Advances in the Biology of Seed and Vegetative Storage Proteins Based on Two-Dimensional Electrophoresis Coupled to Mass Spectrometry
Abstract
1. Introduction
2. Terminology and Classification of Storage Proteins
3. Two-Dimensional-Based Reference Maps of Storage Proteins
4. Advances in the Biology of Storage Proteins
4.1. Seed Development
4.2. Seed Germination
5. Application Areas in Seed Breeding
5.1. Seed Quality
5.2. Gluten Disorders and Allergies
5.3. Seed Longevity
5.4. Other Applications
6. General Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 1-DE | One-dimensional electrophoresis |
| 2-DE | Two-dimensional electrophoresis |
| ABA | Abscisic acid |
| CD | Coeliac disease |
| DIGE | Difference gel electrophoresis |
| EAA | Essential amino acid |
| GA | Gibberellic acid |
| HF-P | Hydrogen fluoride-pyridine |
| IMAC | Immobilized metal affinity chromatography |
| Mr | Relative molecular mass |
| MS | Mass spectrometry |
| pI | Isoelectric point |
| PR | Phosphorylation rate |
| Pro-Q DPS | Pro-Q Diamond phosphoprotein stain |
| PTM | Post-translational modification |
| SSP | Seed storage protein |
| VSP | Vegetative storage protein |
References
- Shewry, P.R.; Napier, J.A.; Tatham, A.S. Seed storage proteins: Structures and biosynthesis. Plant Cell 1995, 7, 945–956. [Google Scholar] [CrossRef] [PubMed]
- Müntz, K. Deposition of storage proteins. Plant Mol. Biol. 1998, 38, 77–99. [Google Scholar] [CrossRef] [PubMed]
- Shewry, P.R.; Halford, N.G. Cereal seed storage proteins: Structures, properties and role in grain utilization. J. Exp. Bot. 2002, 53, 947–958. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, K.; Firnhaber, C.; Zuber, H.; Héricher, D.; Belghazi, M.; Henry, C.; Küster, H.; Thompson, R. A combined proteome and transcriptome analysis of developing Medicago truncatula seeds evidence for metabolic specialization of maternal and filial tissues. Mol. Cell. Proteom. 2007, 6, 2165–2179. [Google Scholar] [CrossRef] [PubMed]
- Tan-Wilson, A.L.; Wilson, K.A. Mobilization of seed protein reserves. Physiol. Plant. 2012, 145, 140–153. [Google Scholar] [CrossRef] [PubMed]
- van Vliet, S.; Burd, N.; van Loon, L. The skeletal muscle anabolic response to plant-versus animal-based protein consumption. J. Nutr. 2015, 145, 1981–1991. [Google Scholar] [CrossRef] [PubMed]
- Pasiakos, S.; Agarwal, S.; Lieberman, H.; Fulgoni, V. Sources and amounts of animal, dairy, and plant protein intake of US adults in 2007–2010. Nutrients 2015, 7, 7058–7069. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT. Statistics Division of the FAO. Available online: http://www.fao.org/faostat/en/ (accessed on 22 June 2018).
- World Bank List of Economies. Available online: http://www.worldbank.org// (accessed on 22 June 2018).
- Aguirrezábal, L.; Martre, P.; Pereyra-Irujo, G.; Echarte, M.M.; Izquierdo, N. Improving grain quality: Ecophysiological and modeling tools to develop management and breeding strategies. In Crop Physiology, Applications for Genetic Improvement and Agronomy, 2nd ed.; Sadras, V., Calderini, D., Eds.; Academic Press: London, UK, 2015; pp. 423–465. ISBN 9780124171046. [Google Scholar]
- Racusen, D. Lipid acyl hydrolase of patatin. Can. J. Bot. 1984, 62, 1640–1644. [Google Scholar] [CrossRef]
- Liu, Y.W.; Han, C.H.; Lee, M.H.; Hsu, F.L.; Hou, W.C. Patatin, the tuber storage protein of potato (Solanum tuberosum L.), exhibits antioxidant activity in vitro. J. Agric. Food Chem. 2003, 51, 4389–4393. [Google Scholar] [CrossRef] [PubMed]
- de Souza Cândido, E.; Pinto, M.F.; Pelegrini, P.B.; Lima, T.B.; Silva, O.N.; Pogue, R.; Grossi-de-Sá, M.F.; Franco, O.L. Plant storage proteins with antimicrobial activity: Novel insights into plant defense mechanisms. FASEB J. 2011, 25, 3290–3305. [Google Scholar] [CrossRef] [PubMed]
- Joshi, J.; Panduranga, S.; Diapari, M.; Marsolais, F. Comparison of Gene Families: Seed Storage and Other Seed Proteins. In The Common Bean Genome; de la Vega, M.P., Santalla, M., Marsolais, F., Eds.; Springer: Cham, Switzerland, 2017; pp. 201–219. ISBN 978-3-319-63524-8. [Google Scholar]
- Girke, T.; Todd, J.; Ruuska, S.; White, J.; Benning, C.; Ohlrogge, J. Microarray analysis of developing Arabidopsis seeds. Plant Physiol. 2000, 12, 1570–1581. [Google Scholar] [CrossRef]
- Tzafrir, I.; Dickerman, A.; Brazhnik, O.; Nguyen, Q.; McElver, J.; Frye, C.; Patton, D.; Meinke, D. The Arabidopsis seedgenes project. Nucleic Acids Res. 2003, 31, 90–93. [Google Scholar] [CrossRef] [PubMed]
- McElver, J.; Tzafrir, I.; Aux, G.; Rogers, R.; Ashby, C.; Smith, K.; Thomas, C.; Schetter, A.; Zhou, Q.; Cushman, M.A.; et al. Insertional mutagenesis of genes required for seed development in Arabidopsis thaliana. Genetics 2001, 159, 1751–1763. [Google Scholar] [PubMed]
- Meinke, D.; Muralla, R.; Sweeney, C.; Dickerman, A. Identifying essential genes in Arabidopsis thaliana. Trends Plant Sci. 2008, 13, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Le, B.H.; Cheng, C.; Bui, A.Q.; Wagmaister, J.A.; Henry, K.F.; Pelletier, J.; Kwong, L.; Belmonte, M.; Kirkbride, R.; Horvath, S.; et al. Global analysis of gene activity during Arabidopsis seed development and identification of seed-specific transcription factors. Proc. Natl. Acad. Sci. USA 2010, 107, 8063–8070. [Google Scholar] [CrossRef] [PubMed]
- Gacek, K.; Bartkowiak-Broda, I.; Batley, J. Genetic and molecular regulation of Seed Storage Proteins (SSPs) to improve protein nutritional value of oilseed rape (Brassica napus L.) seeds. Front. Plant Sci. 2018, 9, 890. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, A.; Xia, X.; Yan, Y.; Appels, R.; Mahmood, T.; He, Z. Wheat seed storage proteins: Advances in molecular genetics, diversity and breeding applications. J. Cereal Sci. 2014, 60, 11–24. [Google Scholar] [CrossRef]
- Finch-Savage, W.; Leubner-Metzger, G. Seed dormancy and the control of germination. New Phytol. 2006, 171, 501–523. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, T.; Shinozaki, K. Perception and transduction of abscisic acid signals: Keys to the function of the versatile plant hormone ABA. Trends Plant Sci. 2007, 12, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, L.; Van Wuytswinkel, O.; Castelain, M.; Bellini, C. Combined networks regulating seed maturation. Trends Plant Sci. 2007, 12, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Yang, P. Studies on the molecular mechanisms of seed germination. Proteomics 2015, 15, 1671–1679. [Google Scholar] [CrossRef] [PubMed]
- Née, G.; Kramer, K.; Nakabayashi, K.; Yuan, B.; Xiang, Y.; Miatton, E.; Finkemeier, I.; Soppe, W.J.J. Delay of germination1 requires PP2C phosphatases of the ABA signalling pathway to control seed dormancy. Nat. Commun. 2017, 8, 72. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.T.; Choi, U.K.; Ryu, H.S.; Lee, S.J.; Kwon, O.S. Mobilization of storage proteins in soybean seed (Glycine max L.) during germination and seedling growth. Biochim. Biophys. Acta 2011, 1814, 1178–1187. [Google Scholar] [CrossRef] [PubMed]
- The Potato Genome Sequencing Consortium. Genome sequence and analysis of the tuber crop potato. Nature 2011, 475, 189–195. [Google Scholar] [CrossRef] [PubMed]
