Preclinical Pharmacokinetics of Scoparone, Geniposide and Rhein in an Herbal Medicine Using a Validated LC-MS/MS Method
Abstract
1. Introduction
2. Results
2.1. LC-MS/MS Optimization Conditions
2.2. Method Validation
2.2.1. Specificity and Selectivity
2.2.2. Linearity and the Limits of Detection and Quantification
2.2.3. Precision and Accuracy
2.2.4. Extraction Recovery and Matrix Effect
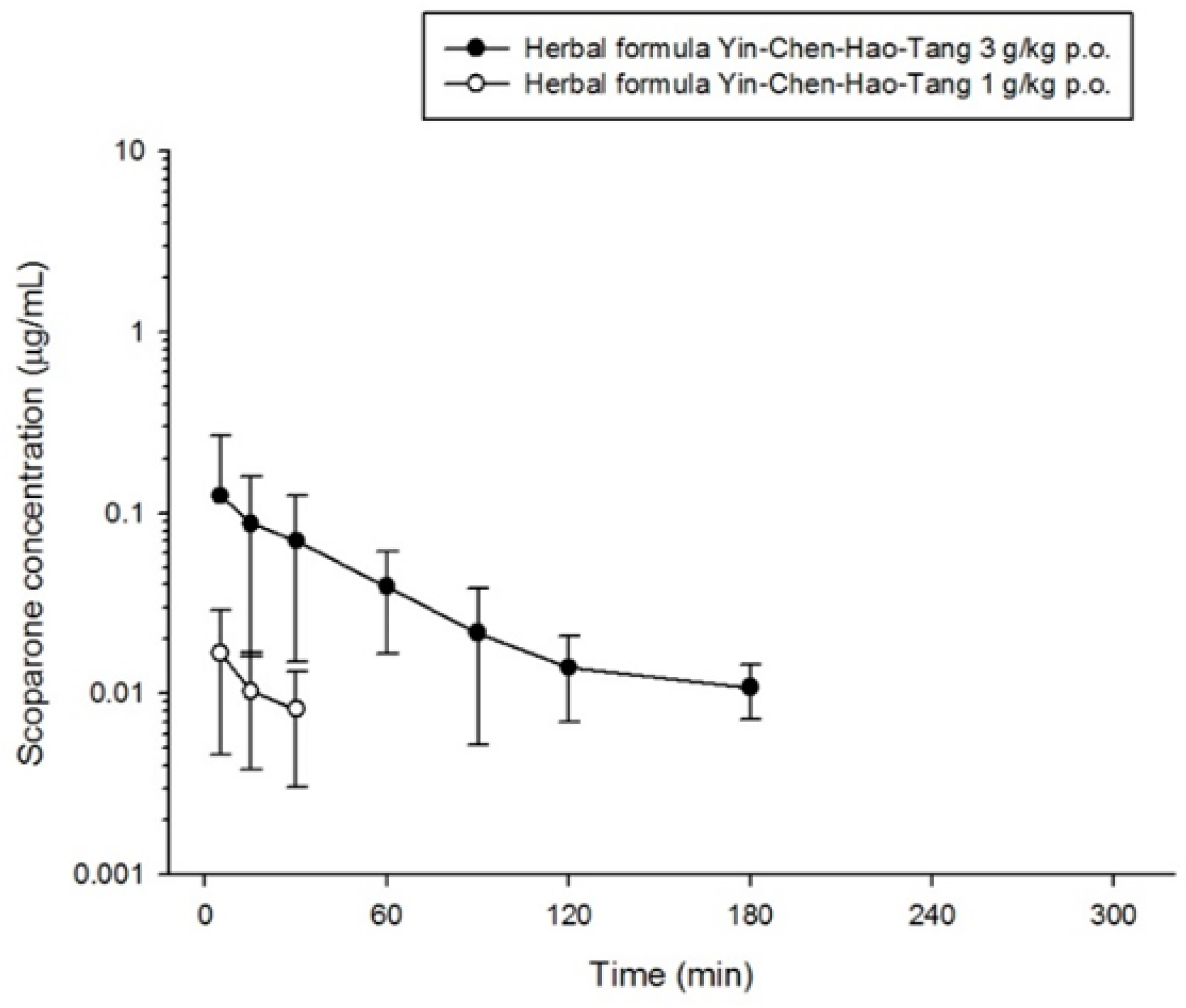
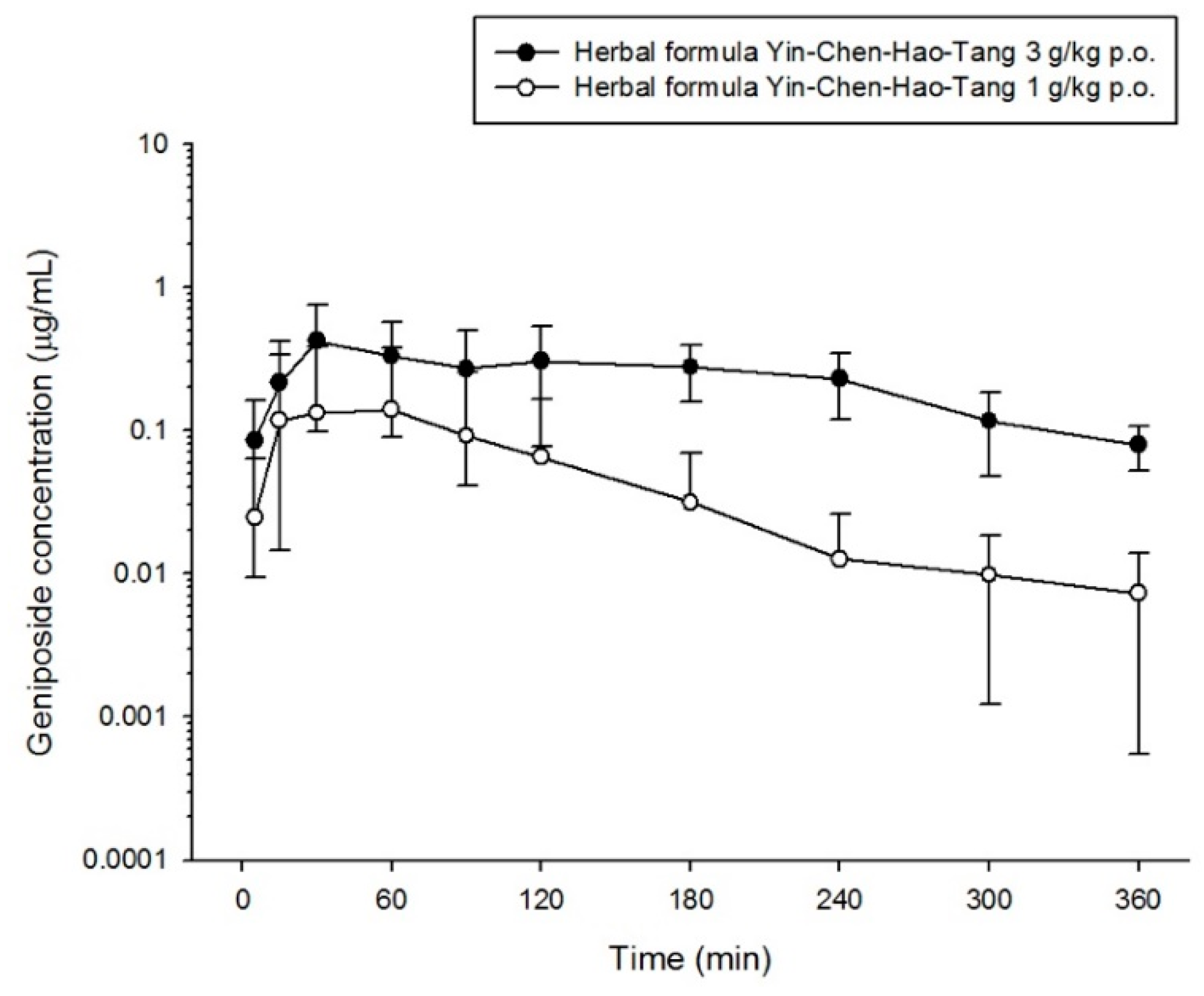
2.3. Pharmacokinetics of Scoparone, Geniposide and Rhein in Rats
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Liquid Chromatography and Tandem Mass Spectrometry
4.3. Working Solutions and Quality Control (QC) Samples
4.4. Sample Preparations
4.5. Determination of Bioactive Contents from the Pharmaceutical Product
4.6. Method Validations
4.6.1. Specificity, Selectivity, LLOQ and Linearity
4.6.2. Accuracy and Precision
4.6.3. Extraction Recovery and Matrix Effect
4.7. Application of the Method to a Pharmacokinetic Study
4.8. PK Parameters and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sanyal, A.J.; Yoon, S.K.; Lencioni, R. The etiology of hepatocellular carcinoma and consequences for treatment. Oncologist 2010, 15, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Mokdad, A.A.; Lopez, A.D.; Shahraz, S.; Lozano, R.; Mokdad, A.H.; Stanaway, J.; Murray, C.J.; Naghavi, M. Liver cirrhosis mortality in 187 countries between 1980 and 2010: A systematic analysis. BMC Med. 2014, 12, 145. [Google Scholar] [CrossRef] [PubMed]
- De Martel, C.; Maucort-Boulch, D.; Plummer, M.; Franceschi, S. World-wide relative contribution of hepatitis b and c viruses in hepatocellular carcinoma. Hepatology 2015, 62, 1190–1200. [Google Scholar] [CrossRef] [PubMed]
- Franssen, B.; Jibara, G.; Tabrizian, P.; Schwartz, M.E.; Roayaie, S. Actual 10-year survival following hepatectomy for hepatocellular carcinoma. HPB 2014, 16, 830–835. [Google Scholar] [CrossRef] [PubMed]
- Gluer, A.M.; Cocco, N.; Laurence, J.M.; Johnston, E.S.; Hollands, M.J.; Pleass, H.C.; Richardson, A.J.; Lam, V.W. Systematic review of actual 10-year survival following resection for hepatocellular carcinoma. HPB 2012, 14, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.P.; Kung, Y.Y.; Chen, Y.C.; Jong, M.S.; Chen, T.J.; Chen, F.J.; Hwang, S.J. Frequency and pattern of Chinese herbal medicine prescriptions for chronic hepatitis in Taiwan. J. Ethnopharmacol. 2008, 117, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.Y.; Chang, H.H.; Lo, W.C.; Lin, H.C. Alleviation of hepatic oxidative stress by Chinese herbal medicine yin-chen-hao-tang in obese mice with steatosis. Int. J. Mol. Med. 2010, 25, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.Y.; Chang, H.H.; Kuo, J.J.; Shen, J.J. Changes of hepatic proteome in bile duct ligated rats with hepatic fibrosis following treatment with yin-chen-hao-tang. Int. J. Mol. Med. 2009, 23, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.Y.; Chang, H.H.; Wu, M.Y.; Lin, H.C. Yin-chen-hao-tang ameliorates obstruction-induced hepatic apoptosis in rats. J. Pharm. Pharmacol. 