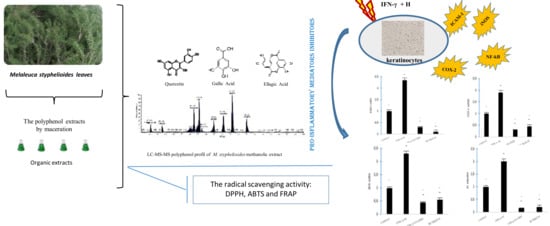
Melaleuca styphelioides Sm. Polyphenols Modulate Interferon Gamma/Histamine-Induced Inflammation in Human NCTC 2544 Keratinocytes
Abstract
1. Introduction
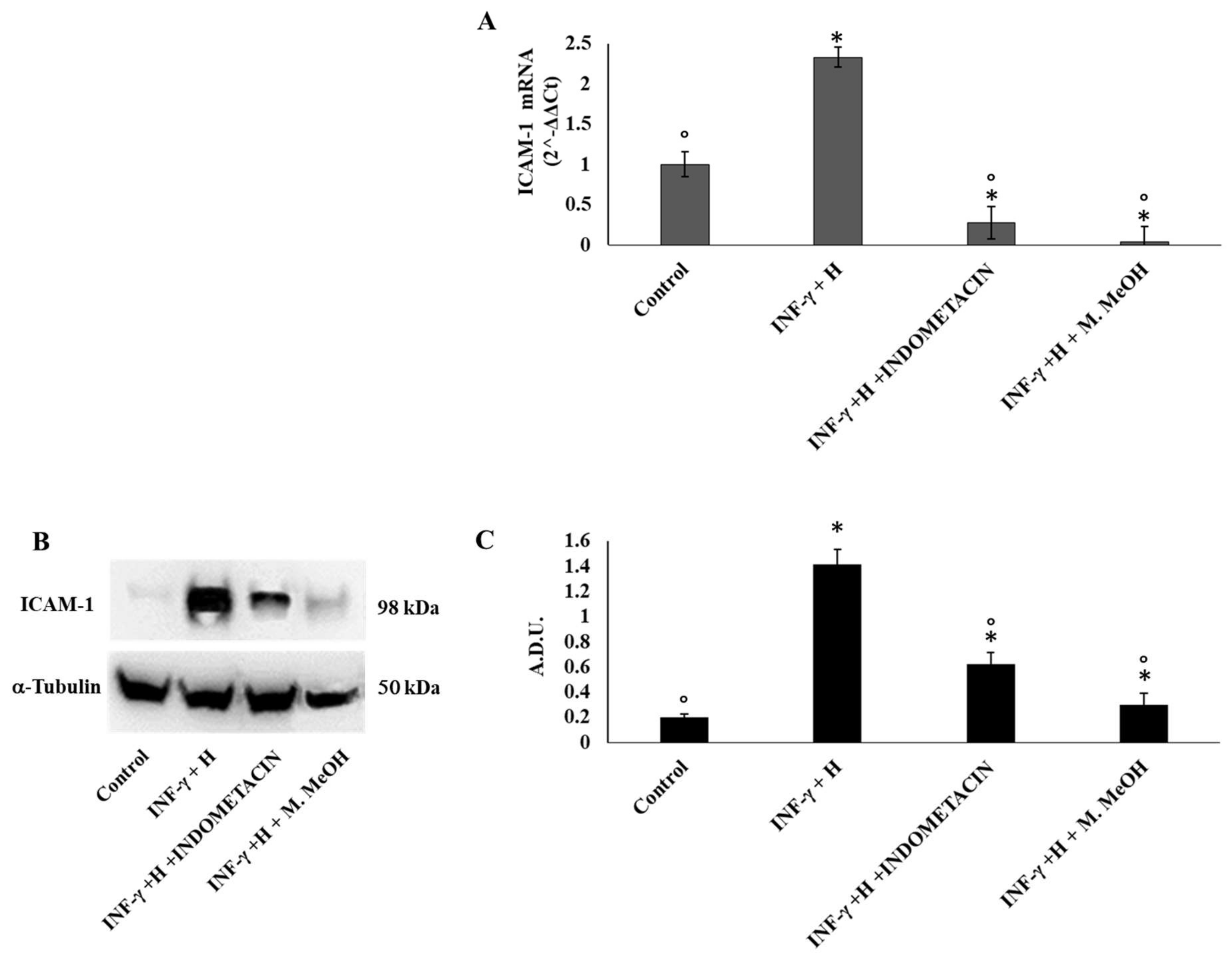
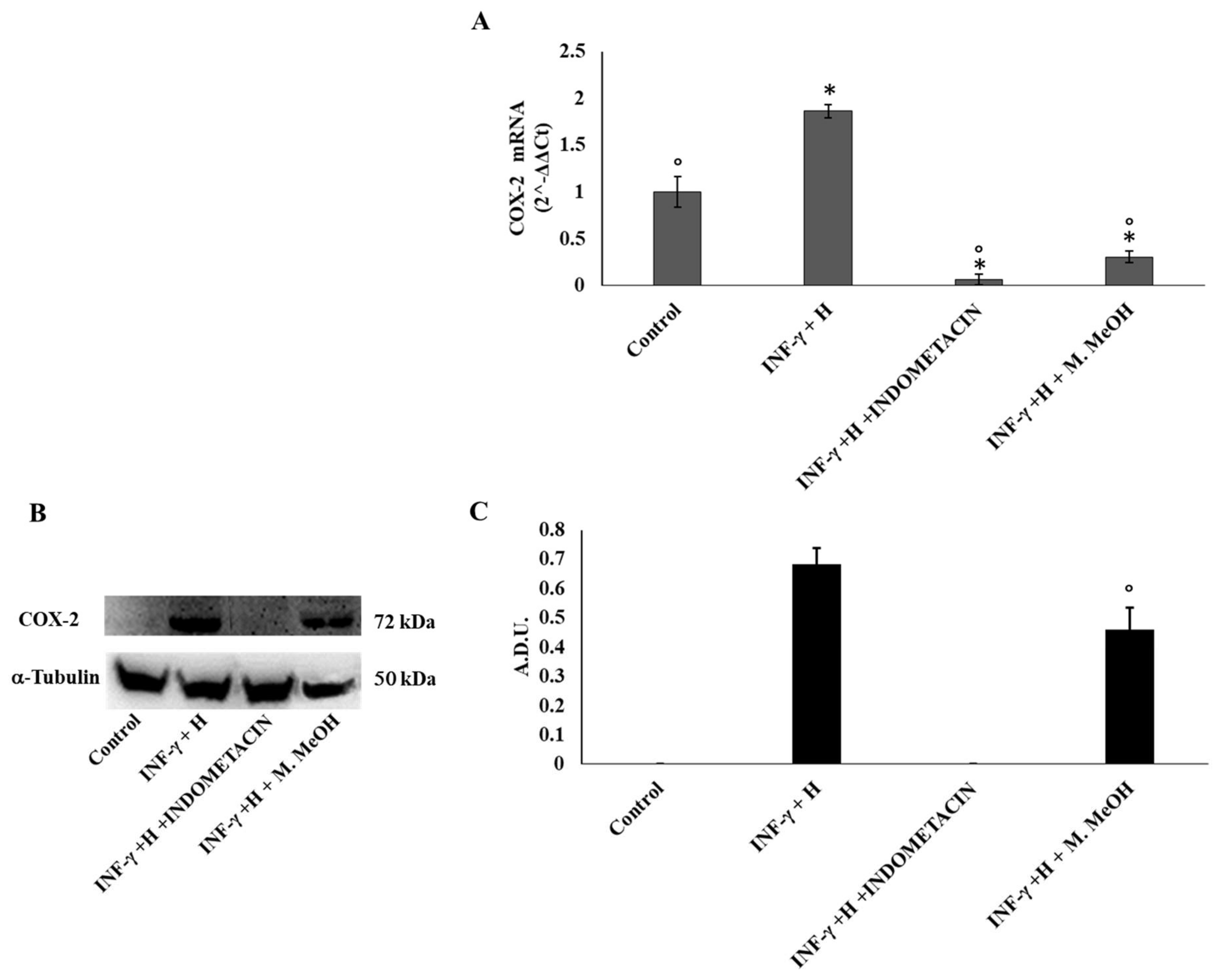
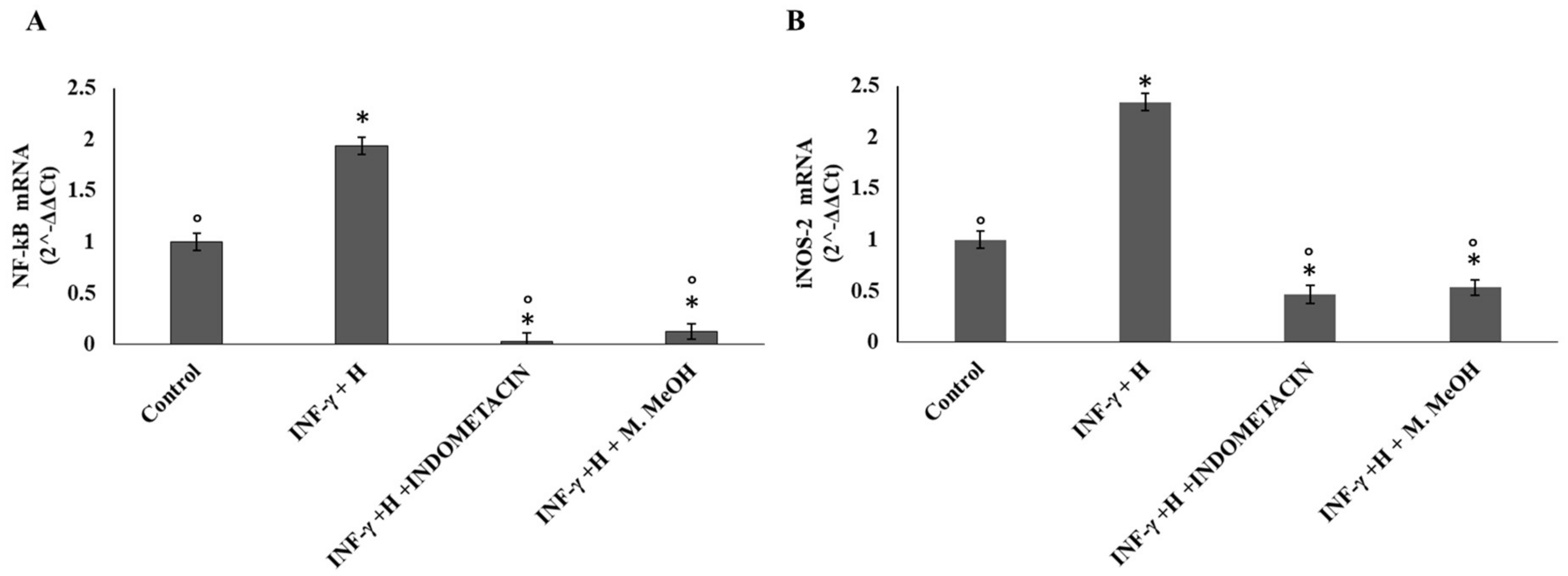
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Plant Material and Extracts Preparation
4.3. Phytochemical Analysis
4.3.1. The Phytochemical Screening
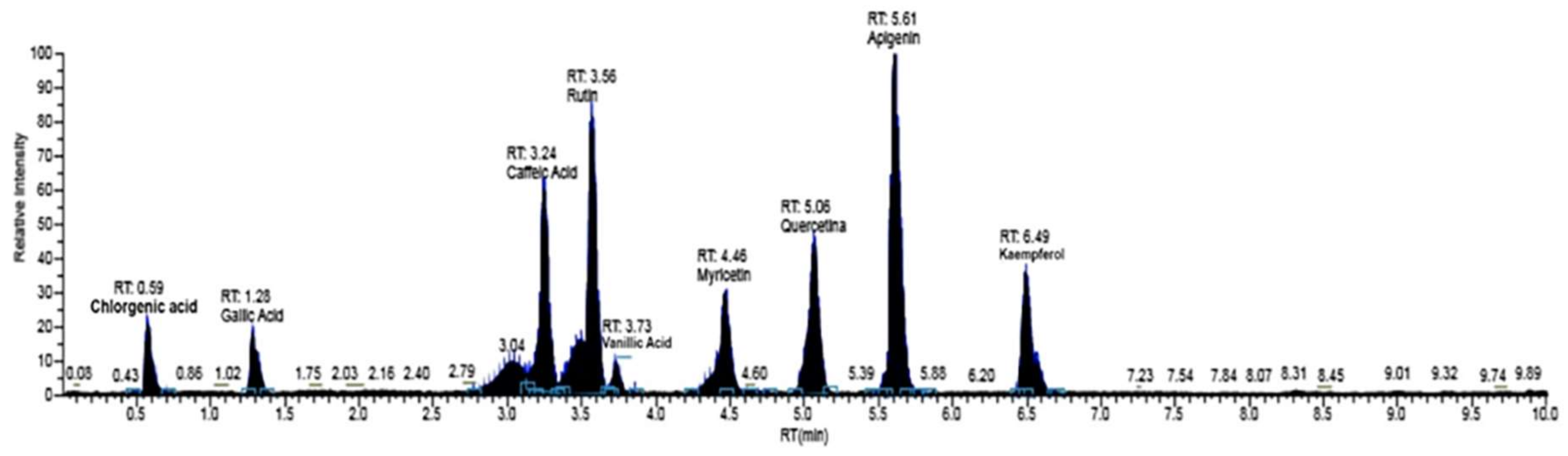
4.3.2. LC/MS-MS Analysis
4.4. Anti-Oxidant Activity Determination
4.5. Anti-Inflammatory Activity Evaluation
4.5.1. Cell Culture and Treatment
4.5.2. Cell Viability
4.5.3. RNA Extraction and RT-PCR
4.5.4. Western Blot
4.6. Statistical Analysis
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| (H) | Histamine |
| (IFN-γ) | interferon gamma |
| (NF-κB) | nuclear factor kappa B |
| (ICAM-1) | inter-cellular adhesion molecule-1 |
| (iNOS) | nitric oxide synthase |
| (COX-2) | cyclooxygenase-2 |
| (DPPH) | 2,2-diphenyl-1-picrylhydrazyl |
| (ABTS) | 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulphonate) |
| (E. hex) | hexane extract |
| (E. Et2O) | diethyl ether extract |
| (E. EtOAc) | ethyl acetate extract |
| (E. MeOH) | methanol extract |
| (M. MeOH) | Melaleuca styphelioides methanolic extract |
References
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Köllisch, G.; Kalali, B.N.; Voelcker, V.; Wallich, R.; Behrendt, H.; Ring, J.; Bauer, S.; Jakob, T.; Mempel, M.; Ollert, M. Various members of the Toll-like receptor family contribute to the innate immune response of human epidermal keratinocytes. Immunology 2005, 114, 531–541. [Google Scholar] [CrossRef] [PubMed]
- McInturff, J.E.; Modlin, R.L.; Kim, J. The role of toll-like receptors in the pathogenesis and treatment of dermatological disease. J. Investig. Dermatol. 2005, 125, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pastore, S.; Mascia, F.; Mariani, V.; Girolomoni, G. The epidermal growth factor receptor system in skin repair and inflammation. J. Investig. Dermatol. 2008, 128, 1365–1374. [Google Scholar] [CrossRef] [PubMed]