- López-Pedrouso, M.; Alonso, J.; Zapata, C. Evidence for phosphorylation of the major seed storage protein of the common bean and its phosphorylation-dependent degradation during germination. Plant Mol. Biol. 2014, 84, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Bernal, J.; López-Pedrouso, M.; Franco, D.; Bravo, S.; García, L.; Zapata, C. Identification and mapping of phosphorylated isoforms of the major storage protein of potato based on two-dimensional electrophoresis. In Advances in Seed Biology; Jimenez-Lopez, J.C., Ed.; InTech: Rijeka, Croatia, 2017; pp. 65–82. ISBN 978-953-51-3621-7. [Google Scholar]
- Jorrín, J.V.; Maldonado, A.M.; Castillejo, M.A. Plant proteome analysis: A 2006 update. Proteomics 2007, 7, 2947–2962. [Google Scholar] [CrossRef] [PubMed]
- Kersten, B.; Agrawal, G.K.; Durek, P.; Neigenfind, J.; Schulze, W.; Walther, D.; Rakwal, R. Plant phosphoproteomics: An update. Proteomics 2009, 9, 964–988. [Google Scholar] [CrossRef] [PubMed]
- Silva-Sanchez, C.; Li, H.; Chen, S. Recent advances and challenges in plant phosphoproteomics. Proteomics 2015, 15, 1127–1141. [Google Scholar] [CrossRef] [PubMed]
- Miernyk, J.A.; Hajduch, M. Seed proteomics. J. Proteom. 2011, 74, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Miernyk, J.A. Seed Proteomics. In Plant Proteomics; Jorrin-Novo, J.J., Komatsu, S., Weckwerth, W., Wienkoop, S., Eds.; Humana Press: New York, NY, USA, 2014; pp. 361–379. ISBN 978-1-62703-630-6. [Google Scholar]
- Narula, K.; Sinha, A.; Haider, T.; Chakraborty, N.; Chakraborty, S. Seed Proteomics: An Overview. In Agricultural Porteomics; Salekdeh, G.H., Ed.; Springer: Cham, Switzerland, 2016; Volume 1, pp. 31–53. ISBN 978-3-319-43273-1. [Google Scholar]
- Zargar, S.M.; Mahajan, R.; Nazir, M.; Nagar, P.; Kim, S.T.; Rai, V.; Masi, A.; Ahmad, S.M.; Shah, R.A.; Ganai, N.A.; et al. Common bean proteomics: Present status and future strategies. J. Proteom. 2017, 169, 239–248. [Google Scholar] [CrossRef] [PubMed]
- O’Farrel, P.H. High resolution two-dimensional electrophoresis of proteins. J. Biol. Chem. 1975, 250, 4007–4021. [Google Scholar]
- Barbier-Brygoo, H.; Joyard, J. Focus on plant proteomics. Plant Physiol. Biochem. 2004, 42, 913–917. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Harmon, A.C. Advances in plant proteomics. Proteomics 2006, 6, 5504–5516. [Google Scholar] [CrossRef] [PubMed]
- Ghatak, A.; Chaturvedi, P.; Weckwerth, W. Cereal crop proteomics: Systemic analysis of crop drought stress responses towards marker-assisted selection breeding. Front. Plant Sci. 2017, 8, 757. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente, M.; López-Pedrouso, M.; Alonso, J.; Santalla, M.; de Ron, A.M.; Alvarez, G.; Zapata, C. In-depth characterization of the phaseolin protein diversity of common bean (Phaseolus vulgaris L.) based on two-dimensional electrophoresis and mass spectrometry. Food Technol. Biotechnol. 2012, 50, 315–325. [Google Scholar]
- Bárta, J.; Bártová, V.; Zdráhal, Z.; Šedo, O. Cultivar variability of patatin biochemical characteristics: Table versus processing potatoes (Solanum tuberosum L). J. Agric. Food Chem. 2012, 60, 4369–4378. [Google Scholar] [CrossRef] [PubMed]
- López-Pedrouso, M.; Bernal, J.; Franco, D.; Zapata, C. Evaluating two-dimensional electrophoresis profiles of the protein phaseolin as markers of genetic differentiation and seed protein quality in common bean (Phaseolus vulgaris L.). J. Agric. Food Chem. 2014, 62, 7200–7208. [Google Scholar] [CrossRef] [PubMed]
- Flores, T.; Alape-Girón, A.; Flores-Díaz, M.; Flores, H.E. Ocatin. A novel tuber storage protein from the andean tuber crop oca with antibacterial and antifungal activities. Plant Physiol. 2002, 128, 1291–1302. [Google Scholar] [CrossRef] [PubMed]
- Palomares, O.; Cuesta-Herranz, J.; Vereda, A.; Sirvent, S.; Villalba, M.; Rodríguez, R. Isolation and identification of an 11S globulin as a new major allergen in mustard seeds. Ann. Allergy Asthma Immunol. 2005, 94, 586–592. [Google Scholar] [CrossRef]
- Collins, R.M.; Afzal, M.; Ward, D.A.; Prescott, M.C.; Sait, S.M.; Rees, H.H.; Tomsett, A.B. Differential proteomic analysis of Arabidopsis thaliana genotypes exhibiting resistance or susceptibility to the insect herbivore, Plutella xylostella. PLoS ONE 2010, 5, e10103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.F.; Huang, X.W.; Wang, L.L.; Wei, L.; Wu, Z.H.; You, M.S.; Li, B.Y. Proteomic analysis of wheat seed in response to drought stress. J. Integr. Agric. 2014, 13, 919–925. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Cueff, G.; Hegedus, D.D.; Rajjou, L.; Bentsink, L. A role for seed storage proteins in Arabidopsis seed longevity. J. Exp. Bot. 2015, 66, 6399–6413. [Google Scholar] [CrossRef] [PubMed]
- Shekhar, S.; Agrawal, L.; Mishra, D.; Buragohain, A.K.; Unnikrishnan, M.; Mohan, C.; Chakraborty, S.; Chakraborty, N. Ectopic expression of amaranth seed storage albumin modulates photoassimilate transport and nutrient acquisition in sweetpotato. Sci. Rep. 2016, 6, 25384. [Google Scholar] [CrossRef] [PubMed]
- García-Molina, M.D.; Muccilli, V.; Saletti, R.; Foti, S.; Masci, S.; Barro, F. Comparative proteomic analysis of two transgenic low-gliadin wheat lines and non-transgenic wheat control. J. Proteom. 2017, 165, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Osborne, T.B. The Vegetable Proteins, 2nd ed.; Longmans, Green and Co.: London, UK, 1924; pp. 1–154. [Google Scholar]
- Marla, S.; Bharatiya, D.; Bala, M.; Singh, V.; Kumar, A. Classification of rice seed storage proteins using neural networks. J. Plant Biochem. Biotechnol. 2010, 19, 123–126. [Google Scholar] [CrossRef]
- Radhika, V.; Rao, V.S. Computational approaches for the classification of seed storage proteins. J. Food Sci. Technol. 2015, 52, 4246–4255. [Google Scholar] [CrossRef] [PubMed]
- Beardmore, T.; Wetzel, S.; Burgess, D.; Charest, P.J. Characterization of seed storage proteins in Populus and their homology with Populus vegetative storage proteins. Tree Physiol. 1996, 16, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, T.; Nambara, E.; Yamagishi, K.; Goto, D.B.; Naito, S. Storage proteins. Arabidopsis Book 2002, 1, e0020. [Google Scholar] [CrossRef] [PubMed]
- Pikaard, C.S.; Brusca, J.S.; Hannapel, D.J.; Park, W.D. The two classes of genes for the major potato tuber protein, patatin, are differentially expressed in tubers and roots. Nucleic Acids Res. 1979. [Google Scholar] [CrossRef]
- Mignery, G.A.; Pikaard, C.; Park, W. Molecular characterization of the patatin multigene family of potato. Gene 1988, 62, 27–44. [Google Scholar] [CrossRef]
- Staswick, P.E. Novel regulation of vegetative storage protein genes. Plant Cell 1990, 2, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Consoli, L.; Damerval, C. Quantification of individual zein isoforms resolved by two-dimensional electrophoresis: Genetic variability in 45 maize inbred lines. Electrophoresis 2001, 22, 2983–2989. [Google Scholar] [CrossRef]
- Lund, G.; Ciceri, P.; Viotti, A. Maternal-specific demethylation and expression of specific alleles of zein genes in the endosperm of Zea mays L. Plant J. 1995, 8, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, C.; Sergeant, K.; Machado, C.M.; Renaut, J.; Ricardo, C.P. Two traditional maize inbred lines of contrasting technological abilities are discriminated by the seed flour proteome. J. Proteome Res. 2013, 12, 3152–3165. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.H.; Messing, J. Organization of the prolamin gene family provides insight into the evolution of the maize genome and gene duplications in grass species. Proc. Natl. Acad. Sci. USA 2008, 105, 14330–14335. [Google Scholar] [CrossRef] [PubMed]
