Intestinal Saturated Long-Chain Fatty Acid, Glucose and Fructose Transporters and Their Inhibition by Natural Plant Extracts in Caco-2 Cells
Abstract
1. Introduction
2. The Role of Intestinal Fatty Acid, Glucose and Fructose Uptake with Regards to Developing Metabolic Disorders
2.1. Fatty Acids and Obesity
2.2. Monosaccharides and Glycemia
3. Cell Models
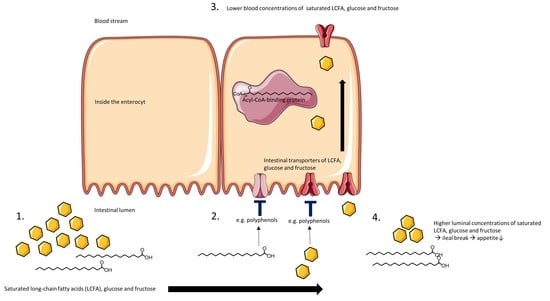
4. Transport Mechanisms and Binding Sites of Intestinal Saturated LCFA, Glucose and Fructose Transporters
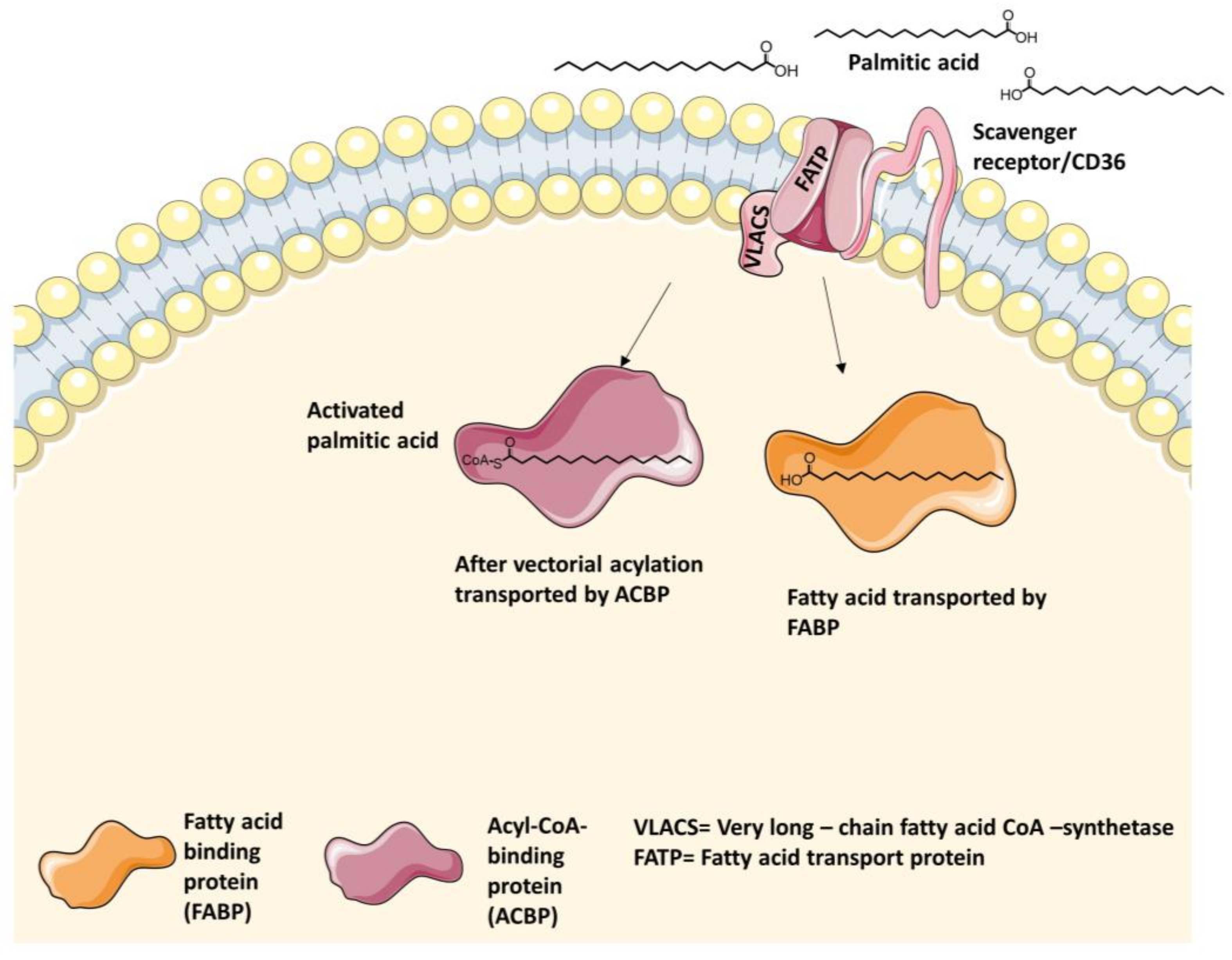
4.1. Intestinal Long-Chain Fatty Acid Transporters
4.1.1. Fatty Acid Transport Protein 2 (FATP2) Inhibitors
4.1.2. Fatty Acid Transport Protein 4 (FATP4) Inhibitors
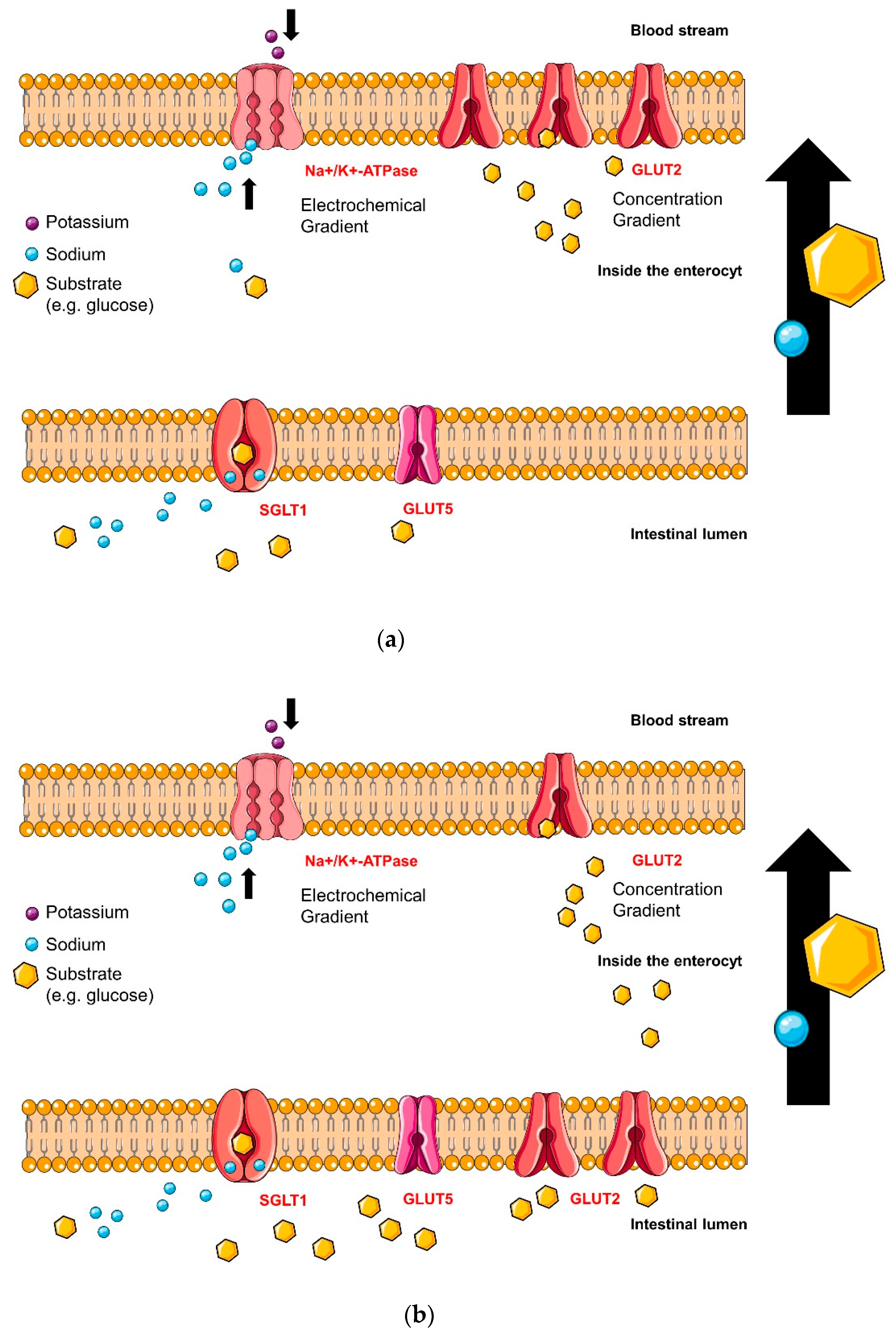
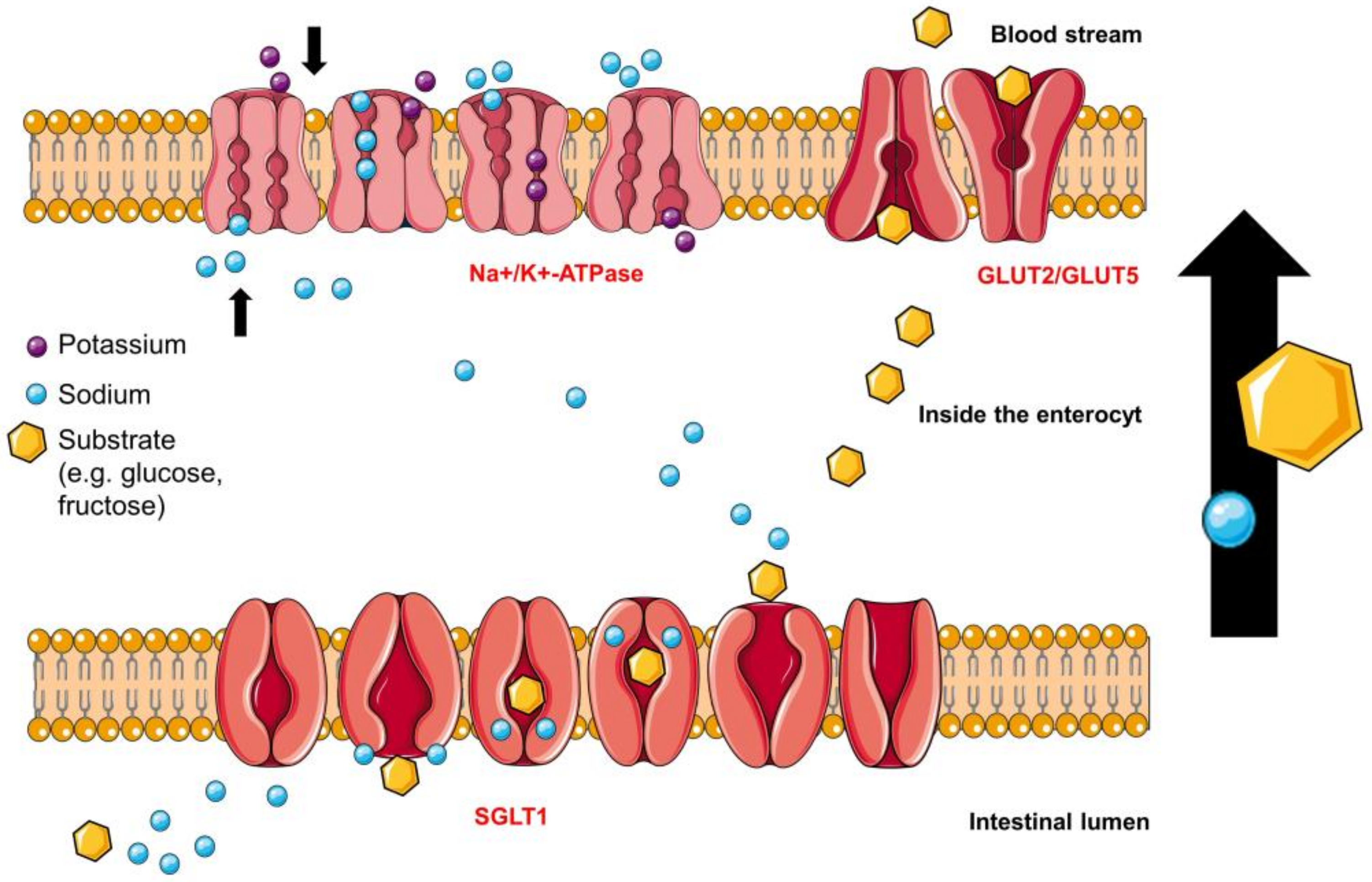
4.2. Intestinal Glucose Transporters
4.2.1. Sodium-Glucose Linked Transporter 1 (SGLT1)
Sodium-Binding Site
Monosaccharide-Binding Site
Phlorizin-Binding Site
4.2.2. Glucose Transporter 2 (GLUT2) Glucose-Binding-Sites
4.3. Intestinal Fructose Transporters
4.3.1. Glucose Transporter 2 (GLUT2) Fructose-Binding Sites
4.3.2. Glucose Transporter 5 (GLUT5)
Rubusoside-Binding Site
5. Plant Extracts with Inhibitory Activity on Intestinal LCFA, Glucose and Fructose Transporters
6. Polyphenols
7. Intestinal Metabolism of Polyphenols
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bullo, M.; Cozar-Torrell, P.; Salas-Salvado, J. Dietary regulation of glucose metabolism in metabolic syndrome. Curr. Vasc. Pharmacol. 2013, 11, 928–945. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Diabetes. World Health Organization: Geneva, Switzerland. Available online: http://www.who.int/mediacentre/factsheets/fs312/en (accessed on 14 March 2018).
- World Health Organization. Infographics on diabetes. World Health Organization: Geneva, Switzerland. Available online: http://www.who.int/diabetes/infographics/en/ (accessed on 14 March 2018).
- World Health Organization; Global Health Observatory. Top 10 causes of death. World Health Organization: Geneva, Switzerland. Available online: http://www.who.int/gho/mortality_burden_disease/causes_death/top_10/en/ (accessed on 14 March 2018).
- World Health Organization. The top 10 causes of death. World Health Organization: Geneva, Switzerland. Available online: http://www.who.int/mediacentre/factsheets/fs310/en/ (accessed on 14 March 2018).
- Williamson, G. Possible effects of dietary polyphenols on sugar absorption and digestion. Mol. Nutr. Food Res. 2013, 57, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Delarue, J.; Magnan, C. Free fatty acids and insulin resistance. Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Boden, G.; Chen, X. Effects of fat on glucose uptake and utilization in patients with non-insulin-dependent diabetes. J. Clin. Invest. 1995, 96, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Palomer, X.; Pizarro-Delgado, J.; Barroso, E.; Vazquez-Carrera, M. Palmitic and oleic acid: The yin and yang of fatty acids in type 2 diabetes mellitus. Trends Endocrinol. Metab. 2018, 29, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Li, J.; Shen, M.; Jia, W.; Chen, N.; Chen, T.; Su, D.; Tian, H.; Zheng, S.; Dai, Y.; et al. Cellular production of n-3 pufas and reduction of n-6-to-n-3 ratios in the pancreatic beta-cells and islets enhance insulin secretion and confer protection against cytokine-induced cell death. Diabetes 2010, 59, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Yazici, D.; Sezer, H. Insulin resistance, obesity and lipotoxicity. Adv. Exp. Med. Biol. 2017, 960, 277–304. [Google Scholar] [PubMed]
- Black, P.N.; Sandoval, A.; Arias-Barrau, E.; DiRusso, C.C. Targeting the fatty acid transport proteins (fatp) to understand the mechanisms linking fatty acid transport to metabolism. Immunol. Endocr. Metab. Agents Med. Chem. 2009, 9, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Black, P.N.; Ahowesso, C.; Montefusco, D.; Saini, N.; DiRusso, C.C. Fatty acid transport proteins: Targeting fatp2 as a gatekeeper involved in the transport of exogenous fatty acids. Medchemcomm 2016, 7, 612–622. [Google Scholar] [CrossRef] [PubMed]
- Doege, H.; Stahl, A. Protein-mediated fatty acid uptake: Novel insights from in vivo models. Physiology (Bethesda, Md.) 2006, 21, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Roder, P.V.; Geillinger, K.E.; Zietek, T.S.; Thorens, B.; Koepsell, H.; Daniel, H. The role of sglt1 and glut2 in intestinal glucose transport and sensing. PLoS ONE 2014, 9, e89977. [Google Scholar] [CrossRef] [PubMed]
- Guillam, M.T.; Hummler, E.; Schaerer, E.; Yeh, J.I.; Birnbaum, M.J.; Beermann, F.; Schmidt, A.; Deriaz, N.; Thorens, B. Early diabetes and abnormal postnatal pancreatic islet development in mice lacking glut-2. Nat. Genet. 1997, 17, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Barone, S.; Fussell, S.L.; Singh, A.K.; Lucas, F.; Xu, J.; Kim, C.; Wu, X.; Yu, Y.; Amlal, H.; Seidler, U.; et al. Slc2a5 (glut5) is essential for the absorption of fructose in the intestine and generation of fructose-induced hypertension. J. Biol. Chem. 2009, 284, 5056–5066. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Onishi, A.; Koepsell, H.; Vallon, V. Sodium glucose cotransporter sglt1 as a therapeutic target in diabetes mellitus. Expert. Opin. Ther. Targets 2016, 20, 1109–1125. [Google Scholar] [CrossRef] [PubMed]
- Thorens, B. Molecular and cellular physiology of glut-2, a high-km facilitated diffusion glucose transporter. Int. Rev. Cytol. 1992, 137, 209–238. [Google Scholar] [PubMed]
- Douard, V.; Ferraris, R.P. Regulation of the fructose transporter glut5 in health and disease. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E227–E237. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, J.E. Lipotoxicity: When tissues overeat. Curr. Opin. Lipidol. 2003, 14, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Salvado, L.; Coll, T.; Gomez-Foix, A.M.; Salmeron, E.; Barroso, E.; Palomer, X.; Vazquez-Carrera, M. Oleate prevents saturated-fatty-acid-induced er stress, inflammation and insulin resistance in skeletal muscle cells through an ampk-dependent mechanism. Diabetologia 2013, 56, 1372–1382. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, Y.A.; Portois, L.; Malaisse, W.J. N-3 fatty acids and the metabolic syndrome. Am. J. Clin. Nutr. 2006, 83, 1499s–1504s. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Cho, H.K.; Kwon, Y.H. Palmitate induces insulin resistance without significant intracellular triglyceride accumulation in hepg2 cells. Metabolism 2010, 59, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Sears, B.; Perry, M. The role of fatty acids in insulin resistance. Lipids Health Dis. 2015, 14, 121. [Google Scholar] [CrossRef] [PubMed]
- Stanhope, K.L. Sugar consumption, metabolic disease and obesity: The state of the controversy. Crit. Rev. Clin. Lab. Sci. 2016, 53, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.A.; Sievenpiper, J.L. Controversies about sugars: Results from systematic reviews and meta-analyses on obesity, cardiometabolic disease and diabetes. Eur. J. Nutr. 2016, 55, 25–43. [Google Scholar] [CrossRef] [PubMed]