- Mascia, F.; Mariani, V.; Girolomoni, G.; Pastore, S. Blockade of the EGF receptor induces a deranged chemokine expression in keratinocytes leading to enhanced skin inflammation. Am. J. Pathol. 2003, 163, 303–312. [Google Scholar] [CrossRef]
- Kerkvliet, N.I. AHR-mediated immunomodulation: The role of altered gene transcription. Biochem. Pharmacol. 2009, 77, 746–760. [Google Scholar] [CrossRef] [PubMed]
- Pastore, S.; Mascia, F.; Mariotti, F.; Dattilo, C.; Mariani, V.; Girolomoni, G. ERK1/2 regulates epidermal chemokine expression and skin inflammation. J. Immunol. 2005, 174, 5047–5056. [Google Scholar] [CrossRef] [PubMed]
- Essafi-Benkhadir, K.; Refai, A.; Riahi, I.; Fattouch, S.; Karoui, H.; Essafi, M. Quince (Cydonia oblonga Miller) peel polyphenols modulate LPS-induced inflammation in human THP-1-derived macrophages through NF-κB, p38MAPK and Akt inhibition. Biochem. Biophys. Res. Commun. 2012, 418, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Korkina, L.G. Phenylpropanoids as naturally occurring antioxidants: From plant defense to human health. Cell. Mol. Biol. 2017, 53, 15–25. [Google Scholar] [CrossRef]
- Yang, H.J.; Kim, M.J.; Kang, S.; Moon, N.R.; Kim, D.S.; Lee, N.R.; Kim, K.S.; Park, S. Topical treatments of Saussurea costus root and Thuja orientalis L. synergistically alleviate atopic dermatitis-like skin lesions by inhibiting protease-activated receptor-2 and NF-κB signaling in HaCaT cells and Nc/Nga mice. J. Ethnopharmacol. 2017, 199, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.O.; Lee, K.W.; Lee, H.J.; Lee, C.Y. Vitamin C equivalent antioxidant capacity (VCEAC) of phenolic phytochemicals. J. Agric. Food Chem. 2002, 50, 3713–3717. [Google Scholar] [CrossRef] [PubMed]