- Ning, F.; Niu, L.; Yang, H.; Wu, X.; Wang, W. Accumulation profiles of embryonic salt-soluble proteins in maize hybrids and parental lines indicate matroclinous inheritance: A proteomic analysis. Front. Plant Sci. 2017, 8, 1824. [Google Scholar] [CrossRef] [PubMed]
- Payne, P.I. Genetics of wheat storage proteins and the effect of allelic variation on bread-making quality. Ann. Rev. Plant Physiol. 1987, 38, 141–153. [Google Scholar] [CrossRef]
- Shewry, P.R.; Tatham, A.S. The prolamin storage proteins of cereal seeds: Structure and evolution. Biochem. J. 1990, 267, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Lv, D.; Yan, X.; Subburaj, S.; Ge, P.; Li, X.; Hu, Y.; Yan, Y. Proteome characterization of developing grains in bread wheat cultivars (Triticum aestivum L.). BMC Plant Biol. 2012, 12, 147. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.H. Nutrient uptake, transport and translocation in cereals: Influences of environmental and farming conditions. Swed. Univ. Agric. Sci. 2009, 1, 1–46. [Google Scholar]
- Zhou, J.; Liu, D.; Deng, X.; Zhen, S.; Wang, Z.; Yan, Y. Effects of water deficit on breadmaking quality and storage protein compositions in bread wheat (Triticum aestivum L.). J. Sci. Food Agric. 2018, 98, 4357–4368. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Wang, C.; Wang, K.; Wang, S.; Li, X.; Zhang, Z.; Ma, W.; Yan, Y. Molecular characterization of the celiac disease epitope domains in α-gliadin genes in Aegilops tauschii and hexaploid wheats (Triticum aestivum L.). Theor. Appl. Genet. 2010, 121, 1239–1251. [Google Scholar] [CrossRef] [PubMed]
- Cavazos, A.; Gonzalez de Mejia, E. Identification of bioactive peptides from cereal storage proteins and their potential role in prevention of chronic diseases. Compr. Rev. Food Sci. Food Saf. 2013, 12, 364–380. [Google Scholar] [CrossRef]
- Ferranti, P.; Mamone, G.; Picariello, G.; Addeo, F. Mass spectrometry analysis of gliadins in celiac disease. J. Mass Spectrom. 2007, 42, 1531–1548. [Google Scholar] [CrossRef] [PubMed]
- Yadav, D.; Singh, N. Wheat triticin: A potential target for nutritional quality improvement. Asian J. Biotechnol. 2011, 3, 1–21. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, J.; Zhao, G.; Wang, J.; Liu, H.; Zheng, H.; Zhao, H.; Zou, D. Association analysis of the glutelin synthesis genes GluA and GluB1 in a Japonica rice collection. Mol. Breed. 2017, 37, 129. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, J.Y.; Yoon, U.H.; Lim, S.H.; Kim, Y.M. Effects of reduced prolamin on seed storage protein composition and the nutritional quality of rice. Int. J. Mol. Sci. 2013, 14, 17073–17084. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, S.; Ding, Y. Identification of novel glutelin subunits and a comparison of glutelin composition between japonica and indica rice (Oryza sativa L.). J. Cereal Sci. 2013, 57, 362–371. [Google Scholar] [CrossRef]
- Bártová, V.; Bárta, J. Chemical composition and nutritional value of protein concentrates isolated from potato (Solanum tuberosum L.) fruit juice by precipitation with ethanol or ferric chloride. J. Agric. Food Chem. 2009, 57, 9028–9034. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, M.; Stensballe, A.; Welinder, K.G. Extensive post-translational processing of potato tuber storage proteins and vacuolar targeting. FEBS J. 2011, 278, 4070–4087. [Google Scholar] [CrossRef] [PubMed]
- Boehm, J.D.; Nguyen, V.; Tashiro, R.M.; Anderson, D.; Shi, C.; Wu, X.; Woodrow, L.; Yu, K.; Cui, Y.; Li, Z. Genetic mapping and validation of the loci controlling 7S α′ and 11S A-type storage protein subunits in soybean [Glycine max (L.) Merr.]. Theor. Appl. Genet. 2018, 131, 659–671. [Google Scholar] [CrossRef] [PubMed]
- Goyal, R.; Sharma, S. Genotypic variability in seed storage protein quality and fatty acid Composition of soybean [Glycine max (L.) Merrill]. Legum. Res. 2015, 38, 297–302. [Google Scholar] [CrossRef]
- Friedman, M.; Brandon, D.L. Nutritional and health benefits of soy proteins. J. Agric. Food Chem. 2001, 49, 1069–1086. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.; Nogueira, L.C.; Gonçalves, C.; Ferreira, A.A.; Ferreira, I.M.P.L.V.O.; Teixeira, N. Electrophoretic and HPLC methods for comparative study of the protein fractions of malts, worts and beers produced from Scarlett and Prestige barley (Hordeum vulgare L.) varieties. Food Chem. 2008, 106, 820–829. [Google Scholar] [CrossRef]
- Quiroga, I.; Regente, M.; Pagnussat, L.; Maldonado, A.; Jorrín, J.; de la Canal, L. Phosphorylated 11S globulins in sunflower seeds. Seed Sci. Res. 2013, 23, 199–204. [Google Scholar] [CrossRef]
- Youle, R.J.; Huang, A.H.C. Occurrence of low molecular weight and high cysteine containing albumin storage protein in oil-seeds of diverse species. Am. J. Bot. 1981, 68, 44–48. [Google Scholar] [CrossRef]
- Žilić, S.; Barać, M.; Pešić, M.; Crevar, M.; Stanojević, S.; Nišavić, A.; Saratlić, G.; Tolimir, M. Characterization of sunflower seed and kernel proteins. Helia 2010, 33, 103–113. [Google Scholar] [CrossRef]
- Montoya, C.A.; Leterme, P.; Victoria, N.F.; Toro, O.; Souffrant, W.B.; Beebe, S.; Lallès, J.P. Susceptibility of phaseolin to in vitro proteolysis is highly variable across common bean varieties (Phaseolus vulgaris). J. Agric. Food Chem. 2008, 56, 2183–2191. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, L.; Valsasina, B.; Daminati, M.G.; Fabbrini, M.S.; Nitti, G.; Bollini, R.; Ceriotti, A.; Vitale, A. Bean homologs of the mammalian glucose regulated proteins: Induction by tunicamycin and interaction with newly synthesized storage proteins in the endoplasmic reticulum. Plant J. 1992, 2, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Mäkienen, O.E.; Sozer, N.; Ercili-Cura, D.; Poutanen, K. Protein form oat: Structure, processes, functionality, and nutrition. In Sustainable Protein Sources; Nadathur, S.R., Wanasundara, J.P.D., Scanlin, L., Eds.; Academic Press: London, UK, 2017; pp. 105–119. ISBN 978-0-12-802779-3. [Google Scholar]
- Chang, Y.W.; Alli, I.; Konishi, Y.; Ziomek, E. Characterization of protein fractions from chickpea (Cicer arietinum L.) and oat (Avena sativa L.) seeds using proteomic techniques. Food Res. Int. 2011, 9, 3094–3104. [Google Scholar] [CrossRef]
- Tulbek, M.C.; Lam, R.S.H.; Wang, Y.; Asavajaru, P.; Lam, A. Pea: A sustainbable vegetable protein crop. In Sustainable Protein Sources; Nadathur, S.R., Wanasundara, J.P.D., Scanlin, L., Eds.; Academic Press: London, UK, 2017; pp. 145–164. ISBN 978-0-12-802779-3. [Google Scholar]
- Barac, M.; Cabrilo, S.; Pesic, M.; Stanojevic, S.; Zilic, S.; Macej, O.; Ristic, N. Profile and Functional Properties of Seed Proteins from Six Pea (Pisum sativum) Genotypes. Int. J. Mol. Sci. 2010, 11, 4973–4990. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Shrivastava, N.; Chaturvedi, K.; Sharma, B.; Bhagyawant, S.S. Characterization of Seed Storage Proteins from Chickpea Using 2D Electrophoresis Coupled with Mass Spectrometry. Biochem. Res. Int. 2016, 12, 1049462. [Google Scholar] [CrossRef] [PubMed]
- Elfalleh, W.; Nasri, N.; Sarraï, N.; Guasmi, F.; Triki, T.; Marzougui, N.; Ferchichi, A. Storage protein contents and morphological characters of some Tunisian pomegranate (Punica granatum L.) cultivars. Acta Bot. Gallica 2010, 157, 401–409. [Google Scholar] [CrossRef]
