Disubstituted 4-Chloro-3-nitrophenylthiourea Derivatives: Antimicrobial and Cytotoxic Studies
Abstract
1. Introduction
2. Results and Discussion
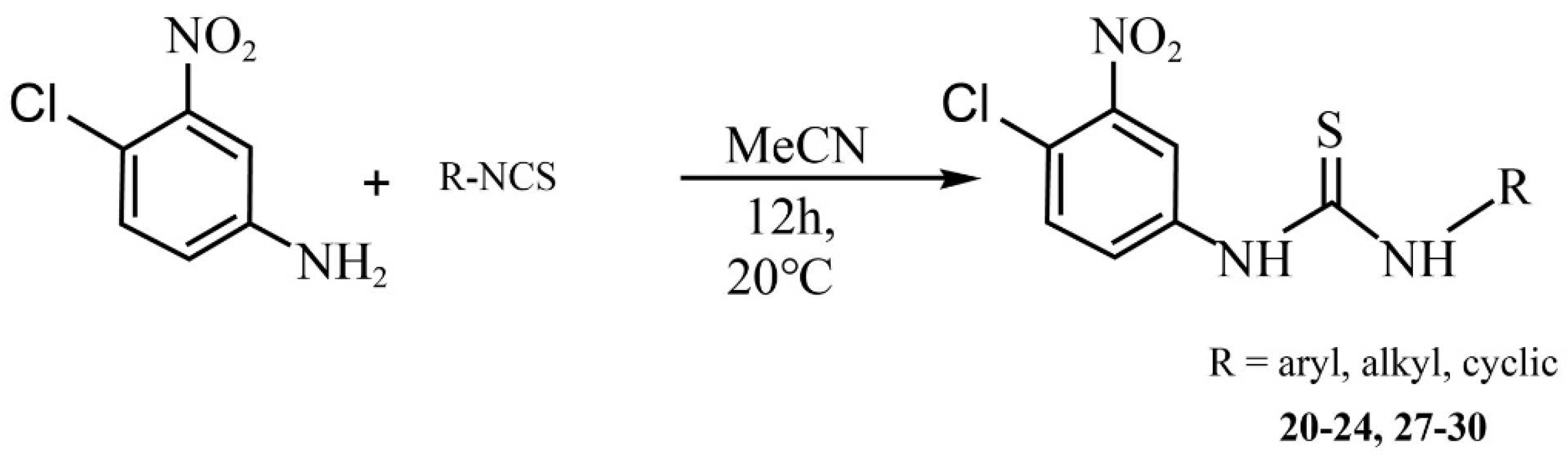
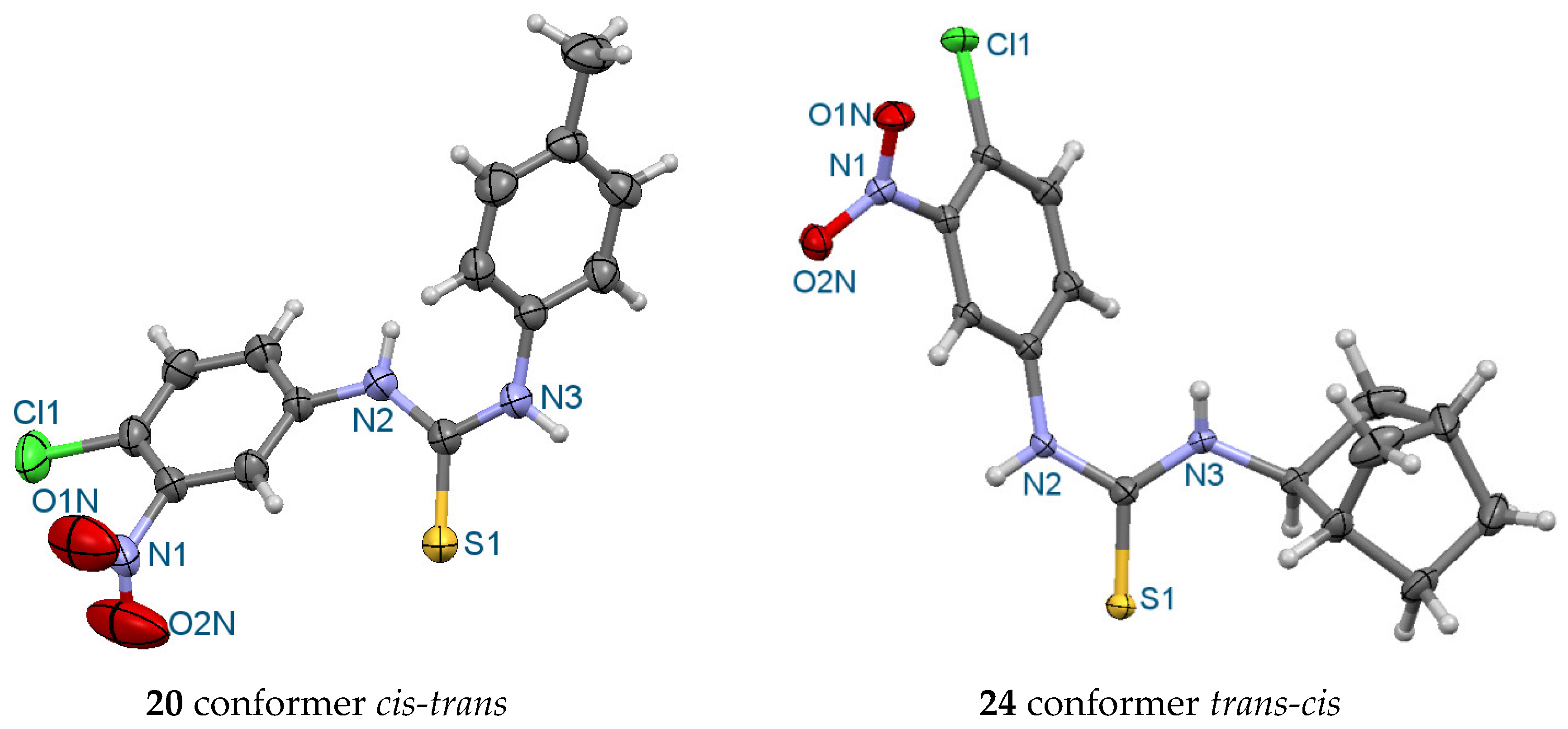
2.1. Chemistry
2.2. Antimicrobial Studies
