Nanoliposomes as Vehicles for Astaxanthin: Characterization, In Vitro Release Evaluation and Structure
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Astaxanthin-Loaded Nanoliposomes
2.1.1. Determination of Encapsulation Efficiency and Particle Size
2.1.2. Morphology of Astaxanthin-Loaded Nanoliposomes
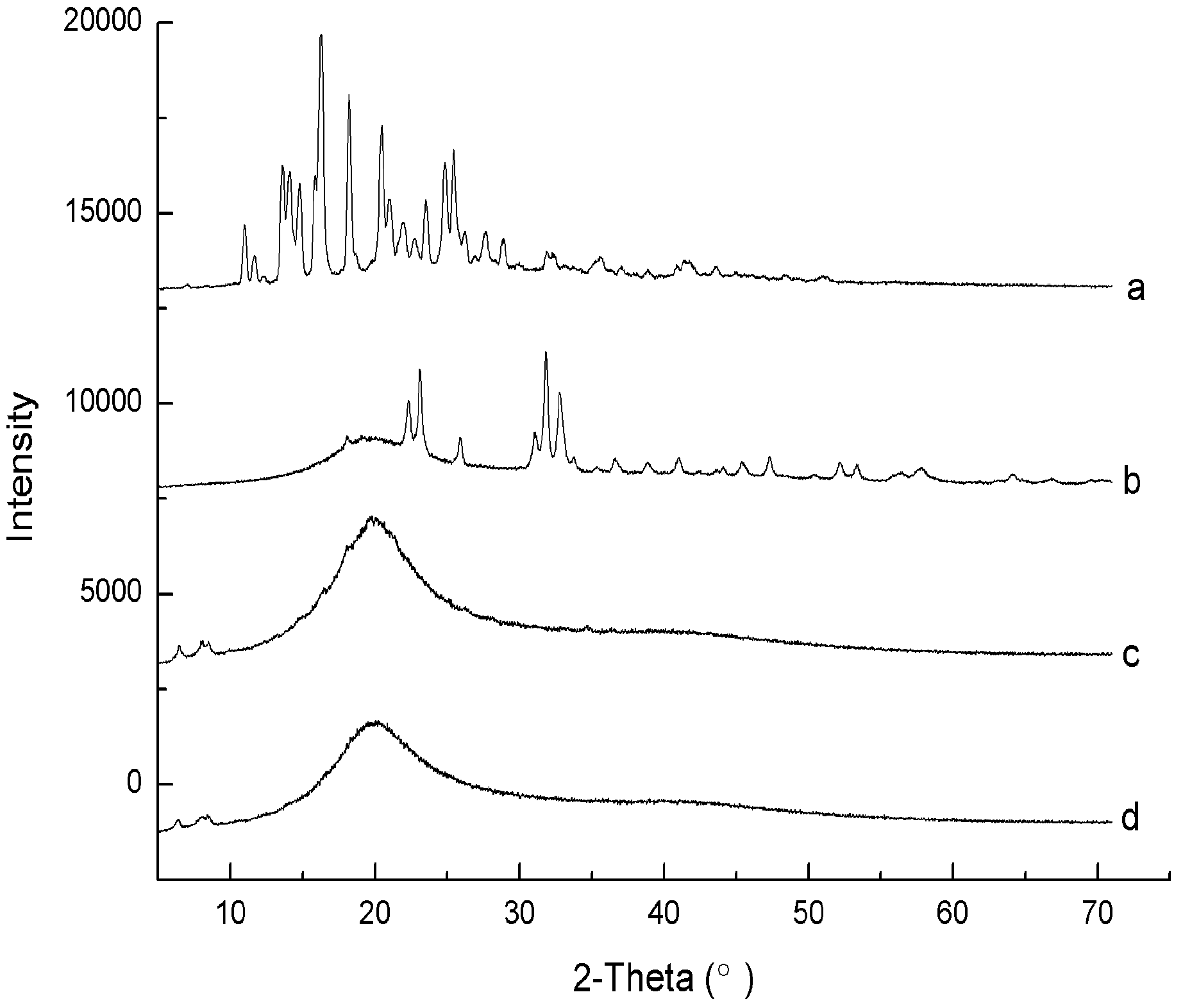
2.1.3. X-ray Diffraction Analysis
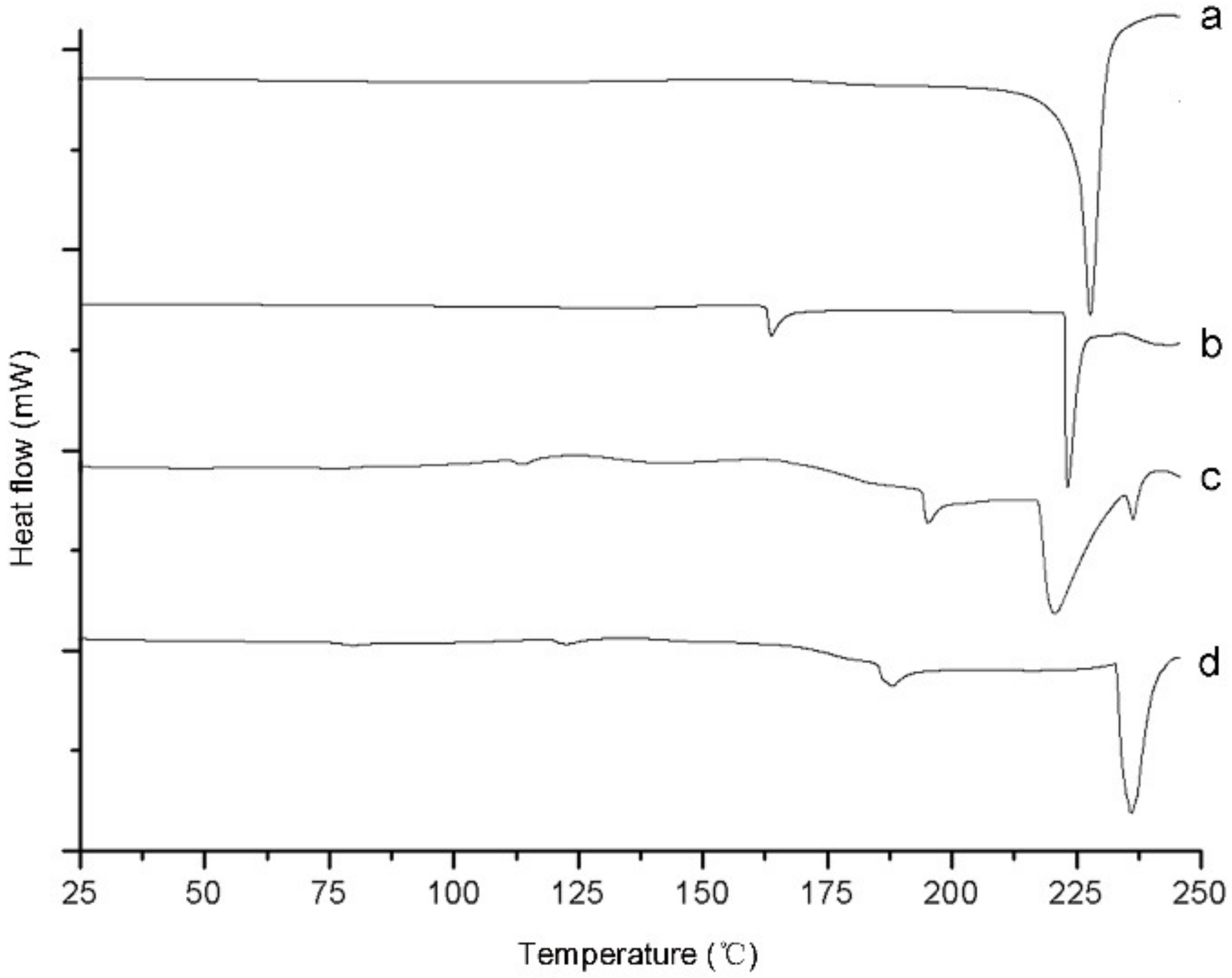
2.1.4. Differential Scanning Calorimetry Analysis
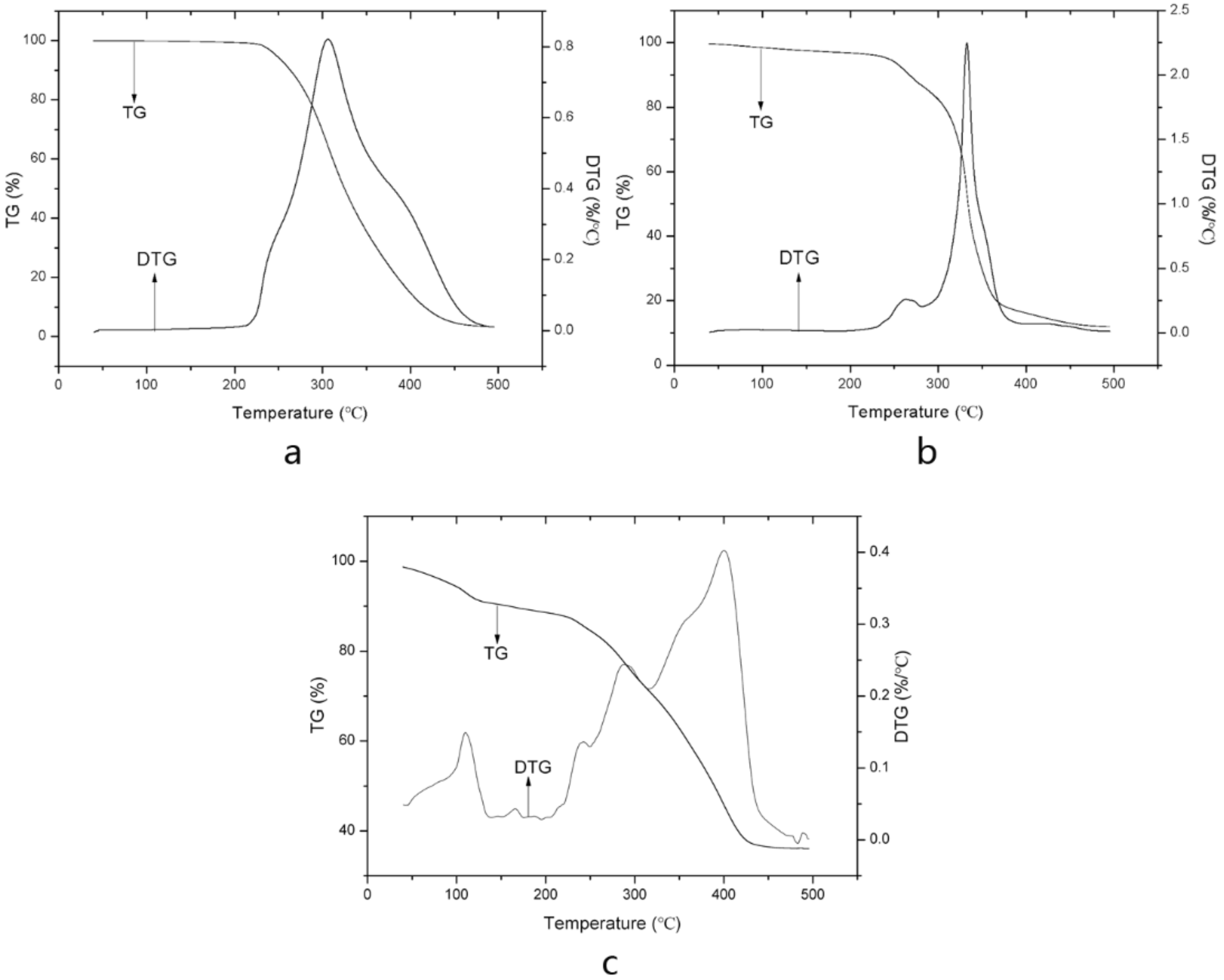
2.1.5. Thermal Gravimetric Analysis
2.1.6. Water Solubility Analysis
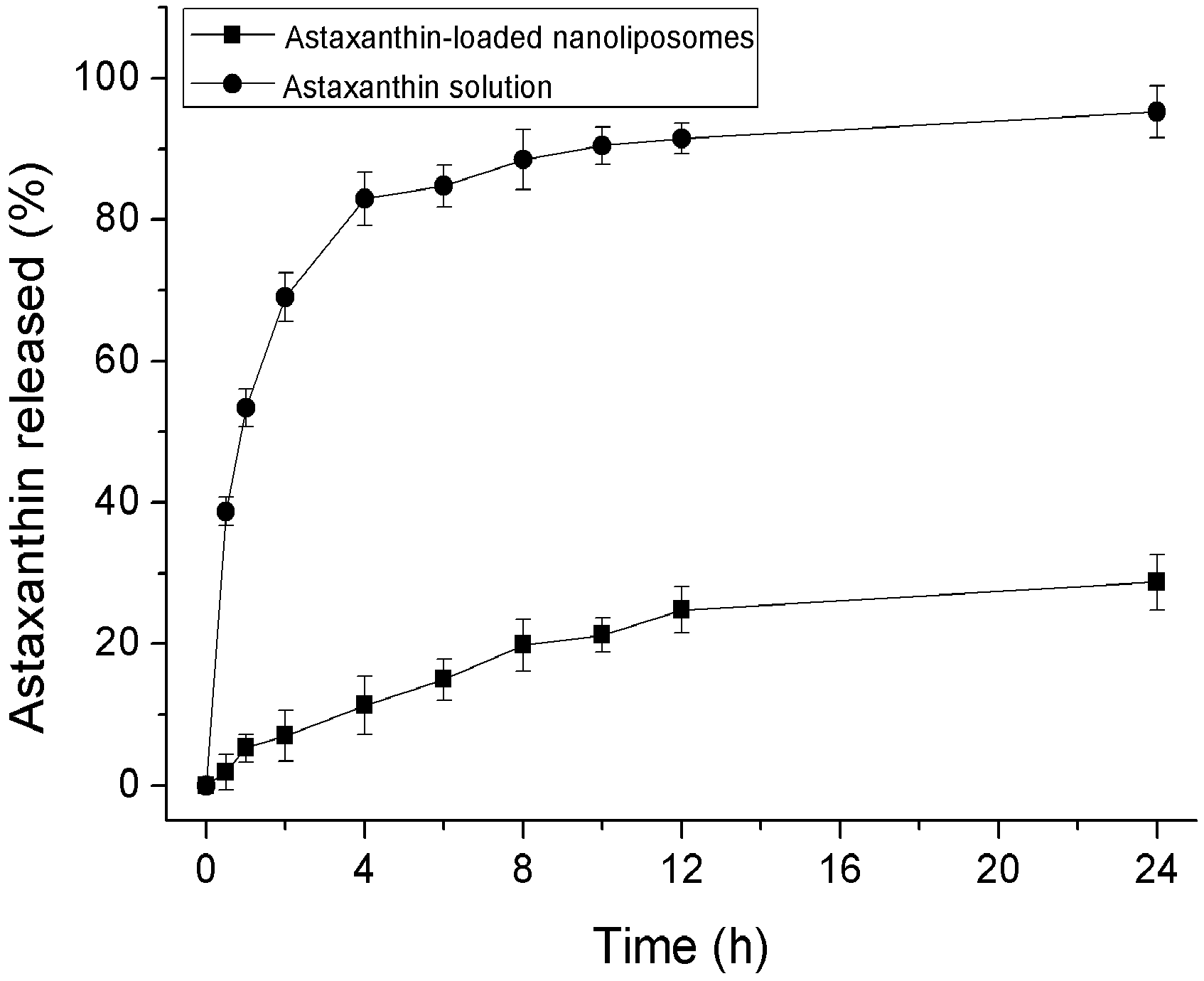
2.2. In Vitro Release
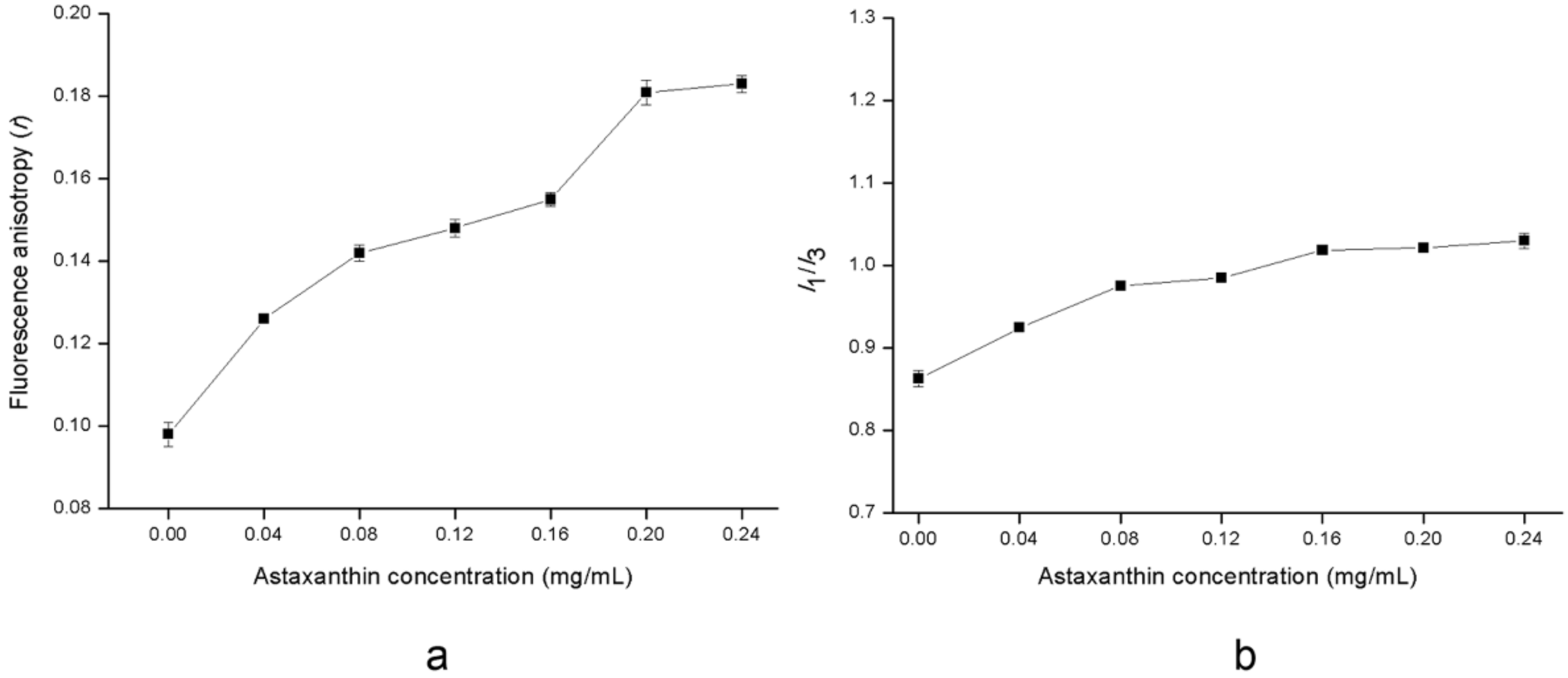
2.3. Effects of Astaxanthin Incorporation on Structural Changes of the Liposomal Membrane
3. Materials and Methods
3.1. Materials
3.2. Preparation of Astaxanthin-Loaded Nanoliposomes
3.3. Characterization of Astaxanthin-Loaded Nanoliposomes
3.3.1. Encapsulation Efficiency Measurement
3.3.2. Particle Size Analysis
3.3.3. Morphological Study
3.3.4. X-ray Diffraction
3.3.5. Differential Scanning Calorimetry
3.3.6. Thermal Gravimetric Analysis
3.3.7. Water-Solubility Study
3.4. In Vitro Release
3.5. Steady-State Fluorescence Determination
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tolasa, S.; Cakli, S.; Ostermeyer, U. Determination of astaxanthin and canthaxanthin in salmonid. Eur. Food Res. Technol. 2005, 221, 787–791. [Google Scholar] [CrossRef]
