Production and Characterization of a Bioflocculant Produced by Bacillus salmalaya 139SI-7 and Its Applications in Wastewater Treatment
Abstract
1. Introduction
2. Results and Discussion
2.1. Selection of the Bacterial Strain for Bioflocculant Production
2.2. Optimization of B. salmalaya Strain 139SI-7 for Bioflocculant Production
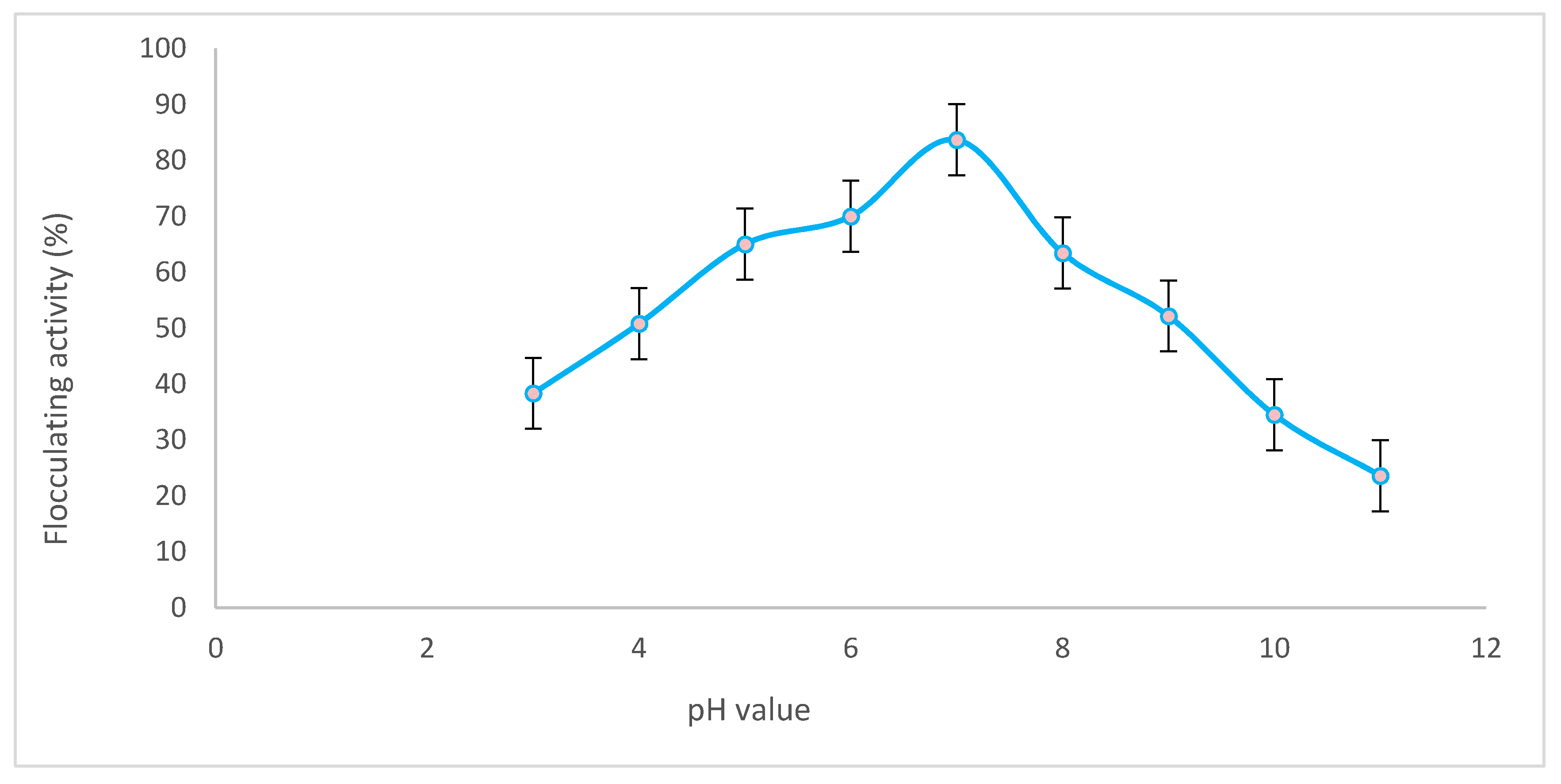
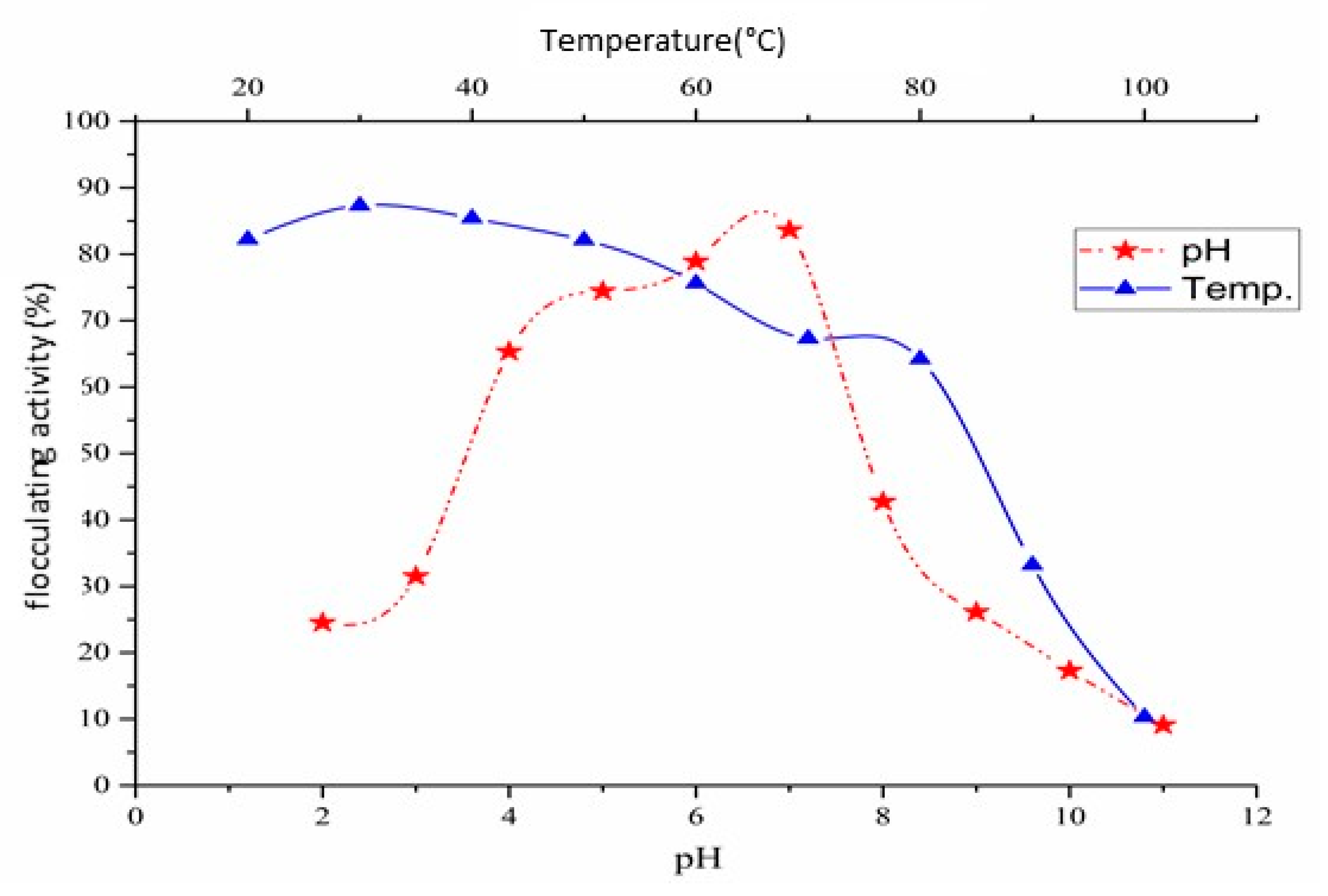
2.2.1. Effect of pH
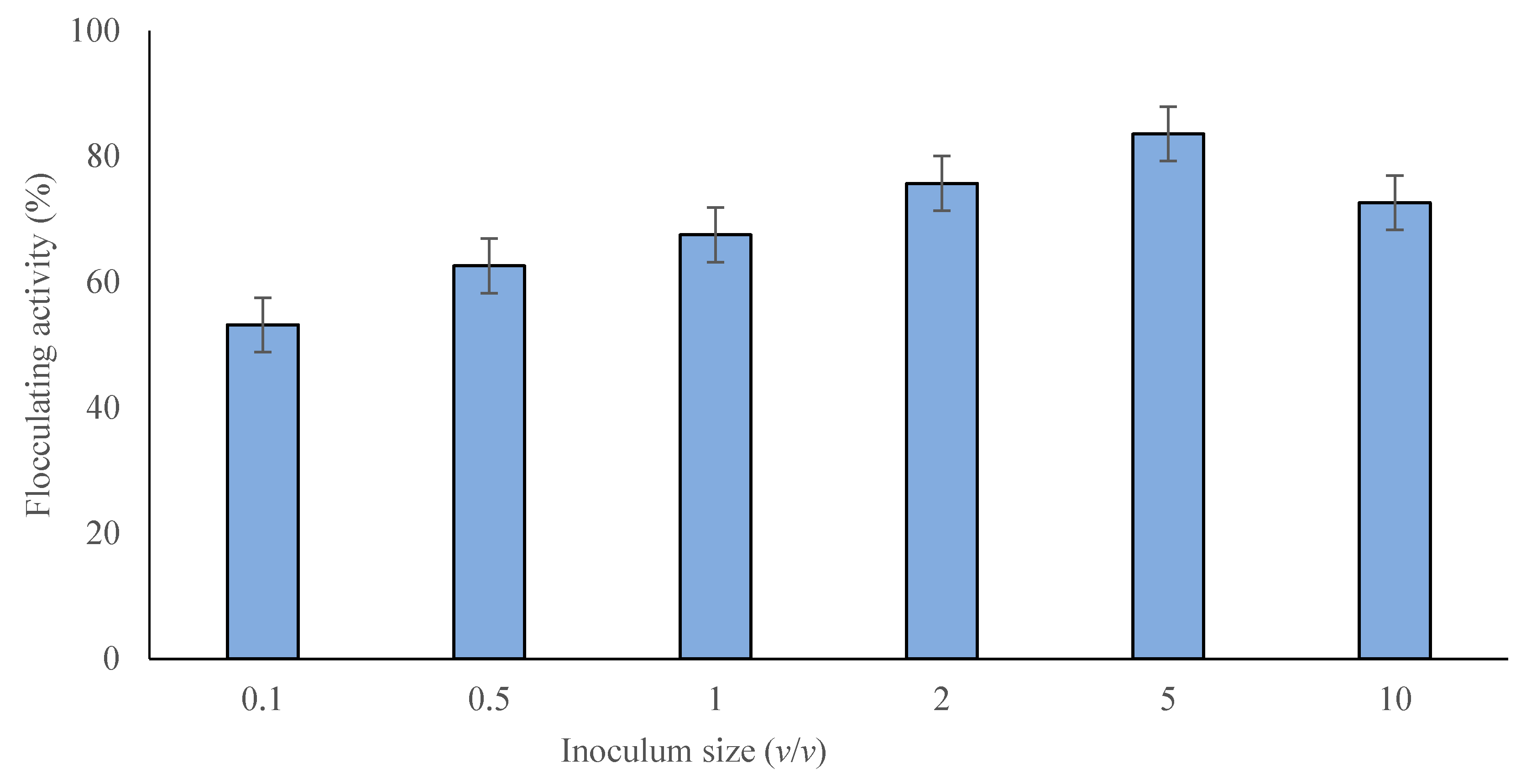
2.2.2. Effect of Inoculum Size
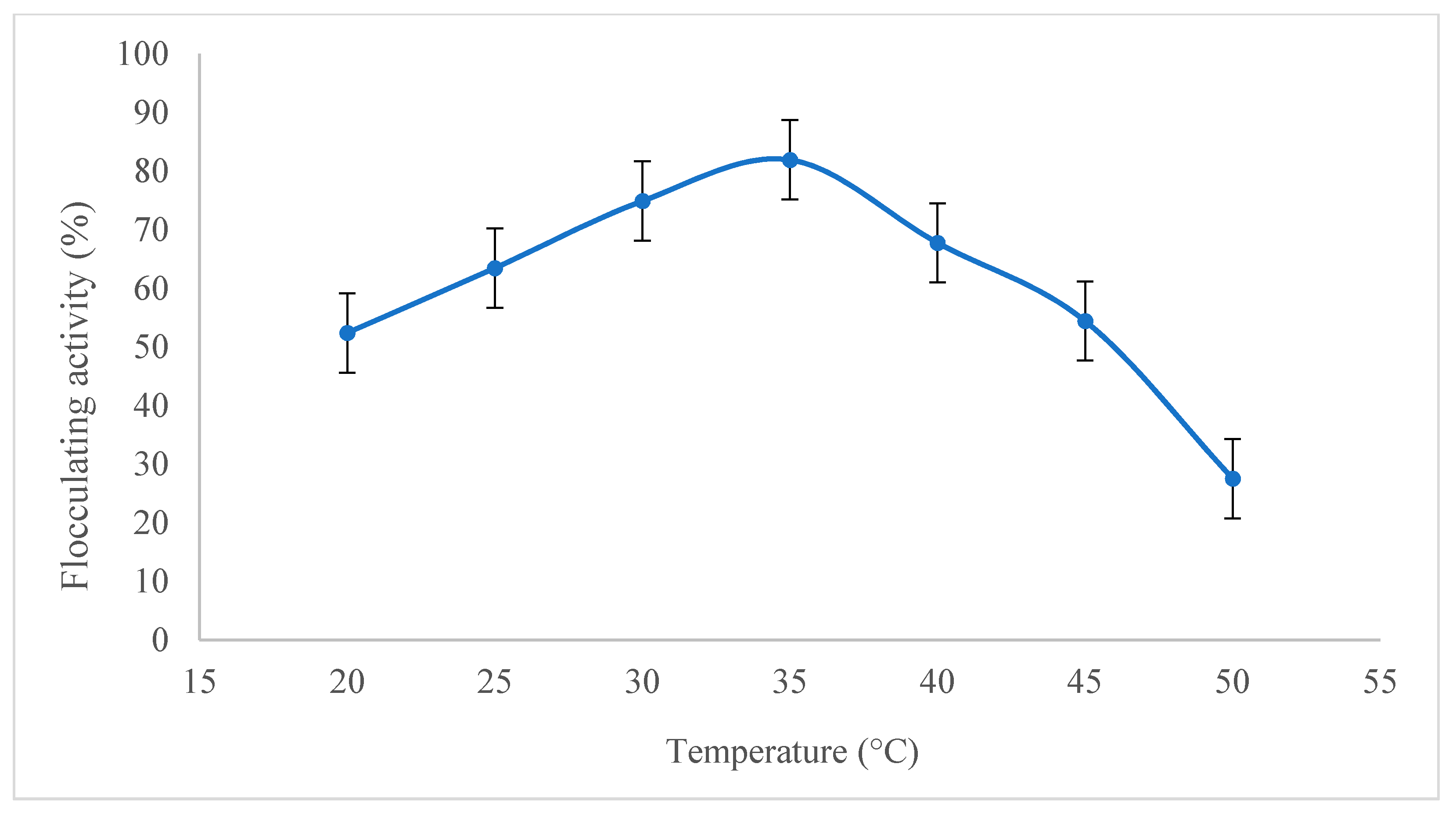
2.2.3. Effect of Temperature
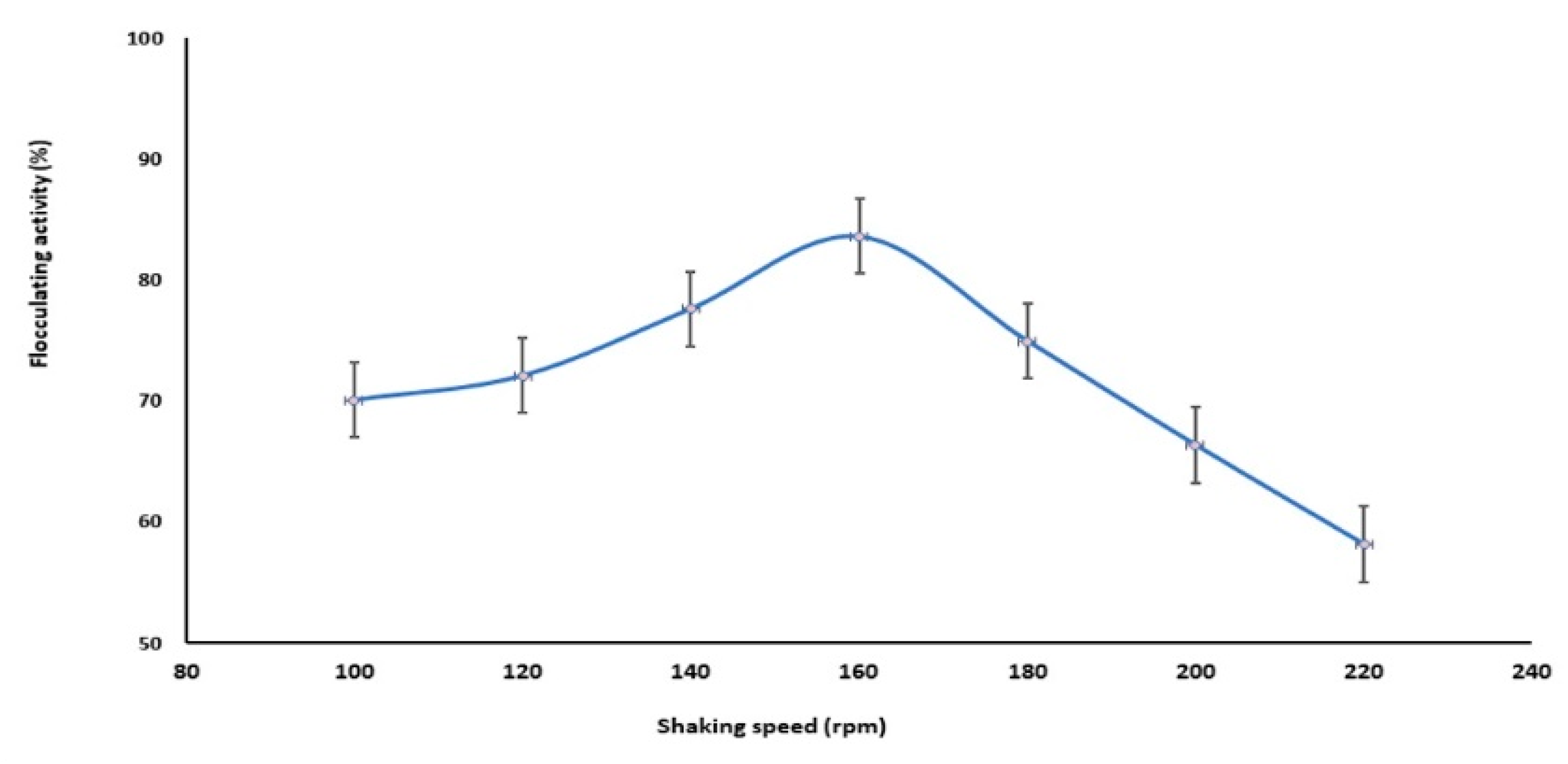
2.2.4. Effect of Shaking Speed
2.2.5. Effect of Carbon Source
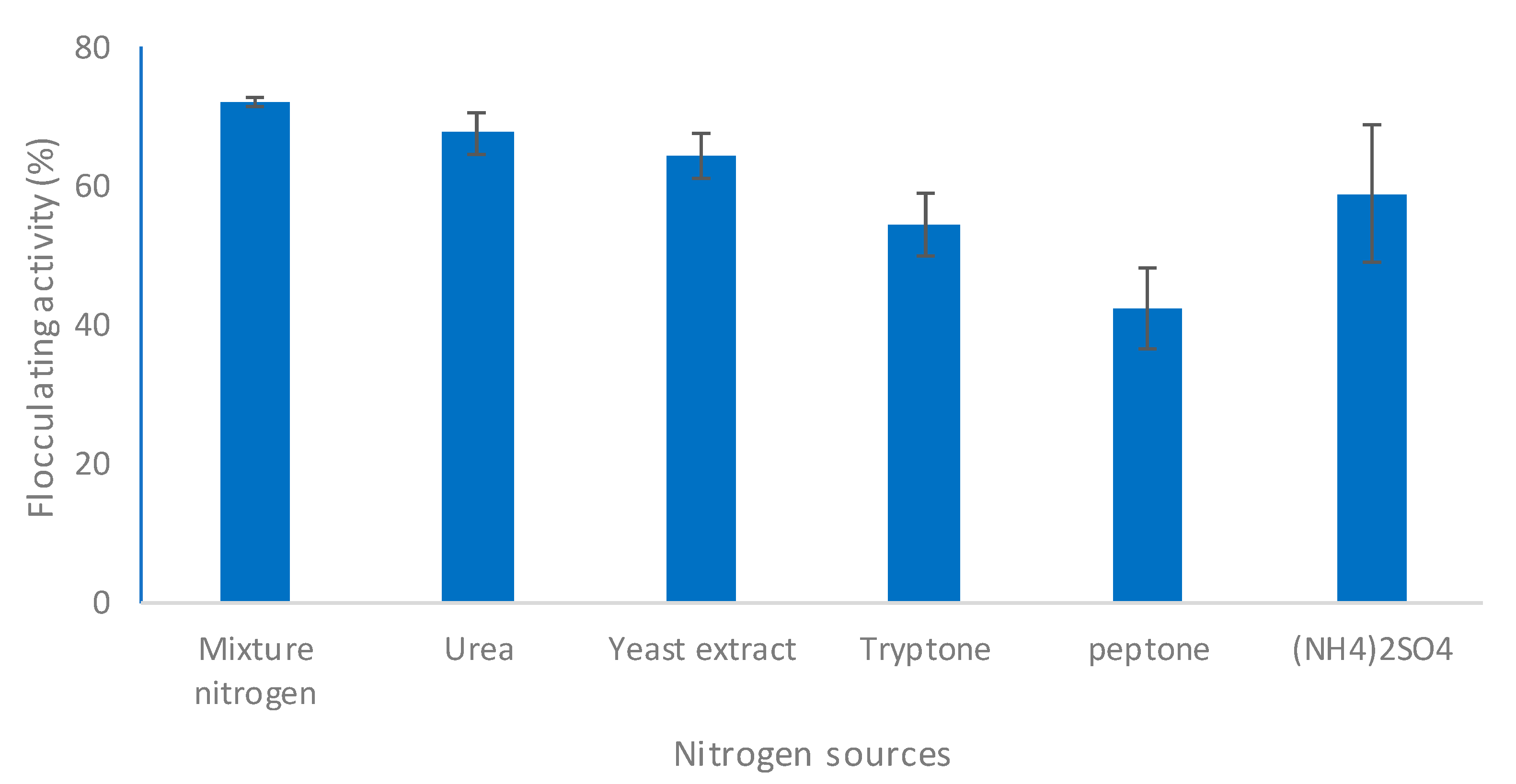
2.2.6. Effect of Nitrogen Source
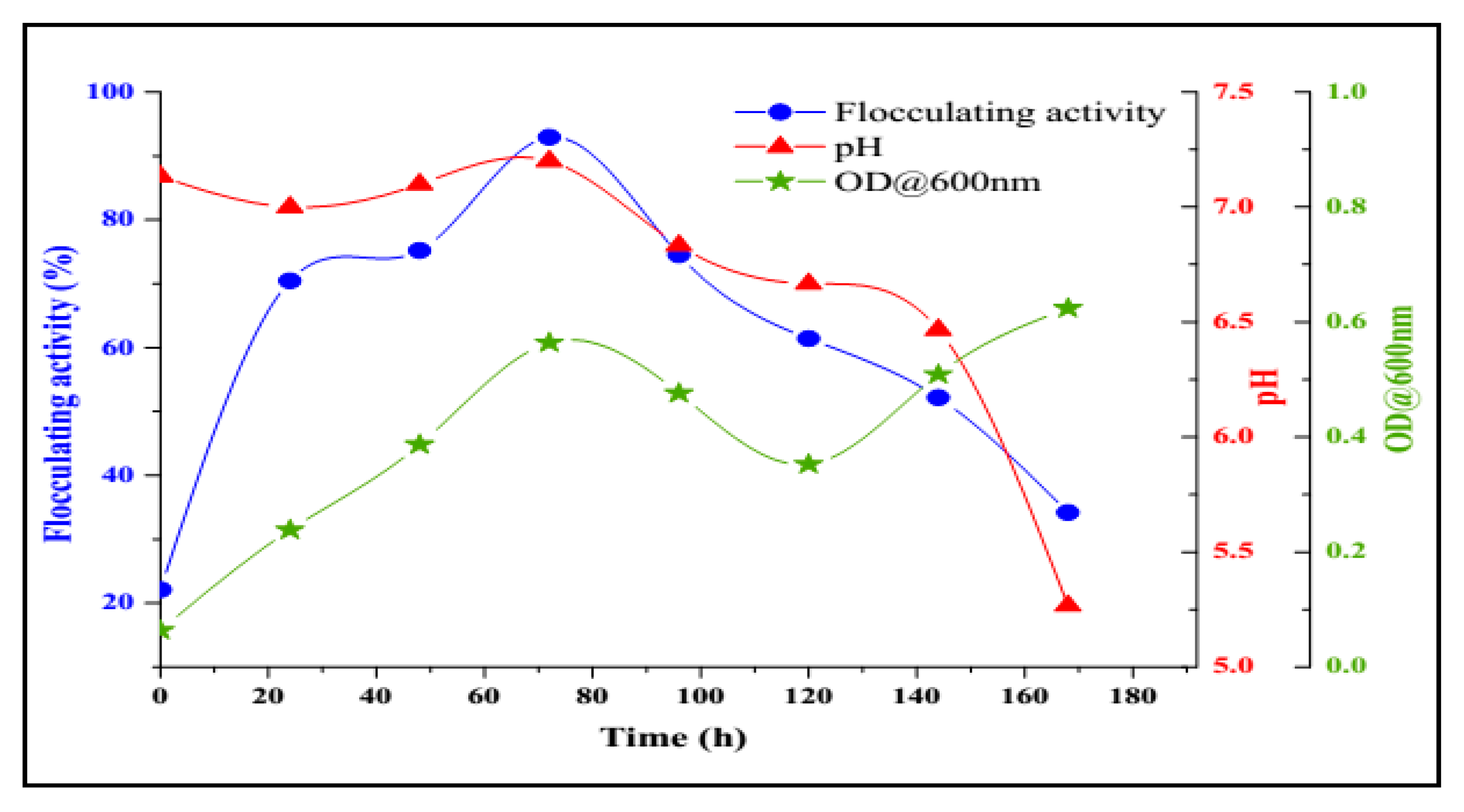
2.3. Bioflocculant Production by B. salmalaya Strain 139SI-7
2.4. Characteristics of Bioflocculant QZ-7
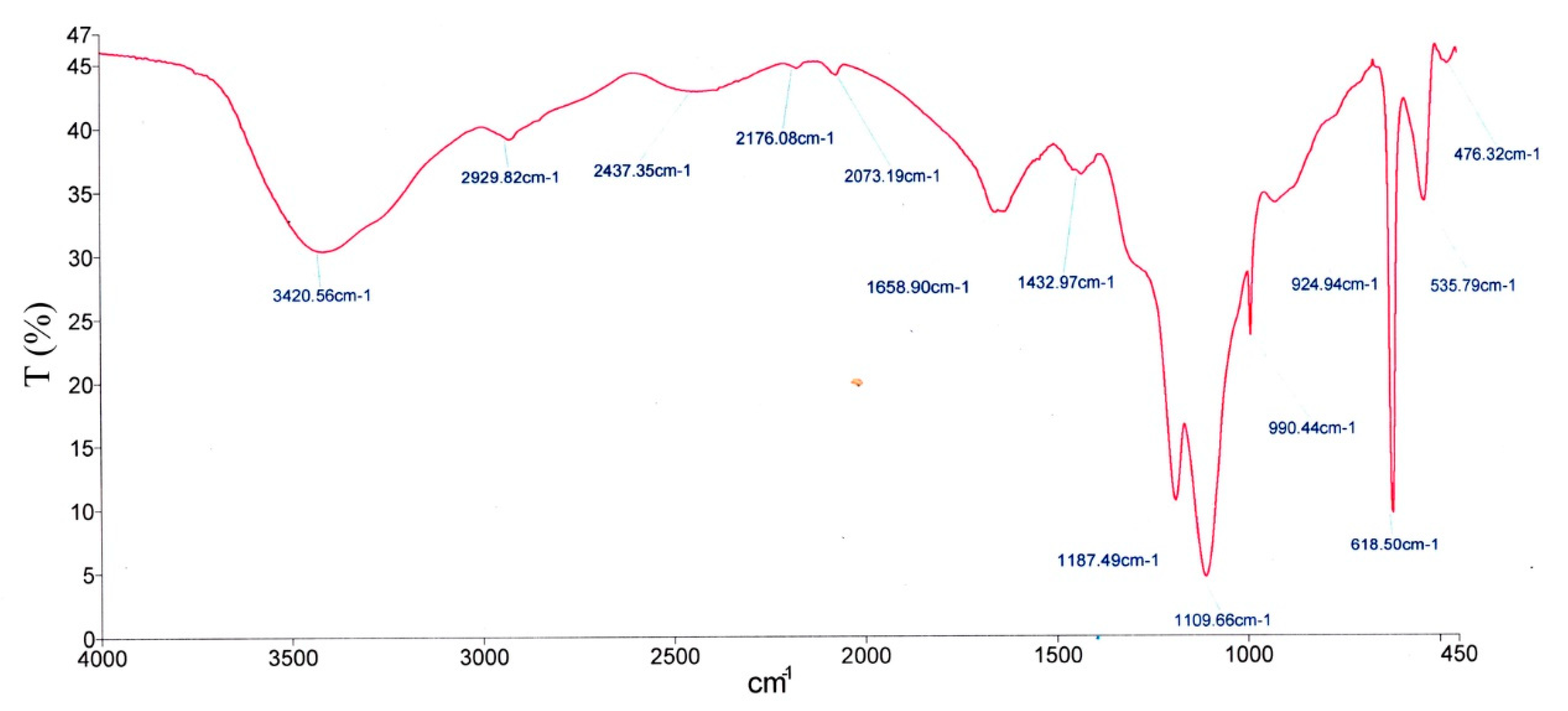
2.5. FTIR
2.6. Molecular Weight Analysis
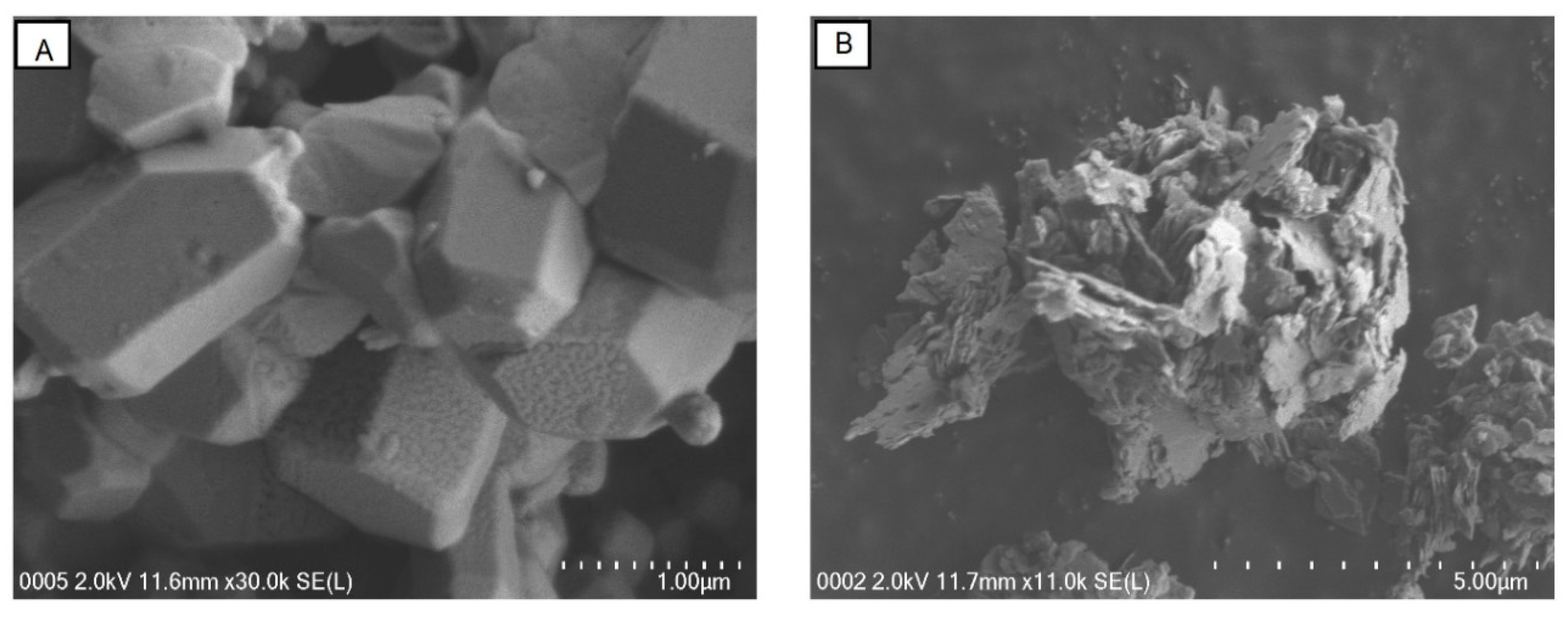
2.7. SEM Imaging
2.8. pH and Thermo-Stability of Pure Bioflocculant QZ-7
2.9. Wastewater Treatment with Bioflocculants
3. Conclusions
4. Materials and Methods
4.1. Cultivation and Isolation of the Bacteria
4.2. Composition of the Used Media
4.3. Screening for Bioflocculant-Producing Bacteria
4.4. Determination of Flocculating Activity
4.5. Optimization of Cultural Conditions for Bioflocculant Production
4.5.1. Effect of pH
4.5.2. Effect of Inoculum Size
4.5.3. Effect of Temperature and Shaking Speed
4.5.4. Effect of Carbon and Nitrogen Sources
4.6. Bioflocculant Production by B. salmalaya Strain 139SI-7
4.7. Extraction and Purification of Bioflocculants
4.8. Characterization of the Bioflocculant QZ-7
4.8.1. Chemical Analysis
4.8.2. pH Stability of Pure Bioflocculant QZ-7
4.8.3. Thermo-Stability of Pure Bioflocculant QZ-7
4.8.4. Fourier-Transform Infrared Spectroscopy (FTIR)
4.8.5. Determination of Molecular Weight of Purified Bioflocculant
4.8.6. Scanning Electron Microscopy (SEM) Remarks
4.9. Removal of COD from Wastewater with Bioflocculant QZ-7
