Synthesis of Novel Benzazole Derivatives and Evaluation of Their Antidepressant-Like Activities with Possible Underlying Mechanisms
Abstract
1. Introduction
2. Results and Discussion
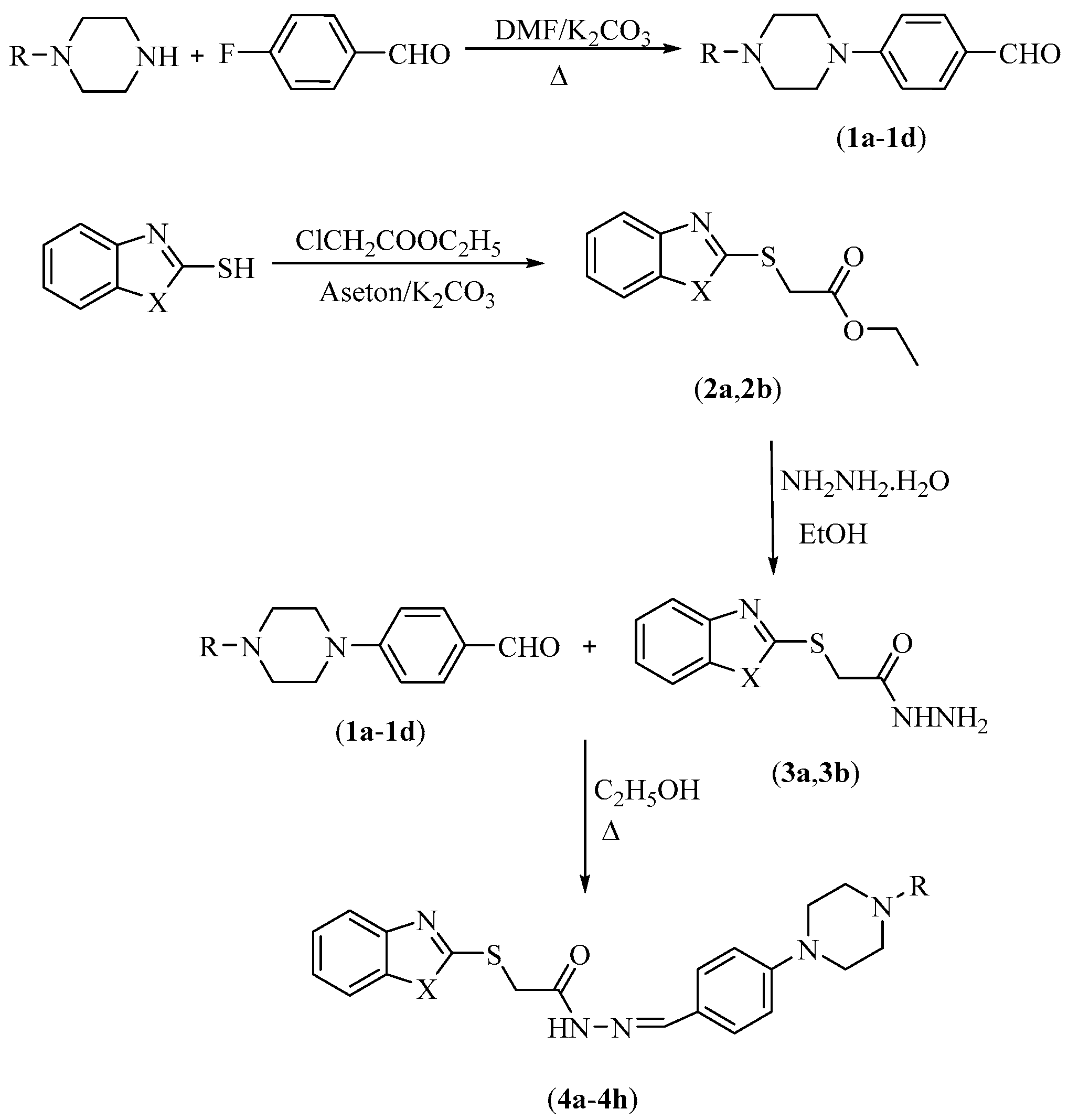
2.1. Chemistry
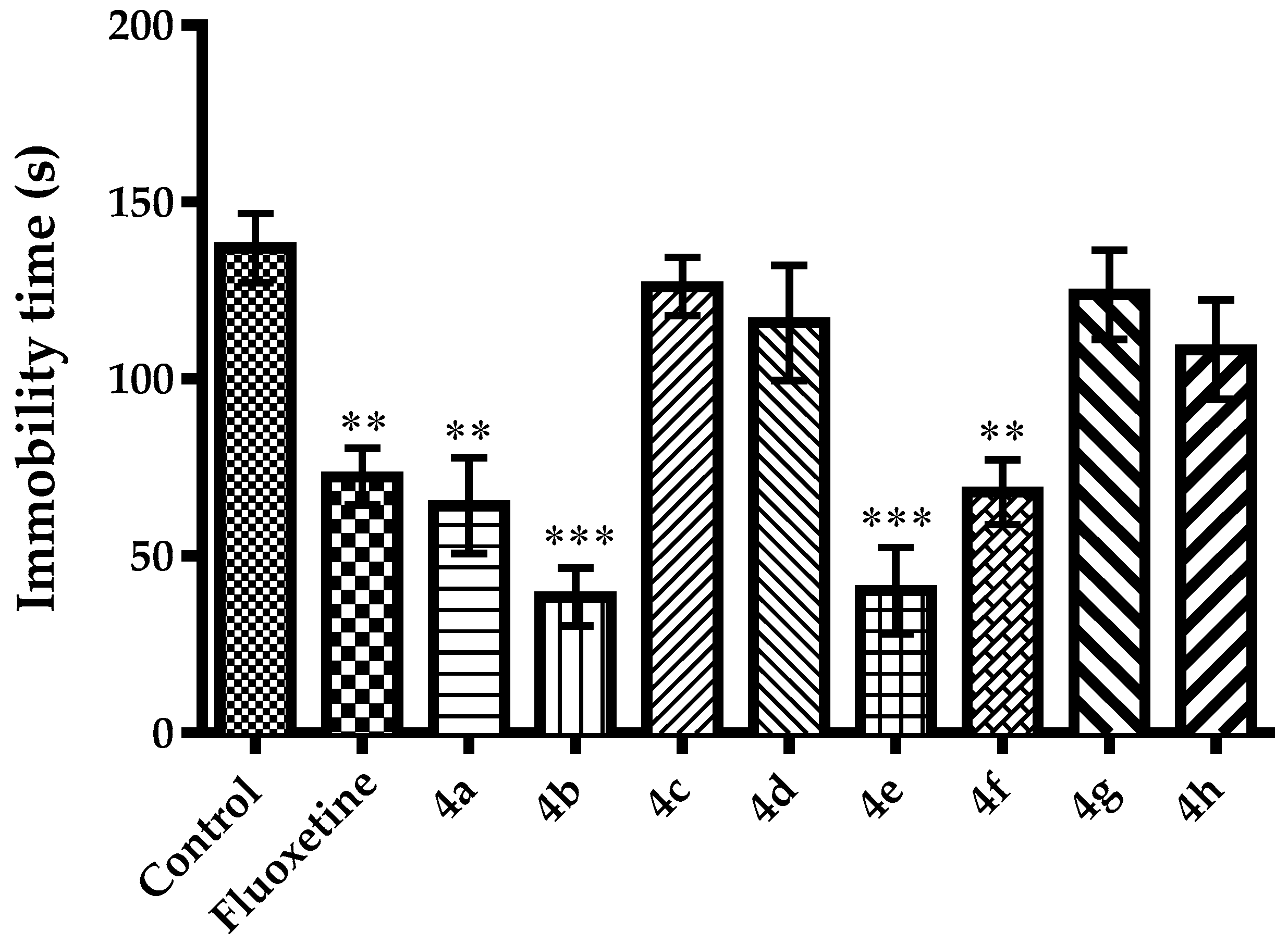
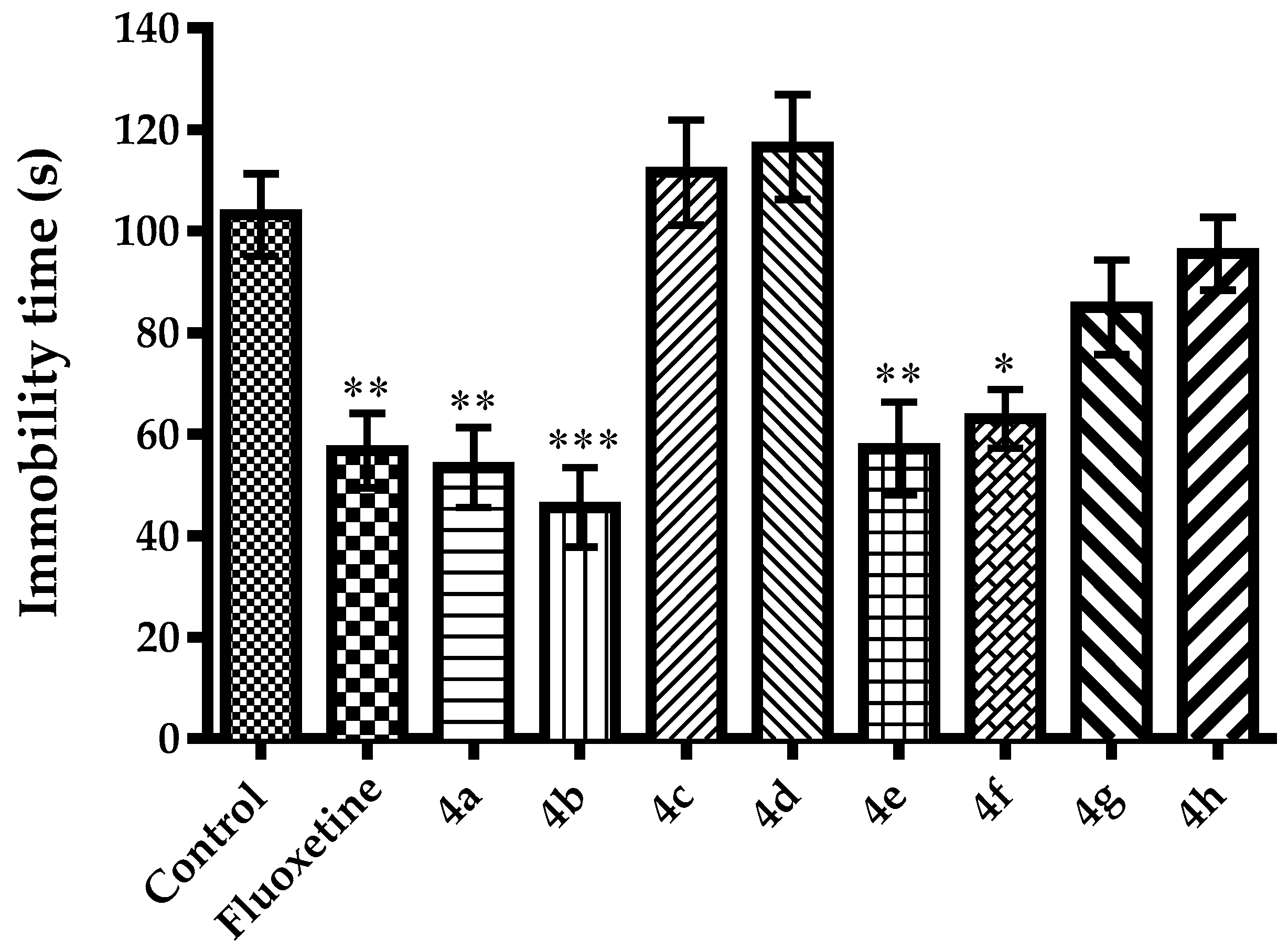