- Bhatt, P.C.; Ahmad, M.; Panda, B.P. Enhanced bioaccumulation of astaxanthin in Phaffia rhodozyma by utilising low-cost agro products as fermentation substrate. Biocatal. Agric. Biotechnol. 2013, 2, 58–63. [Google Scholar] [CrossRef]
- Park, J.S.; Chyun, J.H.; Kim, Y.K.; Line, L.L.; Chew, B.P. Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Nutr. Metab. 2010, 7, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higueraciapara, I.; Félixvalenzuela, L.; Goycoolea, F.M. Astaxanthin: A review of its chemistry and applications. Crit. Rev. Food Sci. Nutr. 2006, 46, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Bustos-Garza, C.; Barragán-Huerta, B.E. Thermal and pH stability of spray-dried encapsulated astaxanthin oleoresin from Haematococcus pluvialis using several encapsulation wall materials. Food Res. Int. 2013, 54, 641–649. [Google Scholar] [CrossRef]
- Park, S.A.; Ahn, J.B.; Choi, S.H.; Lee, J.S.; Lee, H.G. The effects of particle size on the physicochemical properties of optimized astaxanthin-rich Xanthophyllomyces dendrorhous-loaded microparticles. LWT-Food Sci. Technol. 2014, 55, 638–644. [Google Scholar] [CrossRef]
- Li, M.; Zahi, M.R.; Yuan, Q.; Tian, F.; Liang, H. Preparation and stability of astaxanthin solid lipid nanoparticles based on stearic acid. Eur. J. Lipid Sci. Technol. 2016, 118, 592–602. [Google Scholar] [CrossRef]
