell apoptosis via extrinsic and intrinsic pathways. Mol. Med. Rep. 2018, 17, 1071–1076. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.H.; Liao, Z.Y.; Wang, C.L.; Cai, P.; Yang, W.Y.; Lu, M.F.; Huang, G.W. Purification and antitumour activity of a lipopeptide biosurfactant produced by Bacillus natto TK-1. Biotechnol. Appl. Biochem. 2009, 52, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Kuo, P.L.; Lin, C.C. Tetrandrine-induced cell cycle arrest and apoptosis in Hep G2 cells. Life Sci. 2003, 73, 243–252. [Google Scholar] [CrossRef]
- Kkt, G.; Haisman, D.R.; Archer, R.H.; Singh, H. Evaluation and modification of existing methods for the quantification of exopolysaccharides in milk-based media. Food Res. Int. 2005, 38, 605–613. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Gan, D.; Ma, L.; Jiang, C.; Xu, R.; Zeng, X. Production, preliminary characterization and antitumor activity in vitro of polysaccharides from the mycelium of Pholiota dinghuensis Bi. Carbohydr. Polym. 2011, 84, 997–1003. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

| Samples | Carbohydrate (%) | Protein (%) | Sugar Component (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fuc | Rib | Rha | Ara | Xyl | Sor | Man | Glu | Gal | |||
| EPS-1a | 96.32 | - | - | - | - | - | - | 30.18 | 69.82 | - | - |
| EPS-2a | 92.33 | 1.12 | 2.09 | - | - | 1.11 | 0.55 | 15.05 | 57.09 | 23.39 | - |
| EPS-3a | 85.62 | 2.52 | 1.07 | 0.63 | 0.59 | 0.35 | 0.41 | 2.03 | - | 63.93 | 36.07 |
| Time | Apoptosis Cell (%) | G0/G1 (%) | S (%) | G2/M (%) |
|---|---|---|---|---|
| Control | 2.59 ± 0.32 | 63.70 ± 0.45 | 14.90 ± 2.02 | 21.40 ± 0.94 |
| 24 h | 7.45 ± 1.55 * | 75.81 ± 0.33 * | 19.10 ± 1.96 | 5.15 ± 1.86 * |
| 48 h | 14.70 ± 1.24 ** | 89.70 ± 2.01 ** | 5.33 ± 2.04 * | 5.02 ± 1.84 ** |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, N.; Liu, H.; Liu, S.; Zhang, X.; Chen, P.; Li, W.; Xu, X.; Tian, W. Purification, Preliminary Structure and Antitumor Activity of Exopolysaccharide Produced by Streptococcus thermophilus CH9. Molecules 2018, 23, 2898. https://doi.org/10.3390/molecules23112898
Sun N, Liu H, Liu S, Zhang X, Chen P, Li W, Xu X, Tian W. Purification, Preliminary Structure and Antitumor Activity of Exopolysaccharide Produced by Streptococcus thermophilus CH9. Molecules. 2018; 23(11):2898. https://doi.org/10.3390/molecules23112898
Chicago/Turabian StyleSun, Naxin, Huiping Liu, Shaojuan Liu, Xinyuan Zhang, Pei Chen, Weihong Li, Xiangxin Xu, and Wentan Tian. 2018. "Purification, Preliminary Structure and Antitumor Activity of Exopolysaccharide Produced by Streptococcus thermophilus CH9" Molecules 23, no. 11: 2898. https://doi.org/10.3390/molecules23112898
APA StyleSun, N., Liu, H., Liu, S., Zhang, X., Chen, P., Li, W., Xu, X., & Tian, W. (2018). Purification, Preliminary Structure and Antitumor Activity of Exopolysaccharide Produced by Streptococcus thermophilus CH9. Molecules, 23(11), 2898. https://doi.org/10.3390/molecules23112898