- Scippa, G.S.; Rocco, M.; Ialicicco, M.; Trupiano, D.; Viscosi, V.; Di Michele, M.; Arena, S.; Chiatante, D.; Scaloni, A. The proteome of lentil (Lens culinaris Medik.) seeds: Discriminating between landraces. Electrophoresis 2010, 31, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Schatzki, J.; Ecke, W.; Becker, H.C.; Möllers, C. Mapping of QTL for the seed storage proteins cruciferin and napin in a winter oilseed rape doubled haploid population and their inheritance in relation to other seed traits. Theor. Appl. Genet. 2014, 127, 1213–1222. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.G.; Lee, J.S.; Shin, S.H.; Koo, S.C.; Kim, J.T.; Bae, H.H.; Son, B.Y.; Kim, Y.H.; Kim, S.L.; Baek, S.B.; et al. Profiling of differentially expressed proteins in mature kernels of Korean waxy corn cultivars using proteomic analysis. J. Korean Soc. Appl. Biol. Chem. 2015, 58, 293–303. [Google Scholar] [CrossRef]
- Görg, A.; Postel, W.; Günther, S. The current state of two-dimensional electrophoresis with immobilized pH gradients. Electrophoresis 1988, 9, 531–546. [Google Scholar] [CrossRef] [PubMed]
- Görg, A.; Drews, O.; Lück, C.; Weiland, F.; Weiss, W. 2-DE with IPGs. Electrophoresis 2009, 30 (Suppl. 1), 1221–1232. [Google Scholar] [CrossRef]
- Weiss, W.; Görg, A. Two-dimensional electrophoresis for plant proteomics. Methods Mol. Biol. 2007, 355, 121–143. [Google Scholar] [PubMed]
- Wheelock, A.M.; Wheelock, C.E. Bioinformatics in gel-based proteomics. In Plant Proteomics: Technologies, Strategies and Applications; Agrawal, G.K., Rakwal, R., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008; pp. 1–18. ISBN 978-0-470-06976-9. [Google Scholar]
- Chevalier, F. Highlights on the capacities of “Gel-based” proteomics. Proteome Sci. 2010, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Rabilloud, T.; Lelong, C. Two-dimensional gel electrophoresis in proteomics: A tutorial. J. Proteom. 2011, 74, 1829–1841. [Google Scholar] [CrossRef] [PubMed]
- Dowsey, A.W.; Morris, J.S.; Gutstein, H.G.; Yang, G.Z. Informatics and statistics for analyzing 2-D gel electrophoresis images. Methods Mol. Biol. 2010, 604, 239–255. [Google Scholar] [CrossRef] [PubMed]
- Görg, A.; Weiss, W.; Dunn, M.J. Current two-dimensional electrophoresis technology for proteomics. Proteomics 2004, 4, 3665–3685. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Min, C.W.; Wang, Y.; Kim, Y.C.; Agrawal, G.K.; Rakwal, R.; Kim, S.T. Expect the unexpected enrichment of “hidden proteome” of seeds and tubers by depletion of storage proteins. Front. Plant Sci. 2016, 7, 761. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, R.S.; Rose, J.K.C. A critical evaluation of sample extraction techniques for enhanced proteomic analysis of recalcitrant plant tissues. Proteomics 2004, 4, 2522–2532. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, S.C.; Witters, E.; Laukens, K.; Deckers, P.; Swennen, R.; Panis, B. Preparation of protein extracts from recalcitrant plant tissues: An evaluation of different methods for two-dimensional gel electrophoresis analysis. Proteomics 2006, 5, 2497–2507. [Google Scholar] [CrossRef] [PubMed]
- Faurobert, M.; Pelpoir, E.; Chaïb, J. Phenol extraction of proteins for proteomic studies of recalcitrant plan tissues. Methods Mol. Biol. 2007, 355, 9–14. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente, M.; Borrajo, A.; Bermúdez, J.; Lores, M.; Alonso, J.; López, M.; Santalla, M.; de Ron, A.M.; Zapata, C.; Alvarez, G. 2-DE-based proteomic analysis of common bean (Phaseolus vulgaris L.) seeds. J. Proteom. 2011, 74, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Rabilloud, T.; Chevallet, M.; Luche, S.; Lelong, C. Two-dimensional gel electrophoresis in proteomics: Past, present and future. J. Proteom. 2010, 73, 2064–2077. [Google Scholar] [CrossRef] [PubMed]
- López-Pedrouso, M.; Pérez-Santaescolástica, C.; Franco, D.; Fulladosa, E.; Carballo, J.; Zapata, C.; Lorenzo, J.M. Comparative proteomic profiling of myofibrillar proteins in dry-cured ham with different proteolysis indices and adhesiveness. Food Chem. 2018, 244, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Rabilloud, T. How to use 2D gel electrophoresis in plant proteomics. In Plant Proteomics: Methods and Protocols; Jorrin-Novo, J.V., Komatsu, S., Weckwerth, W., Wienkoop, S., Eds.; Humana Press: New York, NY, USA, 2014; pp. 43–50. ISBN 978-1-4939-6029-3. [Google Scholar]
- Agrawal, G.K.; Thelen, J.J. Development of a simplified, economical polyacrylamide gel staining protocol for phosphoproteins. Proteomics 2005, 5, 4684–4688. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Yang, P. Two Dimensional Gel Electrophoresis-Based Plant Phosphoproteomics. Methods Mol. Biol. 2016, 1355, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Kuyama, H.; Toda, C.; Watanabe, M.; Tanaka, K.; Nishimura, O. An efficient chemical method for dephosphorylation of phosphopeptides. Rapid Commun. Mass Spectrom. 2003, 17, 1493–1496. [Google Scholar] [CrossRef] [PubMed]
- Graur, D.; Li, W.H. Fundamentals of Molecular Evolution. Sinauer Associate, 2nd ed.; Sinauer Associates: Sunderland, UK, 2000; pp. 304–322. ISBN 9780878932665. [Google Scholar]
- Mignery, G.A.; Pikaard, C.S.; Hannapel, D.J.; Park, W.D. Isolation and sequence analysis of cDNAs for the major potato tuber protein, patatin. Nucleic Acids Res. 1984, 12, 7987–8000. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Sanchez, M.B.; Lanucara, F.; Helm, M.; Eyers, C.E. Attempting to rewrite history: Challenges with the analysis of histidine-phosphorylated peptides. Biochem. Soc. Trans. 2013, 41, 1089–1095. [Google Scholar] [CrossRef] [PubMed]
- Mehta-D’souza, P. Detection of glycoproteins in polyacrylamide gels using Pro-Q Emerald 300 Dye, a fluorescent periodate schiff-base stain. Methods Mol. Biol. 2012, 869, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Duranti, M.; Scarafoni, A.; Gius, C.; Negri, A.; Faoro, F. Heat-induced synthesis and tunicamycin-sensitive secretion of the putative storage glycoprotein conglutin γ from mature lupin seeds. Eur. J. Biochem. 1994, 222, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Dam, S.; Thaysen-Andersen, M.; Stenkjaer, E.; Lorentzen, A.; Roepstorff, P.; Packer, N.H.; Stougaard, J. Combined N-glycome and N-glycoproteome analysis of the Lotus japonicus seed globulin fraction shows conservation of protein structure and glycosylation in legumes. J. Proteome Res. 2013, 12, 3383–3392. [Google Scholar] [CrossRef] [PubMed]
- Borisjuk, L.; Neuberger, T.; Schwender, J.; Heinzel, N.; Sunderhaus, S.; Fuchs, J.; Hay, J.O.; Tschiersch, H.; Braun, H.P.; Denolf, P.; et al. Seed architecture shapes embryo metabolism in oilseed rape. Plant Cell 2013, 25, 1625–1640. [Google Scholar] [CrossRef] [PubMed]
- Friso, G.; van Wijk, K.J. Posttranslational protein modifications in plant metabolism. Plant Physiol. 2015, 169, 1469–1487. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Wang, X.; Komatsu, S. Phosphoproteomics: Protein phosphorylation in regulation of seed germination and plant growth. Curr. Protein Pept. Sci. 2018, 19, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, G.K.; Thelen, J.J. Large-scale identification and quantitative profiling of phosphoproteins expressed during seed filling in oilseed rape. Mol. Cell Proteom. 2006, 5, 2044–2059. [Google Scholar] [CrossRef] [PubMed]