2007, 59, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.Y.; Chang, H.H.; Chen, J.H.; Hsueh, M.L.; Kuo, J.J. Herb medicine yin-chen-hao-tang ameliorates hepatic fibrosis in bile duct ligation rats. J. Ethnopharmacol. 2007, 109, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, W.; Sun, H.; Lv, H.; Wu, Z.; Wang, P.; Liu, L.; Cao, H. Analysis of the constituents in the rat plasma after oral administration of yin chen hao tang by uplc/q-tof-ms/ms. J. Pharm. Biomed. Anal. 2008, 46, 477–490. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.R.; Choi, D.H.; Ko, B.K.; Nam, C.W.; Park, K.M.; Lee, Y.J.; Lee, S.G.; Lee, J.S.; Lee, K.A.; Lee, E.A.; et al. Cold preservation of rat cultured hepatocytes: The scoparone effect. Transplant. Proc. 2000, 32, 2325–2327. [Google Scholar] [CrossRef]
- Jang, S.I.; Kim, Y.J.; Lee, W.Y.; Kwak, K.C.; Baek, S.H.; Kwak, G.B.; Yun, Y.G.; Kwon, T.O.; Chung, H.T.; Chai, K.Y. Scoparone from artemisia capillaris inhibits the release of inflammatory mediators in raw 264.7 cells upon stimulation cells by interferon-gamma plus lps. Arch. Pharm. Res. 2005, 28, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Sun, H.; Dou, S.; Sun, W.; Wu, X.; Wang, P.; Wang, X. Metabolomics study on the hepatoprotective effect of scoparone using ultra-performance liquid chromatography/electrospray ionization quadruple time-of-flight mass spectrometry. Analyst 2013, 138, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, H.Y.; Lee, S.M. Protective effects of geniposide and genipin against hepatic ischemia/reperfusion injury in mice. Biomol. Ther. (Seoul) 2013, 21, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.J.; Lim, K.H.; Jung, H.J.; Park, E.H. Anti-inflammatory evaluation of gardenia extract, geniposide and genipin. J. Ethnopharmacol. 2006, 103, 496–500. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.J.; Lee, S.; Shin, K.H.; Kim, B.C.; Lim, C.J.; Park, E.H. Geniposide, an anti-angiogenic compound from the fruits of gardenia jasminoides. Planta Med. 2004, 70, 467–469. [Google Scholar] [PubMed]
- Fu, Y.; Liu, B.; Liu, J.; Liu, Z.; Liang, D.; Li, F.; Li, D.; Cao, Y.; Zhang, X.; Zhang, N.; et al. Geniposide, from gardenia jasminoides ellis, inhibits the inflammatory response in the primary mouse macrophages and mouse models. Int. Immunopharmacol. 2012, 14, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Zhu, Y.I.; Ye, S.; Liu, H.; She, X.; Niu, Y.; Ming, Y. Gypenoside attenuates renal ischemia/reperfusion injury in mice by inhibition of erk signaling. Exp. Ther. Med. 2016, 11, 1499–1505. