Anti-Inflammatory Activities of Compounds Isolated from the Rhizome of Anemarrhena asphodeloides
Abstract
1. Introduction
2. Results and Discussion
2.1. Purification and Identification of Chemical Constituents
2.2. Cytotoxic Effects of the Purified Constituents against N9 Microglial Cells
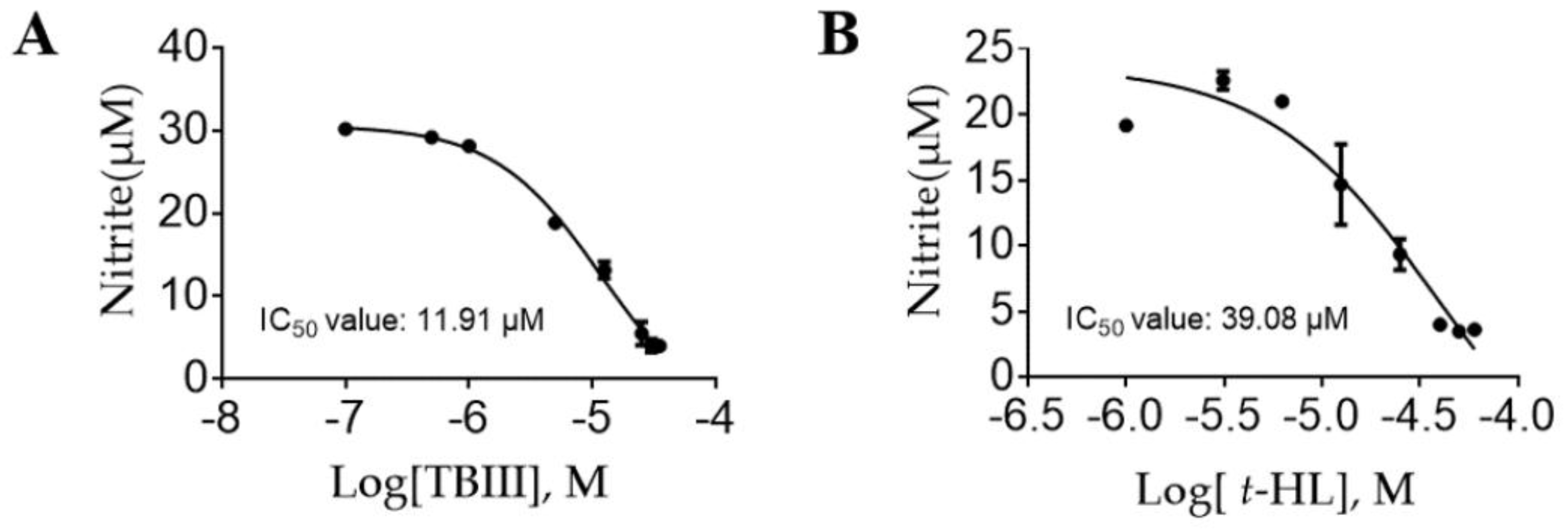
2.3. Inhibitory Effects of the Purified Constituents on Lps-Stimulated NO Production in N9 Microglial Cells
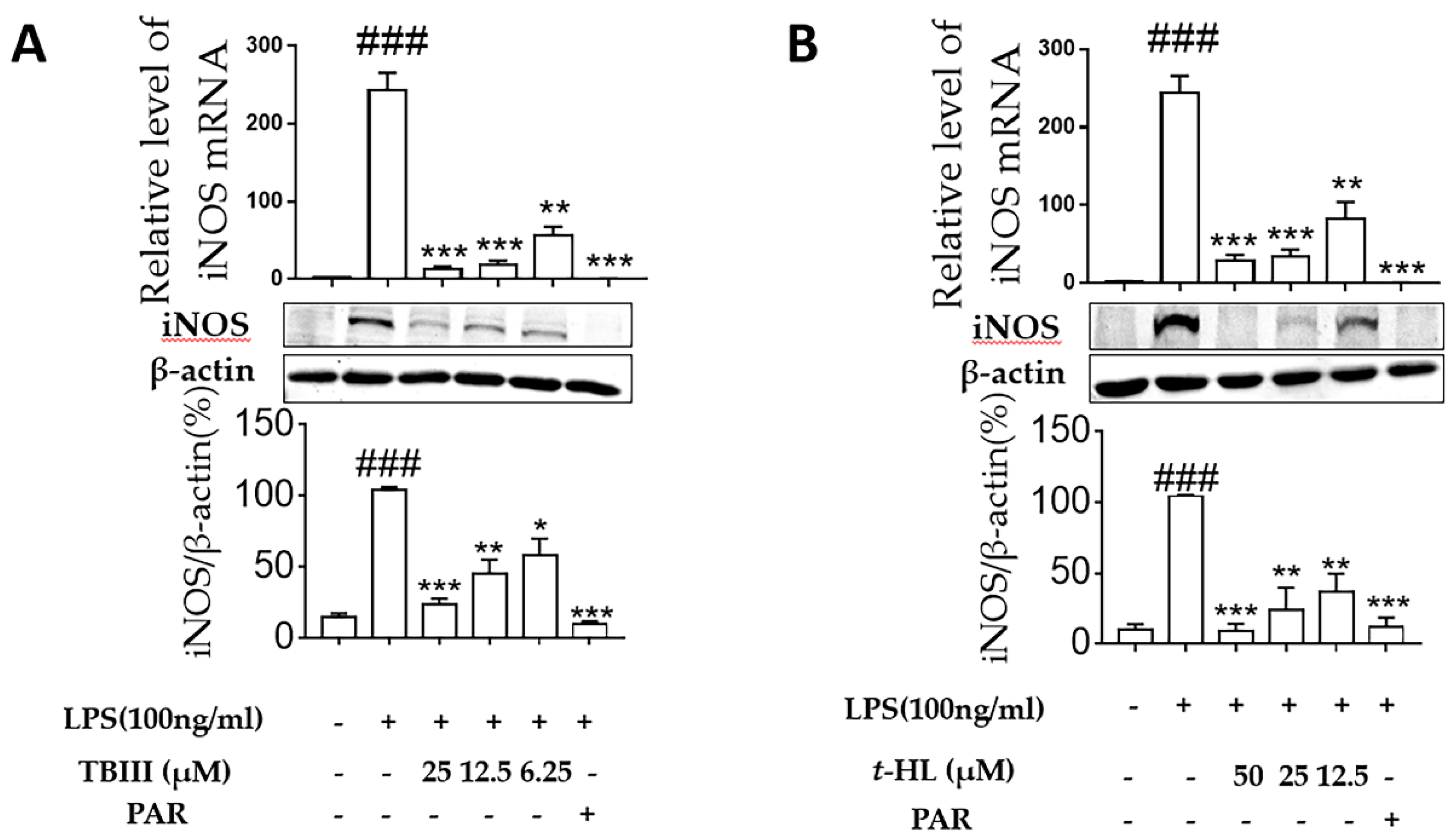
2.4. Effects of TBIII and t-HL on iNOS Expression in LPS-Stimulated N9 Microglial Cells
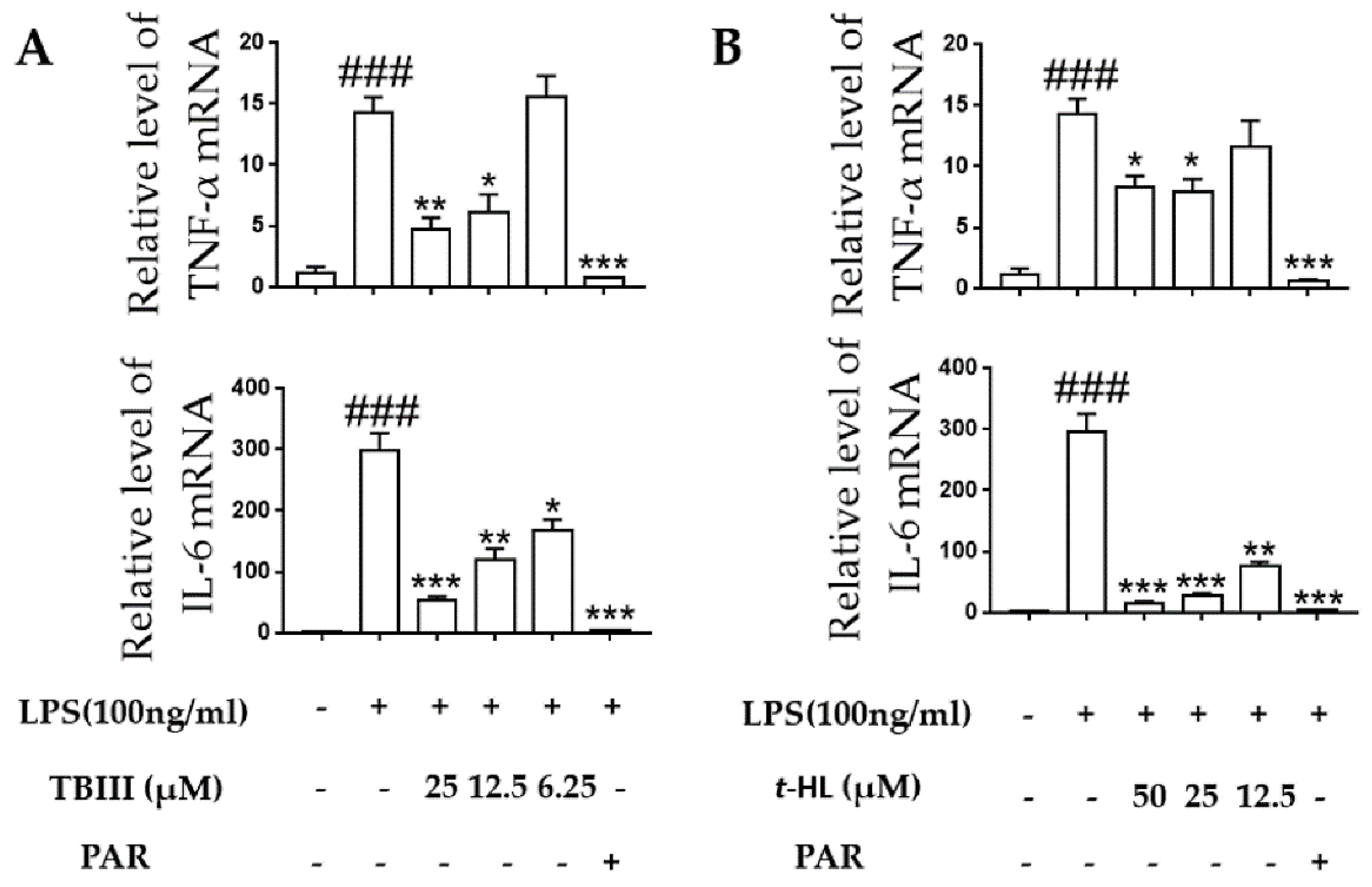
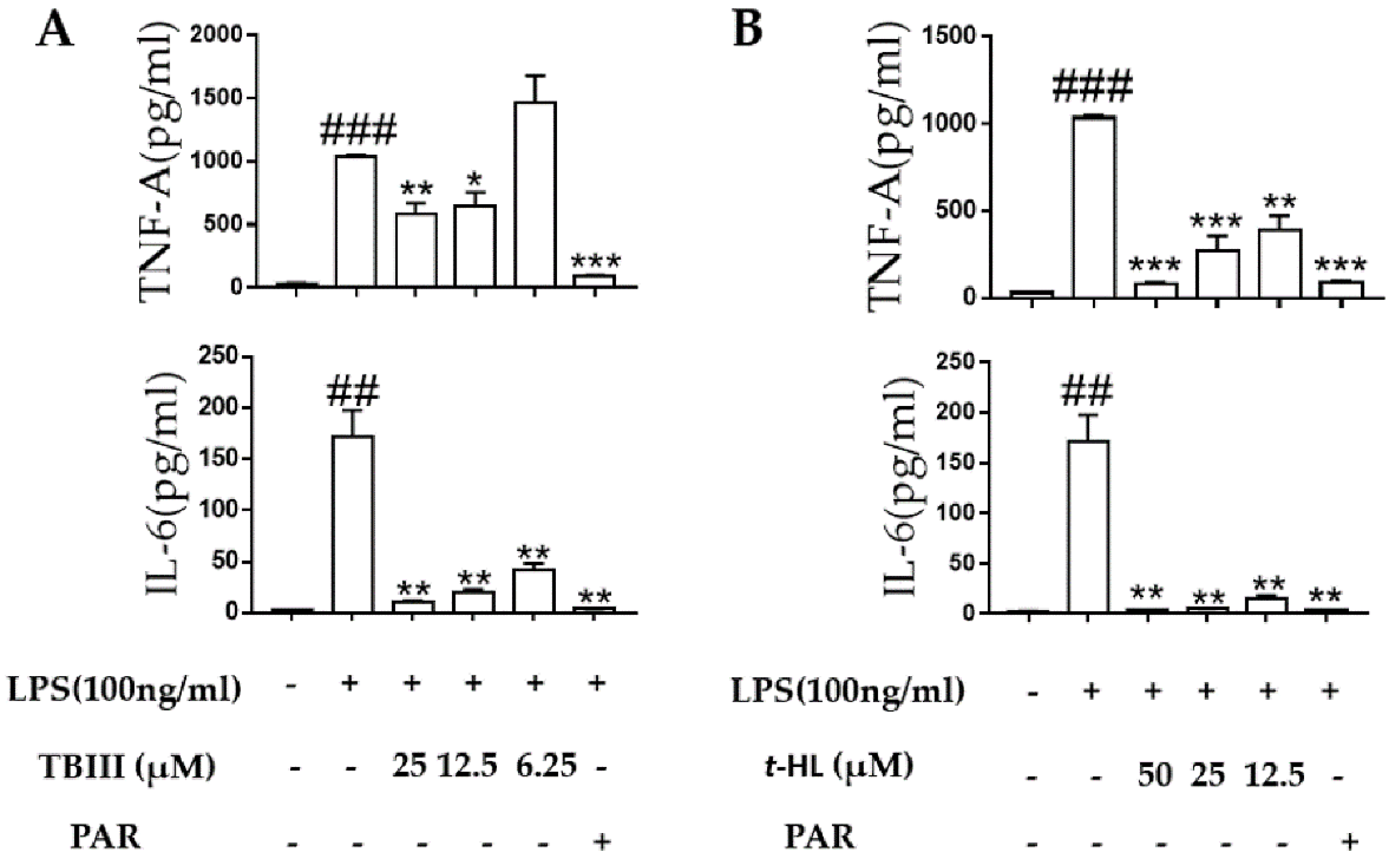
2.5. Effects of TBIII and t-HL on Inflammatory Cytokines in LPS-Stimulated N9 Cells
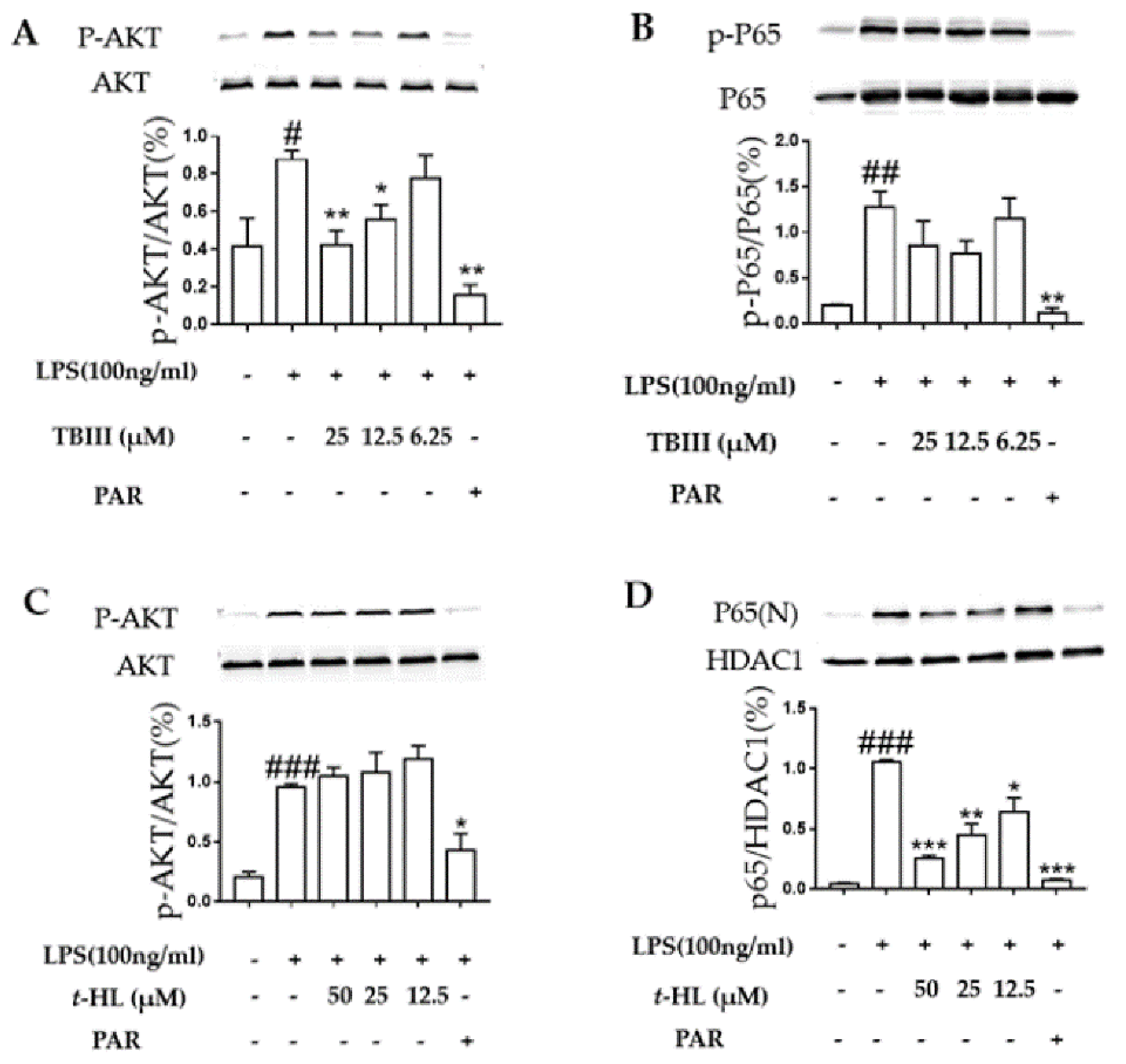
2.6. Effects of TBIII and t-HL on the Activation of Signaling Pathways in LPS-Stimulated N9 Microglial Cells
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Apparatus
3.3. Plant Materials
3.4. Preparation of Saponins-Rich part
3.5. Purification of Compounds from the Saponins-Rich Part
3.6. Cell Culture
3.7. MTT Assay for Cell Viability
3.8. Bioassay for NO Production
3.9. Total RNA Extraction and Real-Time PCR
3.10. Measurements of TNF-α and IL-6 Production by ELISA
3.11. Western Blot Analysis
3.12. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Aloisi, F. The role of microglia and astrocytes in CNS immune surveillance and immunopathology. Adv. Exp. Med. Biol. 1999, 468, 123–133. [Google Scholar] [PubMed]
- Zeinstra, E.; Wilczak, N.; De Keyser, J. Reactive astrocytes in chronic active lesions of multiple sclerosis express co-stimulatory molecules B7-1 and B7-2. J. Neuroimmunol. 2003, 135, 166–171. [Google Scholar] [CrossRef]
- Tang, Y.; Li, T.; Li, J.; Yang, J.; Liu, H.; Zhang, X.J.; Le, W. Jmjd3 is essential for the epigenetic modulation of microglia phenotypes in the immune pathogenesis of Parkinson′s disease. Cell Death Differ. 2014, 21, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Itagaki, S.; McGeer, P.L.; Akiyama, H.; Zhu, S.; Selkoe, D. Relationship of microglia and astrocytes to amyloid deposits of Alzheimer disease. J. Neuroimmunol. 1989, 24, 173–182. [Google Scholar] [CrossRef]