- Irar, S.; Oliveira, E.; Pagès, M.; Goday, A. Towards the identification of late-embryogenic-abundant phosphoproteome in Arabidopsis by 2-DE and MS. Proteomics 2006, 6, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Ross, A.R.S.; Yang, J.; Hegedus, D.D.; Kermode, A.R. Phosphorylation of the 12 S globulin cruciferin in wild-type and abi1-1 mutant Arabidopsis thaliana (thale cress) seeds. Biochem. J. 2007, 404, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Ghelis, T.; Bolbach, G.; Clodic, G.; Habricot, Y.; Miginiac, E.; Sotta, B.; Jeannette, E. Protein tyrosine kinases and protein tyrosine phosphatases are involved in abscisic acid-dependent processes in Arabidopsis seeds and suspension cells. Plant Physiol. 2008, 148, 1668–1680. [Google Scholar] [CrossRef] [PubMed]
- Meyer, L.J.; Gao, J.; Xu, D.; Thelen, J.J. Phosphoproteomic analysis of seed maturation in Arabidopsis, rapeseed, and soybean. Plant Physiol. 2012, 159, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Kovaleva, V.; Cramer, R.; Krynytskyy, H.; Gout, I.; Gout, A. Analysis of tyrosine phosphorylation and phosphotyrosine-binding proteins in germinating seeds from Scots pine. Plant Physiol. Biochem. 2013, 67, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Wang, K.; Yang, P. Gel-based comparative phosphoproteomic analysis on rice during germination. Plant Cell Physiol. 2014, 55, 1376–1394. [Google Scholar] [CrossRef] [PubMed]
- Dong, K.; Zhen, S.; Cheng, Z.; Cao, H.; Ge, P.; Yah, Y. Proteomic analysis reveals key proteins and phosphoproteins upon seed germination of wheat (Triticum aestivum L.). Front. Plant Sci. 2015, 6, 1017. [Google Scholar] [CrossRef] [PubMed]
- Tilley, K.A.; Schofield, J.D. Detection of phosphotyrosine in the high Mr subunits of wheat glutenin. J. Cereal Sci. 1995, 22, 17–19. [Google Scholar] [CrossRef]
- Facchiano, A.M.; Colonna, G.; Chiosi, E.; Illiano, G.; Spina, A.; Lafiandra, D.; Buonocore, F. In vitro phosphorylation of high molecular weight glutenin subunits from wheat endosperm. Plant Physiol. Biochem. 1999, 37, 931–938. [Google Scholar] [CrossRef]
- Lin, S.K.; Chang, M.C.; Tsai, Y.G.; Lur, H.S. Proteomic analysis of the expression of proteins related to rice quality during caryopsis development and the effect of high temperature on expression. Proteomics 2005, 5, 2140–2156. [Google Scholar] [CrossRef] [PubMed]
- Vilela, B.; Pagès, M.; Riera, M. Emerging roles of protein kinase CK2 in abscisic acid signaling. Front. Plant Sci. 2015, 6, 966. [Google Scholar] [CrossRef] [PubMed]
- Mulekar, J.J.; Huq, E. Expanding roles of protein kinase CK2 in regulating plant growth and development. J. Exp. Bot. 2014, 65, 2883–2893. [Google Scholar] [CrossRef] [PubMed]
- Montenarh, M.; Götz, C. Ecto-protein kinase CK2, the neglected form of CK2 (Review). Biomed. Rep. 2018, 8, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Gosti, F.; Beaudoin, N.; Serizet, C.; Webb, A.A.; Vartanian, N.; Giraudat, J. ABI1 protein phosphatase 2C is a negative regulator of abscisic acid signaling. Plant Cell 1999, 11, 1897–1910. [Google Scholar] [CrossRef] [PubMed]
- Ludwików, A. Targeting proteins for proteasomal degradation—A new function of Arabidopsis ABI1 protein phosphatase 2C. Front. Plant Sci. 2015, 6, 310. [Google Scholar] [CrossRef] [PubMed]
- Chibani, K.; Ali-Rachedi, S.; Job, C.; Job, D.; Jullien, M.J.; Grappin, P. Proteomic analysis of seed dormancy in Arabidopsis. Plant Physiol. 2006, 142, 1493–1510. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, K.; Job, C.; Groot, S.P.; Puype, M.; Demol, H.; Vandekerckhove, J.; Job, D. Proteomics of Arabidopsis seed germination. A comparative study of wild-type and gibberellin-deficient seeds. Plant Physiol. 2002, 129, 823–837. [Google Scholar] [CrossRef] [PubMed]
- Lehesranta, S.J.; Davies, H.V.; Shepherd, L.V.T.; Koistinen, K.M.; Massat, N.; Nunan, N.; McNicol, J.W.; Kärenlampi, S.O. Proteomic analysis of the potato tuber life cycle. Proteomics 2006, 6, 6042–6052. [Google Scholar] [CrossRef] [PubMed]
- Bachem, C.; Van der Hoeven, R.; Lucker, J.; Oomen, R.; Casarini, E.; Jacobsen, E.; Visser, R. Functional genomic analysis of potato tuber life-cycle. Potato Res. 2000, 43, 297–312. [Google Scholar] [CrossRef]
- Ronning, C.M.; Stegalkina, S.S.; Ascenzi, R.A.; Bougri, O.; Hart, A.L.; Utterbach, T.R.; Vanaken, S.E.; Riedmuller, S.B.; White, J.A.; Cho, J.; et al. Comparative analyses of potato expressed sequence tag libraries. Plant Physiol. 2003, 131, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.; Segear, E.; Beers, L.; Knauber, D.; Suttle, J. Dormancy in potato tuber meristems: Chemically induced cessation in dormancy matches the natural process based on transcript profiles. Funct. Integr. Genom. 2008, 8, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Baginsky, S. Plant proteomics: Concepts, applications, and novel strategies for data interpretation. Mass Spectrom. Rev. 2009, 28, 93–120. [Google Scholar] [CrossRef] [PubMed]
- Strasser, R. Biological significance of complex N-glycans in plants and their impact on plant physiology. Front. Plant Sci. 2014, 5, 363. [Google Scholar] [CrossRef] [PubMed]
- Strasser, R. Plant protein glycosylation. Glycobiology 2016, 26, 926–939. [Google Scholar] [CrossRef] [PubMed]
- Lerouge, P.; Cabanes-Macheteau, M.; Rayon, C.; Fischette-Laine, A.C.; Gomord, V.; Faye, L. N-glycoprotein biosynthesis in plants: Recent developments and future trends. Plant Mol. Biol. 1998, 38, 31–48. [Google Scholar] [CrossRef] [PubMed]
- Koshiyama, I. Carbohydrate component in 7S protein of soybean casein fraction. Agric. Biol. Chem. 1966, 30, 646–650. [Google Scholar] [CrossRef]
- Ericson, M.C.; Chrispeels, M.J. Isolation and characterization of glucosamine-containing storage glycoproteins from the cotyledons of Phaseolus aureus. Plant Physiol. 1973, 52, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Basha, S.M.M.; Beevers, L. Glycoprotein metabolism in the cotyledons of Pisum sativum during development and germination. Plant. Physiol. 1976, 57, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.C.; Mcleester, R.C.; Bliss, F.A. Equal expression of the maternal and paternal alleles for the polypeptide subunits of the major storage protein of the bean Phaseolus vulgaris L. Plant Physiol. 1977, 59, 1122–1124. [Google Scholar] [CrossRef] [PubMed]
- Eaton-Mordas, C.A.; Moore, K.G. Seed glycoproteins of Lupinus angustifolius. Phytochemistry 1978, 17, 619–621. [Google Scholar] [CrossRef]
- Badenoch-Jones, J.; Spencer, D.; Higgins, T.J.V.; Millerd, A. The role of glycosylation in storage-proteins synthesis in developing pea seeds. Planta 1981, 153, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, C.; Deluca, V.; Bailey, D.S.; Verma, D.P.S. Post-translational processing of 7S and 11S components of soybean storage proteins. Plant Mol. Biol. 1981, 1, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Weber, E.; Manteuffel, R.; Jakubek, M.; Neumann, D. Comparative studies on protein bodies and storage proteins of Pisum sativum L. and Vicia faba L. Biochem. Physiol. Pflanzen 1981, 176, 342–356. [Google Scholar] [CrossRef]