- Korkina, L.G.; Pastore, S.; De Luca, C.; Kostyuk, V.A. Metabolism of plant polyphenols in the skin: Beneficial versus deleterious effects. Curr. Drug Metab. 2008, 9, 710–729. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Kong, Y.; Fu, Y.; Zu, Y.; Yang, Z.; Yang, M.; Peng, X.; Efferth, T. In vitro antioxidant properties, DNA damage protective activity, and xanthine oxidase inhibitory effect of cajaninstilbene acid, a stilbene compound derived from pigeon pea [Cajanus cajan (L.) Millsp.] leaves. J. Agric. Food Chem. 2011, 59, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Salehi, B.; Varoni, E.M.; Sharopov, F.; Yousaf, Z.; Ayatollahi, S.A.; Kobarfard, F.; Sharifi-Rad, M.; Afdjei, M.H.; Sharifi-Rad, M.; et al. Plants of the Melaleuca Genus as Antimicrobial Agents: From Farm to Pharmacy. Phyther. Res. 2017, 31, 1475–1494. [Google Scholar] [CrossRef] [PubMed]
- Al-Sayed, E.; El-Lakkany, N.M.; Seif El-Din, S.H.; Sabra, A.N.A.; Hammam, O.A. Hepatoprotective and antioxidant activity of Melaleuca styphelioides on carbon tetrachloride-induced hepatotoxicity in mice. Pharm. Biol. 2014, 52, 1581–1590. [Google Scholar] [CrossRef] [PubMed]
- Al-Sayed, E.; Esmat, A. Hepatoprotective and antioxidant effect of ellagitannins and galloyl esters isolated from Melaleuca styphelioides on carbon tetrachloride-induced hepatotoxicity in HepG2 cells. Pharm. Biol. 2016, 54, 1727–1735. [Google Scholar] [CrossRef] [PubMed]
- Farag, R.S.; Shalaby, A.S.; El-Baroty, G.A.; Ibrahim, N.A.; Ali, M.A.; Hassan, E.M. Chemical and Biological Evaluation of the Essential Oils of Different Melaleuca Species. Phyther. Res. 2004, 18, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Amri, I.; Mancini, E.; de Martino, L.; Marandino, A.; Lamia, H.; Mohsen, H.; Bassem, J.; Scognamiglio, M.; Reverchon, E.; de Feo, V. Chemical composition and biological activities of the essential oils from three Melaleuca species grown in Tunisia. Int. J. Mol. Sci. 2012, 13, 16580–16591. [Google Scholar] [CrossRef] [PubMed]
