Immobilized Cells of Bacillus circulans ATCC 21783 on Palm Curtain for Fermentation in 5 L Fermentation Tanks
Abstract
:1. Introduction
2. Results and Discussion
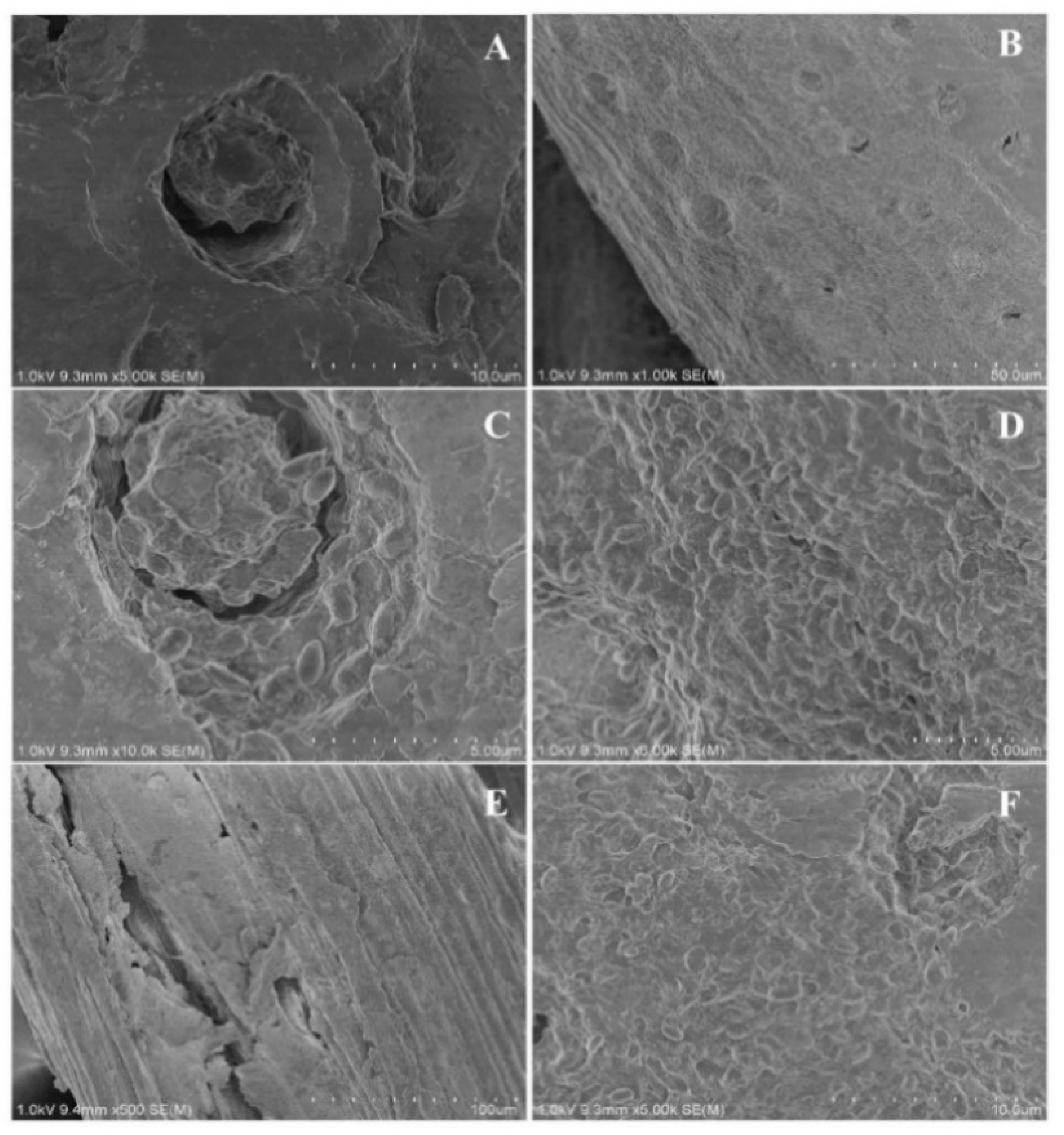
2.1. Morphology of Immobilized Cells
2.2. Immobilization Procedure of ATCC 21783
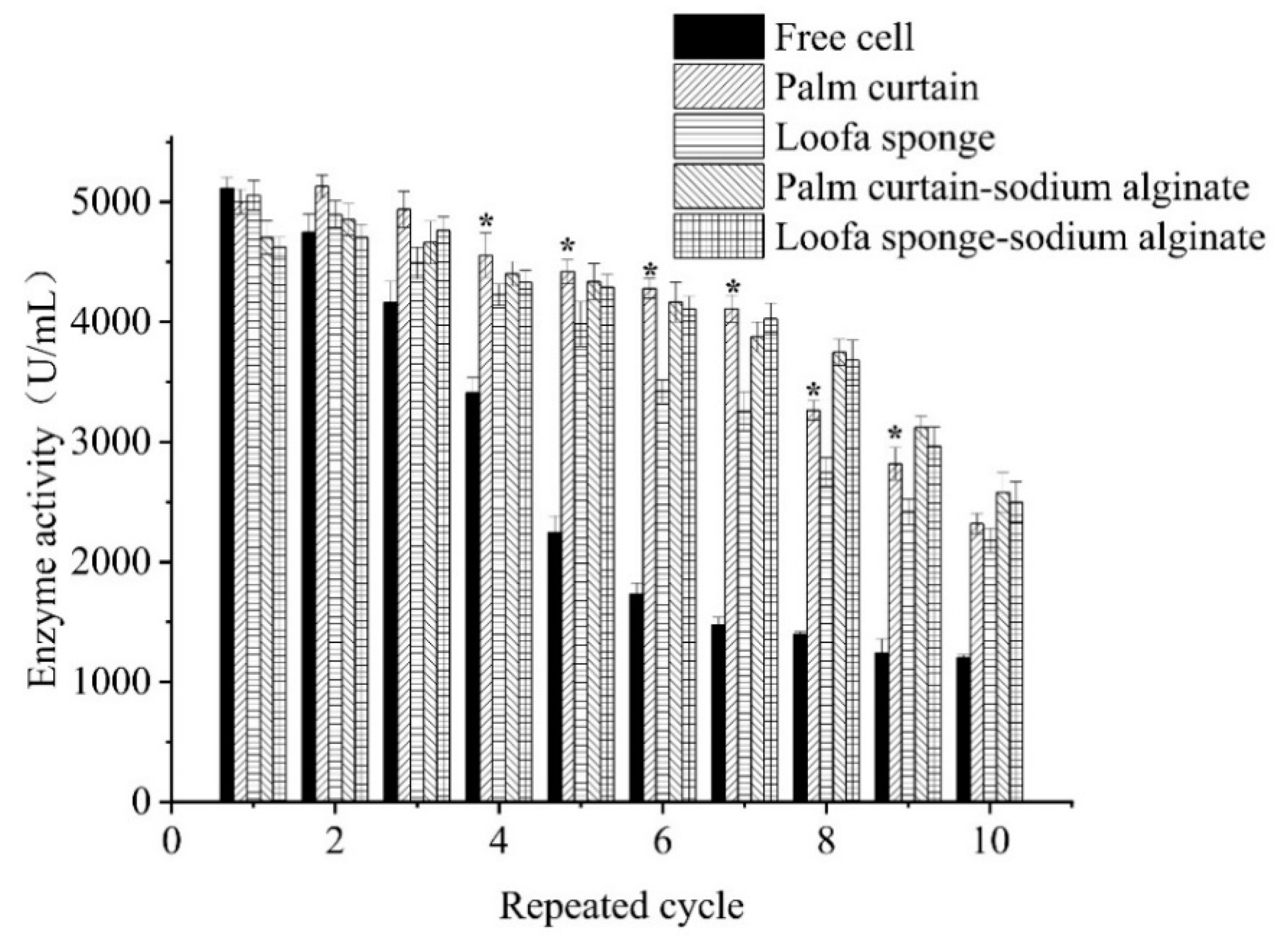
2.3. Operation Stability of Immobilized Cells
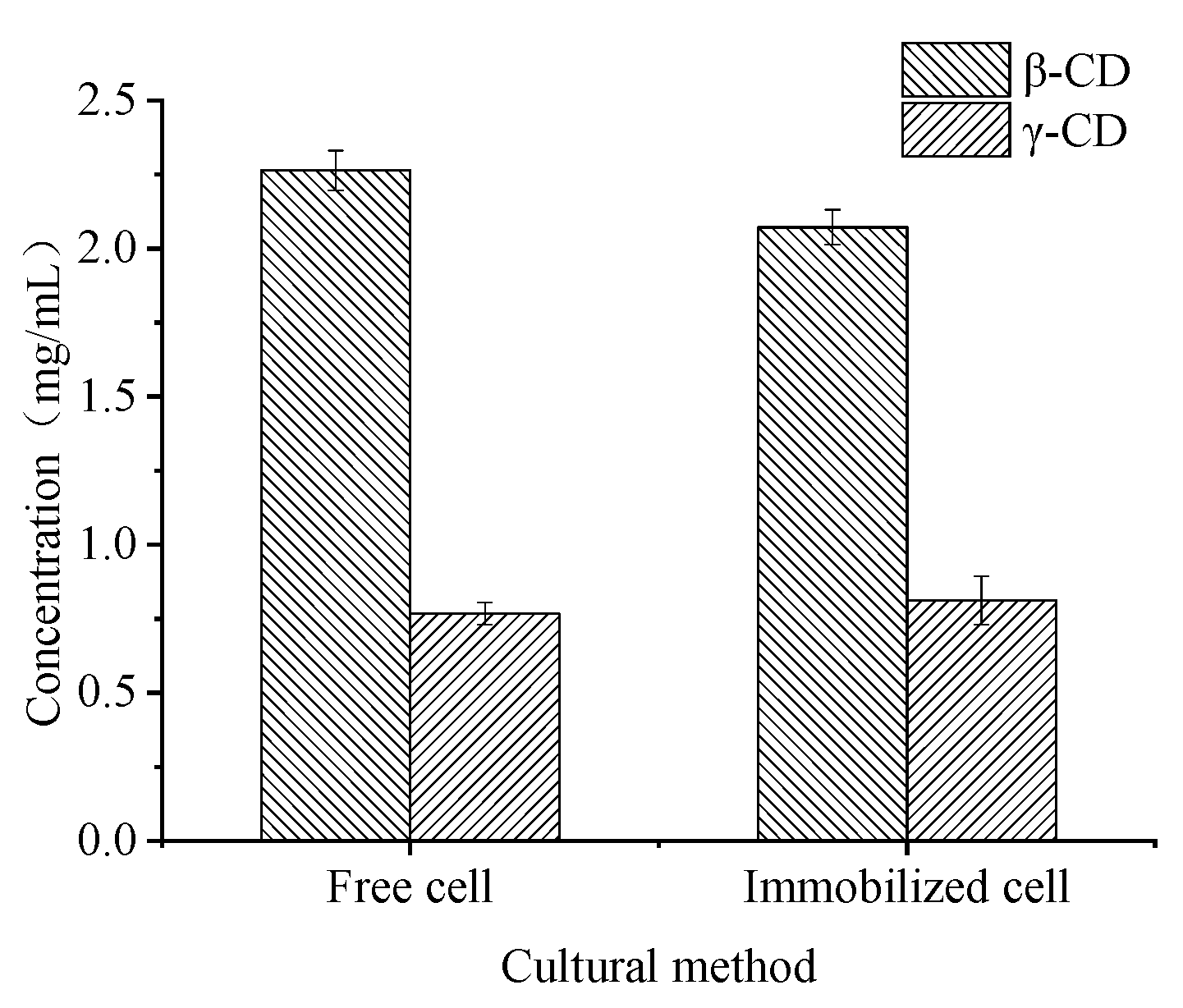
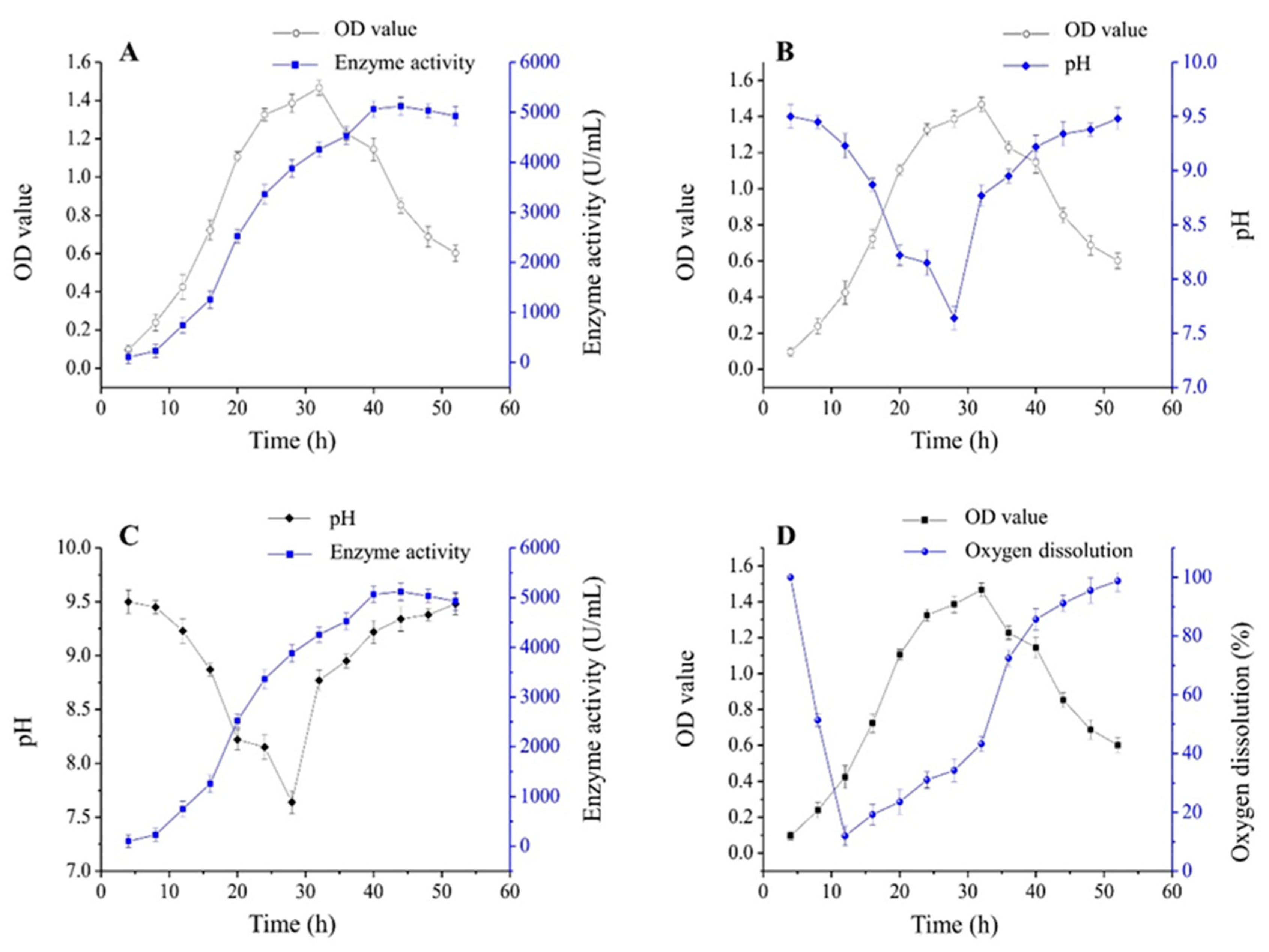
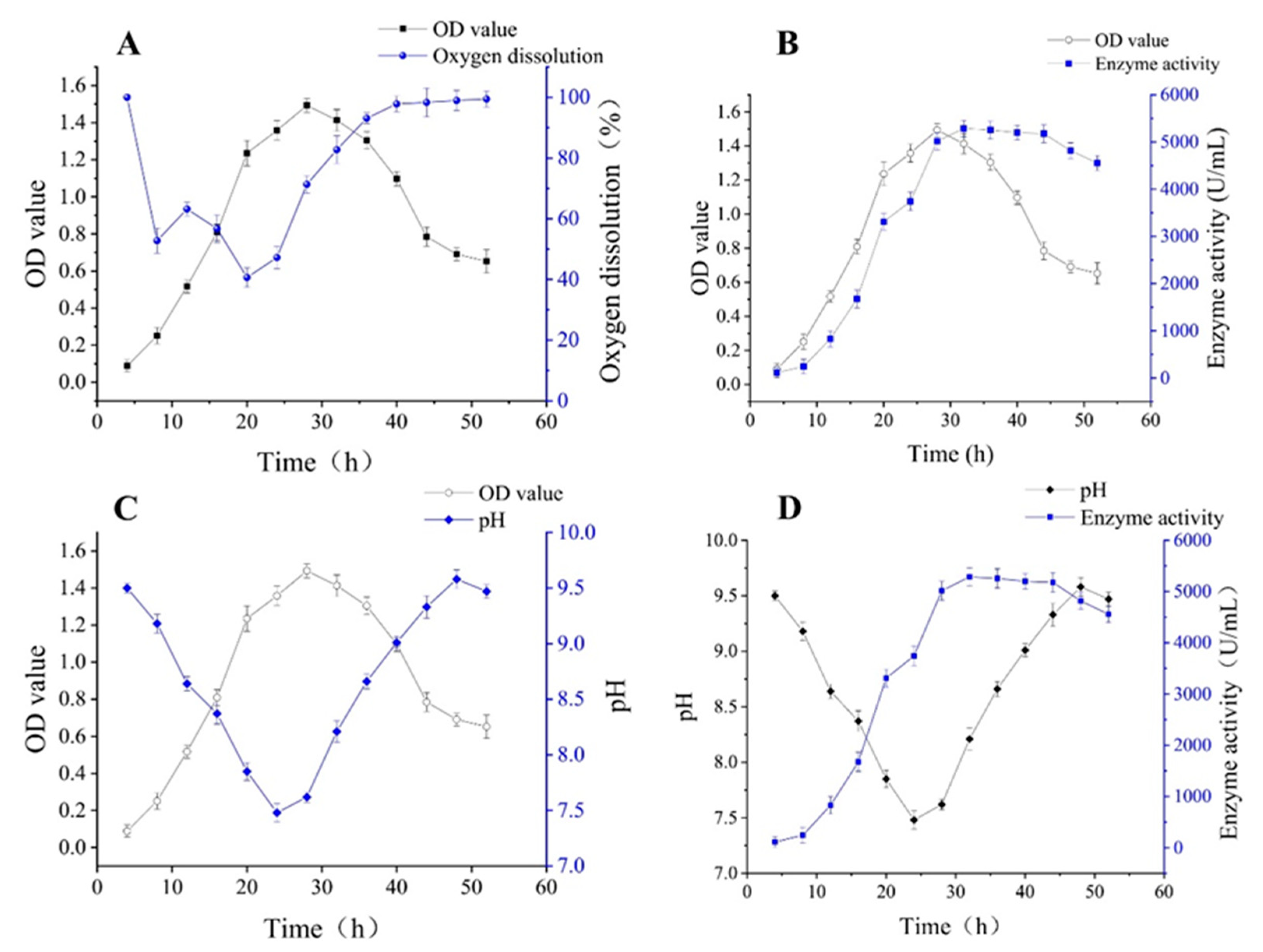
2.4. Biomass and Enzyme Activity during the Growth of Immobilized Cells
2.5. pH Variation during the Growth of Immobilized Cells
2.6. Oxygen Dissolution during the Growth of Immobilized Cells
2.7. Optimization of the Fermentation Condition
2.7.1. Optimization of the Rotation Speed
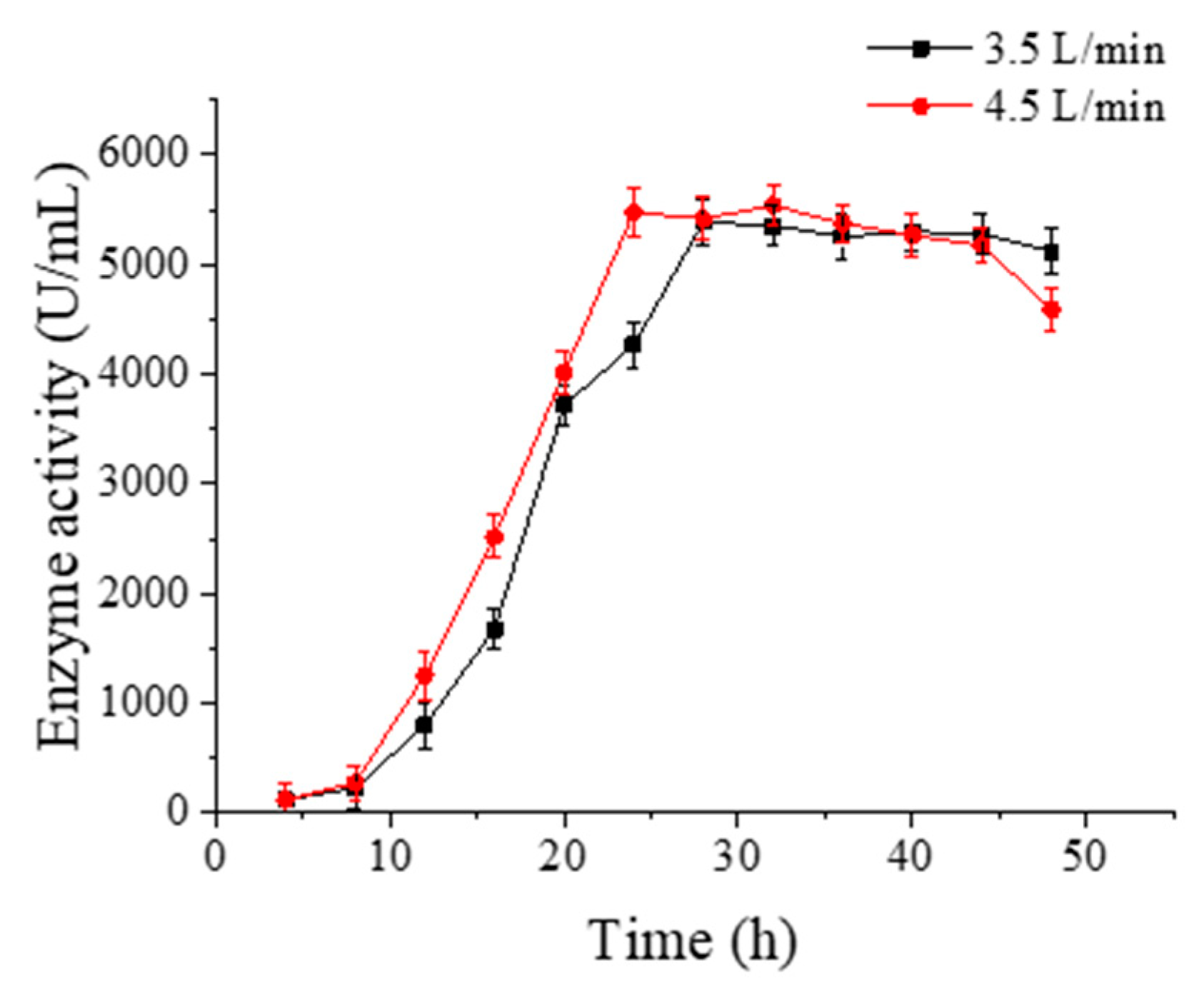
2.7.2. Optimization of Ventilation Capacity
3. Materials and Methods
3.1. Materials
3.2. Pretreatment of the Palm Curtain
3.3. Cell Culture
3.4. Immobilization of ATCC 21783
3.5. HPLC Detection of Cyclodextrins
3.6. Reusability of Immobilized Cells
3.7. Cell. Fermentation in 5 L Tank
3.8. Enzyme Activity Determination
3.9. Scanning Electron Microscopy (SEM) of Immobilized Cells
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Atanasova, N.; Petrova, P.; Ivanova, V.; Yankov, D.; Vassileva, A.; Tonkova, A. Isolation of novel alkaliphilic bacillus strains for cyclodextrin glucanotransferase production. Appl. Biochem. Biotechnol. 2008, 149, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Kitahata, S.; Tsuyama, N.; Okada, S. Purification and some properties of cyclodextrin glycosyltransferase from a strain of Bacillus species. Agric. Biol. Chem. 1974, 38, 387–393. [Google Scholar] [CrossRef]