- Hannou, S.A.; Haslam, D.E.; McKeown, N.M.; Herman, M.A. Fructose metabolism and metabolic disease. J. Clin. Invest. 2018, 128, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Tappy, L.; Le, K.A. Does fructose consumption contribute to non-alcoholic fatty liver disease? Clin. Res. Hepatol. Gastroenterol. 2012, 36, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, I.A. A review of recent evidence relating to sugars, insulin resistance and diabetes. Eur. J. Nutr. 2016, 55, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Blakely, S.R.; Hallfrisch, J.; Reiser, S.; Prather, E.S. Long-term effects of moderate fructose feeding on glucose tolerance parameters in rats. J. Nutr. 1981, 111, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Beck-Nielsen, H.; Pedersen, O.; Lindskov, H.O. Impaired cellular insulin binding and insulin sensitivity induced by high-fructose feeding in normal subjects. Am. J. Clin. Nutr. 1980, 33, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Stanhope, K.L. Role of fructose-containing sugars in the epidemics of obesity and metabolic syndrome. Annu. Rev. Med. 2012, 63, 329–343. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.J.; Segal, M.S.; Sautin, Y.; Nakagawa, T.; Feig, D.I.; Kang, D.H.; Gersch, M.S.; Benner, S.; Sanchez-Lozada, L.G. Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am. J. Clin. Nutr. 2007, 86, 899–906. [Google Scholar] [PubMed]
- Wilcox, G. Insulin and insulin resistance. Clin. Biochem. Rev. 2005, 26, 19–39. [Google Scholar] [PubMed]
- Gkogkolou, P.; Böhm, M. Advanced glycation end products: Key players in skin aging? Dermatoendocrinol 2012, 4, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, T.; Nakagawa, K.; Shimasaki, S.; Nagai, R. Lipid glycation and protein glycation in diabetes and atherosclerosis. Amino Acids. 2012, 42, 1163–1170. [Google Scholar] [CrossRef] [PubMed]
- Makita, Z.; Yanagisawa, K.; Kuwajima, S.; Bucala, R.; Vlassara, H.; Koike, T. The role of advanced glycosylation end-products in the pathogenesis of atherosclerosis. Nephrol. Dial. Transplant. 1996, 11, 31–33. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, A.; Horiuchi, N.; Hasegawa, K.; Uehara, M. Mortality and causes of death in type 2 diabetic patients. A long-term follow-up study in osaka district, japan. Diabetes Res. Clin. Pract. 1989, 7, 33–40. [Google Scholar] [CrossRef]
- Bucala, R. What is the effect of hyperglycemia on atherogenesis and can it be reversed by aminoguanidine? Diabetes Res. Clin. Pract. 1996, 30, S123–S130. [Google Scholar] [CrossRef]
- Genuth, S.; Sun, W.; Cleary, P.; Sell, D.R.; Dahms, W.; Malone, J.; Sivitz, W.; Monnier, V.M. Glycation and carboxymethyllysine levels in skin collagen predict the risk of future 10-year progression of diabetic retinopathy and nephropathy in the diabetes control and complications trial and epidemiology of diabetes interventions and complications participants with type 1 diabetes. Diabetes 2005, 54, 3103–3111. [Google Scholar] [PubMed]
- Chantret, I.; Barbat, A.; Dussaulx, E.; Brattain, M.G.; Zweibaum, A. Epithelial polarity, villin expression, and enterocytic differentiation of cultured human colon carcinoma cells: A survey of twenty cell lines. Cancer Res. 1988, 48, 1936–1942. [Google Scholar] [PubMed]
- Hidalgo, I.J.; Raub, T.J.; Borchardt, R.T. Characterization of the human colon carcinoma cell line (caco-2) as a model system for intestinal epithelial permeability. Gastroenterology 1989, 96, 736–749. [Google Scholar] [CrossRef]
- Jumarie, C.; Malo, C. Caco-2 cells cultured in serum-free medium as a model for the study of enterocytic differentiation in vitro. J. Cell. Physiol. 1991, 149, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Sambuy, Y.; De Angelis, I.; Ranaldi, G.; Scarino, M.L.; Stammati, A.; Zucco, F. The caco-2 cell line as a model of the intestinal barrier: Influence of cell and culture-related factors on caco-2 cell functional characteristics. Cell. Biol. Toxicol. 2005, 21, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Black, P.N.; Chokshi, A.; Sandoval-Alvarez, A.; Vatsyayan, R.; Sealls, W.; DiRusso, C.C. High-throughput screening for fatty acid uptake inhibitors in humanized yeast identifies atypical antipsychotic drugs that cause dyslipidemias. J. Lipid Res. 2008, 49, 230–244. [Google Scholar] [CrossRef] [PubMed]
- Mesonero, J.; Mahraoui, L.; Matosin, M.; Rodolosse, A.; Rousset, M.; Brot-Laroche, E. Expression of the hexose transporters glut1-glut5 and sglt1 in clones of caco-2 cells. Biochem. Soc. Trans. 1994, 22, 681–684. [Google Scholar] [CrossRef] [PubMed]
- Steffansen, B.; Pedersen, M.D.L.; Laghmoch, A.M.; Nielsen, C.U. Sglt1-mediated transport in caco-2 cells is highly dependent on cell bank origin. J. Pharm. Sci. 2017, 106, 2664–2670. [Google Scholar] [CrossRef] [PubMed]
- Naville, D.; Duchampt, A.; Vigier, M.; Oursel, D.; Lessire, R.; Poirier, H.; Niot, I.; Begeot, M.; Besnard, P.; Mithieux, G. Link between intestinal cd36 ligand binding and satiety induced by a high protein diet in mice. PLoS ONE 2012, 7, e30686. [Google Scholar] [CrossRef] [PubMed]
- Storch, J.; McDermott, L. Structural and functional analysis of fatty acid-binding proteins. J. Lipid Res. 2009, 50, S126–S131. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Madrid, P.; Fluitt, A.; Stahl, A.; Xie, X.S. Development and validation of a high-throughput screening assay for human long-chain fatty acid transport proteins 4 and 5. J. Biomol. Screen. 2010, 15, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Black, P.N.; DiRusso, C.C. A live-cell high-throughput screening assay for identification of fatty acid uptake inhibitors. Anal. Biochem. 2005, 336, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Tripp, J.; Essl, C.; Iancu, C.V.; Boles, E.; Choe, J.Y.; Oreb, M. Establishing a yeast-based screening system for discovery of human glut5 inhibitors and activators. Sci. Rep. 2017, 7, 6197. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, C.; Guan, B.; Brown, J.; Cullis, C.; Condon, S.M.; Jenkins, T.J.; Peluso, S.; Ye, Y.; Gimeno, R.E.; Punreddy, S.; et al. Identification and characterization of 4-aryl-3,4-dihydropyrimidin-2(1H)-ones as inhibitors of the fatty acid transporter fatp4. Bioorg. Med. Chem. Lett. 2006, 16, 3504–3509. [Google Scholar] [CrossRef] [PubMed]
- Debnam, E.S.; Levin, R.J. An experimental method of identifying and quantifying the active transfer electrogenic component from the diffusive component during sugar absorption measured in vivo. J. Physiol. 1975, 246, 181–196. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Scow, J.S.; Duenes, J.A.; Sarr, M.G. Mechanisms of glucose uptake in intestinal cell lines: Role of glut2. Surgery 2012, 151, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Spatola, L.; Finazzi, S.; Angelini, C.; Dauriz, M.; Badalamenti, S. Sglt1 and sglt1 inhibitors: A role to be assessed in the current clinical practice. Diabetes Ther. 2018, 9, 427–430. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, N.C.; Ding, Z.M.; Harrison, B.A.; Strobel, E.D.; Harris, A.L.; Smith, M.; Thompson, A.Y.; Xiong, W.; Mseeh, F.; Bruce, D.J.; et al. Discovery of lx2761, a sodium-dependent glucose cotransporter 1 (sglt1) inhibitor restricted to the intestinal lumen, for the treatment of diabetes. J. Med. Chem. 2017, 60, 710–721. [Google Scholar] [CrossRef] [PubMed]