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.Y.; Lee, A.Y.; Kim, H.K. The gardenia jasminoides extract and its constituent, geniposide, elicit anti-allergic effects on atopic dermatitis by inhibiting histamine in vitro and in vivo. J. Ethnopharmacol. 2014, 156, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Nadkarni, J.R.; Vishwakarma, R.A.; Bharate, S.B.; Nivsarkar, M.; Anandjiwala, S. The hydroalcoholic extract of cassia alata (linn.) leaves and its major compound rhein exhibits antiallergic activity via mast cell stabilization and lipoxygenase inhibition. J. Ethnopharmacol. 2012, 141, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Fujii, M.; Hou, D.-X. Rhein induces apoptosis in hl-60 cells via reactive oxygen species-independent mitochondrial death pathway. Arch. Biochem. Biophys. 2003, 418, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Chen, X.; Fang, L.; Liu, F.; Cai, R.; Peng, C.; Qi, Y. Rhein exerts pro- and anti-inflammatory actions by targeting ikkbeta inhibition in lps-activated macrophages. Free Radic. Biol. Med. 2014, 72, 104–112. [Google Scholar] [CrossRef] [PubMed]
- KoraMagazi, A.; Wang, D.; Yousef, B.; Guerram, M.; Yu, F. Rhein triggers apoptosis via induction of endoplasmic reticulum stress, caspase-4 and intracellular calcium in primary human hepatic hl-7702 cells. Biochem. Biophys. Res. Commun. 2016, 473, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Tsang, S.W.; Bian, Z.X. Anti-fibrotic and anti-tumorigenic effects of rhein, a natural anthraquinone derivative, in mammalian stellate and carcinoma cells. Phytother. Res. 2015, 29, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.M.; Lin, L.C.; Tsai, T.H. Determination and pharmacokinetic study of gentiopicroside, geniposide, baicalin and swertiamarin in chinese herbal formulae after oral administration in rats by lc-ms/ms. Molecules 2014, 19, 21560–21578. [Google Scholar] [CrossRef] [PubMed]
- Shaw, L.H.; Lin, L.C.; Tsai, T.H. Hplc-ms/ms analysis of a traditional chinese medical formulation of bu-yang-huan-wu-tang and its pharmacokinetics after oral administration to rats. PLoS ONE 2012, 7, e43848. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, T.-P.; Lin, W.-L.; Tsai, T.-H. Using light microscopy and liquid chromatography tandem mass spectrometry for qualitative and quantitative control of a combined three-herb formulation in different preparations. Molecules 2016, 21, 1673. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, T.-P.; Lin, W.-L.; Tsai, T.