- Brown, G.C.; Neher, J.J. Inflammatory neurodegeneration and mechanisms of microglial killing of neurons. Mol. Neurobiol. 2010, 41, 242–247. [Google Scholar] [CrossRef] [PubMed]
- McCarty, M.F. Down-regulation of microglial activation may represent a practical strategy for combating neurodegenerative disorders. Med. Hypotheses 2006, 67, 251–269. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Chung, J.H.; Yoon, J.S.; Ha, Y.M.; Bae, S.; Lee, E.K.; Jung, K.J.; Kim, M.S.; Kim, Y.J.; Kim, M.K.; et al. Ginsenoside Rd inhibits the expressions of iNOS and COX-2 by suppressing NF-κB in LPS-stimulated RAW264.7 cells and mouse liver. J. Gins. Res. 2013, 37, 54–63. [Google Scholar] [CrossRef]
- Wang, X.; Hu, D.; Zhang, L.; Lian, G.; Zhao, S.; Wang, C.; Yin, J.; Wu, C.; Yang, J. Gomisin A inhibits lipopolysaccharide-induced inflammatory responses in N9 microglia via blocking the NF-κB /MAPKs pathway. Food Chem. Toxicol. 2014, 63, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.T.; Du, Y.G.; He, J.L.; Chen, W.J.; Li, W.M.; Yang, Z.; Wang, Y.X.; Yu, C. Tetramethylpyrazine inhibits production of nitric oxide and inducible nitric oxide synthase in lipopolysaccharide-induced N9 microglial cells through blockade of MAPK and PI3K/Akt signaling pathways, and suppression of intracellular reactive oxygen species. J. Ethnopharmacol. 2010, 129, 335–343. [Google Scholar] [CrossRef]
- Li, R.; Huang, Y.G.; Fang, D.; Le, W.D. (−)-Epigallocatechin gallate inhibits lipopolysaccharide-induced microglial activation and protects against inflammation-mediated dopaminergic neuronal injury. J. Neurosci. Res. 2004, 78, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, Y.O.; Kim, H.; Kim, S.Y.; Noh, H.S.; Kang, S.S.; Cho, G.J.; Choi, W.S.; Suk, K. Flavonoid wogonin from medicinal herb is neuroprotective by inhibiting inflammatory activation of microglia. Faseb J. 2003, 17, 1943–1944. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.K.; Lee, H.S.; Cho, J.Y.; Shin, W.C.; Rhee, M.H.; Kim, T.G.; Kang, J.H.; Kim, S.H.; Hong, S.; Kang, S.Y. Inhibitory effect of curcumin on nitric oxide production from lipopolysaccharide-activated primary microglia. Life Sci. 2006, 79, 2022–2031. [Google Scholar] [CrossRef] [PubMed]
- Ji, D.; Huang, Z.Y.; Fei, C.H.; Xue, W.W.; Lu, T.L. Comprehensive profiling and characterization of chemical constituents of rhizome of Anemarrhena asphodeloides Bge. J. Chromatogr. B 2017, 1060, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.G.; Guo, X.D.; Liang, J.; Yang, B.Y.; Kuang, H.X. Screening and identification of steroidal saponins from Anemarrhena asphodeloides employing UPLC tandem triple quadrupole linear ion trap mass spectrometry. Steroids 2017, 125, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dan, Y.; Yang, D.; Hu, Y.; Zhang, L.; Zhang, C.; Zhu, H.; Cui, Z.; Li, M.; Liu, Y. The genus Anemarrhena Bunge: A review on ethnopharmacology, phytochemistry and pharmacology. J. Ethnopharmacol. 2014, 153, 42–60. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Jin, Y.; Sui, H.J.; Yan, E.Z. Effect and signaling mechanism of SAaB on the Aβ25-35-induced release of inflammatory mediators in cultured macrophages. Chin. Pharmacol. Bull. 2011, 27, 695–700. [Google Scholar]
- Zhao, X.; Liu, C.; Qi, Y.; Fang, L.; Luo, J.; Bi, K.; Jia, Y. Timosaponin B-II ameliorates scopolamine-induced cognition deficits by attenuating acetylcholinesterase activity and brain oxidative damage in mice. Metab. Brain Dis. 2016, 31, 1455–1461. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, B.C.; Kim, J.H.; Sim, G.S.; Lee, D.H.; Lee, K.E.; Yun, Y.P.; Pyo, H.B. The isolation and antioxidative effects of vitexin from Acer palmatum. Arch. Pharmacal Res. 2005, 28, 195–202. [Google Scholar] [CrossRef]