- Corpe, C.P.; Basaleh, M.M.; Affleck, J.; Gould, G.; Jess, T.J.; Kellett, G.L. The regulation of glut5 and glut2 activity in the adaptation of intestinal brush-border fructose transport in diabetes. Pflugers Arch. 1996, 432, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Kellett, G.L.; Brot-Laroche, E. Apical glut2: A major pathway of intestinal sugar absorption. Diabetes 2005, 54, 3056–3062. [Google Scholar] [CrossRef] [PubMed]
- George Thompson, A.M.; Iancu, C.V.; Nguyen, T.T.; Kim, D.; Choe, J.Y. Inhibition of human glut1 and glut5 by plant carbohydrate products; insights into transport specificity. Sci. Rep. 2015, 5, 12804. [Google Scholar] [CrossRef] [PubMed]
- Duttaroy, A.K.; Spener, F. Cellular Proteins and Their Fatty Acids in Health and Disease, 1st ed.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2003; pp. 31–38. [Google Scholar]
- Ordovas, L.; Roy, R.; Zaragoza, P.; Rodellar, C. Structural and functional characterization of the bovine solute carrier family 27 member 1 (slc27a1) gene. Cytogenet Genome Res. 2006, 115, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Kazantzis, M.; Stahl, A. Fatty acid transport proteins, implications in physiology and disease. Biochim. Biophys. Acta 2012, 1821, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Stahl, A.; Gimeno, R.E.; Tartaglia, L.A.; Lodish, H.F. Fatty acid transport proteins: A current view of a growing family. Trends Endocrinol. Metab. 2001, 12, 266–273. [Google Scholar] [CrossRef]
- Dutta-Roy, A.K. Cellular uptake of long-chain fatty acids: Role of membrane-associated fatty-acid-binding/transport proteins. Cell. Mol. Life Sci. 2000, 57, 1360–1372. [Google Scholar] [CrossRef] [PubMed]
- Darimont, C.; Gradoux, N.; Persohn, E.; Cumin, F.; De Pover, A. Effects of intestinal fatty acid-binding protein overexpression on fatty acid metabolism in caco-2 cells. J. Lipid Res. 2000, 41, 84–92. [Google Scholar] [PubMed]
- Noushmehr, H.; D’Amico, E.; Farilla, L.; Hui, H.; Wawrowsky, K.A.; Mlynarski, W.; Doria, A.; Abumrad, N.A.; Perfetti, R. Fatty acid translocase (fat/cd36) is localized on insulin-containing granules in human pancreatic beta-cells and mediates fatty acid effects on insulin secretion. Diabetes 2005, 54, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Kuda, O.; Pietka, T.A.; Demianova, Z.; Kudova, E.; Cvacka, J.; Kopecky, J.; Abumrad, N.A. Sulfo-n-succinimidyl oleate (sso) inhibits fatty acid uptake and signaling for intracellular calcium via binding cd36 lysine 164: Sso also inhibits oxidized low density lipoprotein uptake by macrophages. J. Biol. Chem. 2013, 288, 15547–15555. [Google Scholar] [CrossRef] [PubMed]
- Stahl, A.; Hirsch, D.J.; Gimeno, R.E.; Punreddy, S.; Ge, P.; Watson, N.; Patel, S.; Kotler, M.; Raimondi, A.; Tartaglia, L.A.; et al. Identification of the major intestinal fatty acid transport protein. Mol. Cell. 1999, 4, 299–308. [Google Scholar] [CrossRef]
- Gimeno, R.E. Fatty acid transport proteins. Curr. Opin. Lipidol. 2007, 18, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Ortegon, A.M.; Tsang, B.; Doege, H.; Feingold, K.R.; Stahl, A. Fatp1 is an insulin-sensitive fatty acid transporter involved in diet-induced obesity. Mol. Cell. Biol. 2006, 26, 3455–3467. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.E.; Listenberger, L.L.; Ory, D.S.; Schaffer, J.E. Membrane topology of the murine fatty acid transport protein 1. J. Biol. Chem. 2001, 276, 37042–37050. [Google Scholar] [CrossRef] [PubMed]
- Richards, M.R.; Listenberger, L.L.; Kelly, A.A.; Lewis, S.E.; Ory, D.S.; Schaffer, J.E. Oligomerization of the murine fatty acid transport protein 1. J. Biol. Chem. 2003, 278, 10477–10483. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.; Nemoto, M.; Gelman, L.; Geffroy, S.; Najib, J.; Fruchart, J.C.; Roevens, P.; de Martinville, B.; Deeb, S.; Auwerx, J. The human fatty acid transport protein-1 (slc27a1; fatp-1) cdna and gene: Organization, chromosomal localization, and expression. Genomics 2000, 66, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Grzyb, J.; Latowski, D.; Strzalka, K. Lipocalins—A. family portrait. J. Plant. Physiol. 2006, 163, 895–915. [Google Scholar] [CrossRef] [PubMed]
- Coe, N.R.; Smith, A.J.; Frohnert, B.I.; Watkins, P.A.; Bernlohr, D.A. The fatty acid transport protein (fatp1) is a very long chain acyl-coa synthetase. J. Biol. Chem. 1999, 274, 36300–36304. [Google Scholar] [CrossRef] [PubMed]
- Black, P.N.; DiRusso, C.C. Yeast acyl-coa synthetases at the crossroads of fatty acid metabolism and regulation. Biochim. Biophys. Acta 2007, 1771, 286–298. [Google Scholar] [CrossRef] [PubMed]
- Ahowesso, C.; Black, P.N.; Saini, N.; Montefusco, D.; Chekal, J.; Malosh, C.; Lindsley, C.W.; Stauffer, S.R.; DiRusso, C.C. Chemical inhibition of fatty acid absorption and cellular uptake limits lipotoxic cell death. Biochem. Pharmacol. 2015, 98, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Mueckler, M.; Thorens, B. The slc2 (glut) family of membrane transporters. Mol. Aspects Med. 2013, 34, 121–138. [Google Scholar] [CrossRef] [PubMed]
- Blaschek, W. Natural products as lead compounds for sodium glucose cotransporter (sglt) inhibitors. Planta Med. 2017, 83, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Wright, E.M.; Loo, D.D.; Hirayama, B.A. Biology of human sodium glucose transporters. Physiol. Rev. 2011, 91, 733–794. [Google Scholar] [CrossRef] [PubMed]
- Kellett, G.L. The facilitated component of intestinal glucose absorption. J. Physiol. 2001, 531, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Kellett, G.L.; Brot-Laroche, E.; Mace, O.J.; Leturque, A. Sugar absorption in the intestine: The role of glut2. Annu Rev. Nutr. 2008, 28, 35–54. [Google Scholar] [CrossRef] [PubMed]
- Scow, J.S.; Iqbal, C.W.; Jones, T.W., 3rd; Qandeel, H.G.; Zheng, Y.; Duenes, J.A.; Nagao, M.; Madhavan, S.; Sarr, M.G. Absence of evidence of translocation of glut2 to the apical membrane of enterocytes in everted intestinal sleeves. J. Surg. Res. 2011, 167, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Kellett, G.L.; Helliwell, P.A. The diffusive component of intestinal glucose absorption is mediated by the glucose-induced recruitment of glut2 to the brush-border membrane. Biochem. J. 2000, 350, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Affleck, J.A.; Helliwell, P.A.; Kellett, G.L. Immunocytochemical detection of glut2 at the rat intestinal brush-border membrane. J. Histochem. Cytochem. 2003, 51, 1567–1574. [Google Scholar] [CrossRef] [PubMed]