-H. Pharmacokinetic interactions of herbal medicines for the treatment of chronic hepatitis. J. Food Drug Anal. 2017, 25, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lv, H.; Sun, H.; Sun, W.; Liu, L.; Wang, P.; Cao, H. Simultaneous determination of 6,7-dimethylesculetin and geniposide in rat plasma and its application to pharmacokinetic studies of yin chen hao tang preparation. Arzneimittelforschung 2008, 58, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Sun, H.; Wang, X.; Sun, W.; Jiao, G.; Zhou, D.; Zhao, L.; Cao, H.; Zhang, G. Simultaneous determination by uplc-esi-ms of scoparone, capillarisin, rhein and emodin in rat urine after oral administration of yin chen hao tang preparation. J. Sep. Sci. 2008, 31, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, T.P.; Kao, C.W.; Liang, C.M.; Chen, S.Y.; Hung, Y.C.; Tsai, M.Y. A retrospective analysis of cirrhotic patients receiving chinese herbal medicine in addition to conventional care: Survival and safety. Eur. J. Integr. Med. 2015, 7, 143–150. [Google Scholar] [CrossRef]
- Hou, M.L.; Chang, L.W.; Lin, C.H.; Lin, L.C.; Tsai, T.H. Determination of bioactive components in chinese herbal formulae and pharmacokinetics of rhein in rats by uplc-ms/ms. Molecules 2014, 19, 4058–4075. [Google Scholar] [CrossRef] [PubMed]
- Geng, C.A.; Huang, X.Y.; Chen, X.L.; Ma, Y.B.; Rong, G.Q.; Zhao, Y.; Zhang, X.M.; Chen, J.J. Three new anti-hbv active constituents from the traditional chinese herb of yin-chen (artemisia scoparia). J. Ethnopharmacol. 2015, 176, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Song, Y.H.; Xia, W.J.; Jin, M.W. Aqueous extract of yin-chen-hao decoction, a traditional Chinese prescription, exerts protective effects on concanavalin a-induced hepatitis in mice through inhibition of nf-kappab. J. Pharm. Pharmacol. 2006, 58, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Lu, G.; Shen, H.-M.; Chung, M.C.M.; Ong, C.N. Anti-cancer properties of anthraquinones from rhubarb. Med. Res. Rev. 2007, 27, 609–630. [Google Scholar] [CrossRef] [PubMed]
- Cyong, J.; Matsumoto, T.; Arakawa, K.; Kiyohara, H.; Yamada, H.; Otsuka, Y. Anti-bacteroides fragilis substance from rhubarb. J. Ethnopharmacol. 1987, 19, 279–283. [Google Scholar] [PubMed]
- Cai, Y.; Sun, M.; Xing, J.; Corke, H. Antioxidant phenolic constituents in roots of rheum officinale and rubia cordifolia: Structure-radical scavenging activity relationships. J. Agric. Food Chem. 2004, 52, 7884–7890. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Yin, Q.; Zhang, A.; Wang, X. Uplc-ms/ms performing pharmacokinetic and biodistribution studies of rhein. J. Sep. Sci. 2012, 35, 2063–2068. [Google Scholar] [CrossRef] [PubMed]
- Arai, M.; Yokosuka, O.; Fukai, K.; Kanda, T.; Kojima, H.; Kawai, S.; Imazeki, F.; Hirasawa, H.; Saisho, H. A case of severe acute hepatitis of unknown etiology treated with the Chinese herbal medicine inchinko-to. Hepatol. Res. 2004, 28, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Onji, M. Combined use of ursodeoxycholic acid and inchinko-to in jaundiced patients with primary biliary cirrhosis. Wakan Iyaku Gakkaishi 1990, 7, 161–167. [Google Scholar]