4.10. Removal of BOD from Wastewater with B. salmalaya Strain 139SI-7
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, C.L.; Cui, Y.N.; Wang, Y. Bioflocculant produced from bacteria for decolorization, Cr removal and swine wastewater application. Sustain. Environ. Res. 2012, 22, 129–134. [Google Scholar]
- Zhang, Z.Q.; Lin, B.; Xia, S.Q.; Wang, X.J.; Yang, A.M. Production and application of a novel bioflocculant by multiple-microorganism consortia using brewery wastewater as carbon source. J. Environ. Sci. 2007, 19, 667–673. [Google Scholar] [CrossRef]
- Gao, J.; Bao, H.Y.; Xin, M.X.; Liu, Y.X.; Li, Q.; Zhang, Y.F. Characterization of a bioflocculant from a newly isolated Vagococcus sp. W31. J. Zhejiang Univ. Sci. B 2006, 7, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Czemierska, M.; Szczes, A.; Hołysz, L.; Wiater, A.; Wilkołazka, A.J. Characterization of exopolymer R-202 isolated from Rhodococcus rhodochrous and its flocculating properties. Eur. Polym. J. 2017, 88, 21–33. [Google Scholar] [CrossRef]
- Prasad, K.; Ramanathan, A.; Paul, J.; Subramanian, V.; Prasad, R. Biosorption of arsenite (As+3) and arsenate (As+5) from aqueous solution by Arthrobacter sp. biomass. Environ. Technol. 2013, 34, 2701–2708. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ding, T.; Zhang, Z.; Xu, L.; Zhang, C. Enhanced adsorption of trivalent arsenic from water by functionalized diatom silica shells. PLoS ONE 2015, 10, e0123395. [Google Scholar] [CrossRef] [PubMed]
- Salehizadeh, H.; Shojaosadati, S.A. Extracellular biopolymeric flocculants: Recent trends and biotechnological importance. Biotechnol. Adv. 2001, 19, 371–385. [Google Scholar] [CrossRef]
- Zhong, C.; Cao, G.; Rong, K.; Xia, Z.; Peng, T.; Chen, H.; Zhao, J. Characterization of a microbial polysaccharide-based bioflocculant and its anti-inflammatory and pro-coagulant activity. Colloids Surf. B Biointerfaces 2018, 161, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Salehizadeh, H.; Shojaosadati, S.A. Removal of metal ions from aqueous solution by polysaccharide produced from Bacillus firmus. Water Res. 2003, 37, 4231–4235. [Google Scholar] [CrossRef]