- Al-Abd, N.M.; Mohamed Nor, Z.; Mansor, M.; Azhar, F.; Hasan, M.S.; Kassim, M. Antioxidant, antibacterial activity, and phytochemical characterization of Melaleuca cajuputi extract. BMC Complement. Altern. Med. 2015, 15. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.V.; Bone, D.E.; Carrington, M.F. Antioxidant activity of dulse (Palmaria palmata) extract evaluated in vitro. Food Chem. 2005, 91, 485–494. [Google Scholar] [CrossRef]
- Albouchi, F.; Hassen, I.; Casabianca, H.; Hosni, K. Phytochemicals, antioxidant, antimicrobial and phytotoxic activities of Ailanthus altissima (Mill.) Swingle leaves. S. Afr. J. Bot. 2013, 87, 164–174. [Google Scholar] [CrossRef]
- Lesjak, M.; Beara, I.; Simin, N.; Pintać, D.; Majkić, T.; Bekvalac, K.; Orčić, D.; Mimica-Dukić, N. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J. Funct. Foods 2018, 40, 68–75. [Google Scholar] [CrossRef]
- Graziano, A.C.E.; Cardile, V.; Crascì, L.; Caggia, S.; Dugo, P.; Bonina, F.; Panico, A. Protective effects of an extract from Citrus bergamia against inflammatory injury in interferon-gamma and histamine exposed human keratinocytes. Life Sci. 2012, 90, 968–974. [Google Scholar] [CrossRef] [PubMed]
- Smith, W.L.; DeWitt, D.L.; Garavito, R.M. Cyclooxygenases: Structural, Cellular, and Molecular Biology. Annu. Rev. Biochem. 2000, 69, 145–182. [Google Scholar] [CrossRef] [PubMed]
- Müller-Decker, K.; Scholz, K.; Neufang, G.; Marks, F.; Fürstenberger, G. Localization of prostaglandin-H synthase-1 and -2 in mouse skin: Implications for cutaneous function. Exp. Cell Res. 1998, 242, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Suschek, C.; Schnorr, O.; Kolb-Bachofen, V. The Role of iNOS in Chronic Inflammatory Processes In Vivo: Is it Damage-Promoting, Protective, or Active at all? Curr. Mol. Med. 2004, 4, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Takada, Y.; Boriek, A.M.; Aggarwal, B.B. Nuclear factor-kappaB: Its role in health and disease. J. Mol. Med. 2004, 82, 434–448. [Google Scholar] [CrossRef] [PubMed]