- Van der Veen, B.A.; Uitdehaag, J.C.M.; Dijkstra, B.W.; Dijkhuizen, L. The role of arginine 47 in the cyclization and coupling reactions of cyclodextrin glycosyltransferase from Bacillus circulans strain 251. Eur. J. Biochem. 2000, 267, 3432–3441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vassileva, A.; Atanasova, N.; Ivanova, V.; Dhulster, P.; Tonkova, A. Characterisation of cyclodextrin glucanotransferase from Bacillus circulans ATCC 21783 in terms of cyclodextrin production. Ann. Microbiol. 2007, 57, 609–615. [Google Scholar] [CrossRef]
- Qi, Q.S.; Zimmermann, W. Cyclodextrin glucanotransferase: From gene to applications. Appl. Microbiol. Biotechnol. 2005, 66, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Phillips, C.R.; Poon, Y.C. Immobilization of Cells; Springer: Berlin/Heidelberg, Germany, 1988. [Google Scholar]
- Zajkoska, P.; Rebroš, M.; Rosenberg, M. Biocatalysis with immobilized Escherichia coli. Appl. Microbiol. Biotechnol. 2013, 97, 1441–1455. [Google Scholar] [CrossRef] [PubMed]
- Sekoai, P.T.; Awosusi, A.A.; Yoro, K.O.; Singo, M.; Oloye, O.; Ayeni, A.O.; Bodunrin, M.; Daramola, M.O. Microbial cell immobilization in biohydrogen production: A short overview. Crit. Rev. Biotechnol. 2018, 38, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Min, K.; Yoo, Y.J. Recent progress in nanobiocatalysis for enzyme immobilization and its application. Biotechnol. Bioproc. Eng. 2014, 19, 553–567. [Google Scholar] [CrossRef]