- Chrispeels, M.J.; Higgins, T.J.V.; Craig, S.; Spencer, D. Role of the endoplasmic reticulum in the synthesis of reserve proteins and the kinetics of their transport to protein bodies in developing pea cotyledons. J. Cell Biol. 1982, 93, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Bollini, R.; Vitale, A.; Chrispeels, M.J. In vivo and in vitro processing of seed reserve protein in the endoplasmic reticulum: Evidence for two glycosylation steps. J. Cell Biol. 1983, 96, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Lioi, L.; Bollini, R. Contribution of processing events to the molecular heterogeneity of four banding types of phaseolin, the major storage protein of Phaseolus vulgaris L. Plant Mol. Biol. 1984, 3, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Paaren, H.E.; Slightom, J.L.; Hall, T.C.; Inglis, A.S.; Blagrove, R.J. Purification of a seed glycoprotein: N-terminal and deglycosylation analysis of phaseolin. Phytochemistry 1987, 26, 335–343. [Google Scholar] [CrossRef]
- Sturm, A.; Van Kuik, J.A.; Vliegenthart, J.F.G.; Chrispeels, M.J. Structure, position, and biosynthesis of the high mannose and complex oligosaccharide chains of the bean storage protein phaseolin. J. Biol. Chem. 1987, 262, 13392–13403. [Google Scholar] [PubMed]
- Duranti, M.; Guerrieri, N.; Takajashi, T.; Cerletti, P. The legumin-like storage proteins of Lupinus albus seeds. Phytochemistry 1988, 27, 15–23. [Google Scholar] [CrossRef]
- Duranti, M.; Gorinstein, S.; Cerletti, P. Rapid separation and detection of concanavalin. A reacting glycoproteins: Application to storage proteins of a legume seed. J. Food Biochem. 1990, 14, 327–330. [Google Scholar] [CrossRef]
- Lawrence, M.C.; Suzuki, E.; Varghese, J.N.; Davis, P.C.; Van Donkelaar, A.; Tulloch, P.A.; Colman, P.M. The three-dimensional structure of the seed storage protein phaseolin at 3 Å resolution. EMBO J. 1990, 9, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Duranti, M.; Guerrieri, N.; Cerletti, P.; Vecchio, G. The legumin precursor from white lupin seed. Eur. J. Biochem. 1992, 206, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Duranti, M.; Gius, C.; Sessa, F.; Vecchio, G. The saccharide chain of lupin seed conglutin γ is not responsible for the protection of the native protein from degradation by trypsin, but facilitates the refolding of the acid-treated protein to the resistant conformation. Eur. J. Biochem. 1995, 230, 886–891. [Google Scholar] [CrossRef] [PubMed]
- Duranti, M.; Horstmann, C.; Gilroy, J.; Croy, R.R.D. The molecular basis for N-glycosylation in the 11S globulin (legumin) of lupin seed. J. Protein Chem. 1995, 14, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Kolarich, D.; Altmann, F. N-glycan analysis by matrix-assisted laser desorption/ionization mass spectrometry of electrophoretically separated nonmammalian proteins: Application to peanut allergen Ara h 1 and olive pollen allergen Ole e 1. Anal. Biochem. 2000, 285, 64–75. [Google Scholar] [CrossRef] [PubMed]
- López-Torrejón, G.; Salcedo, G.; Martín-Esteban, M.; Díaz-Perales, A.; Pascual, C.Y.; Sánchez-Monge, R. Len c 1, a major allergen and vicilin from lentil seeds: Protein isolation and cDNA cloning. J. Allergy Clin. Immunol. 2003, 112, 1208–1215. [Google Scholar] [CrossRef] [PubMed]
- Lauer, I.; Foetisch, K.; Kolarich, D.; Ballmer-Weber, B.K.; Conti, A.; Altmann, F.; Vieths, S.; Scheurer, S. Hazelnut (Corylus avellana) vicilin Cor a 11: Molecular characterization of a glycoprotein and its allergenic activity. Biochem. J. 2004, 383, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Vaz, A.C.; Pinheiro, C.; Martins, J.M.N.; Ricardo, C.P.P. Cultivar discrimination of Portuguese Lupinus albus by seed protein electrophoresis: The importance of considering “glutelins” and glycoproteins. Field Crop Res. 2004, 87, 23–34. [Google Scholar] [CrossRef]
- Fukuda, T.; Prak, K.; Fujioka, M.; Maruyama, N.; Utsumi, S. Physicochemical properties of native adzuki bean (Vigna angularis) 7S globulin and the molecular cloning of its cDNA isoforms. J. Agric. Food Chem. 2007, 55, 3667–3674. [Google Scholar] [CrossRef] [PubMed]
- Marsh, J.T.; Tryfona, T.; Powers, S.J.; Stephens, E.; Dupree, P.; Shewry, P.R.; Lovegrove, A. Determination of the N-glycosylation patterns of seed proteins: Applications to determine the authenticity and substantial equivalence of genetically modified (GM) crops. J. Agric. Food Chem. 2011, 59, 8779–8788. [Google Scholar] [CrossRef] [PubMed]
- Picariello, G.; Amigo-Benavent, M.; del Castillo, M.D.; Ferranti, P. Structural characterization of the N-glycosylation of individual soybean β-conglycinin subunits. J. Chromatogr. A 2013, 1313, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.N.; Alves, M.; Oliveira, A.; Ferreira, R.B. β-N-acetylhexosaminidase involvement in α-conglutin mobilization in Lupinus albus. J. Plant Physiol. 2013, 170, 1047–1056. [Google Scholar] [CrossRef] [PubMed]
- Schiarea, S.; Arnoldi, L.; Fanelli, R.; Combarieu, E.; Chiabrando, C. In-depth glycoproteomic characterization of γ-conglutin by high-resolution accurate mass spectrometry. PLoS ONE 2013, 8, e73906. [Google Scholar] [CrossRef] [PubMed]
- Kumari, N.; Kofi, K.J.; Grimbs, S.; D’Souza, R.N.; Kuhnert, N.; Vrancken, G.; Ullrich, M.S. Biochemical fate of vicilin storage protein during fermentation and drying of cocoa beans. Food Res. Int. 2016, 90, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Snégaroff, J.; Bouchez, I.; Smaali, M.I.A.; Pecquet, C.; Raison-Peyron, N.; Jolivet, P.; Laurière, M. Barley γ3-hordein: Glycosylation at an atypical site, disulfide bridge analysis, and reactivity with IgE from patients allergic to wheat. Biochim. Biophys. Acta 2013, 1834, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Sathe, S.K.; Hamaker, B.R.; Sze-Tao, K.W.C.; Venkatachalam, M. Isolation, purification, and biochemical characterization of a novel water soluble protein from inca peanut (Plukenetia volubilis L.). J. Agric. Food Chem. 2002, 50, 4906–4908. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, T.; Watanabe, M.; Mitsui, T.; Mori, H. Glutelin basic subunits have a mammalian mucin type O-linked disaccharide side chain. Arch. Biochem. Biophys. 1999, 370, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Racusen, D.; Foote, M.A. A major soluble glycoprotein from potato tubers. J. Food Biochem. 1980, 4, 43–52. [Google Scholar] [CrossRef]
- Bauw, G.; Nielsen, H.V.; Emmersen, J.; Nielsen, K.L.; Jørgensen, M.; Welinder, K.G. Patatin, Kunitz protease inhibitors and other major proteins in tuber of potato cv. Kuras. FEBS J. 2006, 273, 3569–3584. [Google Scholar] [CrossRef] [PubMed]
- Welinder, K.G.; Jørgensen, M. Covalent structures of potato tuber lipases (patatins) and implications for vacuolar important. J. Biol. Chem. 2009, 284, 9764–9769. [Google Scholar] [CrossRef] [PubMed]
- Lattová, E.; Brabcová, A.; Bártová, V.; Potěšil, D.; Bárta, J.; Zdráhal, Z. N-glycome profiling of patatins from different potato species of Solanum genus. J. Agric. Food Chem. 2015, 63, 3243–3250. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.W.; Svenson, R.H.; Yachnin, S. Purification of mitogenic proteins derived from Phaseolus vulgaris: Isolation of potent and weak phytohemagglutinins possessing mitogenic activity. Proc. Natl. Acad. Sci. USA 1969, 63, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.B.; Hsu, R.; Heinrikson, R.; Yachnin, S. Extensive homology between the subunits of the phytohemagglutinin mitogenic proteins derived from Phaseolus vulgaris. Proc. Natl. Acad. Sci. USA 1975, 72, 1388–1391. [Google Scholar] [CrossRef] [PubMed]