- Zhou, H.B.; Chen, J.M.; Shao, L.M.; Chen, Z.G. Apoptosis of human pancreatic carcinoma cell-1 cells induced by yin chen hao decoction. World J. Gastroenterol. 2015, 21, 8352–8357. [Google Scholar] [CrossRef] [PubMed]
- Ludden, T.M. Nonlinear pharmacokinetics: Clinical implications. Clin. Pharmacokinet. 1991, 20, 429–446. [Google Scholar] [CrossRef] [PubMed]
- Hayes, A.W. Principles and Methods of Toxicology, 15th ed.; Taylor & Francis: London, UK, 2007. [Google Scholar]
- Mehvar, R. Principles of nonlinear pharmacokinetics. Am. J. Pharm. Educ. 2001, 65, 178–184. [Google Scholar]
- Yin, Q.; Sun, H.; Zhang, A.; Wang, X. Pharmacokinetics and tissue distribution study of scoparone in rats by ultraperformance liquid-chromatography with tandem high-definition mass spectrometry. Fitoterapia 2012, 83, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Yang, J.; Shi, D.; Deng, R.; Yan, B. Scoparone potentiates transactivation of the bile salt export pump gene and this effect is enhanced by cytochrome p450 metabolism but abolished by a pkc inhibitor. Br. J. Pharmacol. 2011, 164, 1547–1557. [Google Scholar] [CrossRef] [PubMed]
- Meyer, R.P.; Hagemeyer, C.E.; Knoth, R.; Kurz, G.; Volk, B. Oxidative hydrolysis of scoparone by cytochrome p450 cyp2c29 reveals a novel metabolite. Biochem. Biophys. Res. Commun. 2001, 285, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Mennes, W.C.; van Holsteijn, C.W.; Timmerman, A.; Noordhoek, J.; Blaauboer, B.J. Biotransformation of scoparone used to monitor changes in cytochrome p450 activities in primary hepatocyte cultures derived from rats, hamsters and monkeys. Biochem. Pharmacol. 1991, 41, 1203–1208. [Google Scholar] [CrossRef]
- Akao, T.; KOBASHI, K.; ABURADA, M. Enzymic studies on the animal and intestinal bacterial metabolism of geniposide. Biol. Pharm. Bull. 1994, 17, 1573–1576. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Lin, L.C.; Lin, C.H.; Tsai, T.H. Comparative oral bioavailability of geniposide following oral administration of geniposide, gardenia jasminoides ellis fruits extracts and gardenia herbal formulation in rats. J. Pharm. Pharmacol. 2014, 66, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.Y.; Yang, M.W.; Qian, W.; Lin, H.; Geng, Y.; Zhou, Z.Q.; Xiao, D.W. Quantitative determination of rhein in human plasma by liquid chromatography-negative electrospray ionization tandem mass/mass spectrometry and the application in a pharmacokinetic study. J. Pharm. Biomed. Anal. 2012, 57, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Sun, H.; Sun, W.; Liu, L.; Wang, P.; Wang, X.; Cao, H. Pharmacokinetic studies of a chinese triple herbal drug formula. Phytomedicine 2008, 15, 993–1001. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. Guidance for Industry: Bioanalytical Method Validation; U.S. Department of Health and Human Services Food and Drug Administration: Rockville, MD, USA, 2018.