- Zucca, P.; Sanjust, E. Inorganic materials as supports for covalent enzyme immobilization: Methods and mechanisms. Molecules 2014, 19, 14139–14194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karav, S.; Cohen, J.L.; Barile, D. Recent advances in immobilization strategies for glycosidases. Biotechnol. Prog. 2017, 33, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, S.; Mokhtar, M.N.; Naim, M.N.; Baharuddin, A.S.; Sulaiman, A. A review: Potential usage of cellulose nanofibers (CNF) for enzyme immobilization via covalent interactions. Appl. Biochem. Biotechnol. 2015, 175, 1817–1842. [Google Scholar] [CrossRef] [PubMed]
- Krasňan, V.; Stloukal, R.; Rosenberg, M.; Rebroš, M. Immobilization of cells and enzymes to LentiKats®®. Appl. Microbiol. Biotechnol. 2016, 100, 2535–2553. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Mudhoo, A.; Sivagurunathan, P.; Nagarajan, D.; Ghimire, A.; Lay, C.H.; Lin, C.Y.; Lee, D.J.; Chang, J.S. Recent insights into the cell immobilization technology applied for dark fermentative hydrogen production. Bioresour. Technol. 2016, 219, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Costa, H.; Gastón, J.R.; Lara, J.; Martinez, C.O.; Moriwaki, C.; Matioli, G.; Ferrarotti, S.A. Cyclodextrin glycosyltransferase production by free cells of Bacillus circulans DF 9R in batch fermentation and by immobilized cells in a semi-continuous process. Bioproc. Biosyst. Eng. 2015, 38, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- Mazzer, C.; Ferreira, L.R.; Rodella, J.R.T.; Moriwaki, C.; Matioli, G. Cyclodextrin production by Bacillus firmus strain 37 immobilized on inorganic matrices and alginate gel. Biochem. Eng. J. 2008, 41, 79–86. [Google Scholar] [CrossRef]