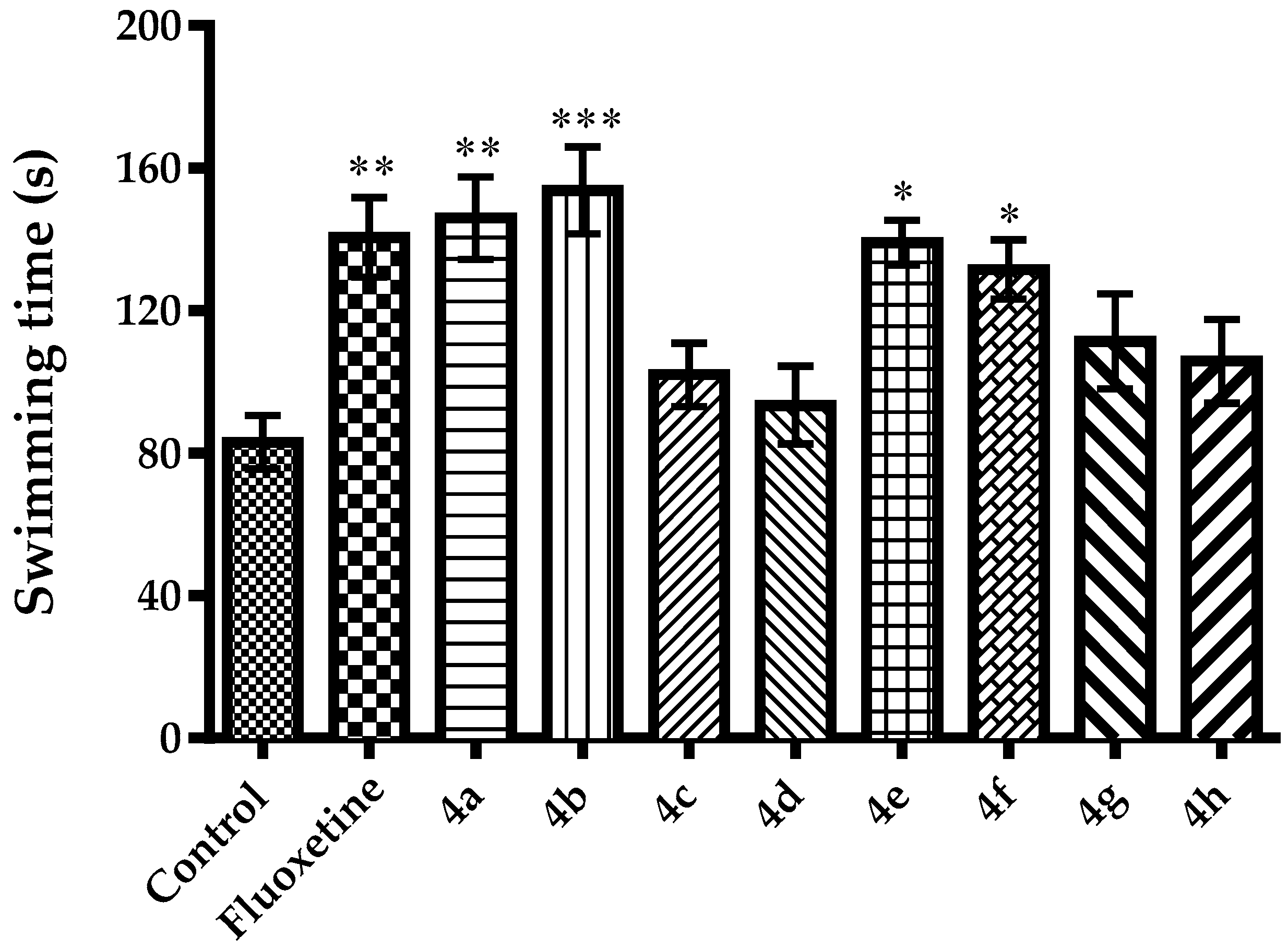
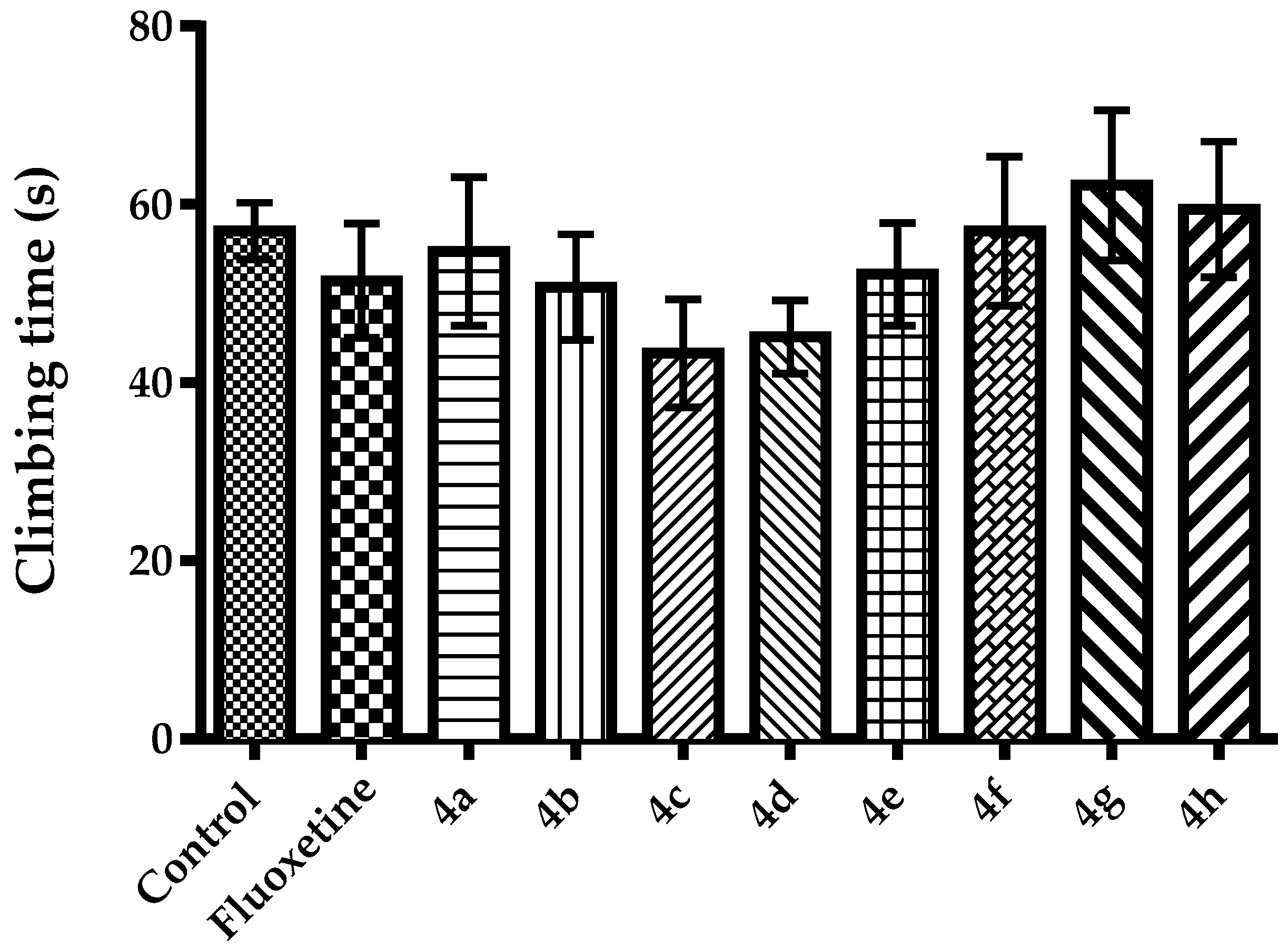
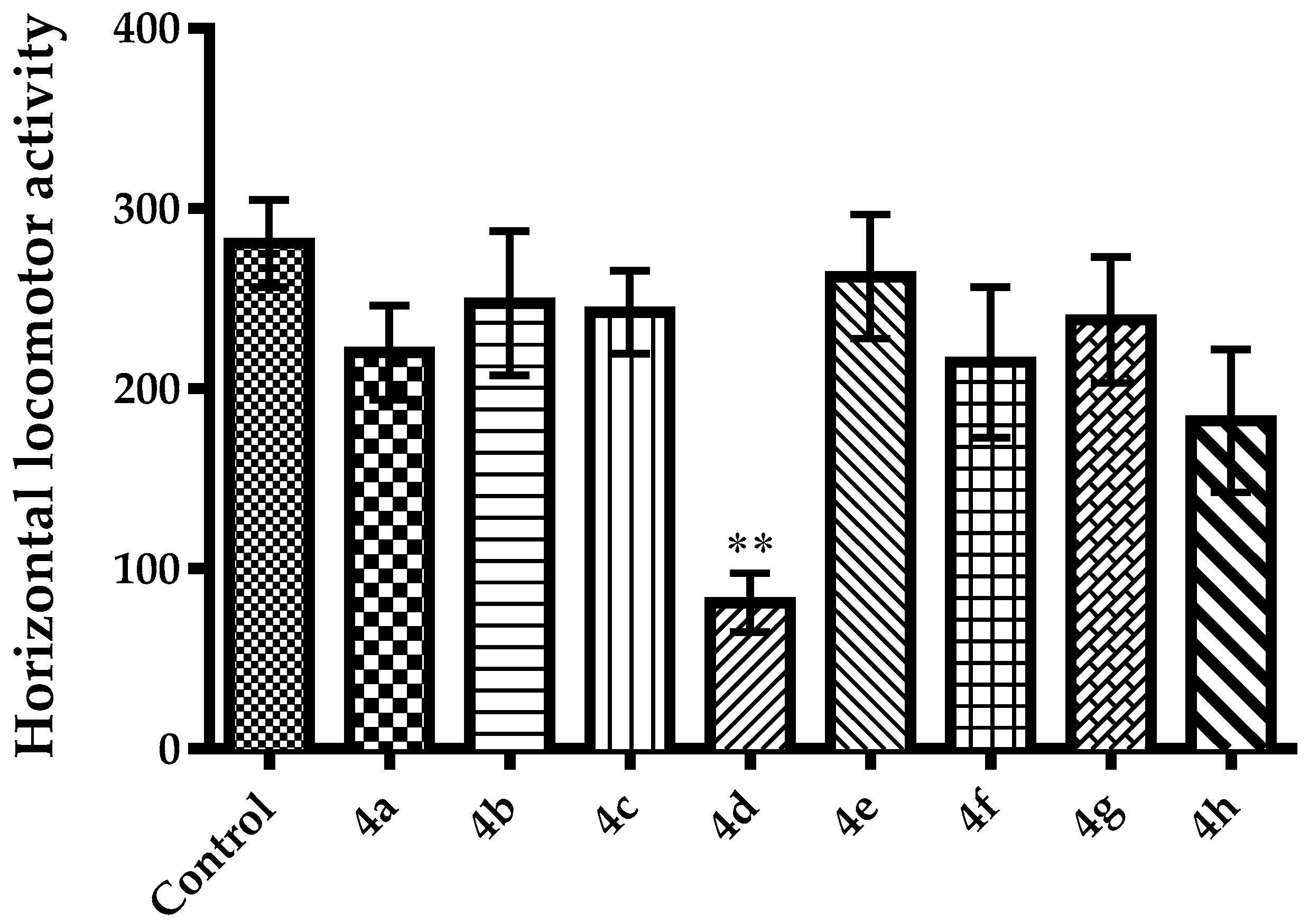
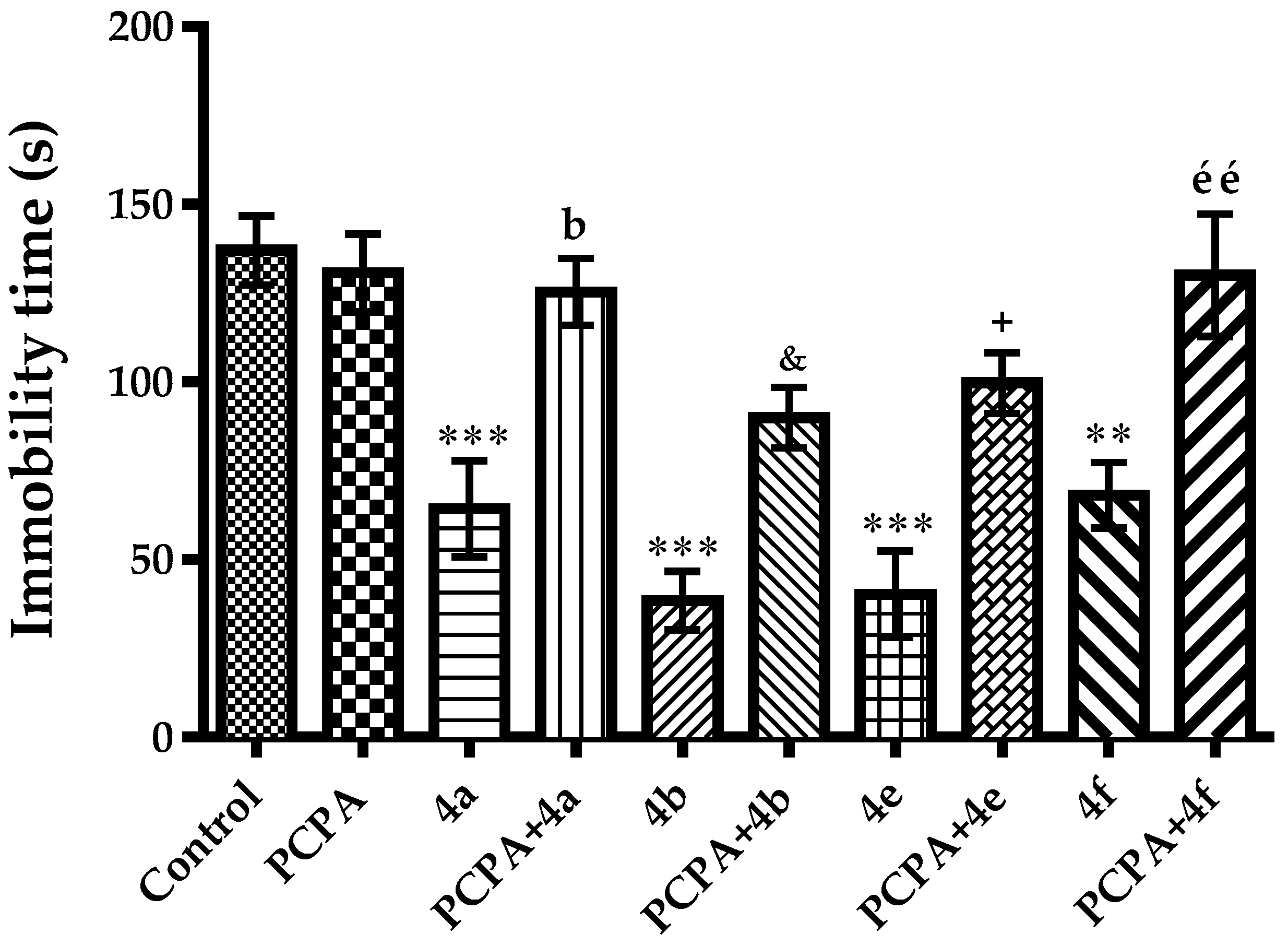
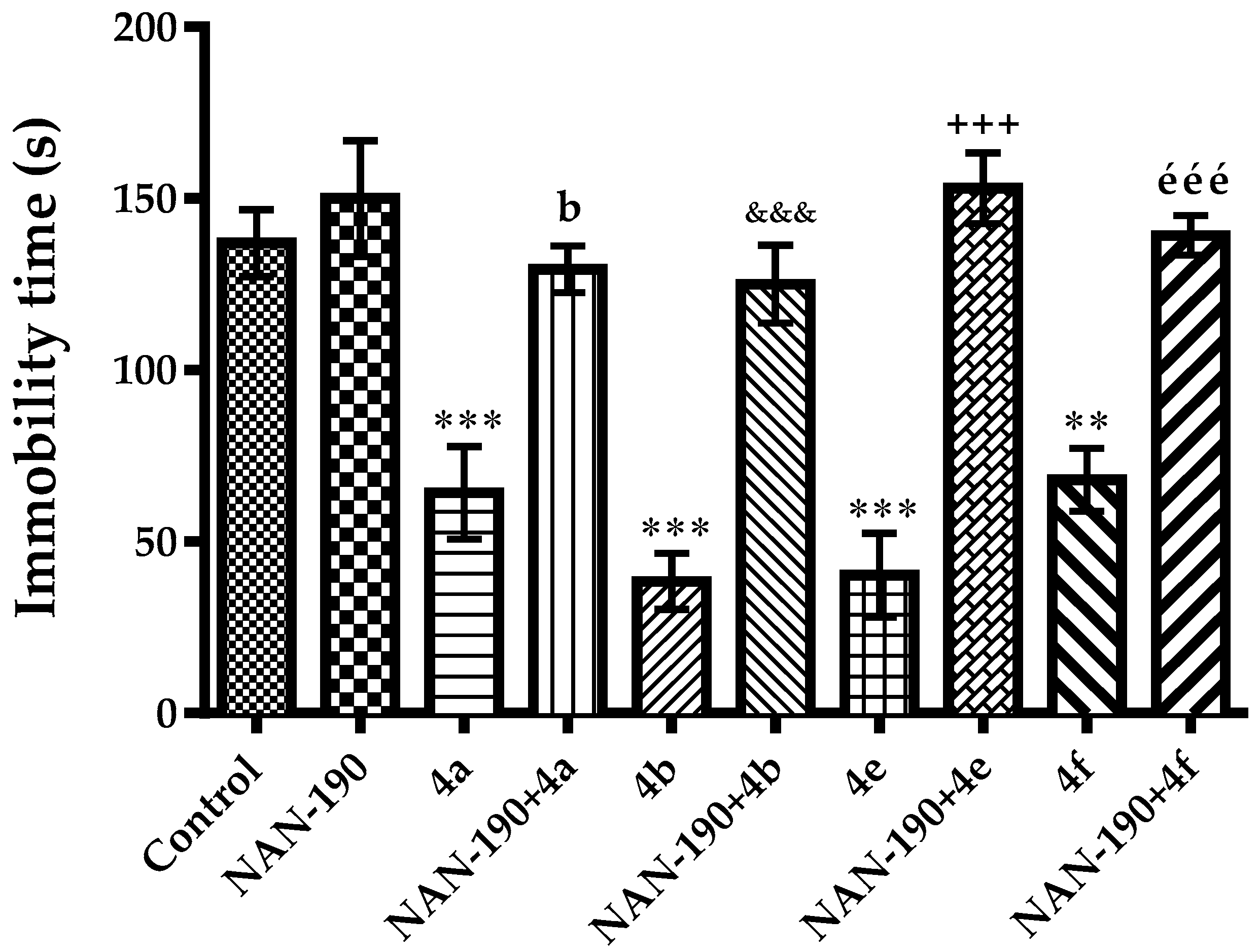
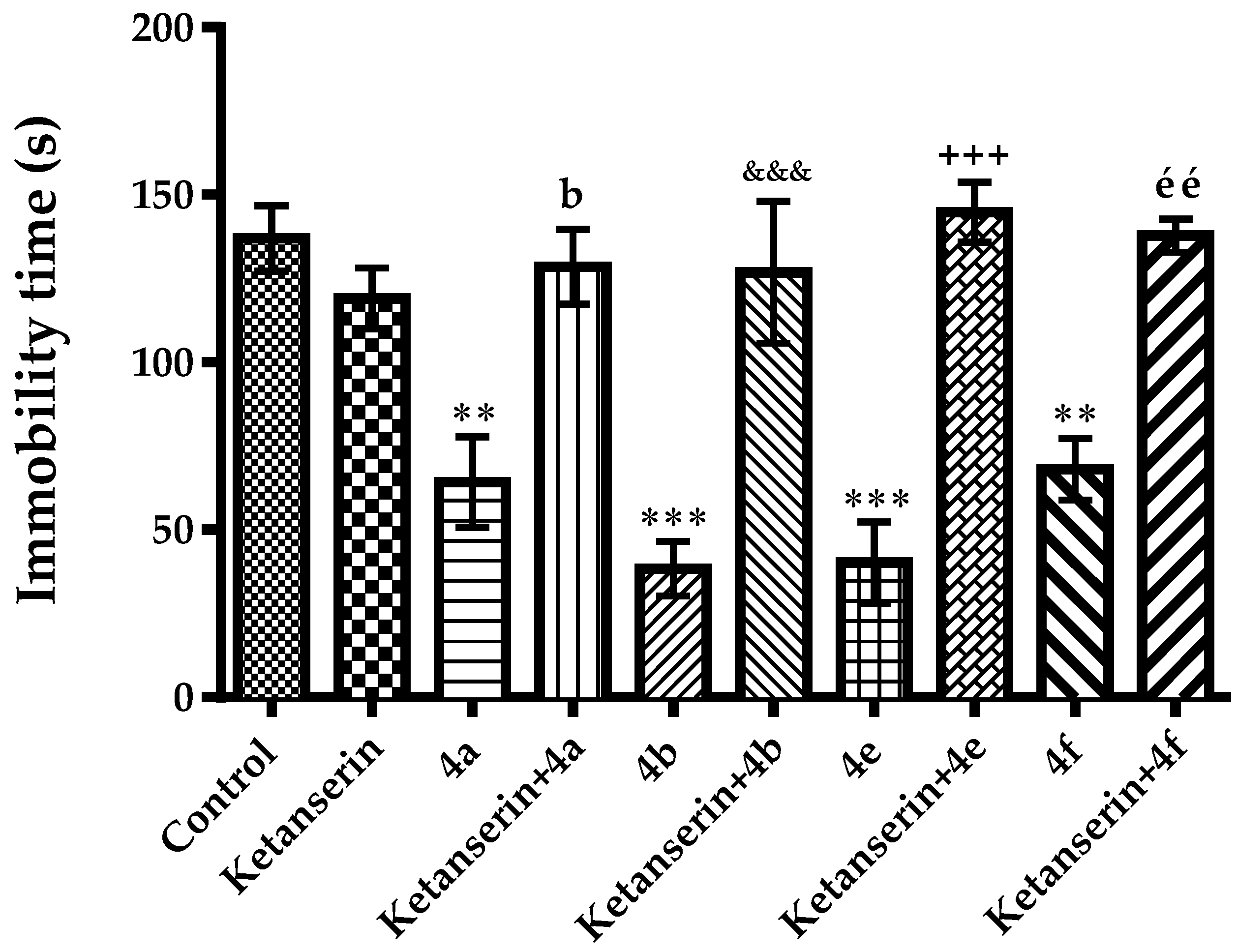
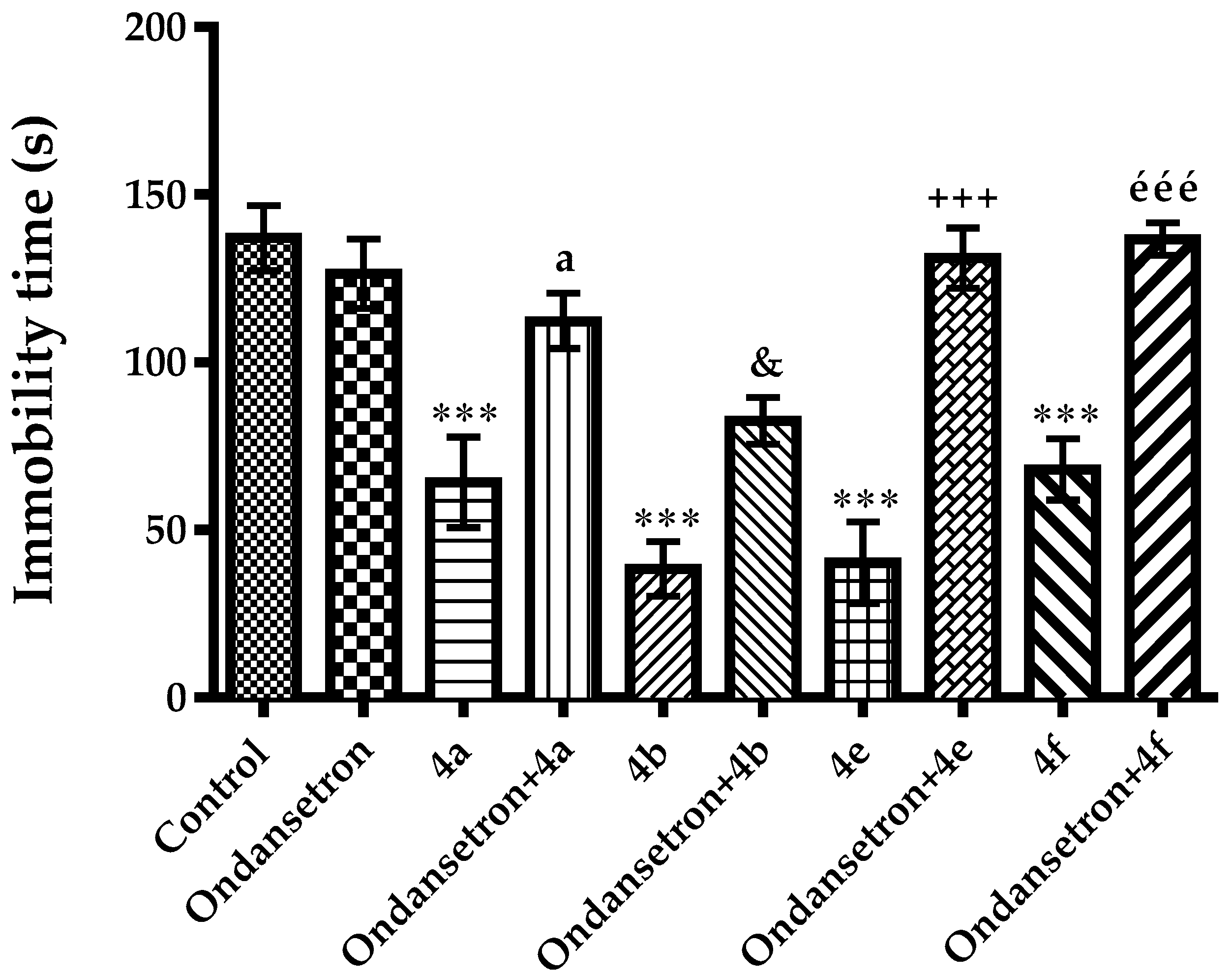
2.2. Pharmacology
3. Materials and Methods
3.1. Chemistry
3.1.1. Synthesis of 4-Substituted Benzaldehydes 1a–1d
3.1.2. Synthesis of Ethyl 2-(Benzazol-2-yl-thio)acetates 2a,2b
3.1.3. Synthesis of 2-((5-Substitutedbenzazol-2-yl)thio)acetohydrazides 3a,3b
3.1.4. General Procedure for the Synthesis of the Target Compounds 4a–4h
3.2. Pharmacology
3.2.1. Animals
3.2.2. Experimental Groups and Treatments
3.2.3. Evaluation of Antidepressant-Like Activity
Tail Suspension Test
Modified Forced Swimming Test
3.2.4. Evaluation of Spontaneous Locomotor Activity
Activity Cage Test
Mechanistic Studies
Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- WHO, mhGAP Intervention Guide for Mental, Neurological and Substance Use Disorders in Non-Specialized Health Settings. 2010. Available online: http://apps.who.int/iris/bitstream/10665/44406/1/9789241548069_eng.pdf (accessed on 1 May 2018).
- WHO, DEPRESSION: A Global Crisis World Mental Health Day. 2012. Available online: http://www.who.int/mental_health/management/depression/wfmh_paper_depression_wmhd_2012.pdf (accessed on 1 May 2018).