- Vitale, A.; Chrispeels, M.J. Transient N-acetylglucosamine in the biosynthesis of phytohemagglutinin: Attachment in the Golgi apparatus and removal in protein bodies. J. Cell Biol. 1984, 99, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Faye, L.; Sturm, A.; Bollini, R.; Vitale, A.; Chrispeels, M.J. The position of the oligosaccharide side-chains of phytohemagglutinin and their accessibility to glycosidases determines their subsequent processing in the Golgi. Eur. J. Biochem. 1986, 158, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Sturm, A.; Chrispeels, M.J. The high mannose oligosaccharide of phytohemagglutinin is attached to asparagine 12 and the modified oligosaccharide to asparagine 60. Plant Physiol. 1986, 80, 320–322. [Google Scholar] [CrossRef]
- Sturm, A.; Bergwerff, A.A.; Vliegenthart, J.F.G. H-NMR structural determination of the N-linked carbohydrate chains on glycopeptides obtained from the bean lectin phytohemagglutinin. Eur. J. Biochem. 1992, 204, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Vishwanathreddy, H.; Sindhura, B.R.; Kamalanathan, A.S.; Swamy, B.M.; Inamdar, S.R. Purification, characterization and biological significance of mannose binding lectin from Dioscorea bulbifera bulbils. Int. J. Biol. Macromol. 2017, 102, 1146–1155. [Google Scholar] [CrossRef] [PubMed]
- Waglay, A.; Karboune, S.; Alli, I. Potato protein isolates: Recovery and characterization of their properties. Food Chem. 2014, 142, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Zarkadas, C.G.; Gagnon, C.; Gleddie, S.; Khanizadeh, S.; Cober, E.R.; Guillemette, R.J.D. Assessment of the protein quality of fourteen soybean [Glycine max (L.) Merr.] cultivars using amino acid analysis and two-dimensional electrophoresis. Food Res. Int. 2007, 40, 129–146. [Google Scholar] [CrossRef]
- Kirihara, J.A.; Hunsperger, J.P.; Mahoney, W.C.; Messing, J.W. Differential expression of a gene for a methionine-rich storage protein in maize. Mol. Gen. Genet. 1988, 211, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Gibbon, B.C.; Wang, X.; Larkins, B.A. Altered starch structure is associated with endosperm modification in Quality Protein Maize. Proc. Natl. Acad. Sci. USA 2003, 100, 15329–15334. [Google Scholar] [CrossRef] [PubMed]
- El-Shemy, H.A.; Khalafalla, M.M.; Fujita, K.; Ishimoto, M. Improvement of protein quality in transgenic soybean plants. Biol. Plant. 2007, 51, 277–284. [Google Scholar] [CrossRef]
- Jiang, C.; Cheng, Z.; Zhang, C.; Yu, T.; Zhong, Q.; Shen, J.; Huang, X. Proteomic analysis of seed storage proteins in wild rice species of the Oryza genus. Proteome Sci. 2014, 12, 51. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.X.; Zhai, L.J.; Yang, H.; Zhai, S.M.; Zhai, C.K. Analysis of active components and proteomics of chinese wild rice (Zizania latifolia (Griseb) Turcz) and Indica rice (Nagina22). J. Med. Food. 2016, 19, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Hedrick, P.H. Genetics of Populations, 3rd ed.; Jones and Bartlett: Sudbury, MA, USA, 2005; pp. 525–595. ISBN 0-7637-4772-6. [Google Scholar]
- Bárta, J.; Bártová, V. Patatin, the major protein of potato (Solanum tuberosum L.) tubers, and its occurrence as genotype effect: Processing versus table potatoes. Czech J. Food Sci. 2008, 26, 347–359. [Google Scholar] [CrossRef]
- Bártová, V.; Bárta, J.; Brabcová, A.; Zdráhal, Z.; Horáčková, V. Amino acid composition and nutritional value of four cultivated South American potato species. J. Food Compos. Anal. 2015, 40, 78–85. [Google Scholar] [CrossRef]
- Ogawa, T.; Tayama, E.; Kitamura, K.; Kaizuma, N. Genetic improvement of seed storage proteins using three variant alleles of 7S globulin subunits in soybean (Glycine max L.). Jpn. J. Breed. 1989, 39, 137–147. [Google Scholar] [CrossRef]
- Gobbetti, M.; Giuseppe Rizzello, C.; Di Cagno, R.; De Angelis, M. Sourdough lactobacilli and celiac disease. Food Microbiol. 2007, 24, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Tanner, G.J.; Blundell, M.J.; Colgrave, M.L.; Howitt, C.A. Creation of the first ultra-low gluten barley (Hordeum vulgare L.) for coeliac and gluten-intolerant populations. Plant Biotechnol. J. 2016, 14, 1139–1150. [Google Scholar] [CrossRef]
- Wild, D.; Robins, G.G.; Burley, V.J.; Howdle, P.D. Evidence of high sugar intake, and low fibre and mineral intake, in the gluten-free diet. Aliment. Pharmacol. Ther. 2010, 32, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Öhlund, K.; Olsson, C.; Hernell, O.; Öhlund, I. Dietary shortcomings in children on a gluten-free diet. J. Hum. Nutr. Diet. 2010, 23, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Kawaura, K.; Miura, M.; Kamei, Y.; Ikeda, T.M.; Ogihara, Y. Molecular characterization of gliadins of Chinese Spring wheat in relation to celiac disease elicitors. Genes Genet. Syst. 2018. [Google Scholar] [CrossRef] [PubMed]
- van den Broeck, H.C.; Gilissen, L.J.W.J.; Smulders, M.J.M.; van der Meer, I.M.; Hamer, R.J. Dough quality of bread wheat lacking α-gliadins with celiac disease epitopes and addition of celiac-safe avenins to improve dough quality. J. Cereal Sci. 2011, 53, 206–216. [Google Scholar] [CrossRef]
- Rizzello, C.G.; Curiel, J.A.; Nionelli, L.; Vincentini, O.; Di Cagno, R.; Silano, M.; Gobbetti, M.; Coda, R. Use of fungal proteases and selected sourdough lactic acid bacteria for making wheat bread with an intermediate content of gluten. Food Microbiol. 2014, 37, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Bewley, J.D.; Black, M. Seeds: Physiology of Development and Germination, 2nd ed.; Plenum Press: New York, NY, USA, 1994; pp. 377–416. ISBN 978-1-4899-1002-8. [Google Scholar]
- Sugliani, M.; Rajjou, L.; Clerkx, E.J.M.; Koornneef, M.; Soppe, W.J.J. Natural modifiers of seed longevity in the Arabidopsis mutants abscisic acid insensitive3-5(abi3-5) and leafy cotyledon1-3(lec1-3). New Phytol. 2009, 184, 898–908. [Google Scholar] [CrossRef] [PubMed]
- Dobiesz, M.; Piotrowicz-Cieślak, A.I.; Michalczyk, D.J. Physiological and biochemical parameters of lupin seed subjected to 29 years of storage. Crop Sci. 2017, 57, 2149–2159. [Google Scholar] [CrossRef]
- Rajjou, L.; Lovigny, Y.; Groot, S.P.C.; Belghazi, M.; Job, C.; Job, D. Proteome-wide characterization of seed aging in Arabidopsis: A comparison between artificial and natural aging protocols. Plant Physiol. 2008, 148, 620–641. [Google Scholar] [CrossRef] [PubMed]
- Sano, N.; Rajjou, L.; North, H.M.; Debeaujon, I.; Marion-Poll, A.; Seo, M. Staying alive: Molecular aspects of seed longevity. Plant Cell Physiol. 2015, 57, 660–674. [Google Scholar] [CrossRef] [PubMed]
- Kalemba, E.M.; Pukacka, S. Carbonylated proteins accumulated as vitality decreases during long-term storage of beech (Fagus sylvatica L.) seeds. Trees 2014, 28, 503–515. [Google Scholar] [CrossRef]
- Senakoon, W.; Nuchadomrong, S.; Chiou, R.Y.Y.; Senawong, G.; Jogloy, S.; Songsri, P.; Patanothai, A. Identification of peanut seed prolamins with an antifungal role by 2D-GE and drought treatment. Biosci. Biotechnol. Biochem. 2015, 79, 1771–1778. [Google Scholar] [CrossRef] [PubMed]