- Iyer, J.L.; Shetty, P.; Pai, J.S. Immobilisation of cyclodextrin glucanotransferase from Bacillus circulans ATCC 21783 on purified seasand. J. Ind. Microbiol. Biotechnol. 2003, 30, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Naby, M.A.; Reyad, R.M.; Abdel-Fattah, A.F. Biosynthesis of cyclodextrin glucosyltransferase by immobilized Bacillus amyloliquefaciens in batch and continuous cultures. Biochem. Eng. J. 2000, 5, 1–9. [Google Scholar] [CrossRef]
- Kunamneni, A.; Prabhakar, T.; Jyothi, B.; Ellaiah, P. Investigation of continuous cyclodextrin glucanotransferase production by the alginate-immobilized cells of alkalophilic Bacillus sp. in an airlift reactor. Enzyme Microb. Technol. 2007, 40, 1538–1542. [Google Scholar] [CrossRef]
- Vassileva, A.; Burhan, N.; Beschkov, V.; Spasova, D.; Radoevska, S.; Ivanova, V.; Tonkova, A. Cyclodextrin glucanotransferase production by free and agar gel immobilized cells of Bacillus circulans ATCC 21783. Process. Biochem. 2003, 38, 1585–1591. [Google Scholar] [CrossRef]
- Abdel-Naby, M.A.; El-Refai, H.A.; Abdel-Fattah, A.F. Biosynthesis of cyclodextrin glucosyltransferase by the free and immobilized cells of Bacillus cereus NRC7 in batch and continuous cultures. J. Appl. Microbiol. 2011, 111, 1129–1137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, T.; Horikoshi, K. Immobilized cyclomaltodextrin glucanotransferase of an alkalophilic Bacillus sp. No. 38-2. Biotechnol. Bioeng. 1984, 26, 595–598. [Google Scholar] [CrossRef] [PubMed]