- Paschos, K.A.; Veletza, S.; Chatzaki, E. Neuropeptide and sigma receptors as novel therapeutic targets for the pharmacotherapy of depression. CNS Drugs 2009, 23, 755–772. [Google Scholar] [CrossRef] [PubMed]
- Yue, N.; Li, B.; Yang, L.; Han, Q.Q.; Huang, H.J.; Wang, Y.L.; Wang, J.; Yu, R.; Wu, G.C.; Liu, Q.; et al. Electro-Acupuncture Alleviates Chronic Unpredictable Stress-Induced Depressive- and Anxiety-Like Behavior and Hippocampal Neuroinflammation in Rat Model of Depression. Front. Mol. Neurosci. 2018. [Google Scholar] [CrossRef] [PubMed]
- Ajani, O.O.; Tolu-Bolaji, O.O.; Olorunshola, S.J.; Zhao, Y.; Aderohunmu, D.V. Structure-based design of functionalized 2-substituted and 1,2-disubstituted benzimidazole derivatives and their in vitro antibacterial efficacy. J. Adv. Res. 2017, 8, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.A.; Awadallah, F.M.; Refaat, H.M.; Amin, K.M. Molecular docking simulation, synthesis and 3D pharmacophore studies of novel 2-substituted-5-nitro-benzimidazole derivatives as anticancer agents targeting VEGFR-2 and c-Met. Bioorg. Chem. 2018, 77, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Yadav, D.; Yadav, M.; Dhamanage, A.; Kulkarni, S.; Singh, R.K. Molecular modeling, synthesis and biological evaluation of N-heteroaryl compounds as reverse transcriptase inhibitors against HIV-1. Chem. Biol. Drug Des. 2015, 85, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Noor, A.; Qazi, N.G.; Nadeem, H.; Khan, A.U.; Paracha, R.Z.; Ali, F.; Saeed, A. Synthesis, characterization, anti-ulcer action and molecular docking evaluation of novel benzimidazole-pyrazole hybrids. Chem. Cent. J. 2017, 11, 85. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, S.D.; Kumar, B.V.S.; Kumar, R.V.; Bhise, U.N.; Mashelkar, U.C. Synthesis, anti-bacterial, anti-asthmatic and anti-diabetic activities of novel N-substituted-2-(benzo[d]isoxazol-3-ylmethyl)-1H-benzimidazoles. J. Heterocycl. Chem. 2007, 44, 685–691. [Google Scholar] [CrossRef]
- Shingalapur, R.V.; Hosamani, K.M.; Keri, R.S.; Hugar, M.H. Derivatives of benzimidazole pharmacophore: Synthesis, anticonvulsant, antidiabetic and DNA cleavage studies. Eur. J. Med. Chem. 2010, 45, 1753–1759. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, I.N.; Hosamani, K.M.; Seetharamareddy, H.R.; Hugar, M.H. Synthesis and in-vivo evaluation of carbonyl-amide linkage based new benzimidazole derivatives. Arch. Pharm. 2012, 345, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Da, Y.; Wu, D.; Zheng, H.; Zhu, L.; Wang, L.; Yan, Y.; Chen, Z. Design, synthesis and biological evaluation of new 5-nitro benzimidazole derivatives as AT1 antagonists with anti-hypertension activities. Bioorg. Med. Chem. 2014, 22, 2294–2302. [Google Scholar] [CrossRef] [PubMed]
- Alpan, A.S.; Sarıkaya, G.; Çoban, G.; Parlar, S.; Armagan, G.; Alptüzün, V. Mannich-Benzimidazole Derivatives as Antioxidant and Anticholinesterase Inhibitors: Synthesis, Biological Evaluations, and Molecular Docking Study. Arch. Pharm. 2017. [Google Scholar] [CrossRef] [PubMed]
- Lad, N.P.; Manohar, Y.; Mascarenhas, M.; Pandit, Y.B.; Kulkarni, M.R.; Sharma, R.; Salkar, K.; Suthar, A.; Pandit, S.S. Methylsulfonyl benzothiazoles (MSBT) derivatives: Search for new potential antimicrobial and anticancer agents. Bioorg. Med. Chem. Lett. 2017, 27, 1319–1324. [Google Scholar] [CrossRef] [PubMed]
- Karali, N.; Güzel, O.; Ozsoy, N.; Ozbey, S.; Salman, A. Synthesis of new spiroindolinones incorporating a benzothiazole moiety as antioxidant agents. Eur. J. Med. Chem. 2010, 45, 1068–1077. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.J.; Khan, A.A.; Ali, Z.; Dewangan, R.P.; Rafi, M.; Hassan, M.Q.; Akhtar, M.S.; Siddiqui, A.A.; Partap, S.; Pasha, S.; et al. Synthesis of stable benzimidazole derivatives bearing pyrazole as anticancer and EGFR receptor inhibitors. Bioorg. Chem. 2018, 78, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Netalkar, P.P.; Netalkar, S.P.; Budagumpi, S.; Revankar, V.K. Synthesis, crystal structures and characterization of late first row transition metal complexes derived from benzothiazole core: Anti-tuberculosis activity and special emphasis on DNA binding and cleavage property. Eur. J. Med. Chem. 2014, 79, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Meltzer-Mats, E.; Babai-Shani, G.; Pasternak, L.; Uritsky, N.; Getter, T.; Viskind, O.; Eckel, J.; Cerasi, E.; Senderowitz, H.; Sasson, S.; et al. Synthesis and mechanism of hypoglycemic activity of benzothiazole derivatives. J. Med. Chem. 2013, 56, 5335–5350. [Google Scholar] [CrossRef] [PubMed]
- Azam, M.A.; Dharanya, L.; Mehta, C.C.; Sachdeva, S. Synthesis and biological evaluation of some novel pyrazolopyrimidines incorporating a benzothiazole ring system. Acta Pharm. 2013, 63, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Mute, V.M.; Bodhankar, S.L. Antidepressant like effect of newly synthesized compound 2[(N-benzylacetamido) mercapto] benzimidazole (vs 25) and its possible mechanism by inhibition of monoamine oxidase enzyme in mice. Int. J. Pharm. Pharm. Sci. 2015, 7, 407–410. [Google Scholar]
- Khan, I.; Tantray, M.A.; Hamid, H.; Alam, M.S.; Kalam, A.; Dhulap, A. Synthesis of benzimidazole based thiadiazole and carbohydrazide conjugates as glycogen synthase kinase-3β inhibitors with anti-depressant activity. Bioorg. Med. Chem. Lett. 2016, 26, 4020–4024. [Google Scholar] [CrossRef] [PubMed]
- Tantray, M.A.; Khan, I.; Hamid, H.; Alam, M.S.; Dhulap, A.; Kalam, A. Synthesis of benzimidazole-based 1,3,4-oxadiazole-1,2,3-triazole conjugates as glycogen synthase kinase-3b inhibitors with antidepressant activity in in vivo models. SC Adv. 2016, 6, 43345–43355. [Google Scholar]
- Kamil, A.; Akhtar, S.; Khan, A.; Farooq, E.; Nishan, U.; Uddin, R.; Farooq, U. Synthesis, structure–activity relationship and antinociceptive activities of some 2-(2′-pyridyl) benzimidazole derivatives. Med. Chem. Res. 2016, 25, 1216–1228. [Google Scholar] [CrossRef]