- García-Mediavilla, V.; Crespo, I.; Collado, P.S.; Esteller, A.; Sánchez-Campos, S.; Tuñón, M.J.; González-Gallego, J. The anti-inflammatory flavones quercetin and kaempferol cause inhibition of inducible nitric oxide synthase, cyclooxygenase-2 and reactive C-protein, and down-regulation of the nuclear factor kappaB pathway in Chang Liver cells. Eur. J. Pharmacol. 2007, 557, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Shi, D.; Liu, L.; Wang, J.; Xie, X.; Kang, T.; Deng, W. Quercetin suppresses cyclooxygenase-2 expression and angiogenesis through inactivation of P300 signaling. PLoS ONE 2011, 6, e22934. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Chatterjee, S.; Das, S.; Saha, A.; Chattopadhyay, S.; Bandyopadhyay, S.K. Ellagic acid facilitates indomethacin-induced gastric ulcer healing via COX-2 up-regulation. Acta Biochim. Biophys. Sin. 2012, 44, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Ahad, A.; Ganai, A.A.; Mujeeb, M.; Siddiqui, W.A. Ellagic acid, an NF-κB inhibitor, ameliorates renal function in experimental diabetic nephropathy. Chem. Biol. Interact. 2014, 219, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Chen, F.; Wu, C.; Wang, X.; Chung, H.Y.; Jin, Z. Evaluation of antioxidant activity of Australian tea tree (Melaleuca alternifolia) oil and its components. J. Agric. Food Chem. 2004, 52, 2849–2854. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.C.; Kim, S.J.; Kim, D.S.; Jeon, Y.D.; Park, S.J.; Lee, H.S.; Um, J.Y.; Hong, S.H. Vanillic acid inhibits inflammatory mediators by suppressing NF-κB in lipopolysaccharide-stimulated mouse peritoneal macrophages. Immunopharmacol. Immunotoxicol. 2011, 33, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, Y.T.; Xiao, L.; Zhu, L.; Wang, Q.; Yan, T. Anti-Inflammatory Effects of Apigenin in Lipopolysaccharide-Induced Inflammatory in Acute Lung Injury by Suppressing COX-2 and NF-kB Pathway. Inflammation 2014, 37, 2085–2090. [Google Scholar] [CrossRef] [PubMed]