- Moriwaki, C.; Mangolim, C.S.; Ruiz, G.B.; de Morais, G.R.; Baesso, M.L.; Matioli, G. Biosynthesis of CGTase by immobilized alkalophilic Bacilli and crystallization of beta-cyclodextrin: Effective techniques to investigate cell immobilization and the production of cyclodextrins. Biochem. Eng. J. 2014, 83, 22–32. [Google Scholar] [CrossRef]
- Moriwaki, C.; Pelissari, F.M.; Gonçalves, R.A.C.; Gonçalves, J.E.; Matioli, G. Immobilization of Bacillus firmus strain 37 in inorganic matrix for cyclodextrin production. J. Mol. Catal. B Enzym. 2007, 49, 1–7. [Google Scholar] [CrossRef]
- Pazzetto, R.; de Oliveira Delani, T.C.; Fenelon, V.C.; Matioli, G. Cyclodextrin production by Bacillus firmus strain 37 cells immobilized on loofa sponge. Process. Biochem. 2011, 46, 46–51. [Google Scholar] [CrossRef]
- Melo, A.D.Q.; Silva, F.F.M.; dos Santos, J.C.S.; Fernández-Lafuente, R.; Lemos, T.L.G.; Dias Filho, F.A. Synthesis of benzyl acetate catalyzed by lipase immobilized in nontoxic chitosan-polyphosphate beads. Molecules 2017, 22, 2165. [Google Scholar] [CrossRef] [PubMed]
- DiCosimo, R.; McAuliffe, J.; Poulose, A.J.; Bohlmann, G. Industrial use of immobilized enzymes. Chem. Soc. Rev. 2013, 42, 6437–6474. [Google Scholar] [CrossRef] [PubMed]
- Zur, J.; Wojcieszynska, D.; Guzik, U. Metabolic responses of bacterial cells to immobilization. Molecules 2016, 21, 958. [Google Scholar] [CrossRef] [PubMed]