- Palin, R.; Clark, J.K.; Evans, L.; Houghton, A.K.; Jones, P.S.; Prosser, A.; Wishart, G.; Yoshiizumi, K. Structure-activity relationships and CoMFA of N-3 substituted phenoxypropyl piperidine benzimidazol-2-one analogues as NOP receptor agonists with analgesic properties. Bioorg. Med. Chem. 2008, 16, 2829–2851. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Sharma, P.K.; Rajak, H.; Pawar, R.S.; Patil, U.K.; Singour, P.K. Design, synthesis and biological evaluation of some novel benzimidazole derivatives for their potential anticonvulsant activity. Arch. Pharm. Res. 2010, 33, 971–980. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, N.; Rana, A.; Khan, S.A.; Ahsan, W.; Alam, M.S.; Ahmed, S. Analgesic and antidepressant activities of benzothiazole-benzamides. Biomed. Pharmacol. J. 2008, 1, 297–300. [Google Scholar]
- Wang, S.; Chen, Y.; Zhao, S.; Xu, X.; Liu, X.; Liu, B.F.; Zhang, G. Synthesis and biological evaluation of a series of benzoxazole/benzothiazole-containing 2,3-dihydrobenzo[b][1,4]dioxine derivatives as potential antidepressants. Bioorg. Med. Chem. Lett. 2014, 24, 1766–1770. [Google Scholar] [CrossRef] [PubMed]
- Ali, R.; Siddiqui, N. New benzo[d]thiazol-2-yl-aminoacetamides as potential anticonvulsants: Synthesis, activity and prediction of molecular properties. Arch. Pharm. 2015, 348, 254–265. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.C.; Zhang, H.J.; Jin, C.M.; Quan, Z.S. Synthesis and biological evaluation of novel benzothiazole derivatives as potential anticonvulsant agents. Molecules 2016, 21, 164. [Google Scholar] [CrossRef] [PubMed]
- Kaplancikli, Z.A.; Altintop, M.D.; Turan-Zitouni, G.; Ozdemir, A.; Can, O.D. Synthesis and analgesic activity of some acetamide derivatives. J. Enzyme Inhib. Med. Chem. 2012, 27, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, N.A.; Abdel-Aziz, H.A.; Kamel, G.M.; Fakhr, I.M. Convenient synthesis, anti-inflammatory, analgesic and ulcerogenic activites of some new bis-hydrazones and pyrazole derivatives. Acta Pol. Pharm. 2013, 70, 469–480. [Google Scholar] [PubMed]
- Gökce, M.I.; Cakir, B.; Erol, K.; Sahin, M.F. Synthesis and antinociceptive activity of [(2-oxobenzothiazolin-3-yl)methyl]-4-alkyl/aryl-1,2,4-triazoline-5-thiones. Arch. Pharm. 2001, 334, 279–283. [Google Scholar] [CrossRef]
- Ozkay, U.D.; Can, O.D.; Özkay, Y.; Öztürk, Y. Effect of benzothiazole/piperazine derivatives on intracerebroventricular streptozotocin-induced cognitive deficits. Pharmacol. Rep. 2012, 64, 834–847. [Google Scholar] [CrossRef]
- Keri, R.S.; Quintanova, C.; Marques, S.M.; Esteves, A.R.; Cardoso, S.M.; Santos, M.A. Design, synthesis and neuroprotective evaluation of novel tacrine-benzothiazole hybrids as multi-targeted compounds against Alzheimer’s disease. Bioorg. Med. Chem. 2013, 21, 4559–4569. [Google Scholar] [CrossRef] [PubMed]
- Carboni, S.; Hiver, A.; Szyndralewiez, C.; Gaillard, P.; Gotteland, J.P.; Vitte, P.A. AS601245 (1,3-benzothiazol-2 -yl(2-[[2-(3-pyridinyl)ethyl]amino]-4-pyrimidinyl)acetonitrile): A c-Jun NH2-terminal protein kinase inhibitor with neuroprotective properties. J. Pharmacol. Exp. Ther. 2004, 310, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Can, Ö.D.; Turan, N.; Demir Özkay, Ü.; Öztürk, Y. Antidepressant-like effect of gallic acid in mice: Dual involvement of serotonergic and catecholaminergic systems. Life Sci. 2017, 190, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Demir Özkay, Ü.; Yurttas, L.; Özkay, Y.; Üçel, U.I.; Can, Ö.D.; Öztürk, Y. Synthesis of new 1-phenyl-2-(4-substituted-piperazin-1-yl)-propanol derivatives and evaluation of their antidepressant-like effects. Arch. Pharm. Res. 2013, 36, 802–811. [Google Scholar] [CrossRef] [PubMed]
- Can, A.; Dao, D.T.; Terrillion, C.E.; Piantadosi, S.C.; Bhat, S.; Gould, T.D. The tail suspension test. J. Vis. Exp. 2012. [Google Scholar] [CrossRef] [PubMed]
- Slattery, D.A.; Cryan, J.F. Using the rat forced swim test to assess antidepressant-like activity in rodents. Nat. Protoc. 2012, 7, 1009–1014. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; Mombereau, C.; Vassout, A. The tail suspension test for assessing antidepressant activity: Review of pharmacological and genetic studies in mice. Neurosci. Biobehav. Rev. 2005, 29, 571–625. [Google Scholar] [CrossRef] [PubMed]
- Steru, L.; Chermat, R.; Thierry, B.; Simon, P. The tail suspension test: A new method for screening antidepressants in mice. Psychopharmacology 1985, 85, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Brocardo, P.S.; Budni, J.; Kaster, M.P.; Santos, A.R.; Rodrigues, A.L. Folic acid administration produces an antidepressant-like effect in mice: Evidence for the involvement of the serotonergic and noradrenergic systems. Neuropharmacology 2008, 54, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Girish, C.; Raj, V.; Arya, J. Balakrishnan, S. Evidence for the involvement of the monoaminergic system, but not the opioid system in the antidepressant-like activity of ellagic acid in mice. Eur. J. Pharmacol. 2012, 682, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Porsolt, R.D.; Bertin, A.; Jalfre, M. Behavioral despair in mice: A primary screening test for antidepressants. Arch. Int. Pharmacodyn. Ther. 1977, 229, 327–336. [Google Scholar] [PubMed]
- Detke, M.J.; Lucki, I. Detection of serotonergic and noradrenergic antidepressants in the rat forced swimming test: The effects of water depth. Behav. Brain Res. 1996, 73, 43–46. [Google Scholar] [CrossRef]
- Aksoz, E.; Aksoz, T.; Bilge, S.S.; Ilkaya, F.; Celik, S.; Diren, H.B. Antidepressant-like effects of echo-planar magnetic resonance imaging in mice determined using the forced swimming test. Brain Res. 2008, 1236, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; Markou, A.; Lucki, I. Assessing antidepressant activity in rodents: Recent developments and future needs. Trends Pharmacol. Sci. 2002, 23, 238–245. [Google Scholar] [CrossRef]