- Salehizadeh, H.; Yan, N. Recent advances in extracellular biopolymer flocculants. Biotechnol. Adv. 2014, 32, 1506–1522. [Google Scholar] [CrossRef] [PubMed]
- Campbell, A. The potential role of aluminium in Alzheimer’s disease. Nephrol. Dial. Transplant. 2002, 17, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Yu, G.; Ting, Y.P. Production of a bioflocculant by Aspergillus parasiticus and its application in dye removal. Colloids Surf. B Biointerfaces 2005, 44, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Takagi, H.; Kadowake, K. Purification and chemical properties of a flocculant produced paecilomyces. Agric. Biol. Chem. 1985, 49, 3159–3164. [Google Scholar] [CrossRef]
- Shih, L.; Van, Y.T.; Yeh, L.C.; Lin, H.G.; Chang, Y.N. Production of a biopolymer flocculant from Bacillus licheniformis and its flocculation properties. Bioresour. Technol. 2001, 78, 267–272. [Google Scholar] [CrossRef]
- Takeda, M.; Kurane, R.; Kozumi, J.; Nakamura, I. Bioflocculant produced by Rhodococcus erythroplis. Agric. Biol. Chem. 1992, 55, 2663–2664. [Google Scholar]
- Takeda, M.; Kurane, R. Localization of a biopolymer produced by Rhodococcus erythropolis grown on n-Pentadecane. Agric. Biol. Chem. 1991, 55, 2665–2666. [Google Scholar] [CrossRef]
- Yokoi, H.; Arima, T.; Hayashi, S.; Takasaki, Y. Flocculation properties of poly (γ-glutamic acid) produced by Bacillus subtilis. J. Ferment. Bioeng. 1996, 82, 84–87. [Google Scholar] [CrossRef]
- Toeda, K.; Kuranem, R. Microbial flocculant from Alkaligenes cupidus KT201. Agric. Biol. Chem. 1991, 55, 2793–2799. [Google Scholar]
- Wang, Z.; Wang, K.; Xie, Y. Bioflocculant producing microorganism. Chin. Sci. Abstr. Ser. B 1995, 3, 14. [Google Scholar]
- Lee, S.H.; Lee, S.O.; Jang, K.L.; Lee, T.H. Microbial flocculant from Arcuadendron sp. Ts-49. Biotechnol. Lett. 1995, 17, 95–100. [Google Scholar] [CrossRef]