- Ham, J.R.; Lee, H.-I.; Choi, R.-Y.; Sim, M.-O.; Seo, K.-I.; Lee, M.-K. Anti-steatotic and anti-inflammatory roles of syringic acid in high-fat diet-induced obese mice. Food Funct. 2016, 7, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Kadioglu, O.; Nass, J.; Saeed, M.E.M.; Schuler, B.; Efferth, T. Kaempferol is an anti-inflammatory compound with activity towards NF-κB pathway proteins. Anticancer Res. 2015, 35, 2645–2650. [Google Scholar] [PubMed]
- Yoo, H.; Ku, S.K.; Baek, Y.D.; Bae, J.S. Anti-inflammatory effects of rutin on HMGB1-induced inflammatory responses in vitro and in vivo. Inflamm. Res. 2014, 63, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, D.; Al-Sayed, E.; Albert, A.; Paul, E.; Singab, A.N.B.; Govindan Sadasivam, S.; Saso, L. Antioxidant activity of phenolic compounds from extracts of Eucalyptus globulus and Melaleuca styphelioides and their protective role on d-glucose-induced hyperglycemic stress and oxalate stress in NRK-49Fcells. Nat. Prod. Res. 2018, 32, 1274–1280. [Google Scholar] [CrossRef] [PubMed]
- El Euch, S.K.; Bouajila, J.; Bouzouita, N. Chemical composition, biological and cytotoxic activities of Cistus salviifolius flower buds and leaves extracts. Ind. Crops Prod. 2015, 76, 1100–1105. [Google Scholar] [CrossRef]
- López-Gutiérrez, N.; del MAguilera-Luiz, M.; Romero-González, R.; Vidal, J.L.M.; Garrido Frenich, A. Fast analysis of polyphenols in royal jelly products using automated TurboFlowTM-liquid chromatography-Orbitrap high resolution mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014, 973, 17–28. [Google Scholar] [CrossRef] [PubMed]
- International Standard Organization. ISO/IEC 17025 General Requirements for the Competence of Testing and Calibration Laboratories; ISO: Geneva, Switzerland, 2005; pp. 1–36. [Google Scholar]
- Eurachem, a Laboratory Guide to Method Validation and Related Topics. 2014. Available online: http://www.eurachem.org/images/stories/Guides/pdf/valid.pdf (accessed on 25 July 2017).