- Tachaprutinun, A.; Udomsup, T.; Luadthong, C.; Wanichwecharungruang, S. Preventing the thermal degradation of astaxanthin through nanoencapsulation. Int. J. Pharm. 2009, 374, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Anarjan, N.; Tan, C.P. Chemical stability of astaxanthin nanodispersions in orange juice and skimmed milk as model food systems. Food Chem. 2013, 139, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Quek, S.Y. Microencapsulation of astaxanthin with blends of milk protein and fiber by spray drying. J. Food Eng. 2014, 123, 165–171. [Google Scholar] [CrossRef]
- Apanasenko, I.E.; Selyutina, O.Y.; Polyakov, N.E.; Suntsova, L.P.; Meteleva, E.S.; Dushkin, A.V.; Vachali, P.; Bernstein, P.S. Solubilization and stabilization of macular carotenoids by water soluble oligosaccharides and polysaccharides. Arch. Biochem. Biophys. 2015, 572, 58–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polyakov, N.E.; Kispert, L.D. Water soluble biocompatible vesicles based on polysaccharides and oligosaccharides inclusion complexes for carotenoid delivery. Carbohydr. Polym. 2015, 128, 207–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polyakov, N.E.; Magyar, A.; Kispert, L.D. Photochemical and optical properties of water-soluble xanthophyll antioxidants: Aggregation vs complexation. J. Phys. Chem. B. 2013, 117, 10173–10182. [Google Scholar] [CrossRef] [PubMed]