- Yaeger, R.G. Protozoa: Structure, Classification, Growth, and Development. In Medical Microbiology, 4th ed.; Baron, S., Ed.; University of Texas Medical Branch: Galveston, TX, USA, 1996. [Google Scholar]
- Bunk, B.; Biedendieck, R.; Jahn, D.; Vary, P.S. Bacillus Megaterium and other Bacilli: Industrial Applications. Encycl. Ind. Biotechnol. 2010. [Google Scholar] [CrossRef]
- Perez-Garcia, A.; Romero, D.; de Vicente, A. Plant protection and growth stimulation by microorganisms: Biotechnological applications of Bacilli in agriculture. Curr. Opin. Biotechnol. 2011, 22, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Priest, F.G.; Goodfellow, M.; Shute, L.A.; Berkeley, R.C.W. Bacillus amyloliquefaciens sp. nov. norn. rev. Int. J. Syst. Bacteriol. 1987, 37, 69–71. [Google Scholar] [CrossRef]
- He, J.; Zou, J.; Shao, Z.; Zhang, J. Characteristics and flocculating mechanism of a novel bioflocculant HBF-3 produced by deep-sea bacterium mutant Halomona sp. V3a. World J. Microbiol. Biotechnol. 2010, 26, 1135–1141. [Google Scholar] [CrossRef]
- Zhao, G.; Ma, F.; Wei, L.; Chua, H. Using rice straw fermentation liquor to produce bioflocculants during an anaerobic dry fermentation process. Bioresour. Technol. 2012, 113, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Xiao, J.; Wang, F.; Zhang, L. Adsorption–flocculation of Rhodococcus erythropolis on micro- fine hemalitic. J. Cent. South Univ. 2013, 44, 874–879. [Google Scholar]
- Okaiyeto, K.; Nwodo, U.V.; Mabinya, L.V.; Okoh, A.I. Evaluation of the flocculation potential and characterization of bioflocculant produced by Micrococcus sp. Leo. Appl. Biochem. Microbial. 2014, 50, 601–608. [Google Scholar] [CrossRef]
- Tang, J.; Qi, S.; Li, Z.; An, Q.; Xie, M.; Yang, B.; Wang, Y. Production, purification and application of polysaccharide-based bioflocculant by Paenibacillus mucilaginosus. Carbohydr. Polym. 2014, 113, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Ike, M.; Tachibana, S.; Kitada, G.; Kim, S.M.; Inoue, Z. Charactrisation of bioflocculant produced by Citrobactor sp. TKF04 from acetic and propionic acids. J. Biosci. Bioeng. 2000, 89, 40–46. [Google Scholar] [CrossRef]