- Lo Dico, G.M.; Cammilleri, G.; Macaluso, A. Simultaneous Determination of As, Cu, Cr, Se, Sn, Cd, Sb and Pb Levels in Infant Formulas by ICP-MS after Microwave-Assisted Digestion: Method Validation. J. Environ. Anal. Toxicol. 2015, 5, 1–5. [Google Scholar] [CrossRef]
- Le Man, H.; Behera, S.K.; Park, H.S. Optimization of operational parameters for ethanol production from korean food waste leachate. Int. J. Environ. Sci. Technol. 2010, 7, 157–164. [Google Scholar] [CrossRef]
- Graziano, A.C.E.; Parenti, R.; Avola, R.; Cardile, V. Krabbe disease: Involvement of connexin43 in the apoptotic effects of sphingolipid psychosine on mouse oligodendrocyte precursors. Apoptosis 2016, 21, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Avola, R.; Graziano, A.C.E.; Pannuzzo, G.; Albouchi, F.; Cardile, V. New insights on Parkinson’s disease from differentiation of SH-SY5Y into dopaminergic neurons: An involvement of aquaporin4 and 9. Mol. Cell. Neurosci. 2018, 88, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Avola, R.; Graziano, A.C.E.; Pannuzzo, G.; Cardile, V. Human Mesenchymal Stem Cells from Adipose Tissue Differentiated into Neuronal or Glial Phenotype Express Different Aquaporins. Mol. Neurobiol. 2017, 54, 8308–8320. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
| M. styphelioides Extracts | Total Phenolic mg GAE/g Dry Extract | Total Flavonoid mg QE/g Dry Extract | Total Tannins mg Eq Catéchine/g Dry Extract |
|---|---|---|---|
| E. MeOH | 142.7 ± 3.15 | 31.54 ± 1.99 | 15.2 ± 1.9 |
| E. EtOAc | 97.39 ± 7.69 | 26.8 ± 2.4 | 19.9 ± 2.9 |
| E. Et2O | 22.95 ± 0.4 | 7.83 ± 1.11 | 4.1 ± 1.3 |
| E. Hex | 3.27 ± 2.1 | nd | nd |
| C.A.S. Number | RT (min) | Mass (amu) [M − H−] | Fragments (m/z) | Compounds | Phenolic Family | Concentration (µg/kg DW) |
|---|---|---|---|---|---|---|
| 327-97-9 | 0.59 | 353.8 | 191.20 | Chlorgenic Acid | Phenolic acids | 36 ± 7 |
| 149-91-7 | 1.28 | 169.01 | 125.00 | Gallic Acid | Phenolic acids | 1116 ± 127 |
| 1135-24-6 | 3.04 | 193.05 | 143.00 | Ferulic Acid | Phenolic acids | 86 ± 15 |
| 331-39-5 | 3.24 | 179.03 | 135.02 | Caffeic Acid | Phenolic acids | 92 ± 17 |
| 530-57-4 | 3.51 | 197.04 | 121.00 | Syringic Acid | Phenolic acids | 292 ± 35 |
| 207671-50-9 | 3.56 | 610.01 | 300.30 | Rutin | Flavonoids | 259 ± 31 |
| 520-26-3 | 3.59 | 609.20 | 301.00 | Hesperidina | Flavonoids | 177 ± 27 |
| 121-34-6 | 3.73 | 167.04 | 108.00 | Vanillic Acid | Phenolic acids | 359 ± 36 |
| 491-70-3 | 4.36 | 285.04 | 133.00 | Luteolin | Flavonoids | 56 ± 10 |