- Cláudia, S.; Martins, S.; Martins, C.M.; Maria, L.; Fiúza, C.G.; Santaella, S.T. Immobilization of microbial cells: A promising tool for treatment of toxic pollutants in industrial wastewater. Afr. J. Biotechnol. 2013, 12, 4412–4418. [Google Scholar] [CrossRef]
- Gungormusler-Yilmaz, M.; Cicek, N.; Levin, D.B.; Azbar, N. Cell immobilization for microbial production of 1,3-propanediol. Crit. Rev. Biotechnol. 2016, 36, 482–494. [Google Scholar] [CrossRef] [PubMed]
- Saeed, A.; Iqbal, M. Loofa (Luffa cylindrica) sponge: Review of development of the biomatrix as a tool for biotechnological applications. Biotechnol. Prog. 2013, 29, 573–600. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Garcia, J.; Garcia-Martinez, T.; Mauricio, J.C.; Moreno, J. Yeast immobilization systems for alcoholic wine fermentations: Actual trends and future perspectives. Front. Microbiol. 2018, 9, 241. [Google Scholar] [CrossRef] [PubMed]
- Kilonzo, P.; Margaritis, A.; Bergougnou, M. Airlift-driven fibrous-bed bioreactor for continuous production of glucoamylase using immobilized recombinant yeast cells. J. Biotechnol. 2009, 143, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Gotovtsev, P.M.; Yuzbasheva, E.Y.; Gorin, K.V.; Butylin, V.V.; Badranova, G.U.; Perkovskaya, N.I.; Mostova, E.B.; Namsaraev, Z.B.; Rudneva, N.I.; Komova, A.V.; et al. Immobilization of microbial cells for biotechnological production: Modern solutions and promising technologies. Appl. Biochem. Microbiol. 2015, 51, 792–803. [Google Scholar] [CrossRef]
- Kourkoutas, Y.; Bekatorou, A.; Banat, I.M.; Marchant, R.; Koutinas, A.A. Immobilization technologies and support materials suitable in alcohol beverages production: A review. Food Microbiol. 2004, 21, 377–397. [Google Scholar] [CrossRef]