- Polyakov, N.E.; Leshina, T.V.; Meteleva, E.S.; Dushkin, A.V.; Konovalova, T.A.; Kispert, L.D. Water soluble complexes of carotenoids with arabinogalactan. J. Phys. Chem. B 2009, 113, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhao, Y.; Guan, L.; Zhang, Y.; Dang, Q.; Dong, P.; Li, J.; Liang, X. Preparation of astaxanthin-loaded DNA/chitosan nanoparticles for improved cellular uptake and antioxidation capability. Food Chem. 2017, 227, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Zhang, Y.; Abbas, S.; Feng, B.; Zhang, X.; Xia, S. Modulation of the carotenoid bioaccessibility through liposomal encapsulation. Colloids Surf. B 2014, 123, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Feng, B.; Zhang, X.; Xia, W.; Xia, S. Biopolymer-coated liposomes by electrostatic adsorption of chitosan (chitosomes) as novel delivery systems for carotenoids. Food Hydrocoll. 2016, 52, 774–784. [Google Scholar] [CrossRef]
- Taksima, T.; Limpawattana, M.; Klaypradit, W. Astaxanthin encapsulated in beads using ultrasonic atomizer andapplication in yogurt as evaluated by consumer sensory profile. LWT-Food Sci. Technol. 2015, 62, 431–437. [Google Scholar] [CrossRef]
- Tamjidi, F.; Shahedi, M.; Varshosaz, J.; Nasirpour, A. Design and characterization of astaxanthin-loaded nanostructured lipid carriers. Innov. Food Sci. Emerg. 2014, 26, 366–374. [Google Scholar] [CrossRef]
- Zhao, L.; Temelli, F.; Curtis, J.M.; Chen, L. Preparation of liposomes using supercritical carbon dioxide technology: Effects of phospholipids and sterols. Food Res. Int. 2015, 77, 63–72. [Google Scholar] [CrossRef]