- Sala-Rabanal, M.; Hirayama, B.A.; Loo, D.D.; Chaptal, V.; Abramson, J.; Wright, E.M. Bridging the gap between structure and kinetics of human sglt1. Am. J. Physiol. Cell. Physiol. 2012, 302, C1293–C1305. [Google Scholar] [CrossRef] [PubMed]
- Loo, D.D.; Jiang, X.; Gorraitz, E.; Hirayama, B.A.; Wright, E.M. Functional identification and characterization of sodium binding sites in na symporters. Proc. Natl. Acad. Sci. USA 2013, 110, E4557–4566. [Google Scholar] [CrossRef] [PubMed]
- Longpre, J.P.; Lapointe, J.Y. Determination of the na(+)/glucose cotransporter (sglt1) turnover rate using the ion-trap technique. Biophys. J. 2011, 100, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, A.; Choe, S.; Chaptal, V.; Rosenberg, J.M.; Wright, E.M.; Grabe, M.; Abramson, J. The mechanism of sodium and substrate release from the binding pocket of vsglt. Nature 2010, 468, 988–991. [Google Scholar] [CrossRef] [PubMed]
- Faham, S.; Watanabe, A.; Besserer, G.M.; Cascio, D.; Specht, A.; Hirayama, B.A.; Wright, E.M.; Abramson, J. The crystal structure of a sodium galactose transporter reveals mechanistic insights into na+/sugar symport. Science 2008, 321, 810–814. [Google Scholar] [CrossRef] [PubMed]
- Abramson, J.; Wright, E.M. Structure and function of na(+)-symporters with inverted repeats. Curr. Opin. Struct. Biol. 2009, 19, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Puntheeranurak, T.; Kasch, M.; Xia, X.; Hinterdorfer, P.; Kinne, R.K. Three surface subdomains form the vestibule of the na+/glucose cotransporter sglt1. J. Biol. Chem. 2007, 282, 25222–25230. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, D.G.; Bissonnette, P.; Lapointe, J.Y. Identification of a disulfide bridge linking the fourth and the seventh extracellular loops of the na+/glucose cotransporter. J. Gen. Physiol. 2006, 127, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Sasseville, L.J.; Morin, M.; Coady, M.J.; Blunck, R.; Lapointe, J.Y. The human sodium-glucose cotransporter (hsglt1) is a disulfide-bridged homodimer with a re-entrant c-terminal loop. PLoS ONE 2016, 11, e0154589. [Google Scholar] [CrossRef] [PubMed]
- Raja, M.; Kinne, R.K. Identification of phlorizin binding domains in sodium-glucose cotransporter family: Sglt1 as a unique model system. Biochimie 2015, 115, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, F. Hypothesis for the interaction of phlorizin and phloretin with membrane carriers for sugars. Biochim. Biophys. Acta 1967, 135, 483–495. [Google Scholar] [CrossRef]
- Crespy, V.; Aprikian, O.; Morand, C.; Besson, C.; Manach, C.; Demigne, C.; Remesy, C. Bioavailability of phloretin and phloridzin in rats. J. Nutr. 2001, 131, 3227–3230. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, U. Flavonoids as drugs at the small intestinal level. Curr. Opin. Pharmacol. 2013, 13, 864–868. [Google Scholar] [CrossRef] [PubMed]
- Leturque, A.; Brot-Laroche, E.; Le Gall, M. Glut2 mutations, translocation, and receptor function in diet sugar managing. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E985–992. [Google Scholar] [CrossRef] [PubMed]
- Naftalin, R.J. Does apical membrane glut2 have a role in intestinal glucose uptake? F1000Res. 2014, 3, 304. [Google Scholar] [CrossRef] [PubMed]
- Gouyon, F.; Caillaud, L.; Carriere, V.; Klein, C.; Dalet, V.; Citadelle, D.; Kellett, G.L.; Thorens, B.; Leturque, A.; Brot-Laroche, E. Simple-sugar meals target glut2 at enterocyte apical membranes to improve sugar absorption: A study in glut2-null mice. J. Physiol. 2003, 552, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Manolescu, A.; Salas-Burgos, A.M.; Fischbarg, J.; Cheeseman, C.I. Identification of a hydrophobic residue as a key determinant of fructose transport by the facilitative hexose transporter slc2a7 (glut7). J. Biol. Chem. 2005, 280, 42978–42983. [Google Scholar] [CrossRef] [PubMed]
- Duddela, S.; Pagadala, N.; Padmavati, G.V.; Banerjee, A.K.; Murty, U.S.N. Probing the structure of human glucose transporter 2 and analysis of protein ligand interactions. Med. Chem. Res. 2010, 19, 836–853. [Google Scholar] [CrossRef]
- Cohen, M.; Kitsberg, D.; Tsytkin, S.; Shulman, M.; Aroeti, B.; Nahmias, Y. Live imaging of glut2 glucose-dependent trafficking and its inhibition in polarized epithelial cysts. Open Biol. 2014, 4, 140091. [Google Scholar] [CrossRef] [PubMed]
- Burant, C.F.; Takeda, J.; Brot-Laroche, E.; Bell, G.I.; Davidson, N.O. Fructose transporter in human spermatozoa and small intestine is glut5. J. Biol. Chem. 1992, 267, 14523–14526. [Google Scholar] [PubMed]
- Ebert, K.; Ewers, M.; Bisha, I.; Sander, S.; Rasputniac, T.; Daniel, H.; Antes, I.; Witt, H. Identification of essential amino acids for glucose transporter 5 (glut5)-mediated fructose transport. J. Biol. Chem. 2018, 293, 2115–2124. [Google Scholar] [CrossRef] [PubMed]
- Naira, G.; Maureen, J.C. What we know about facilitative glucose transporters: Lessons from cultured cells, animal models, and human studies. Biochem. Mol. Biol. Edu. 2003, 31, 163–172. [Google Scholar]
- Barrett, M.P.; Walmsleyt, A.R.; Gould, G.W. Structure and function of facultative sugar transporters. Curr. Opin. Cell. Biol. 1999, 11, 496–502. [Google Scholar] [CrossRef]
- Seatter, M.J.; De la Rue, S.A.; Porter, L.M.; Gould, G.W. Qls motif in transmembrane helix vii of the glucose transporter family interacts with the c-1 position of d-glucose and is involved in substrate selection at the exofacial binding site. Biochemistry 1998, 37, 1322–1326. [Google Scholar] [CrossRef] [PubMed]
- Cura, A.J.; Carruthers, A. Role of monosaccharide transport proteins in carbohydrate assimilation, distribution, metabolism, and homeostasis. Compr. Physiol. 2012, 2, 863–914. [Google Scholar] [PubMed]
- Colville, C.A.; Seatter, M.J.; Gould, G.W. Analysis of the structural requirements of sugar binding to the liver, brain and insulin-responsive glucose transporters expressed in oocytes. Biochem. J. 1993, 294, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Nomura, N.; Verdon, G.; Kang, H.J.; Shimamura, T.; Nomura, Y.; Sonoda, Y.; Hussien, S.A.; Qureshi, A.A.; Coincon, M.; Sato, Y.; et al. Structure and mechanism of the mammalian fructose transporter glut5. Nature 2015, 526, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.T.; Wang, T.Z.; Chen, Y.; Liu, J.B.; Liu, Y.; Wang, W.J. Pollen typhae total flavone improves insulin-induced glucose uptake through the beta-arrestin-2-mediated signaling in c2c12 myotubes. Int. J. Mol. Med. 2012, 30, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Nistor Baldea, L.A.; Martineau, L.C.; Benhaddou-Andaloussi, A.; Arnason, J.T.; Levy, E.; Haddad, P.S. Inhibition of intestinal glucose absorption by anti-diabetic medicinal plants derived from the james bay cree traditional pharmacopeia. J. Ethnopharmacol. 2010, 132, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Eid, H.M.; Haddad, P.S. Mechanisms of action of indigenous antidiabetic plants from the boreal forest of northeastern canada. Adv. Endocrinol. 2014, 2014, 11. [Google Scholar] [CrossRef]