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic. Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
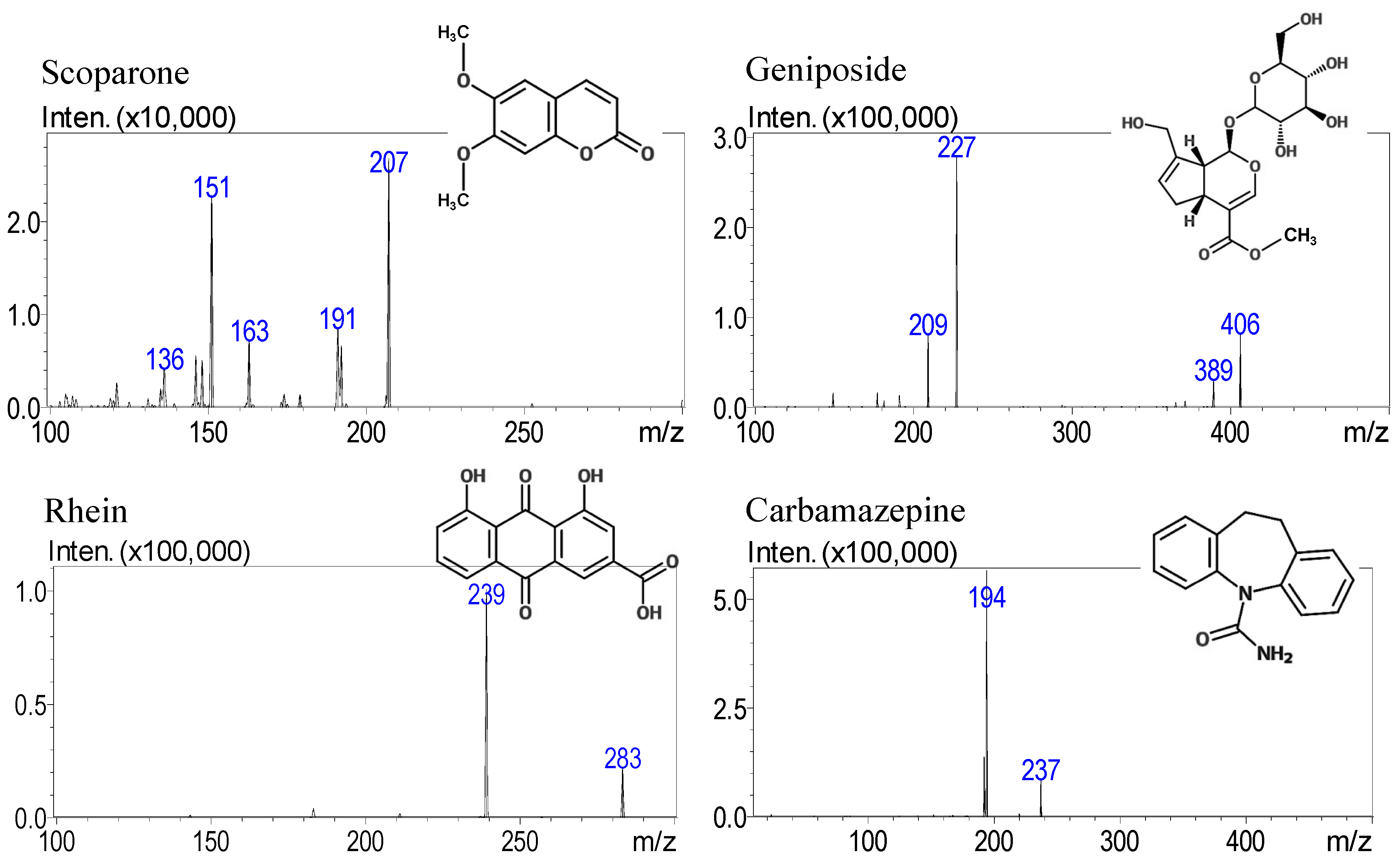


| Constituents | Molecular Weight | RT 1 (min) | Selected m/z | Collision Energy (eV) | |
|---|---|---|---|---|---|
| Q1 m/z | Q3 m/z | ||||
| Scoparone | 206.57 | 5.3 | 207 | 151 | −22 |
| Geniposide | 388.14 | 4.7 | 406 | 227 | −10 |
| Rhein | 284.03 | 6.9 | 283 | 239 | 14 |
| Carbamazepine | 236.10 | 5.8 | 237 | 194 | −20 |
| Constituents | Calibration Curve | Range (ng/mL) | R2 | LOD (ng/mL) |
|---|---|---|---|---|
| Scoparone | y = 0.0195x − 0.0136 | 5–500 | 0.9999 | 1 |
| Geniposide | y = 0.0075x − 0.0066 | 10–1000 | 0.9998 | 5 |
| Rhein | y = 0.007x − 0.001 | 10–1000 | 0.9999 | 5 |
| Nominal Concentration (ng/mL) | Observed Concentration (ng/mL) 1 | Precision (%) | Accuracy (%) | Observed Concentration (ng/mL) 1 | Precision (%) | Accuracy (%) |
|---|---|---|---|---|---|---|
| Intra-Day | Inter-Day | |||||
| Scoparone | ||||||
| 5 | 5.2 ± 0.1 | 2.6 | 3.4 | 5.1 ± 0.1 | 1.7 | 2.0 |
| 10 | 10.6 ± 0.8 | 7.9 | 5.6 | 10.0 ± 0.4 | 4.1 | 0.2 |
| 50 | 49.9 ± 1.2 | 2.4 | −0.2 | 48.1 ± 1.3 | 2.7 | −3.7 |
| 100 | 99.9 ± 0.7 | 0.7 | −0.1 | 100.4 ± 3.0 | 3.0 | 0.4 |
| 500 | 500.3 ± 0.4 | 0.1 | 0.1 | 499.4 ± 8.3 | 1.7 | −0.1 |
| Geniposide | ||||||
| 10 | 10.7 ± 0.7 | 6.9 | 7.2 | 10.7 ± 0.4 | 3.6 | 6.6 |
| 50 | 49.7 ± 0.9 | 1.8 | −0.7 | 50.6 ± 2.5 | 5.0 | 1.2 |
| 100 | 99.6 ± 1.4 | 1.4 | −0.5 | 100.0 ± 3.0 | 3.0 | −0.1 |
| 500 | 500.7 ± 1.5 | 0.3 | 0.1 | 505.2 ± 5.9 | 1.2 | 5.2 |
| 1000 | 999.3 ± 1.8 | 0.2 | −0.1 | 1014.1 ± 31.6 | 3.1 | 1.4 |
| Rhein | ||||||