- Fernandez-Lopez, L.; Pedrero, S.G.; Lopez-Carrobles, N.; Gorines, B.C.; Virgen-Ortíz, J.J.; Fernandez-Lafuente, R. Effect of protein load on stability of immobilized enzymes. Enzyme Microb. Technol. 2017, 98, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Castro, H.F.; Silva, M.L.C.P.; Silva, G.L.J.P. Evaluation of inorganic matrixes as supports for immobilization of microbial lipase. Braz. J. Chem. Eng. 2000, 17, 849–858. [Google Scholar] [CrossRef]
- Villalba, M.; Verdasco-Martín, C.M.; dos Santos, J.C.S.; Fernandez-Lafuente, R.; Otero, C. Operational stabilities of different chemical derivatives of Novozym 435 in an alcoholysis reaction. Enzyme Microb. Technol. 2016, 90, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, A.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Glutaraldehyde in bio-catalysts design: A useful crosslinker and a versatile tool in enzyme immobilization. RSC Adv. 2014, 4, 1583–1600. [Google Scholar] [CrossRef]
- Sanchez, A.; Cruz, J.; Rueda, N.; dos Santos, J.C.S.; Torres, R.; Ortiz, C.; Villalonga, R.; Fernandez-Lafuente, R. Inactivation of immobilized trypsin under dissimilar conditions produces trypsin molecules with different structures. RSC Adv. 2016, 6, 27329–27334. [Google Scholar] [CrossRef]
- Vassileva, A.; Beschkov, V.; Ivanova, V.; Tonkova, A. Continuous cyclodextrin glucanotransferase production by free and immobilized cells of Bacillus circulans ATCC 21783 in bioreactors. Process. Biochem. 2005, 40, 3290–3295. [Google Scholar] [CrossRef]