- Watanabe, K.; Kamata, K.; Sato, J.; Takahashi, T. Fundamental studies on the inhibitory action of acanthopanax senticosus harms on glucose absorption. J. Ethnopharmacol. 2010, 132, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Baek, S.S.; Cho, H.Y. Inhibitory effect of pomegranate on intestinal sodium dependent glucose uptake. Am. J. Chin. Med. 2011, 39, 1015–1027. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Koecher, K.; Hansen, L.; Ferruzzi, M.G. Phenolics from whole grain oat products as modifiers of starch digestion and intestinal glucose transport. J. Agric. Food Chem. 2017, 65, 6831–6839. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Kobayashi, Y.; Suzuki, M.; Satsu, H.; Miyamoto, Y. Regulation of intestinal glucose transport by tea catechins. BioFactors 2000, 13, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Villa-Rodriguez, J.A.; Aydin, E.; Gauer, J.S.; Pyner, A.; Williamson, G.; Kerimi, A. Green and chamomile teas, but not acarbose, attenuate glucose and fructose transport via inhibition of glut2 and glut5. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Satoh, T.; Igarashi, M.; Yamada, S.; Takahashi, N.; Watanabe, K. Inhibitory effect of black tea and its combination with acarbose on small intestinal alpha-glucosidase activity. J. Ethnopharmacol. 2015, 161, 147–155. [Google Scholar] [CrossRef] [PubMed]
- De la Garza, A.L.; Etxeberria, U.; Lostao, M.P.; San Roman, B.; Barrenetxe, J.; Martinez, J.A.; Milagro, F.I. Helichrysum and grapefruit extracts inhibit carbohydrate digestion and absorption, improving postprandial glucose levels and hyperinsulinemia in rats. J. Agric. Food Chem. 2013, 61, 12012–12019. [Google Scholar] [CrossRef] [PubMed]
- Esatbeyoglu, T.; Rodriguez-Werner, M.; Schlosser, A.; Liehr, M.; Ipharraguerre, I.; Winterhalter, P.; Rimbach, G. Fractionation of plant bioactives from black carrots (daucus carota subspecies sativus varietas atrorubens alef.) by adsorptive membrane chromatography and analysis of their potential anti-diabetic activity. J. Agric. Food Chem. 2016, 64, 5901–5908. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Clifford, M.N.; Sharp, P. Analysis of chlorogenic acids in beverages prepared from chinese health foods and investigation, in vitro, of effects on glucose absorption in cultured caco-2 cells. Food Chem. 2008, 108, 369–373. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Wang, F.; Yang, D.; Ding, K.; Fan, J. The ethanol extract of eucommia ulmoides oliv. Leaves inhibits disaccharidase and glucose transport in caco-2 cells. J. Ethnopharmacol. 2015, 163, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Manzano, S.; Williamson, G. Polyphenols and phenolic acids from strawberry and apple decrease glucose uptake and transport by human intestinal caco-2 cells. Mol. Nutr. Food. Res. 2010, 54, 1773–1780. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Yang, X.; Cai, Q.; Ren, B.; Qiu, H.; Yao, Z. Lycium barbarum l. Polysaccharide (lbp) reduces glucose uptake via down-regulation of sglt-1 in caco2 cell. Molecules 2017, 22, 341. [Google Scholar] [CrossRef] [PubMed]
- Hanamura, T.; Mayama, C.; Aoki, H.; Hirayama, Y.; Shimizu, M. Antihyperglycemic effect of polyphenols from acerola (malpighia emarginata dc.) fruit. Biosci. Biotechnol. Biochem. 2006, 70, 1813–1820. [Google Scholar] [CrossRef] [PubMed]
- Jaber, H.; Baydoun, E.; O, E.L.-Z.; Kreydiyyeh, S.I. Anti-hyperglycemic effect of the aqueous extract of banana infructescence stalks in streptozotocin-induced diabetic rats. Plant. Foods Hum. Nutr. 2013, 68, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Kushida, H.; Makino, T. Ginsenosides, ingredients of the root of panax ginseng, are not substrates but inhibitors of sodium-glucose transporter 1. J. Nat. Med. 2017, 71, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.C.; Huang, S.F.; Yang, T.C.; Chan, F.N.; Lin, H.C.; Chang, W.L. Effect of ginsenosides on glucose uptake in human caco-2 cells is mediated through altered na+/glucose cotransporter 1 expression. J. Agric. Food Chem. 2007, 55, 1993–1998. [Google Scholar] [CrossRef] [PubMed]
- El-Zein, O.; Kreydiyyeh, S.I. Pine bark extract inhibits glucose transport in enterocytes via mitogen-activated kinase and phosphoinositol 3-kinase. Nutrition 2011, 27, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Fowler, M.I.; Messenger, D.J.; Terry, L.A.; Gu, X.; Zhou, L.; Liu, R.; Su, J.; Shi, S.; Ordaz-Ortiz, J.J.; et al. Homoisoflavonoids are potent glucose transporter 2 (glut2) inhibitors: A potential mechanism for the glucose-lowering properties of polygonatum odoratum. J. Agric. Food Chem. 2018, 66, 3137–3145. [Google Scholar] [CrossRef] [PubMed]
- Kerimi, A.; Nyambe-Silavwe, H.; Gauer, J.S.; Tomas-Barberan, F.A.; Williamson, G. Pomegranate juice, but not an extract, confers a lower glycemic response on a high-glycemic index food: Randomized, crossover, controlled trials in healthy subjects. Am. J. Clin. Nutr. 2017, 106, 1384–1393. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Ha, T.; Gao, M. Prunella vulgaris l. Extract decreasing mrna expressions of α-glucosidase sglt-1, glut-2 and na+-k+-atpase in caco-2 cells. Chinese J. Biochem. Pharm. 2010, 31, 373–376. [Google Scholar]
- Muller, U.; Stubl, F.; Schwarzinger, B.; Sandner, G.; Iken, M.; Himmelsbach, M.; Schwarzinger, C.; Ollinger, N.; Stadlbauer, V.; Hoglinger, O.; et al. In vitro and in vivo inhibition of intestinal glucose transport by guava (psidium guajava) extracts. Mol. Nutr. Food Res. 2018, e1701012. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Horita, M.; Nagai, H.; Nagatomo, A.; Nishida, N.; Matsuura, Y.; Nagaoka, S. Tiliroside, a glycosidic flavonoid, inhibits carbohydrate digestion and glucose absorption in the gastrointestinal tract. Mol. Nutr. Food Res. 2012, 56, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Terazono, Y.; Hirasaki, N.; Tatemichi, Y.; Kinoshita, E.; Obata, A.; Matsui, T. Inhibition of glucose transport by tomatoside a, a tomato seed steroidal saponin, through the suppression of glut2 expression in caco-2 cells. J. Agric. Food Chem. 2018, 66, 1428–1434. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.N.; Zhao, D.D.; Gao, B.; Zhong, K.; Zhu, R.X.; Zhang, Y.; Xie, W.J.; Jia, L.R.; Gao, H. Anti-hyperglycemic effect of chebulagic acid from the fruits of terminalia chebula retz. Int. J. Mol. Sci. 2012, 13, 6320–6333. [Google Scholar] [CrossRef] [PubMed]
- Malunga, L.N.; Eck, P.; Beta, T. Inhibition of intestinal alpha-glucosidase and glucose absorption by feruloylated arabinoxylan mono- and oligosaccharides from corn bran and wheat aleurone. J. Nutr. Metab. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Moser, S.; Lim, J.; Chegeni, M.; Wightman, J.D.; Hamaker, B.R.; Ferruzzi, M.G. Concord and niagara grape juice and their phenolics modify intestinal glucose transport in a coupled in vitro digestion/caco-2 human intestinal model. Nutrients 2016, 8, 414. [Google Scholar] [CrossRef] [PubMed]