- Hajheidari, M.; Eivazi, A.; Buchanan, B.B.; Wong, J.H.; Majidi, I.; Salekdeh, G.H. Proteomics uncovers a role for redox in drought tolerance in wheat. J. Proteome Res. 2007, 6, 1451–1460. [Google Scholar] [CrossRef] [PubMed]
- Yahata, E.; Maruyama-Funatsuki, W.; Nishio, Z.; Tabiki, T.; Takata, K.; Yamamoto, Y.; Tanida, M.; Saruyama, H. Wheat cultivar-specific proteins in grain revealed by 2-DE and their application to cultivar identification of flour. Proteomics 2005, 5, 3942–3953. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Lee, Y.H.; Kim, H.S.; Kim, M.S.; Hahn, K.W.; Ko, J.H.; Joung, H.; Jeon, J.H. Development of patatin knockdown potato tubers using RNA interference (RNAi) technology, for the production of human-therapeutic glycoproteins. BMC Biotechnol. 2008, 8, 36. [Google Scholar] [CrossRef] [PubMed]
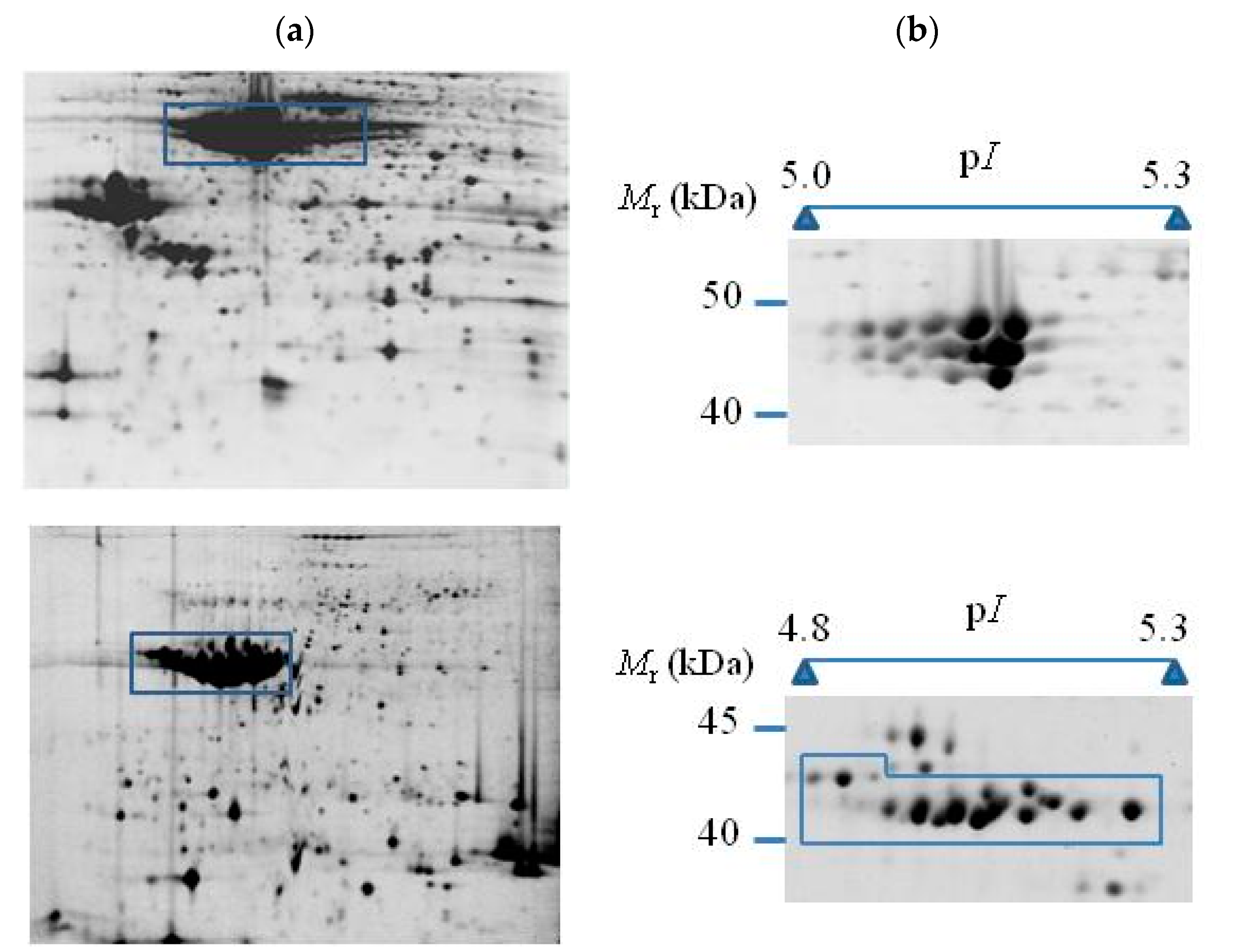
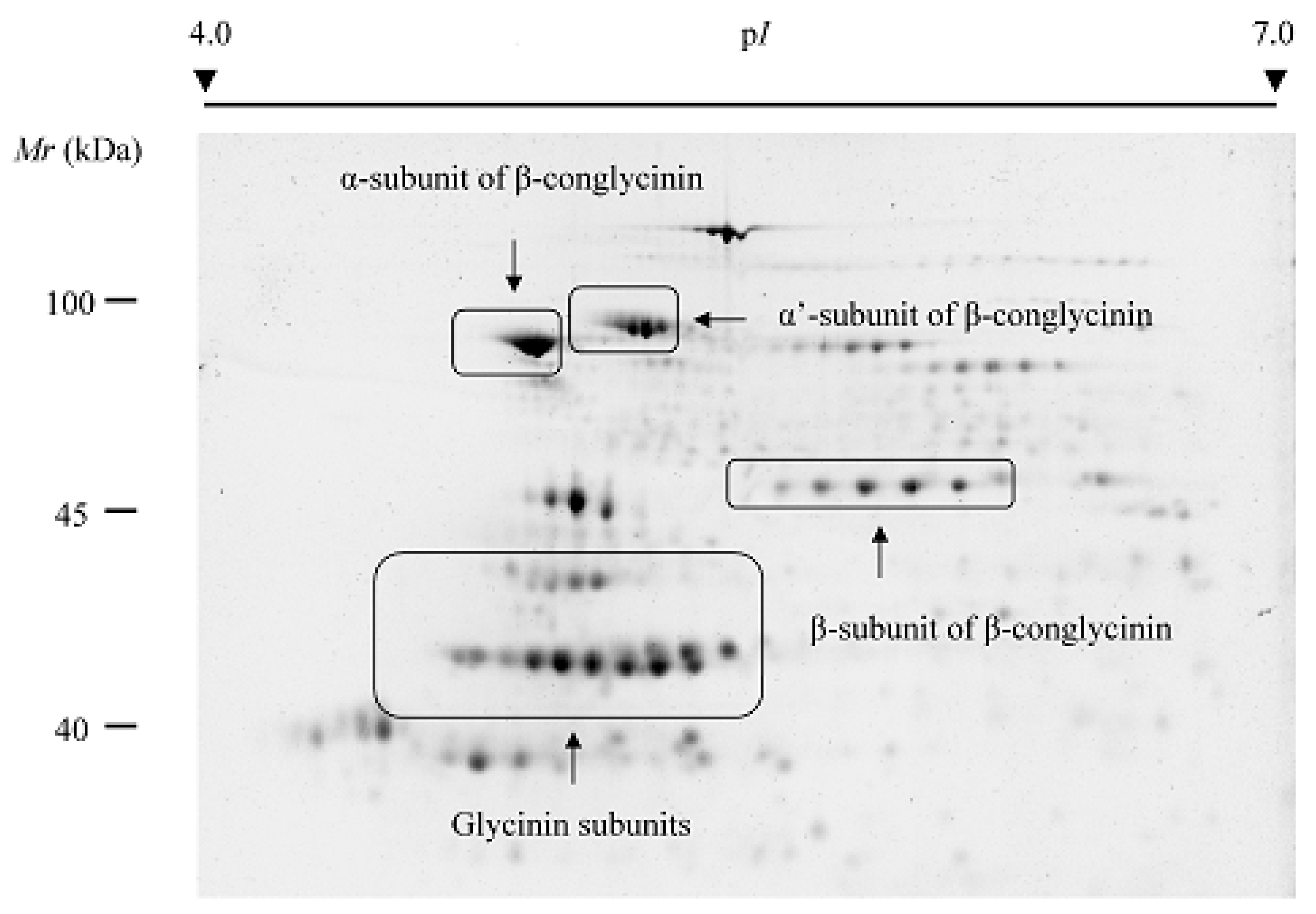
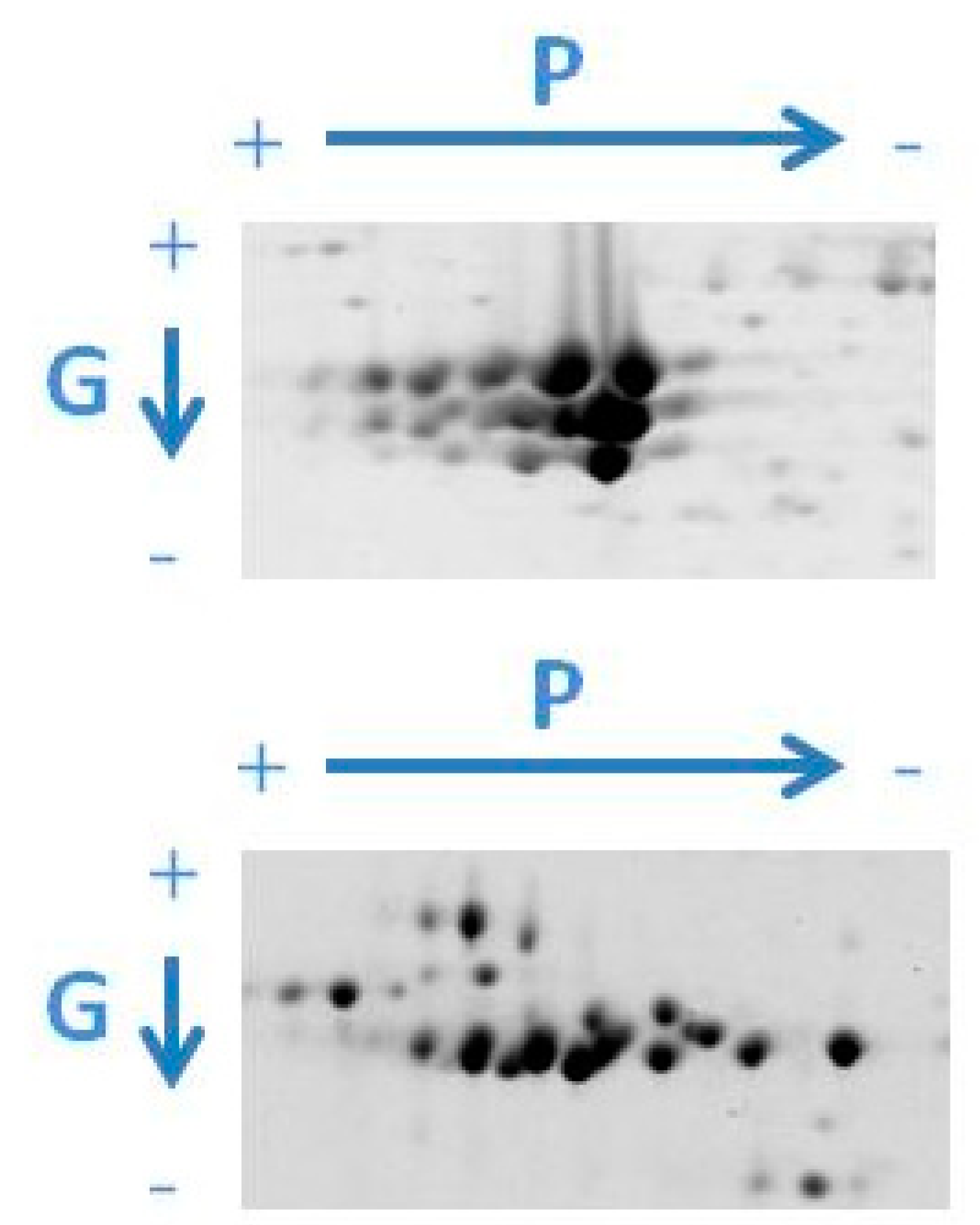
| Crop | Storage Proteins | Percentage of Total Protein | Molecular Weight Subunits (kDa) | References |
|---|---|---|---|---|
| Maize (Zea mays L.) | Globulins | 12–16 | [3,60,61,62,63,64] | |
| globulin-1 | 63, 45, 26, 23 | |||
| globulin-2 | ||||
| Prolamins | 50–70 | |||
| α-zeins | 25–49 | 22, 19 | ||
| β-zeins | 1–4 | 14–16 | ||
| γ-zeins | 6–13 | 27, 16, 50 | ||
| δ-zeins | 1–4 | 10 | ||
| Wheat (Triticum aestivum L.) | Prolamins | 80 | [65,66,67,68,69,70,71,72,73] | |
| gliadins | 30–50 | 30–80 | ||
| α-gliadins | 15–30 | |||
| β-gliadins | ||||
| γ-gliadins | ||||
| ω-gliadins | ||||
| glutenins | ||||
| LMW-GS | 12 | 42–51 (B), 30–40 (C), 58 (D) | ||
| HMW-GS | 80–130 (A) | |||
| Globulins | ||||
| 11-12S triticins | 5 | 58 (D), 22 (δ), 52 (A), 23 (α) | ||
| Rice (Oryza sativa L.) | Glutelins | 60–80 | 35–40, 20–22 | [74,75,76] |
| Prolamins | 20–30 | 10, 13, 16 | ||
| Globulins | ||||
| α-globulins | 2–8 | 26 | ||
| Potato (Solanum tuberosum L.) | Patatins | 45 | 39–45 | [30,77,78] |
| Kunitz protease inhibitors | 20 | |||
| Protease inhibitors 1 | 45 | |||
| Protease inhibitors 2 | ||||
| Carboxypeptidase inhibitors | 10 | |||
| Lipoxygenases | 97 | |||
| Soybean (Glycine max L.) | Globulins | [79,80,81] | ||
| α-conglycinins | ||||
| 7S vicilin/β-conglycinins | 40 | 76 (α), 72 (α’), 52 (β) | ||
| γ-conglycinins | ||||
| 11S legumin/glycinins | 25 | 56 (G1), 54 (G2), 54 (G3), 64 (G4), 58 (G5) | ||
| Barley (Hordeum vulgare L.) | Prolamins | [68,82] | ||
| hordeins | 35–55 | |||
| B-hordeins | 15–44 | 30–45 | ||
| C-hordeins | 4–11 | 45–75 | ||
| D-hordeins | 45 | |||
| γ-hordeins | ||||
| Sunflower (Helianthus annuus L.) | Globulins | [83,84,85] | ||
| 11S helianthinins | 38 | 37–43 (α), 31–35 (α’), 21–30 (β) | ||
| Albumins | ||||
| 2S | 62 | 12–20 | ||
| Common Bean (Phaseolus vulgaris L.) | Globulins | [14,44,86,87] | ||
| 7S phaseolins | 40–50 | |||
| 11S legumins | 3 | |||
| Lectins | ||||
| phytohemagglutinins | 5–10 | |||
| α-amylase inhibitors | ||||
| Oat (Avena sativa L.) | Globulins | 10–55 | [71,88,89] | |
| 3S | 48–52 | |||
| 7S | 50–70 | |||
| 11S | 60 | |||
| 12S avenalins | 32–43 (α), 19–25 (β) | |||
| Albumins | 10–20 | |||
| Prolamins | 12–14 | |||
| Glutelins | 23–54 | |||
| Pea (Pisum sativum L.) | Globulins | [90,91] | ||
| 7S vicilins | 47, 50, 34, 30 | |||
| 11S legumins | 41 (α), 22 (β), 23 (β’) | |||
| convincilins | 78, 72 | |||
| Chickpea (Cicer arietinum L.) | Albumins | [89,92] | ||
| 2S | 12 | |||
| Globulins | 50 | |||
| 7S vicilins | ||||
| 11S legumins | 40–47 (α), 24–25 (β) | |||
| Glutelins | 18.1 | |||
| Prolamins | 2.8 | |||
| Pomegranate (Punica granatum L.) | Globulins | 40.5 | 38–54, 13–18 | [93] |
| Albumins | 32.2 | 58–116, 33–46, 15–23 | ||
| Glutelins | 15.6 | 37, 21–23, 14 | ||
| Prolamins | 9.7 | 15, 20, 24 | ||
| Lentils (Lens culinaris Medik.) | Globulins | [94] | ||
| 11S legumins | 21 | 38–43 | ||
| 7S vicilin/ convicilins | 72 | 15–59 | ||
| Albumins | ||||
| 2S | ||||
| Rapeseed (Brassica napus L) | Globulins | [95] | ||
| 12S cruciferins | 20 | 29–33 (α), 21–23 (β) | ||
| Albumins | ||||
| 2S napins | 60 | 4–9 |
| Storage Protein Type | Storage Protein Subtype | Seed Stage | Additional Techniques | Species | References |
|---|---|---|---|---|---|
| Globulin | 12S cruciferin | Development | Pro-Q DPS | Rapeseed (Brassica napus L.) | [113] |
| LC-MS/MS | |||||
| 12S triticin Globulin 3 | Development | Pro-Q DPS | Wheat (Triticum aestivum L.) | [67] | |
| MALDI-TOF | |||||
| MALDI-TOF/TOF | |||||
| 12S cruciferin | Dormancy | 1-DE, Pro-Q DPS | Arabidopsis thaliana L. | [126,127] | |
| immunoblotting | |||||
| LC-MS/MS | |||||
| 7S phaseolin | Dormancy/ Germination | Pro-Q DPS, HF-P | Common bean (Phaseolus vulgaris L.) | [29] | |
| MALDI-TOF | |||||
| MALDI-TOF/TOF | |||||
| 12S cruciferin | Germination | Western blotting | Arabidopsis thaliana L. | [128] | |
| MALDI-TOF | |||||
| MALDI-TOF/TOF | |||||
| Cupin | Germination | Pro-Q DPS | Rice (Oryza sativa L.) | [131] | |
| MALDI-TOF/TOF | |||||
| Globulin 3 | Germination | Pro-Q DPS | Wheat (Triticum aestivum L.) | [132] | |
| LC-MS/MS | |||||
| Albumin | 2S napin | Dormancy | 1-DE, Pro-Q DPS | Arabidopsis thaliana L. | [126,127] |
| immunoblotting | |||||
| LC-MS/MS | |||||
| Glutelin | N/A | Development | Pro-Q DPS | Rice (Oryza sativa L.) | [135] |
| LC-MS/MS | |||||
| Vegetative | Patatin | Dormancy | Pro-Q DPS, HF-P | Potato (Solanum tuberosum L.) | [30] |
| MALDI-TOF | |||||
| MALDI-TOF/TOF |
| Storage Protein Type | Storage Protein Subtype | Additional Techniques | Species | References |
|---|---|---|---|---|
| Globulin | 7S vicilin | 1-DE, Glycoprotein staining | Cocoa bean (Theobroma cacao L.) | [179] |
| 7S phaseolin | 1-DE, Fluorography | Common bean (Phaseolus vulgaris L.) | [42,161,162] | |
| Radioactive labelling of sugars, | ||||
| Concanavalin A binding | ||||
| Immunoaffinity chromatography | ||||
| N-deglycosylation | ||||
| 7S convicilin | N-deglycosylation | Lotus (Lotus japonicus L.) | [121] | |
| Glutelin | N/A | Glycoprotein staining, | Rice (Oryza sativa L.) | [135] |
| LC-MS/MS | ||||
| Lectin | N/A | N-deglycosylation | Lotus (Lotus japonicus L.) | [121] |
| Vegetative | Patatin | N-deglycosylation | Potato (Solanum tuberosum L.) | [30,43,184] |
| MALDI-TOF, MALDI-TOF/TOF |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mouzo, D.; Bernal, J.; López-Pedrouso, M.; Franco, D.; Zapata, C. Advances in the Biology of Seed and Vegetative Storage Proteins Based on Two-Dimensional Electrophoresis Coupled to Mass Spectrometry. Molecules 2018, 23, 2462. https://doi.org/10.3390/molecules23102462
Mouzo D, Bernal J, López-Pedrouso M, Franco D, Zapata C. Advances in the Biology of Seed and Vegetative Storage Proteins Based on Two-Dimensional Electrophoresis Coupled to Mass Spectrometry. Molecules. 2018; 23(10):2462. https://doi.org/10.3390/molecules23102462
Chicago/Turabian StyleMouzo, Daniel, Javier Bernal, María López-Pedrouso, Daniel Franco, and Carlos Zapata. 2018. "Advances in the Biology of Seed and Vegetative Storage Proteins Based on Two-Dimensional Electrophoresis Coupled to Mass Spectrometry" Molecules 23, no. 10: 2462. https://doi.org/10.3390/molecules23102462
APA StyleMouzo, D., Bernal, J., López-Pedrouso, M., Franco, D., & Zapata, C. (2018). Advances in the Biology of Seed and Vegetative Storage Proteins Based on Two-Dimensional Electrophoresis Coupled to Mass Spectrometry. Molecules, 23(10), 2462. https://doi.org/10.3390/molecules23102462