- Ju, C.; Hwang, S.; Cho, G.S.; Kondaji, G.; Song, S.; Prather, P.L.; Choi, Y.; Kim, W.K. Differential anti-ischemic efficacy and therapeutic time window of trans- and cis-hinokiresinols: Stereo-specific antioxidant and anti-inflammatory activities. Neuropharmacology 2013, 67, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Li, S.M.; Yang, X.W.; Li, Y.L.; Shen, Y.H.; Feng, L.; Wang, Y.H.; Zeng, H.W.; Liu, X.H.; Zhang, C.S.; Long, C.L.; et al. Chemical constituents of Dracocephalum forrestii. Planta Med. 2009, 75, 1591–1596. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.B.; Peng, Y.; Li, L.Z.; Gao, P.Y.; Sun, Y.; Yu, L.H.; Song, S.J. Steroidal saponins from Anemarrhena asphodeloides. J. Asian Nat. Prod. Res. 2013, 15, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Ishii, H.; Tori, K.; Tozyo, T.; Yoshimura, Y. Saponins from roots of platycodon grandiflorum. Part 2. Isolation and structure of new triterpene glycosides. J. Chem. Soc., Perkin Trans. 1 1984, 15, 661–668. [Google Scholar] [CrossRef]
- Sang, S.; Mao, S.; Lao, A.; Chen, Z.; Ho, C.T. Four new steroidal saponins from the seeds of Allium tuberosum. J. Agric. Food. Chem. 2001, 49, 1475–1478. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Kim, J.S.; Choi, S.U.; Kim, J.S.; Lee, H.S.; Roh, S.H.; Jeong, Y.C.; Kim, Y.K.; Ryu, S.Y. Isolation of a new saponin and cytotoxic effect of saponins from the root of Platycodon grandiflorum on human tumor cell lines. Planta Med. 2005, 71, 566–568. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhao, M.; Zheng, W.; Liu, Y. Platycodin D a triterpenoid saponin from platycodon grandiflorum, suppresses the growth and invasion of human oral squamous cell carcinoma cells via the NF-κB pathway. J. Biochem. Mol. Toxicol. 2017, 31, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.S.; Kang, O.H.; Kong, R.; Zhou, T.; Kim, S.A.; Ryu, S.; Kim, H.R.; Kwon, D.Y. Polygalacin D induces apoptosis and cell cycle arrest via the PI3K/Akt pathway in non-small cell lung cancer. Oncol. Rep. 2018, 39, 1702–1710. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Chen, X.; Chen, X.; Ma, D.L.; Leung, C.H.; Lu, J.J. Platycodin D potentiates proliferation inhibition and apoptosis induction upon AKT inhibition via feedback blockade in non-small cell lung cancer cells. Sci. Rep. 2016, 6, 37997. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; He, J.L.; Xie, X.Y.; Yu, C. LPS-induced iNOS expression in N9 microglial cells is suppressed by geniposide via ERK, p38 and nuclear factor-κB signaling pathways. Int. J. Mol. Med. 2012, 30, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.L.; Wang, L.; Xiong, L.; Huang, F.H.; Xue, H. Timosaponin B-III exhibits antidepressive activity in a mouse model of postpartum depression by the regulation of inflammatory cytokines, BNDF signaling and synaptic plasticity. Exp. Ther. Med. 2017, 14, 3856–3861. [Google Scholar] [CrossRef] [PubMed]
- Giles, J.A.; Greenhalgh, A.D.; Davies, C.L.; Denes, A.; Shaw, T.; Coutts, G.; Rothwell, N.J.; McColl, B.W.; Allan, S.M. Requirement for interleukin-1 to drive brain inflammation reveals tissue-specific mechanisms of innate immunity. Eur. J. Immunol. 2015, 45, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Zhao, L.; Zhao, Q.; Zhao, Y.; Sun, Y.; Zhang, Y.; Miao, H.; You, Q.D.; Hu, R.; Guo, Q.L. NF-κB and Nrf2 signaling pathways contribute to wogonin-mediated inhibition of inflammation-associated colorectal carcinogenesis. Cell Death Dis. 2014, 5, 1283. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.N.; Son, M.S.; Park, J.H.; Lee, E.H. Shikonins attenuate microglial inflammatory responses by inhibition of ERK, Akt, and NF-κB: Neuroprotective implications. Neuropharmacology 2008, 55, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, C.; Wang, J.; Zhao, S.; Zhang, K.; Wang, J.; Zhang, W.; Wu, C.; Yang, J. Pseudoginsenoside-F11 (PF11) exerts anti-neuroinflammatory effects on LPS-activated microglial cells by inhibiting TLR4-mediated TAK1/IKK/ NF-κB, MAPKs and Akt signaling pathways. Neuropharmacology 2014, 79, 642–656. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Guo, Z.; Xiao, Y.; Xue, X.; Zhang, X.; Liang, X. Evaluation and comparison of n-alkyl chain and polar ligand bonded stationary phases for protein separation in reversed-phase liquid chromatography. J. Sep. Sci. 2014, 37, 2467–2473. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Xin, H.; Cheng, L.; Guo, Z.; Feng, J.; Fu, Q.; Jin, Y.; Liang, X. Selective separation of xanthones and saponins from the rhizomes of Anemarrhena asphodeloides by modulating the density of surface charges in C18-bonded stationary phases. Anal. Methods 2017, 9, 5604–5610. [Google Scholar] [CrossRef]
- Yang, Y.Y.; He, H.Q.; Cui, J.H.; Nie, Y.J.; Wu, Y.X.; Wang, R.; Wang, G.; Zheng, J.N.; Ye, R.D.; Wu, Q.; et al. Shikonin Derivative DMAKO-05 Inhibits Akt Signal Activation and Melanoma Proliferation. Chem. Biol. Drug Des. 2016, 87, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Fan, Y.; Fan, C.; Yu, Y.; Sun, L.; Jin, Y.; Zhang, Y.; Ye, R.D. Licocoumarone isolated from Glycyrrhiza uralensis selectively alters LPS-induced inflammatory responses in RAW 264.7 macrophages. Eur. J. Pharmacol. 2017, 801, 46–53. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
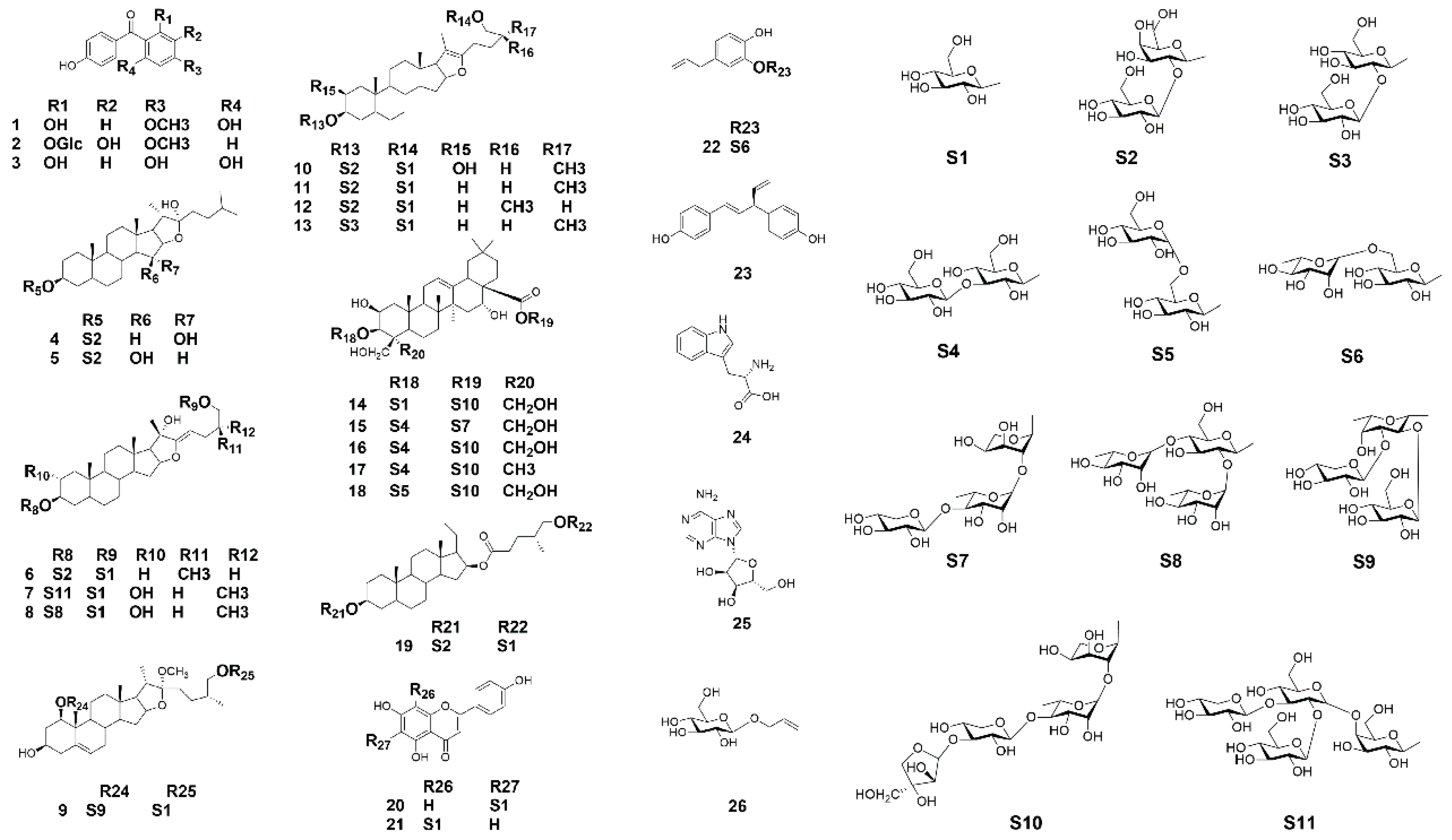
| Compound | Name | % Cell Viability | Nitrite (μM) | % Inhibition of NO Production | |||
|---|---|---|---|---|---|---|---|
| 50 μM | 25 μM | 50 μM | 25 μM | 50 μM | 25 μM | ||
| 1 | 2,6,4′-Trihydroxy-4-methoxybenzo-phenone | 97.49 ± 1.85 | 98.73 ± 8.27 | 19.29 ± 0.10 * | 26.78 ± 1.27 * | 41.74 ± 3.90 * | 15.35 ± 9.40 |
| 2 | Zimoside A | 94.62 ± 5.47 | 97.24 ± 3.27 | 21.41 ± 0.92 * | 26.64 ± 1.13 * | 32.77 ± 2.88 * | 16.32 ± 3.55 * |
| 3 | Iriflophene | 112.72 ± 5.32 | 107.03 ± 3.62 | 20.41 ± 0.38 * | 26.06 ± 1.24 * | 35.89 ± 1.20 * | 18.14 ± 3.88 * |