- Bai, C.; Peng, H.; Xiong, H.; Liu, Y.; Zhao, L.; Xiao, X. Carboxymethylchitosan-coated proliposomes containing coix seed oil: Characterisation, stability and in vitro release evaluation. Food Chem. 2011, 129, 1695–1702. [Google Scholar] [CrossRef]
- Bochicchio, S.; Barba, A.A.; Grassi, G.; Lamberti, G. Vitamin delivery: Carriers based on nanoliposomes produced via ultrasonic irradiation. LWT-Food Sci. Technol. 2016, 69, 9–16. [Google Scholar] [CrossRef]
- Pan, L.; Zhang, S.; Gu, K.; Zhang, N. Preparation of astaxanthin-loaded liposomes: Characterization, storage stability and antioxidant activity. CyTA-J. Food. 2018, 16, 607–618. [Google Scholar] [CrossRef]
- Formariz, T.P.; Chiavacci, L.A.; Sarmento, V.H.V.; Franzini, C.M.; Silva, A.A., Jr.; Scarpa, M.V.; Santilli, C.V.; Egito, E.S.T.; Oliveira, A.G. Structural changes of biocompatible neutral microemulsions stabilized by mixed surfactant containing soya phosphatidylcholine and their relationship with doxorubicin release. Colloids Surf. B 2008, 63, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Luo, Z.; Xiao, Z. Preparation, characterization, and thermal stability of β-cyclodextrin/soybean lecithin inclusion complex. Carbohydr. Polym. 2014, 101, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Jin, Z.; Xu, X.; Zhuang, H.; Shen, W. Preparation and stability of the inclusion complex of astaxanthin with hydroxypropyl-β-cyclodextrin. Food Chem. 2008, 109, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Bai, C.; Peng, H.; Xiong, H.; Zhu, X.; Hu, J.; Zhao, S.; Luo, G.; Zhao, Q. Characterisation, stability and in vitro degradation of microcapsules containing Chinese yak (Poephagus grunniens L.) butter. Int. J. Food Sci. Technol. 