- Meleigy, S.A.; Khalaf, M.A. Biosynthesis of gibberellic acid from milk permeate in repeated batch operation by a mutant Fusarium moniliforme cells immobilized on loofa sponge. Bioresour. Technol. 2009, 100, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Phisalaphong, M.; Budiraharjo, R.; Bangrak, P.; Mongkolkajit, J.; Limtong, S. Alginate-loofa as carrier matrix for ethanol production. J. Biosci. Bioeng. 2007, 104, 214–217. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Hu, Y.; Qiu, C.; Fan, H.; Yue, Y.; Jiao, A.; Xu, X.; Jin, Z. Immobilized Cells of Bacillus circulans ATCC 21783 on Palm Curtain for Fermentation in 5 L Fermentation Tanks. Molecules 2018, 23, 2888. https://doi.org/10.3390/molecules23112888
Wang J, Hu Y, Qiu C, Fan H, Yue Y, Jiao A, Xu X, Jin Z. Immobilized Cells of Bacillus circulans ATCC 21783 on Palm Curtain for Fermentation in 5 L Fermentation Tanks. Molecules. 2018; 23(11):2888. https://doi.org/10.3390/molecules23112888
Chicago/Turabian StyleWang, Jinpeng, Yao Hu, Chao Qiu, Haoran Fan, Yan Yue, Aiquan Jiao, Xueming Xu, and Zhengyu Jin. 2018. "Immobilized Cells of Bacillus circulans ATCC 21783 on Palm Curtain for Fermentation in 5 L Fermentation Tanks" Molecules 23, no. 11: 2888. https://doi.org/10.3390/molecules23112888
APA StyleWang, J., Hu, Y., Qiu, C., Fan, H., Yue, Y., Jiao, A., Xu, X., & Jin, Z. (2018). Immobilized Cells of Bacillus circulans ATCC 21783 on Palm Curtain for Fermentation in 5 L Fermentation Tanks. Molecules, 23(11), 2888. https://doi.org/10.3390/molecules23112888