- Faria, A.; Pestana, D.; Azevedo, J.; Martel, F.; de Freitas, V.; Azevedo, I.; Mateus, N.; Calhau, C. Absorption of anthocyanins through intestinal epithelial cells—Putative involvement of glut2. Mol. Nutr. Food Res. 2009, 53, 1430–1437. [Google Scholar] [CrossRef] [PubMed]
- Farrell, T.L.; Ellam, S.L.; Forrelli, T.; Williamson, G. Attenuation of glucose transport across caco-2 cell monolayers by a polyphenol-rich herbal extract: Interactions with sglt1 and glut2 transporters. Biofactors 2013, 39, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Alzaid, F.; Cheung, H.M.; Preedy, V.R.; Sharp, P.A. Regulation of glucose transporter expression in human intestinal caco-2 cells following exposure to an anthocyanin-rich berry extract. PLoS ONE 2013, 8, e78932. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Lim, Y.; Kwon, O. Selected phytochemicals and culinary plant extracts inhibit fructose uptake in caco-2 cells. Molecules 2015, 20, 17393–17404. [Google Scholar] [CrossRef] [PubMed]
- Drozdowski, L.A.; Reimer, R.A.; Temelli, F.; Bell, R.C.; Vasanthan, T.; Thomson, A.B. Beta-glucan extracts inhibit the in vitro intestinal uptake of long-chain fatty acids and cholesterol and down-regulate genes involved in lipogenesis and lipid transport in rats. J. Nutr. Biochem. 2010, 21, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Cazarolli, L.H.; Zanatta, L.; Alberton, E.H.; Figueiredo, M.S.; Folador, P.; Damazio, R.G.; Pizzolatti, M.G.; Silva, F.R. Flavonoids: Cellular and molecular mechanism of action in glucose homeostasis. Mini Rev. Med. Chem. 2008, 8, 1032–1038. [Google Scholar] [CrossRef] [PubMed]
- Marin, M.; Manez, S. Recent trends in the pharmacological activity of isoprenyl phenolics. Curr. Med. Chem. 2013, 20, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Kozlowska, A.; Szostak-Wegierek, D. Flavonoids--food sources and health benefits. Rocz Panstw Zakl Hig. 2014, 65, 79–85. [Google Scholar] [PubMed]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef] [PubMed]
- Rechner, A. Influence of processing techniques on polyphenols and antioxidative capacity of apple- and berry juices. Ph.D. Thesis, Justus-Liebig University of Gießen, Germany, 2001. Available online: http://geb.uni-giessen.de/geb/volltexte/2001/421/pdf/RechnerAndreas-2001-03-19.pdf (accessed on 24 May 2018).
- Kwon, O.; Eck, P.; Chen, S.; Corpe, C.P.; Lee, J.H.; Kruhlak, M.; Levine, M. Inhibition of the intestinal glucose transporter glut2 by flavonoids. FASEB J. 2007, 21, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Johnston, K.; Sharp, P.; Clifford, M.; Morgan, L. Dietary polyphenols decrease glucose uptake by human intestinal caco-2 cells. FEBS Lett. 2005, 579, 1653–1657. [Google Scholar] [CrossRef] [PubMed]
- Hanhineva, K.; Torronen, R.; Bondia-Pons, I.; Pekkinen, J.; Kolehmainen, M.; Mkkanen, H.; Poutanen, K. Impact of dietary polyphenols on carbohydrate metabolism. Int. J. Mol. Sci. 2010, 11, 1365–1402. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Jin, J.Y.; Baek, W.K.; Park, S.H.; Sung, H.Y.; Kim, Y.K.; Lee, J.; Song, D.K. Ambivalent role of gallated catechins in glucose tolerance in humans: A novel insight into non-absorbable gallated catechin-derived inhibitors of glucose absorption. J. Physiol. Pharmacol. 2009, 60, 101–109. [Google Scholar] [PubMed]
- Peluso, I.; Serafini, M. Antioxidants from black and green tea: From dietary modulation of oxidative stress to pharmacological mechanisms. Br. J. Pharmacol. 2017, 174, 1195–1208. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.E.; Breinholt, V.M. Non-nutritive bioactive food constituents of plants: Bioavailability of flavonoids. Int J. Vitam. Nutr. Res. 2003, 73, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Day, A.J.; Canada, F.J.; Diaz, J.C.; Kroon, P.A.; McLauchlan, R.; Faulds, C.B.; Plumb, G.W.; Morgan, M.R.; Williamson, G. Dietary flavonoid and isoflavone glycosides are hydrolysed by the lactase site of lactase phlorizin hydrolase. FEBS Lett. 2000, 468, 166–170. [Google Scholar] [CrossRef]
- Gee, J.M.; DuPont, M.S.; Day, A.J.; Plumb, G.W.; Williamson, G.; Johnson, I.T. Intestinal transport of quercetin glycosides in rats involves both deglycosylation and interaction with the hexose transport pathway. J. Nutr. 2000, 130, 2765–2771. [Google Scholar] [CrossRef] [PubMed]
- Hollman, P.C.; Bijsman, M.N.; van Gameren, Y.; Cnossen, E.P.; de Vries, J.H.; Katan, M.B. The sugar moiety is a major determinant of the absorption of dietary flavonoid glycosides in man. Free Radic Res. 1999, 31, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Tsuchihashi, R.; Sakamoto, S.; Kodera, M.; Nohara, T.; Kinjo, J. Microbial metabolism of soy isoflavones by human intestinal bacterial strains. J. Nat. Med. 2008, 62, 456–460. [Google Scholar] [CrossRef] [PubMed]
- Schantz, M.; Erk, T.; Richling, E. Metabolism of green tea catechins by the human small intestine. Biotechnol. J. 2010, 5, 1050–1059. [Google Scholar] [CrossRef] [PubMed]
- Lafay, S.; Morand, C.; Manach, C.; Besson, C.; Scalbert, A. Absorption and metabolism of caffeic acid and chlorogenic acid in the small intestine of rats. Br. J. Nutr. 2006, 96, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Couteau, D.; McCartney, A.L.; Gibson, G.R.; Williamson, G.; Faulds, C.B. Isolation and characterization of human colonic bacteria able to hydrolyse chlorogenic acid. J. Appl. Microbiol. 2001, 90, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Maljaars, P.W.; Peters, H.P.; Mela, D.J.; Masclee, A.A. Ileal brake: A sensible food target for appetite control. A review. Physiol. Behav. 2008, 95, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Noelting, J.; DiBaise, J.K. Mechanisms of fructose absorption. Clin. Transl. Gastroenterol. 2015, 6, e120. [Google Scholar] [CrossRef]
- Thomson, A.B.; De Pover, A.; Keelan, M.; Jarocka-Cyrta, E.; Clandinin, M.T. Inhibition of lipid absorption as an approach to the treatment of obesity. Methods Enzymol. 1997, 286, 3–44. [Google Scholar] [PubMed]


| FATP2 | FATP4 | SGLT1 | GLUT2 | GLUT5 |
|---|---|---|---|---|
| Tissue expression | ||||
| Liver, kidney, intestine [13,14], pancreas, placenta [13] | Small intestine, adipose tissue, brain, liver, skin, heart [14] | Small intestine, kidney, heart, prostate [18] | β-cells, liver, intestine, kidney [19] | Intestine, testis, kidney, skeletal muscle, fat tissue, brain [20] |
| FATP2 | FATP4 | SGLT1 | GLUT2 | GLUT5 | |
|---|---|---|---|---|---|
| Specific inhibitors | |||||
| Grassofermata (I), Lipofermata (II) [13] | 4-Aryl-3,4-dihydro- Pyrimidin-2(1H)- ones (III) [54] | Phlorizin (IV) [55,56], LX2761 (V) [57,58] | Phloretin (VI) [59,60] | Astragalin-6-glucoside (VII), N-[4-(methylsulfonyl)- 2-nitrophenyl]- 1,3-benzodioxol-5-amine (MSNBA) (VIII) [53,61] | |
 | 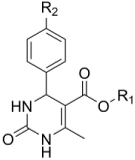 III |  | 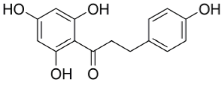 VI | 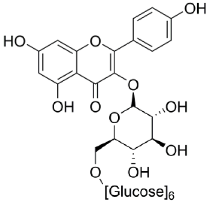 | |
| I | IV | VII | |||
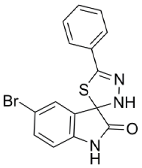 | 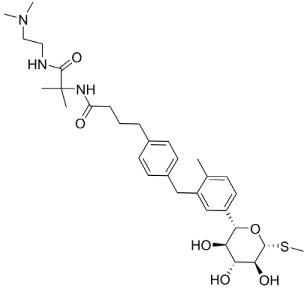 |  | |||
| II | V | VIII | |||