| 476-66-4 | 4.40 | 302.20 | 131.98 | Ellagic Acid | Phenolic acids | 522 ± 47 |
| 529-44-2 | 4.46 | 317.04 | 151.00 | Myricetin | Flavonoids | 160 ± 24 |
| 117-39-5 | 5.06 | 447.09 | 151.00 | Quercetin | Flavonoids | 4440 ± 355 |
| 67604-48-2 | 5.55 | 272.06 | 119.00 | Naringenin | Flavonoids | 23 ± 5 |
| 520-36-5 | 5.61 | 271.08 | 117.00 | Apigenin | Flavonoids | 336 ± 38 |
| 520-18-3 | 6.49 | 285.04 | 108.00 | Kaempferol | Flavonoids | 271 ± 35 |
| M. styphelioides Extracts | DPPH IC50 μg/mL | ABTS IC50 μg/mL | FRAP mM FeSO4/g DE |
|---|---|---|---|
| E. MeOH | 22.13 ± 2.17 | 21.39 ± 0.62 | 3.66 ± 0.014 |
| E. EtOAc | 119.15 ± 1.669 | 75.84 ± 1.22 | 0.85 ± 0.002 |
| E. Et2O | 73.24 ± 2.811 | 52.22 ± 1.40 | nd |
| E. Hexane | 229.9 ± 5.8 | 201.35 ± 9.4 | nd |
| Trolox IC50 | 13.69 ± 0.04 | 64.37 ± 1.28 | |
| BHT IC50 | 19.33 ± 0.32 |
| Primers | Forward (5′→3′) | Reverse (5′→3′) |
|---|---|---|
| ICAM-1 | GGCCGGCCAGCTTATACAC | TAGACACTTGAGCTCGGGCA |
| iNOS | GTTCTCAAGGCACAGGTCTC | GCAGGTCACTTATGTCACTTATC |
| NF-κB | ATGGCTTCTATGAGGCTGAG | GTTGTTGTTGGTCTGGATGC |
| COX-2 | ATCATTCACCAGGCAAATTGC | GGCTTCAGCATAAAGCGTTTG |
| GAPDH | TCAACAGCGACACCCAC | GGGTCTCTCTCTTCCTCTTGTG |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albouchi, F.; Avola, R.; Dico, G.M.L.; Calabrese, V.; Graziano, A.C.E.; Abderrabba, M.; Cardile, V. Melaleuca styphelioides Sm. Polyphenols Modulate Interferon Gamma/Histamine-Induced Inflammation in Human NCTC 2544 Keratinocytes. Molecules 2018, 23, 2526. https://doi.org/10.3390/molecules23102526
Albouchi F, Avola R, Dico GML, Calabrese V, Graziano ACE, Abderrabba M, Cardile V. Melaleuca styphelioides Sm. Polyphenols Modulate Interferon Gamma/Histamine-Induced Inflammation in Human NCTC 2544 Keratinocytes. Molecules. 2018; 23(10):2526. https://doi.org/10.3390/molecules23102526
Chicago/Turabian StyleAlbouchi, Ferdaous, Rosanna Avola, Gianluigi Maria Lo Dico, Vittorio Calabrese, Adriana Carol Eleonora Graziano, Manef Abderrabba, and Venera Cardile. 2018. "Melaleuca styphelioides Sm. Polyphenols Modulate Interferon Gamma/Histamine-Induced Inflammation in Human NCTC 2544 Keratinocytes" Molecules 23, no. 10: 2526. https://doi.org/10.3390/molecules23102526
APA StyleAlbouchi, F., Avola, R., Dico, G. M. L., Calabrese, V., Graziano, A. C. E., Abderrabba, M., & Cardile, V. (2018). Melaleuca styphelioides Sm. Polyphenols Modulate Interferon Gamma/Histamine-Induced Inflammation in Human NCTC 2544 Keratinocytes. Molecules, 23(10), 2526. https://doi.org/10.3390/molecules23102526