- Lachhwani, P. Studies on Polymeric Bioflocculant Producing Microorganisms. Master’s Thesis, Thapar Institute of Engineering and Technology, Patiala, India, 2005. [Google Scholar]
- Xia, S.; Zhang, Z.; Wang, X.; Yang, A.; Chen, L.; Zhao, J.; Leonard, D.; Renault, N.J. Production and characterization of a bioflocculant by Proteus mirabilis Tj-1. Bioresour. Technol. 2008, 99, 6520–6527. [Google Scholar] [CrossRef] [PubMed]
- Nakata, K.; Kurane, R. Production of an extracellular polysaccharides on bioflocculant producing by Klebsiella pneumoniae. Biosci. Biotechnol. Biochem. 1999, 63, 2064–2068. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-G.; Gong, W.-X.; Liu, X.-W.; Tian, L.; Yue, Q.-Y.; Gao, B.-Y. Production of a novel bioflocculant by culture of klebsiella mobilis using dairy wastewater. Biochem. Eng. J. 2007, 36, 81–86. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, C.; Wu, Y.; Hu, C. Comparison of coagulation performance and floc properties of a novel zirconium-glycine complex coagulant with traditional coagulants. Environ. Sci. Pollut. Res. 2014, 21, 6632–6639. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ma, F.; Zuo, H. Production of a novel bioflocculant and its flocculation performance in aluminum removal. Bioengineered 2016, 7, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Aljuboori, A.H.R.; Idris, A.; Abdullah, N.; Mohamad, R. Production and characterization of a bioflocculant produced by Aspergillus flavus. Bioresour. Technol. 2013, 127, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Rasulov, B.; Li, L.; Liu, Y.; Mohammed, O.; Xiao, M.; Ma, J. Production, characterization and structural modification of exopolysaccharide-based bioflocculant by Rhizobium radiobacter SZ4S7S14 and media optimization. Biotechnology 2017, 179. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Ye, Z.-L.; Fang, X.-L.; Li, Y.-H.; Cai, W.-M. Production and characteristics of a bioflocculant produced by Bacillus sp. F19. Bioresour. Technol. 2008, 99, 7686–7691. [Google Scholar] [CrossRef] [PubMed]