| 4 | Anemarrhena-saponin II | 8.57 ± 0.11 # | 63.92 ± 5.98 # | 3.80 ± 0.53 * | 9.52 ± 0.84 * | 87.98 ± 2.99 * | 69.91 ± 4.86 * |
| 5 | Anemarrhena-saponin I | 115.47 ± 6.83 | 110.42 ± 4.67 | 19.14 ± 0.59 * | 29.82 ± 0.24 * | 39.90 ± 1.85 * | 6.36 ± 0.76 * |
| 6 | Anemarnoside B | 104.33 ± 16.47 | 115.27 ± 5.52 | 26.38 ± 2.63 | 40.89 ± 2.04 | 17.23 ± 8.26 | −28.28 ± 6.40 |
| 7 | Hostaplantagineo-side C | 79.71 ± 6.03 | 84.89 ± 1.82 | 23.52 ± 0.37 * | 32.76 ± 0.85 | 25.75 ± 2.40 * | −3.57 ± 8.75 |
| 8 | Tuberoside G | 99.29 ± 2.46 | 112.82 ± 4.14 | 27.65 ± 0.74 * | 35.05 ± 0.68 * | 13.20 ± 2.32 * | −10.83 ± 2.14 * |
| 9 | Spicatoside B | 94.29 ± 3.21 | 99.70 ± 3.13 | 24.20 ± 0.29 * | 34.46 ± 0.41 * | 24.01 ± 0.90 * | −8.22 ± 1.30 * |
| 10 | Timosaponin D | 110.25 ± 2.57 | 111.11 ± 4.39 | 20.78 ± 2.00 * | 29.22 ± 0.60 | 34.75 ± 6.27 * | 8.57 ± 1.87 |
| 11 | Timosaponin BIII | 74.93 ± 3.15 # | 119.63 ± 11.75 | 4.24 ± 0.10 * | 8.55 ± 0.25 * | 86.67 ± 0.37 * | 73.16 ± 0.80 * |
| 12 | Macrostemono-side F | 105.77 ± 2.84 | 104.74 ± 3.16 | 11.82 ± 1.03 * | 19.64 ± 1.21 * | 62.89 ± 3.22 * | 38.33 ± 3.80 * |
| 13 | Timosaponin C | 97.19 ± 6.46 | 109.04 ± 4.85 | 13.36 ± 0.56 * | 24.24 ± 1.32 | 58.10 ± 1.75 * | 23.94 ± 4.13 |
| 14 | Platycodin D | 17.93 ± 1.99 # | 40.16 ± 9.18 # | 4.29 ± 0.59 * | 28.94 ± 0.40 * | 86.54 ± 2.71 * | 8.65 ± 2.24 |
| 15 | Platycoside A | 108.08 ± 1.17 | 103.09 ± 3.81 | 22.55 ± 0.24 * | 29.14 ± 1.22 | 29.19 ± 0.74 * | 8.49 ± 3.84 |
| 16 | Platycodin D2 | 101.05 ± 1.83 | 113.54 ± 4.62 | 24.94 ± 0.45 * | 33.68 ± 1.44 | 21.67 ± 1.40 * | −5.78 ± 4.51 |
| 17 | Polygalacin D2 | 88.00 ± 8.92 | 97.50 ± 1.83 | 26.21 ± 0.82 * | 31.14 ± 0.78 | 17.74 ± 3.14 * | 2.25 ± 2.46 |
| 18 | Platycodin D3 | 9.07 ± 0.72 # | 9.23 ± 0.22 # | 3.50 ± 0.21 * | 8.49 ± 0.36 * | 89.01 ± 0.67 * | 73.37 ± 1.14 * |
| 19 | Anemarnside | 125.85 ± 3.00 # | 124.43 ± 3.13 # | 9.83 ± 0.30 * | 23.63 ± 1.13 * | 66.91 ± 2.82 * | 25.23 ± 8.72 * |