- Nakatomi, Y.; Yokoyama, C.; Kinoshita, S.; Masaki, D.; Tsuchida, H.; Onoe, H.; Yoshimoto, K.; Fukui, K. Serotonergic mediation of the antidepressant-like effect of the green leaves odor in mice. Neurosci. Lett. 2008, 436, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Abreu, T.M.; Monteiro, V.S.; Martins, A.B.S.; Teles, F.B.; da Conceição Rivanor, R.L.; Mota, É.F.; Macedo, D.S.; De Vasconcelos, S.M.M.; Júnior, J.E.R.H.; Benevides, N.M.B. Involvement of the dopaminergic system in the antidepressant-like effect of the lectin isolated from the red marine alga Solieria filiformis in mice. Int. J. Biol. Macromol. 2018, 111, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Castagné, V.; Moser, P.; Roux, S.; Porsolt, R.D. Rodent models of depression: Forced swim and tail suspension behavioral despair tests in rats and mice. Curr. Protoc. Neurosci. 2011. [Google Scholar] [CrossRef]
- Koe, B.K.; Weissman, A. p-Chlorophenylalanine: A specific depletor of brain serotonin. J. Pharmacol. Exp. Ther. 1966, 154, 499–516. [Google Scholar] [PubMed]
- Redrobe, J.P.; Bourin, M.; Colombel, M.C.; Baker, G.B. Dose-dependent noradrenergic and serotonergic properties of venlafaxine in animal models indicative of antidepressant activity. Psychopharmacology 1998, 138, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Redrobe, J.P.; Bourin, M.; Colombel, M.C.; Baker, G.B. Psychopharmacological profile of the selective serotonin reuptake inhibitor, paroxetine: Implication of noradrenergic and serotonergic mechanisms. J. Psychopharmacol. 1998, 12, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Lee, B.; Kim, M.; Lee, H.; Park, H.J.; Hahm, D.H. Antidepressant-like effect of the methanolic extract from Bupleurum falcatum in the tail suspension test. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2010, 34, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Can, Ö.D.; Demir Özkay, Ü.; Üçel, U.İ. Anti-depressant-like effect of vitexin in BALB/c mice and evidence for the involvement of monoaminergic mechanisms. Eur J. Pharmacol. 2013, 699, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Lian, L.; Xu, Y.; Zhang, J.; Yu, Y.; Zhu, N.; Guan, X.; Huang, H.; Chen, R.; Chen, J.; Shi, G.; et al. Antidepressant-like effects of a novel curcumin derivative J147: Involvement of 5-HT1A receptor. Neuropharmacology 2018, 135, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Xu, Y.; Wu, H.L.; Li, Y.B.; Li, Y.H.; Guo, J.B.; Li, X.J. The antidepressant effects of curcumin in the forced swimming test involve 5-HT1 and 5-HT2 receptors. Eur. J. Pharmacol. 2008, 578, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Karim, N.; Khan, I.; Abdelhalim, A.; Khan, A.; Halim, S.A. Antidepressant potential of novel flavonoids derivatives from sweet violet (Viola odorata L): Pharmacological, biochemical and computational evidences for possible involvement of serotonergic mechanism. Fitoterapia 2018, 128, 148–161. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.L.; Wang, Y.P.; Zheng, J.Y.; Zhang, L.X. Monoaminergic and aminoacidergic receptors are involved in the antidepressant-like effect of ginsenoside Rb1 in mouse hippocampus (CA3) and prefrontal cortex. Brain Res. 2018, 1699, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Jin, Y.; Fu, W.; Xiao, S.; Feng, C.; Fang, B.; Gu, Y.; Li, C.; Zhao, Y.; Liu, Z.; et al. Design, Synthesis, and Structure-Activity Relationship Analysis of Thiazolo [3,2-a]pyrimidine Derivatives with Anti-inflammatory Activity in Acute Lung Injury. Chem. Med. Chem. 2017, 12, 1022–1032. [Google Scholar] [CrossRef] [PubMed]
- Faraji, L.; Shahkarami, S.; Nadri, H.; Moradi, A.; Saeedi, M.; Foroumadi, A.; Ramazani, A.; Haririan, I.; Ganjali, M.R.; Shafiee, A.; et al. Synthesis of novel benzimidazole and benzothiazole derivatives bearing a 1,2,3-triazole ring system and their acetylcholinesterase inhibitory activity. J. Chem. Res. 2017, 41, 1–63. [Google Scholar] [CrossRef]
- Al Omran, F.; Abou El-Khair, A. Synthesis, spectroscopy and X-ray characterization of novel derivatives of substituted 2-(benzothiazol-2′-ylthio)acetohydrazide. IJOC 2016, 6, 31–43. [Google Scholar] [CrossRef]
- Bhatt, J.D.; Nimavat, K.S.; Vyas, K.B. Studies on novel thiazolidinone and their biological studies. J. Chem. Pharm. Res. 2013, 5, 327–331. [Google Scholar]
- Sanmukhani, J.; Anovadiya, A.; Tripathi, C.B. Evaluation of antidepressant like activity of curcumin and its combination with fluoxetine and imipramine: An acute and chronic study. Acta Pol. Pharm. 2011, 68, 769–775. [Google Scholar] [PubMed]
- Tanaka, M.; Telegdy, G. Involvement of adrenergic and serotonergic receptors in antidepressant-like effect of urocortin 3 in a modified forced swimming test in mice. Brain Res. Bull. 2008, 77, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Votava, M.; Hess, L.; Slíva, J.; Krsiak, M.; Agová, V. Dexmedetomidine selectively suppresses dominant behaviour in aggressive and sociable mice. Eur. J. Pharmacol. 2005, 523, 79–85. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
| Comp. | X | R |
| 4a | -NH | Methyl |
| 4b | -NH | Ethyl |
| 4c | -NH | Isopropyl |
| 4d | -NH | Cyclopropyl |
| 4e | -S | Methyl |
| 4f | -S | Ethyl |
| 4g | -S | Isopropyl |
| 4h | -S | Cyclopropyl |
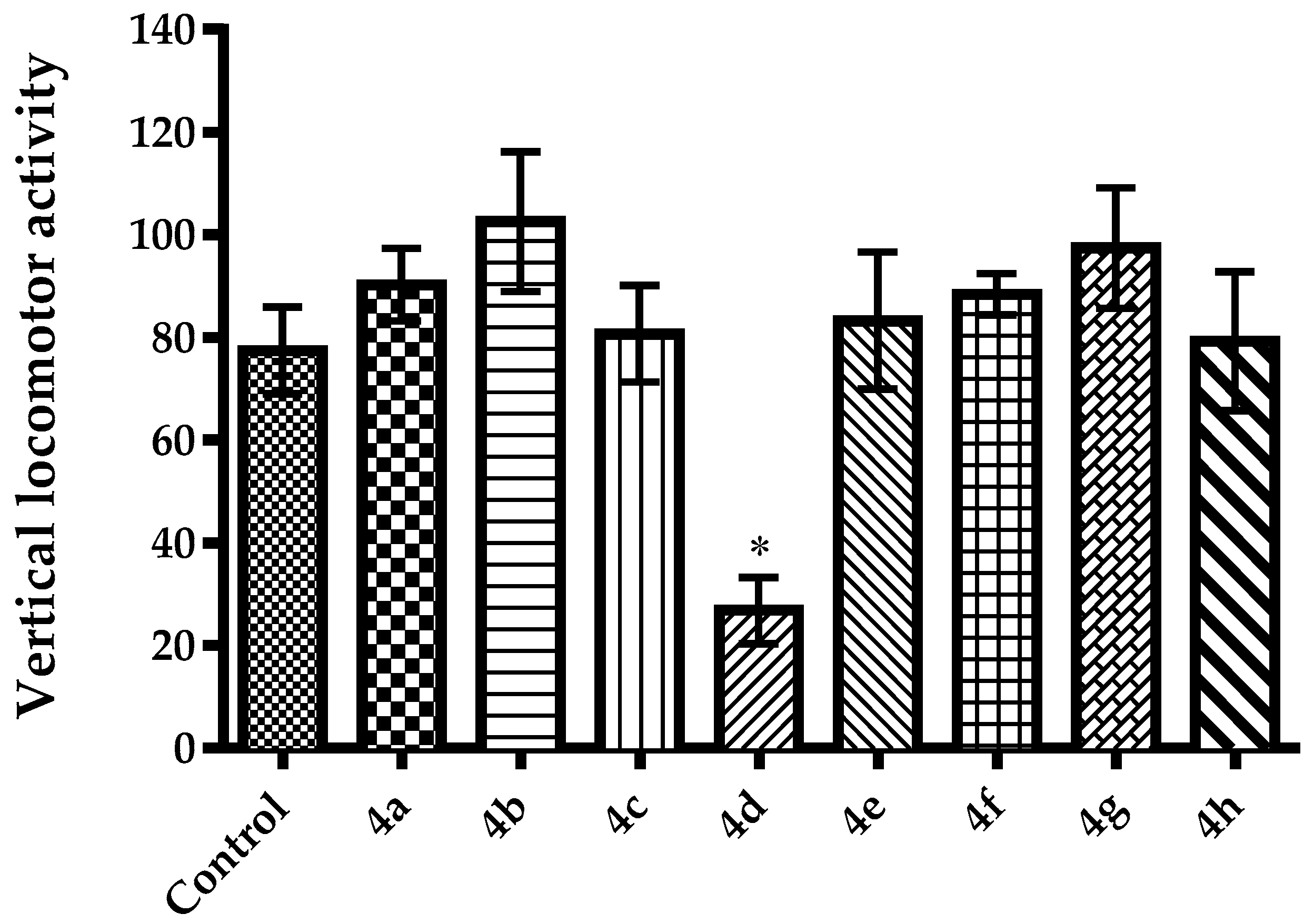
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tokgöz, G.; Demir Özkay, Ü.; Osmaniye, D.; Turan Yücel, N.; Can, Ö.D.; Kaplancıklı, Z.A. Synthesis of Novel Benzazole Derivatives and Evaluation of Their Antidepressant-Like Activities with Possible Underlying Mechanisms. Molecules 2018, 23, 2881. https://doi.org/10.3390/molecules23112881
Tokgöz G, Demir Özkay Ü, Osmaniye D, Turan Yücel N, Can ÖD, Kaplancıklı ZA. Synthesis of Novel Benzazole Derivatives and Evaluation of Their Antidepressant-Like Activities with Possible Underlying Mechanisms. Molecules. 2018; 23(11):2881. https://doi.org/10.3390/molecules23112881
Chicago/Turabian StyleTokgöz, Gamze, Ümide Demir Özkay, Derya Osmaniye, Nazlı Turan Yücel, Özgür Devrim Can, and Zafer Asım Kaplancıklı. 2018. "Synthesis of Novel Benzazole Derivatives and Evaluation of Their Antidepressant-Like Activities with Possible Underlying Mechanisms" Molecules 23, no. 11: 2881. https://doi.org/10.3390/molecules23112881
APA StyleTokgöz, G., Demir Özkay, Ü., Osmaniye, D., Turan Yücel, N., Can, Ö. D., & Kaplancıklı, Z. A. (2018). Synthesis of Novel Benzazole Derivatives and Evaluation of Their Antidepressant-Like Activities with Possible Underlying Mechanisms. Molecules, 23(11), 2881. https://doi.org/10.3390/molecules23112881