- Ugbenyen, A.M.; Cosa, S.; Mabinya, L.V.; Okoh, A.I. Bioflocculant production by Bacillus sp. Gilbert isolated from a marine environment in South Africa. Appl. Biochem. Microbiol. 2014, 50, 49–54. [Google Scholar] [CrossRef]
- Ismail, B.; Nampoothiri, K.M. Production, purification and structural characterization of an exopolysaccharide produced by a probiotic Lactobacillus plantarum MTCC 9510. Arch. Microbiol. 2010, 192, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhong, S.; Lei, H.; Chen, R.; Yu, Q.; Li, H. Production of a novel bioflocculant by Bacillus licheniformis X14 and its application to low temperature drinking water treatment. Bioresour. Technol. 2009, 100, 3650–3656. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.Y.; Zhang, T.; Zhang, D.Y.; Li, C.H.; Wen, J.P.; Du, L.X. A novel bioflocculant produced by Enterobacter aerogenes and its use in defecating the trona suspension. Biochem. Eng. J. 2005, 27, 1–7. [Google Scholar] [CrossRef]
- Wu, J.Y.; Ye, H.F. Characteristic and flocculating properties of an extracellular biopolymers from Bacillus subtilis D4U, isolate. Process. Biochem. 2007, 42, 1114–1123. [Google Scholar] [CrossRef]
- Chaplin, M.F.; Kennedy, J.F. Carbohydrate Analysis, 2nd ed.; Oxford University Press: New York, NY, USA, 1994. [Google Scholar]
- Aguilera, M.; Quesada, M.T.; Aguila, V.G.; Morillo, J.A.; Rivadeneyra, M.A.; Cormenzana, A.R.; Sanchez, M.M. Charaterization of paenibacillus jamilae stains that produce exopolysaccharides during growth on and detoxification of olive mill wastewater. Bioresour. Technol. 2008, 99, 5640–5644. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Hui, C.; Bai, N.; Yang, S.; Wan, L.; Zhang, Q.; Zhao, Y. Revealing the characteristics of a novel bioflocculant and its flocculation performance in Microcystis aeruginosa removal. Sci. Rep. 2015. [Google Scholar] [CrossRef] [PubMed]
- Suh, H.H.; Kwon, G.S.; Lee, C.H.; Kim, H.S.; Oh, H.M.; Yoon, B.D. Characterisation of bioflocculant produced by Bacillus sp. DP-152. Ferment. Bioeng. 1997, 84, 108–112. [Google Scholar] [CrossRef]