| Characteristics of Intestinal Glucose Transporters | SGLT1 | GLUT2 |
|---|---|---|
| Type of transporter | Sodium-dependent active cotransporter | Sodium-independent passive transporter |
| Family | SLC5 (SLC5A1) | SLC2 (SLC2A2) |
| Substrates | Glucose, galactose | Glucose, galactose, fructose, mannose, glucosamine |
| Localization | Apical membrane | Basolateral/apical membrane |
| Total length of amino acid sequence | 664 amino acids | 524 amino acids |
| Number of transmembrane segments | 14 | 12 |
| Affinity to glucose | High (KM = 0.5–2 mM) | Low (KM = 17 mM) |
| Saturation | Yes, >10 mM glucose (capacity low) | No (capacity high) |
| Scientific Plant Name | Part of Plant | Influence on Intestinal Glucose Transporters | Effect on the Expression of Intestinal Glucose Transporters | Discussed Active Compounds | Ref |
|---|---|---|---|---|---|
| Abies balsamea (L.) Mill. | Bark | None | None | N.s. | [116,117] |
| Acanthopanax senticosus (Rupr. & Maxim.) Harms | Stem bark | Uptake inhibition | N.s. | Isofraxidin, eleutherosides, senticosides, chlorogenic acid | [118] |
| Adenophora tryphilla var. japonica (Regel.) Hara | N.s. | Uptake inhibition | N.s. | N.s. | [119] |
| Alnus incana (L.) Moench | Bark | Uptake inhibition | None | Oregonin | [116,117] |
| Angelica gigas Nakai | N.s. | Uptake inhibition | N.s. | N.s. | [119] |
| Astragalus membranaceus (Fisch.) Bunge | N.s. | Uptake inhibition | N.s. | N.s. | [119] |
| AvenaL. sp. | Grains | Uptake inhibition | N.s. | β-glucans, phenolic acids (caffeic, gallic, p-coumaric, ferulic and sinapic acid), flavonoids, lignans, avenanthramides (AVE A, AVE B, AVE C) | [120] |
| Camellia sinensis (L.) Kuntze | N.s. 1 | Uptake inhibition | N.s. | Catechins (epicatchin gallate) | [121] |
| Camellia sinensis (L.) Kuntze | Leaf | Uptake inhibition | N.s. | (−)-epigallocatechin gallate, (−)-epigallocatechin, (−)-epicatechin, (+)-catechin | [122] |
| Camellia sinensis (L.) Kuntze | Leaf | None | N.s. | Catechins, theaflavins, caffeine, polysaccharides | [123] |
| Capsella bursa-pastoris (L.) Medik. | N.s. | Uptake inhibition | N.s | N.s. | [119] |
| Capsicum annuum L. | N.s. | Uptake inhibition | N.s. | N.s. | [119] |
| Cinnamomum camphora (L.) J.Presl | N.s. | Uptake inhibition | N.s. | N.s. | [119] |
| Citrus junos Siebold ex Tanaka | N.s. | Uptake inhibition | N.s. | N.s. | [119] |
| Citrus paradisi Macfad | N.s. | Inhibition of SGLT1 | N.s. | kaempferol rutinoside, naringenin-7-O-rutinoside | [124] |
| Citrus unshiu (Yu.Tanaka ex Swingle) Marcow | N.s. | Uptake inhibition | N.s. | N.s. | [119] |
| Codonopsis lanceolata (Siebold & Zucc.) Benth. & Hook.f. ex Trautv. | N.s. | Uptake inhibition | N.s. | N.s. | [119] |
| Cornus officinalis Siebold & Zucc. | N.s. | Uptake inhibition | N.s. | N.s. | [119] |
| Crataegus pinnatifida var. typica C.K.Schneid. | N.s. | Uptake inhibition | N.s. | N.s. | [119] |
| Cuscuta japonica Choisy | N.s. | Uptake inhibition | N.s. | N.s. | [119] |
| Daucus carota ssp. sativus var. atrorubens Alef. | Root | Uptake inhibition | N.s. | Anthocyanins (cyanidin-3-xylosyl-(feruloylglucosyl)-Galactoside, chlorogenic acid | [125] |
| Dendranthema morifolium (Ramat.) Tzvelev | N.s. | Moderate inhibition of SGLT1 and GLUT2 | N.s. | 1,3-dicaffeoylquinic acid, 5-caffeoylquinic acid, 1,5-dicaffeoylquinic acid, 3,5-dicaffeoylquinic acid | [126] |
| Diospyros kaki L.f. | N.s. | Uptake inhibition | N.s. | N.s. | [119] |
| Eucommia ulmoides Oliv. | Leaf | Uptake inhibition | N.s. | Lignans, iridoids, polyphenols (catechin, caffeic acid, 2,6-dihydroxy-benzoic acid, mandelic acid), steroids, triterpenes, organicacids, polysaccharides, flavonoids, amino acids | [127] |
| Eucommia ulmoides OLIV. | N.s. | Uptake inhibition | N.s. | N.s. | [119] |
| FragariaL. sp. ‘Albion’ | Fruit | Predominant inhibition of GLUT2 | N.s. | Polyphenols (pelargonidin-3-O-glucoside), phenolic acids and tannins | [128] |
| Gaultheria hispidula (L.) Muhl. Ex Bigelow | N.s. | Uptake inhibition | None | N.s. | [116] |
| Ginkgo biloba L. | N.s. | Uptake inhibition | N.s. | N.s. | [119] |
| Helichrysum italicum (ROTH) G. Don | N.s. | Inhibition of SGLT1 | N.s. | kaempferol-3-O-glucoside, chlorogenic acid-3-O-glucoside, naringenin-7-O-glucoside, naringenin diglycoside | [124] |
| Ipomoea batatas (L.) Lam. | Stem | Moderate inhibition of SGLT1 and GLUT2 | N.s. | 5-caffeoylquinic acid, 3,5-dicaffeoylquinic acid | [126] |
| Juniperus communis L. | N.s. | Uptake inhibition | None | N.s. | [116] |
| Kalmia angustifolia L. | N.s. | None | None | N.s. | [116] |
| Larix laricina (Du Roi) K.Koch | Bark | Mild to moderate inhibition | None | N.s. | [116,117] |
| Lonicera japonica Thunb. | N.s. | None | N.s. | 5-caffeoylquinic acid, | [126] |
| Lycium barbarum L. | N.s. | Uptake inhibition | Downregulation of SGLT1 | Polysaccharides (average weight of 10 to 30 kDa) | [129] |
| Lycopodium clavatum L. | N.s. | Uptake inhibition | None | N.s. | [116] |
| Malpighia emarginata DC. | Fruit | Uptake inhibition | N.s. | cyanidin-3-α-O- rhamnoside, pelargonidin-3-α-O-rhamnoside, Quercetin-3-α-O-rhamnoside | [130] |
| Malus domestica Borkh. ‘Golden delicious’ | Fruit | Predominant inhibition of GLUT2 | N.s. | Polyphenols (Quercetin-3-O-rhamnoside, phlorizin) phenolic acids (5-caffeoylquinic acid) and tannins | [128] |
| Matricaria recutita L. | N.s. | Predominant inhibition of GLUT2 | N.s. | Apigenin-7-O-glucoside, apigenin | [122] |
| Mentha arvensis L. var. japonica | N.s. | Uptake inhibition | N.s. | N.s. | [119] |
| Musa x sapientum L. | Infructescence stalks | Indirect Inhibition caused by reduction of the Na+-gradient due to the decrease of Na+/K+-ATPase activity | None | cycloartenol, cycloeucalenol, 24-methylene cycloartanol, campesterol, β-sitosterol and stigmasterol, serotonin and norepinephrine | [131] |
| Opuntia ficus-indica (L.) Mill. | N.s. | Uptake inhibition | N.s. | N.s. | [119] |
| Panax ginseng C.A.Mey | Root | Inhibition of SGLT1 | N.s. | Protopanaxadiol-type Ginsenosides (Rd, Rg3, Rh2, F2, compound K) | [132] |
| Panax notoginseng (Burkill) F.H.Chen | Root | Uptake inhibition | Downregulation of SGLT1 | Protopanaxatriol ginsenoside Rg1 | [133] |
| Picea glauca (Moench) Voss | Needle, cone, bark. | Uptake inhibition | None | Phenolic acids, stilbene, flavonoids | [116,117] |
| Picea mariana (Mill.) Britton, Sterns & Poggenb. | N.s. | Uptake inhibition | Downregulation of GLUT2 | N.s. | [116] |
| Pinus banksianaLamb. | N.s. | Uptake inhibition | None | N.s. | [116] |
| Pinus pinea L. | Bark | Predominant inhibition of SGLT1 | Downregulation of SGLT1 and GLUT2 | N.s. | [134] |
| Polygonatum odoratum (Mill.) Druce | Root | Uptake inhibition | N.s. | Sappanin-type homoisoflavonoids (5,7-dihydroxy-3-(4′-hydroxybenzyl)-6-methylchroman-4-one (EA-1), 5,7-dihydroxy-3-(4′-hydroxybenzyl)-6-methyl-8-methoxychroman-4-one (EA-2), and 5,7-dihydroxy-3-(4′-hydroxybenzyl)-6, 8-dimethylchroman-4-one (EA-3) | [135] |
| Populus balsamifera L. | N.s. | Uptake inhibition | None | N.s. | [116,117] |