| 10 | 9.8 ± 0.7 | 6.8 | −2.3 | 9.8 ± 0.2 | 1.6 | −1.7 |
| 50 | 48.8 ± 0.7 | 1.4 | −2.4 | 49.4 ± 0.9 | 1.7 | −1.1 |
| 100 | 100.0 ± 2.8 | 2.8 | 0.0 | 97.0 ± 1.7 | 1.8 | −3.0 |
| 500 | 502.4 ± 2.7 | 0.5 | 0.5 | 489.2 ± 10.6 | 2.2 | −2.2 |
| 1000 | 998.5 ± 2.5 | 0.3 | −0.2 | 988.1 ± 30.4 | 3.1 | −1.2 |
| Nominal Conc. (ng/mL) | R 1 | R 2 | R 3 | ME (%) 1 | RE (%) 2 |
|---|---|---|---|---|---|
| Scoparone | |||||
| 10 | 44,912 ± 1152 | 48,721 ± 2536 | 41,883 ± 1236 | 113.0 ± 16.7 | 99.8 ± 13.1 |
| 100 | 427,813 ± 14,929 | 478,693 ± 2688 | 424,339 ± 15,638 | 109.5 ± 2.0 | 99.5 ± 3.5 |
| 1000 | 4,400,014 ± 56,880 | 4,332,780 ± 178,478 | 3,636,719 ± 220,707 | 97.5 ± 1.5 | 99.7 ± 5.2 |
| mean ± SD | 106.7 ± 6.7 | 99.7 ± 7.2 | |||
| Geniposide | |||||
| 10 | 4690 ± 482 | 7474 ± 582 | 5863 ± 412 | 167.7 ± 34.5 | 91.2 ± 10.6 |
| 100 | 49,536 ± 2178 | 67,386 ± 1831 | 54,524 ± 2940 | 133.5 ± 11.3 | 90.9 ± 7.1 |
| 1000 | 649,073 ± 7233 | 771,802 ± 15,246 | 607,190 ± 24,396 | 117.9 ± 5.9 | 93.5 ± 3.5 |
| mean ± SD | 139.7 ± 17.2 | 91.9 ± 7.1 | |||
| Rhein | |||||
| 50 | 15,161 ± 597 | 12,484 ± 309 | 12,858 ± 653 | 85.1 ± 5.2 | 121.5 ± 11.1 |
| 100 | 27,608 ± 879 | 24,708 ± 1230 | 23,412 ± 1000 | 87.6 ± 3.7 | 106.5 ± 5.8 |
| 1000 | 330,092 ± 2490 | 285,450 ± 3193 | 241,434 ± 977 | 85.8 ± 5.1 | 100.5 ± 2.0 |
| mean ± SD | 86.2 ± 4.7 | 109.5 ± 6.3 | |||
| Carbamazepine (IS) | |||||
| 10 | 289,244 ± 6018 | 282,335 ± 3741 | 244,125 ± 3117 | 99.9 ± 2.9 | 85.7 ± 5.2 |
| Parameters | YCHT 1 g/kg | YCHT 3 g/kg | ||||
|---|---|---|---|---|---|---|
| Scoparone | Geniposide | Rhein | Scoparone | Geniposide | Rhein | |