- Gupta, S.; Madan, R.N.; Bansal, M.C. Chemical composition of pinus carbaea hemicellulose. Tappi J. 1987, 70, 113–114. [Google Scholar]
- Yang, Q.; Ming, H.; Zhao, X.; Zhang, J.; Zou, W.; Zhao, C.; Guan, X. Screening of bioflocculant and preliminary application to treatment of tannery wastewater. J. Resid. Sci. Technol. 2015, 12, 177–181. [Google Scholar] [CrossRef]
- Lian, B.; Chen, Y.; Zhao, J.; Teng, H.H.; Zhu, L.J.; Yuan, S. Microbial flocculation by Bacillus mucilaginosus; application and mechanism. Bioresour. Technol. 2008, 99, 4825–4831. [Google Scholar] [CrossRef] [PubMed]
- Yong, P.; Bo, S.; Yu, Z. Research on flocculation property of bioflocculant. PG. a21 ca. Mod. Appl. Sci. 2009, 3, 106–112. [Google Scholar]
- Alehizadeh, H.; Yan, N.; Farnood, R. Recent advances in polysaccharide bio-based flocculants. Biotechnol. Adv. 2018, 36, 92–119. [Google Scholar] [CrossRef] [PubMed]
- Ismail, S.; Dadrasnia, A. Biotechnological potential of Bacillus salmalaya 139SI: A novel strain for remediating water polluted with Crude Oil Waste. PLoS ONE 2015, e0120931. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Wang, Y.; Yu, Y.; Li, Q.; Wang, H.; Chen, R.; He, N. Production and characterisation of a novel bioflocculant from Bacillus Licheniformis. Appl. Environ. Microbial. 2010, 76, 2778–2782. [Google Scholar] [CrossRef] [PubMed]
- Kurane, R.; Nohata, Y. A new water-absorbing polysaccharide from Alcaligenes latus. Biosci. Biotechnol. Biochem. 1994, 58, 235–238. [Google Scholar] [CrossRef]
- Cosa, S.; Mabinya, L.V.; Olaniran, A.O.; Okoh, A.I. Production and characterization of bioflocculant produced by Halobacillus sp. Mvuyo isolated from bottem sediment of Algoa Bay. Environ. Technol. 2012, 33, 967–973. [Google Scholar] [CrossRef] [PubMed]
- He, N.; Li, Y.; Chen, J. Production of a novel polygalacturoic acid bioflocculant REA-11 by Corynebacterium glutamicum. Bioresour. Technol. 2004, 94, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, E.Z. Production and characteristics of a heavy metals removing bioflocculant produced by Pseudomonas aeruginosa. Pol. J. Microbiol. 2012, 61, 281–289. [Google Scholar]