2013, 48, 826–834. [Google Scholar] [CrossRef]
- Gong, K.J.; Shi, A.M.; Liu, H.Z.; Liu, L.; Hu, H.; Yang, Y.; Adhikari, B.; Wang, Q. Preparation of nanoliposome loaded with peanut peptide fraction: Stability and bioavailability. Food Funct. 2016, 7, 2034–2042. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Li, D.C.; Jiang, J.G. Preparation of a tea polyphenol nanoliposome system and its physicochemical properties. J. Agric. Food Chem. 2011, 59, 13004. [Google Scholar] [CrossRef] [PubMed]
- Shibata, A.; Kiba, Y.; Akati, N.; Fukuzawa, K.; Terada, H. Molecular characteristics of astaxanthin and β-carotene in the phospholipid monolayer and their distributions in the phospholipid bilayer. Chem. Phys. Lipids 2001, 113, 11–22. [Google Scholar] [CrossRef]
- Gruszecki, W.I.; Strzałka, K. Carotenoids as modulators of lipid membrane physical properties. Biochim. Biophys. Acta 2005, 1740, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Ioffe, V.; Gorbenko, G.P. Lysozyme effect on structural state of model membranes as revealed by pyrene excimerization studies. Biophys. Chem. 2005, 114, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Qiu, D.; An, X.; Chen, Z.; Ma, X. Microstructure study of liposomes decorated by hydrophobic magnetic nanoparticles. Chem. Phys. Lipids 2012, 165, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Asprea, M.; Leto, I.; Bergonzi, M.C.; Bilia, A.R. Thyme essential oil loaded in nanocochleates: Encapsulation efficiency, in vitro release study and antioxidant activity. LWT-Food Sci. Technol. 2017, 77, 497–502. [Google Scholar] [CrossRef]
- Zou, L.; Zheng, B.; Zhang, R.; Zhang, Z.; Liu, W.; Liu, C.; Zhang, G.; Xiao, H.; Mcclements, D.J. Influence of lipid phase composition of excipient emulsions on curcumin solubility, stability, and bioaccessibility. Food Biophys. 2016, 11, 213–225. [Google Scholar] [CrossRef]
- Suzuki, M.; Miura, T. Effect of amyloid β-peptide on the fluidity of phosphatidylcholine membranes: Uses and limitations of diphenylhexatriene fluorescence anisotropy. Biochim. Biophys. Acta 2015, 1848, 753–759. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, L.; Wang, H.; Gu, K. Nanoliposomes as Vehicles for Astaxanthin: Characterization, In Vitro Release Evaluation and Structure. Molecules 2018, 23, 2822. https://doi.org/10.3390/molecules23112822
Pan L, Wang H, Gu K. Nanoliposomes as Vehicles for Astaxanthin: Characterization, In Vitro Release Evaluation and Structure. Molecules. 2018; 23(11):2822. https://doi.org/10.3390/molecules23112822
Chicago/Turabian StylePan, Li, Hongyan Wang, and Keren Gu. 2018. "Nanoliposomes as Vehicles for Astaxanthin: Characterization, In Vitro Release Evaluation and Structure" Molecules 23, no. 11: 2822. https://doi.org/10.3390/molecules23112822