| Cmax (μg/mL) | 0.018 ± 0.012 | 0.145 ± 0.251 | 0.311 ± 0.166 | 0.132 ± 0.137 * | 0.604 ± 0.256 ** | 1.659 ± 0.805 ** |
| Tmax (min) | 14 ± 20 | 81 ± 71 | 12 ± 5 | 21 ± 20 | 81 ± 77 | 23 ± 19 |
| AUC (min μg/mL) | 0.955 ± 1.168 | 18.76 ± 28.45 | 29.11 ± 13.13 | 7.123 ± 3.379 ** | 94.88 ± 32.28 ** | 198.5 ± 51.3 ** |
| AUC/dose | - | - | - | 2.374 ± 1.126 | 31.63 ± 10.76 | 66.18 ± 17.09 |
| t1/2 (min) | 26 ± 5 | 80 ± 18 | 121 ± 46 | 69 ± 55 | 86 ± 29 | 104 ± 69 |
| CL (mL/min/kg) | 394.7 ± 209.4 | 1403 ± 1018 | 3.710 ± 1.366 | 131.5 ± 123.6 * | 259.8 ± 109.2 * | 1.483 ± 0.357 ** |
| MRT (min) | 19 ± 14 | 115 ± 31 | 106 ± 12 | 51 ± 18 ** | 152 ± 20 * | 116 ± 20 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsueh, T.-P.; Tsai, T.-H. Preclinical Pharmacokinetics of Scoparone, Geniposide and Rhein in an Herbal Medicine Using a Validated LC-MS/MS Method. Molecules 2018, 23, 2716. https://doi.org/10.3390/molecules23102716
Hsueh T-P, Tsai T-H. Preclinical Pharmacokinetics of Scoparone, Geniposide and Rhein in an Herbal Medicine Using a Validated LC-MS/MS Method. Molecules. 2018; 23(10):2716. https://doi.org/10.3390/molecules23102716
Chicago/Turabian StyleHsueh, Tun-Pin, and Tung-Hu Tsai. 2018. "Preclinical Pharmacokinetics of Scoparone, Geniposide and Rhein in an Herbal Medicine Using a Validated LC-MS/MS Method" Molecules 23, no. 10: 2716. https://doi.org/10.3390/molecules23102716
APA StyleHsueh, T.-P., & Tsai, T.-H. (2018). Preclinical Pharmacokinetics of Scoparone, Geniposide and Rhein in an Herbal Medicine Using a Validated LC-MS/MS Method. Molecules, 23(10), 2716. https://doi.org/10.3390/molecules23102716