- Chen, Z.; Li, Z.; Liu, P.; Wang, Y.; Li, Q.; He, N. Characterization of a novel bioflocculant from a marine bacterium and its application in dye wastewater treatment. BMC Biotechnol. 2017, 17, 84. [Google Scholar] [CrossRef] [PubMed]
- Rice, W.; Baird, R.B.; Eaton, A.D.; Clesceri, L.S. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association (APHA), American Water Works Association (AWWA) and Water Environment Federation (WEF): Washington, DC, USA, 2005. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |

| Strain Code No | Flocculating Activity (%) | Standard Deviation (SD) |
|---|---|---|
| BS * 139SI-1 | 67.5 | 0.655 |
| BS 139SI-5 | 54.2 | 0.770 |
| BS 139SI-7 | 83.3 | 0.75 |
| BS 139SI-8 | 72.2 | 1.93 |
| BS 139SI-13 | 63.4 | 0.45 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abu Tawila, Z.M.; Ismail, S.; Dadrasnia, A.; Usman, M.M. Production and Characterization of a Bioflocculant Produced by Bacillus salmalaya 139SI-7 and Its Applications in Wastewater Treatment. Molecules 2018, 23, 2689. https://doi.org/10.3390/molecules23102689
Abu Tawila ZM, Ismail S, Dadrasnia A, Usman MM. Production and Characterization of a Bioflocculant Produced by Bacillus salmalaya 139SI-7 and Its Applications in Wastewater Treatment. Molecules. 2018; 23(10):2689. https://doi.org/10.3390/molecules23102689
Chicago/Turabian StyleAbu Tawila, Zayed M., Salmah Ismail, Arezoo Dadrasnia, and Mohammed Maikudi Usman. 2018. "Production and Characterization of a Bioflocculant Produced by Bacillus salmalaya 139SI-7 and Its Applications in Wastewater Treatment" Molecules 23, no. 10: 2689. https://doi.org/10.3390/molecules23102689
APA StyleAbu Tawila, Z. M., Ismail, S., Dadrasnia, A., & Usman, M. M. (2018). Production and Characterization of a Bioflocculant Produced by Bacillus salmalaya 139SI-7 and Its Applications in Wastewater Treatment. Molecules, 23(10), 2689. https://doi.org/10.3390/molecules23102689