| Pueraria thunbergiana (Siebold & Zucc.) Benth | N.s. | Uptake inhibition | N.s. | N.s. | [119] |
| Punica granatum L. | Fruit | Inhibition of SGLT1 | Downregulation of SGLT1 | Polyphenols (anthocyanins, hydrolysable tannins) | [119] |
| Punica granatum L. ‘Mollar’ | Fruit | None (in Caco-2 model) | None (in Caco-2 model) | Punicalagin, Punicalin, ellagic acid | [136] |
| Prunella vulgaris L. | N.s. | None | Downregulation | N.s. | [137] |
| Prunus armeniaca L. var. ansu | N.s. | Uptake inhibition | N.s. | N.s. | [119] |
| Psidium guajava L. | Leaf, fruit | Inhibition of SGLT1 and GLUT2 | N.s. | Phloridzin, phloretin, quercetin, quercitrin, isoquercitrin, hyperoside, avicularin, guaijaverin, procyanidin B1, B2, (+)-catechin, (−)-epicatechin, gallocatechin, epicatechin gallate, gallic and ellagic acid | [138] |
| Rhododendron groenlandicum (Oeder) Kron & Judd | Leaf | Moderate to strong inhibition | None | N.s. | [116,117] |
| Rhododendron tomentosum Harmaja | N.s. | Strong inhibition | Downregulation of SGLT1 | N.s. | [116,117] |
| Raphanus sativus L. | N.s. | Uptake inhibition | N.s. | N.s. | [119] |
| Rosa canina L. | Seed | Inhibition of SGLT1 and GLUT2 | N.s. | Tiliroside | [139] |
| Rosa laevigata Michx. | N.s. | Uptake inhibition | N.s. | N.s. | [119] |
| Rosa rugosa Thunb. | N.s. | Uptake inhibition | N.s. | N.s. | [119] |
| Rubus coreanus Miq. | N.s. | Uptake inhibition | N.s. | N.s. | [119] |
| Salix planifolia Pursh | N.s. | Uptake inhibition | None | N.s. | [116] |
| Saposhnikovia divaricata (Turcz.) Schischk. | N.s. | Uptake inhibition | N.s. | N.s. | [119] |
| Sarracenia purpurea L. | N.s. | Uptake inhibition | None | N.s. | [116,117] |
| Solanum lycopersicum L. | Seed | Uptake inhibition | Downregulation of GLUT2 | Saponins (Tomatoside A) | [140] |
| Sorbus decora (Sarg.) C.K.Schneid. | N.s. | Moderate inhibition | None | N.s. | [116,117] |
| Terminalia chebula Retz. | Fruit | None | N.s. | Chebulagic acid | [141] |
| Triticum L. sp. | Wheat aleurone | Uptake inhibition | N.s. | Ferulic acid, feruloylated arabinoxylan mono- and oligosaccharides | [142] |
| Vaccinium vitis-idaea L. | Fruit | Uptake inhibition | None | N.s. | [116] |
| Vitis labruscaL. ‘Concord’ | Fruit | Uptake inhibition | Downregulation of GLUT2 | Flavan-3-ols, flavonols (quercetin), stilbenes (resveratrol), phenolic acids (gallic, caffeic acids), anthocyanins (cyanidin-3,5-O-diglucoside delphinidin-3-O-glucoside) | [143] |
| Vitis labruscaL. ‘Niagara’ | Fruit | Uptake inhibition | Downregulation of GLUT2 | Flavan-3-ols, flavonols (quecetin), stilbenes (resveratrol), phenolic acids (gallic, caffeic acids) | [143] |
| Vitis vinifera L. | Grape skin | Uptake inhibition | Upregulation of GLUT2 | Malvidin-3-glucose | [144] |
| Zea mays L. | Corn bran | Uptake inhibition | N.s. | Ferulic acid, feruloylated arabinoxylan mono- and oligosaccharides | [142] |
| Ziziphus jujuba var. inermis (Bunge) Rehder | N.s. | Uptake inhibition | N.s. | N.s. | [119] |
| Mixture of Gymnema sylvestre (Retz.) R. Br. Ex Sm., Coffea L. sp., Vitis L. sp., Hibiscus L. sp., Cinnamomum Schaeff. sp., Ocimum L. sp., Artemisia dracunculus L., Zingiber Mill. sp., Curcuma L. sp. | N.s. | Predominant inhibition of GLUT2 | N.s. | flavonoid aglycones (quercetin, kaempferol), caffeic acid, and p-coumaric acid | [145] |
| Mixture of Ilex latifoliaThunb. and Camellia sinensis (L.) Kuntze var. sinensis | N.s. | Strong inhibition of SGLT1 and GLUT2 | N.s. | Chlorogenic acids (5-caffeoylquinic acid, 3,5-dicaffeoylquinic acid), (–)-epicatechin gallate, (–)-epigallocatechin gallate, flavonols, flavonol glycosides (rutin) | [126] |
| Mixture of Vaccinium L. sp., Sambucus L. sp., Rubus L. sp., Fragaria L. sp. | Fruit, Seed | Inhibition of SGLT1 and GLUT2 | Downregulation of SGLT1 and GLUT2 | Flavonoids, anthocyanins (cyanidin-3-glucoside, cyanidin-3-rutinoside) | [146] |
| Scientific Plant Name | Part of Plant | Influence on Intestinal Fructose Transporters | Effect on the Expression of Intestinal Fructose Transporters | Discussed Active Compounds | Ref |
|---|---|---|---|---|---|
| Allium cepa L. | N.s. 1 | N.s. | N.s. | N.s. | [147] |
| Camellia sinensis (L.) Kuntze | Leaf | Uptake inhibition | N.s. | (−)-epigallocatechin gallate, (−)-epigallocatechin, (−)-epicatechin, (+)- catechin | [122] |
| Chrysanthemum L. sp. | N.s. | N.s. | N.s. | N.s. | [147] |
| Curcuma longa L. | N.s. | Uptake inhibition | N.s. | Curcumin, bisdemethoxycurcumin, dimethoxycurcumin | [147] |
| Glycine max (L.) Merr. | N.s. | N.s. | N.s. | N.s. | [147] |
| Matricaria recutita L. | N.s. | Predominant inhibition of GLUT2 | N.s. | Phenolic glucosides (apigenin-7-O-glucoside, apigenin) | [122] |
| Myrica L. sp. | Bark | N.s. | N.s. | N.s. | [147] |
| Panax ginseng C.A. Mey. | N.s. | N.s. | N.s. | N.s. | [147] |
| Passiflora L. sp. | N.s. | N.s. | N.s. | N.s | [147] |
| Psidium guajava L. | Leaf | Uptake inhibition | N.s. | Quercetin, catechin | [147] |
| Rosmarinus officinalis L. | N.s. | Uptake inhibition | N.s. | N.s. | [147] |
| Vitis labrusca L. ‘Concord’ | Fruit | Uptake inhibition | Downregulation of GLUT2 | Flavan-3-ols, flavonols (quercetin), stilbenes (resveratrol), phenolic acids (gallic, caffeic acids), anthocyanins (cyanidin-3,5-O-diglucoside delphinidin-3-O-glucoside) | [143] |
| Vitis labrusca L. ‘Niagara’ | Fruit | Uptake inhibition | Downregulation of GLUT2 | Flavan-3-ols, flavonols (quercetin), stilbenes (resveratrol), phenolic acids (gallic, caffeic acids) | [143] |
| Scientific Plant Name | Part of Plant | Influence on Intestinal Fatty Acid Transporters | Effect on the Expression of Intestinal Fatty Acid Transporters | Discussed Active Compounds | Ref |
|---|---|---|---|---|---|
| Avena L. sp. | Grains | Uptake inhibition | Downregulation of FABP and FATP4 | Β-glucan | [148] |
| Hordeum L. sp. | Grains | Uptake inhibition | Downregulation of FABP and FATP4 | Β-glucan | [148] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schreck, K.; Melzig, M.F. Intestinal Saturated Long-Chain Fatty Acid, Glucose and Fructose Transporters and Their Inhibition by Natural Plant Extracts in Caco-2 Cells. Molecules 2018, 23, 2544. https://doi.org/10.3390/molecules23102544
Schreck K, Melzig MF. Intestinal Saturated Long-Chain Fatty Acid, Glucose and Fructose Transporters and Their Inhibition by Natural Plant Extracts in Caco-2 Cells. Molecules. 2018; 23(10):2544. https://doi.org/10.3390/molecules23102544
Chicago/Turabian StyleSchreck, Katharina, and Matthias F. Melzig. 2018. "Intestinal Saturated Long-Chain Fatty Acid, Glucose and Fructose Transporters and Their Inhibition by Natural Plant Extracts in Caco-2 Cells" Molecules 23, no. 10: 2544. https://doi.org/10.3390/molecules23102544
APA StyleSchreck, K., & Melzig, M. F. (2018). Intestinal Saturated Long-Chain Fatty Acid, Glucose and Fructose Transporters and Their Inhibition by Natural Plant Extracts in Caco-2 Cells. Molecules, 23(10), 2544. https://doi.org/10.3390/molecules23102544