| 20 | Isovitexin | 95.68 ± 2.75 | 135.43 ± 11.78 | 22.82 ± 0.27 * | 28.39 ± 0.07 * | 29.77 ± 0.60 * | 10.21 ± 4.73 |
| 21 | Vitexin | 81.75 ± 4.38 # | 90.52 ± 2.51 | 17.81 ± 1.13 * | 28.88 ± 1.38 | 44.11 ± 3.56 * | 9.39 ± 4.33 |
| 22 | 3,4-dihydroxyallyl-benzene-3-O-α-L-rhamnopyranosyl (1→6)-β-d-glucopy-ranoside | 95.17 ± 4.91 | 99.63 ± 5.82 | 19.43 ± 1.79 * | 27.23 ± 0.21 * | 38.97 ± 5.62 * | 14.48 ± 0.65 * |
| 23 | trans-Hinokiresinol | 98.29 ± 11.55 | 108.44 ± 4.39 | 5.18 ± 0.06 * | 14.93 ± 0.41 * | 83.73 ± 0.22 * | 53.12 ± 1.30 * |
| 24 | Tryptophan | 92.44 ± 8.71 | 104.52 ± 6.13 | 24.19 ± 0.72 * | 30.49 ± 0.93 | 24.03 ± 2.27 * | 4.24 ± 2.92 |
| 25 | Adenosine | 80.54 ± 6.19 | 81.56 ± 1.26 | 20.69 ± 1.74 * | 27.43 ± 1.35 | 35.09 ± 5.47 * | 13.93 ± 4.23 |
| 26 | α-d-Glucose-monoallyl ether | 105.47 ± 16.13 | 107.17 ± 0.63 | 22.60 ± 2.19 * | 29.57 ± 0.71 | 29.03 ± 8.43 * | 7.13 ± 2.23 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Cai, J.; Fu, Q.; Cheng, L.; Wu, L.; Zhang, W.; Zhang, Y.; Jin, Y.; Zhang, C. Anti-Inflammatory Activities of Compounds Isolated from the Rhizome of Anemarrhena asphodeloides. Molecules 2018, 23, 2631. https://doi.org/10.3390/molecules23102631
Wang Z, Cai J, Fu Q, Cheng L, Wu L, Zhang W, Zhang Y, Jin Y, Zhang C. Anti-Inflammatory Activities of Compounds Isolated from the Rhizome of Anemarrhena asphodeloides. Molecules. 2018; 23(10):2631. https://doi.org/10.3390/molecules23102631
Chicago/Turabian StyleWang, Zeyuan, Jianfeng Cai, Qing Fu, Lingping Cheng, Lehao Wu, Weiyue Zhang, Yan Zhang, Yu Jin, and Chunzhi Zhang. 2018. "Anti-Inflammatory Activities of Compounds Isolated from the Rhizome of Anemarrhena asphodeloides" Molecules 23, no. 10: 2631. https://doi.org/10.3390/molecules23102631
APA StyleWang, Z., Cai, J., Fu, Q., Cheng, L., Wu, L., Zhang, W., Zhang, Y., Jin, Y., & Zhang, C. (2018). Anti-Inflammatory Activities of Compounds Isolated from the Rhizome of Anemarrhena asphodeloides. Molecules, 23(10), 2631. https://doi.org/10.3390/molecules23102631