2.3. Cytotoxicity and Anti-HIV Activity
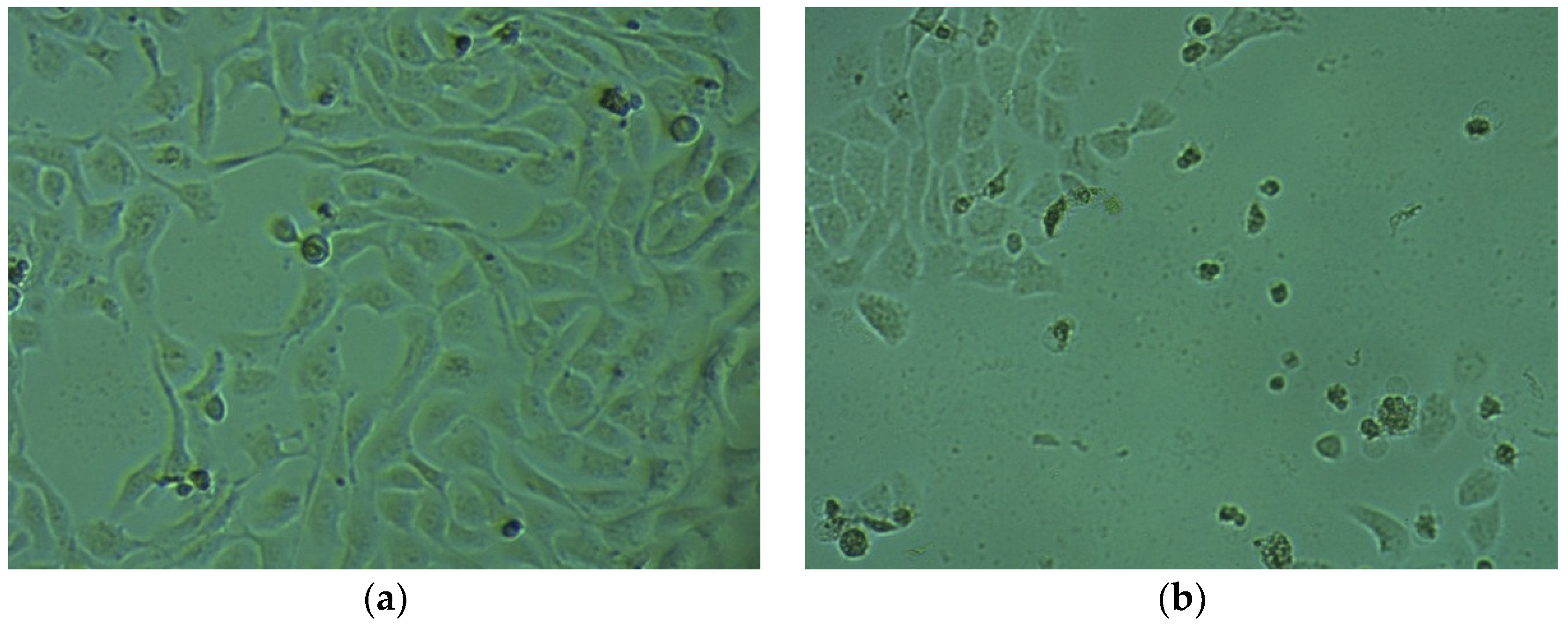
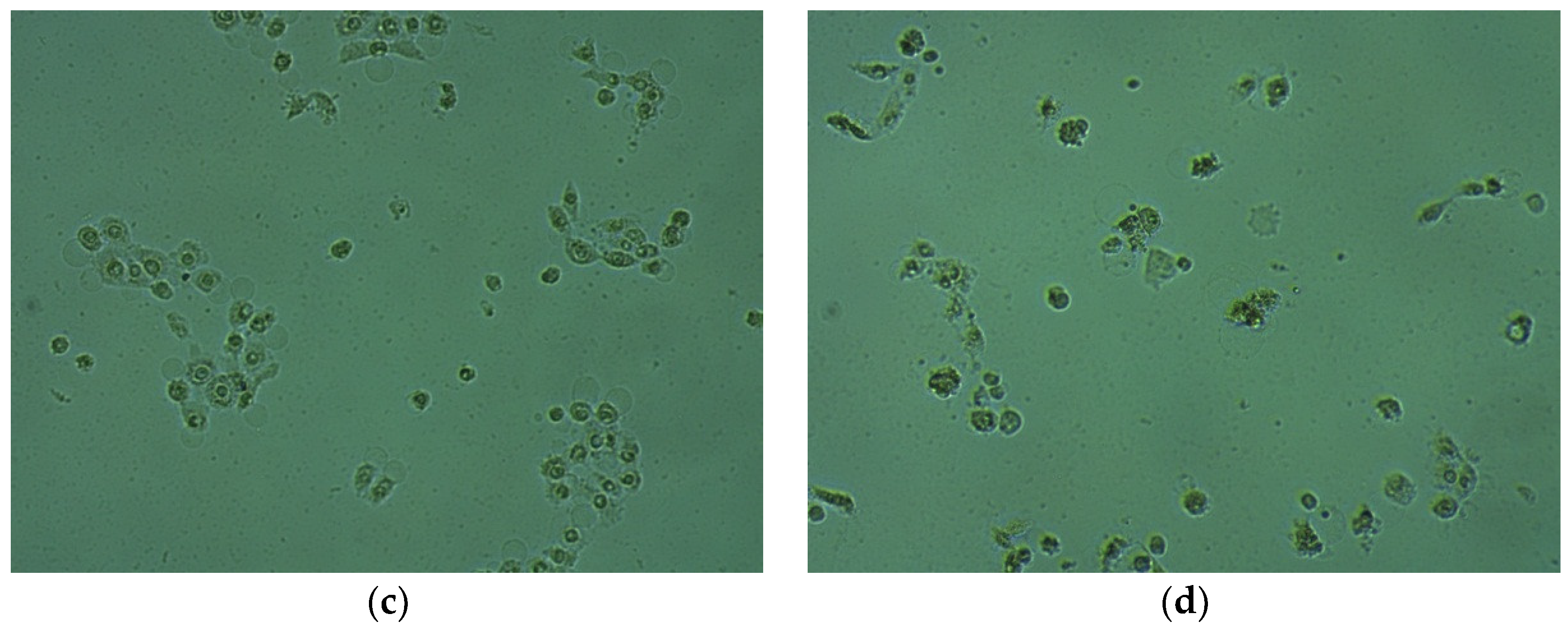
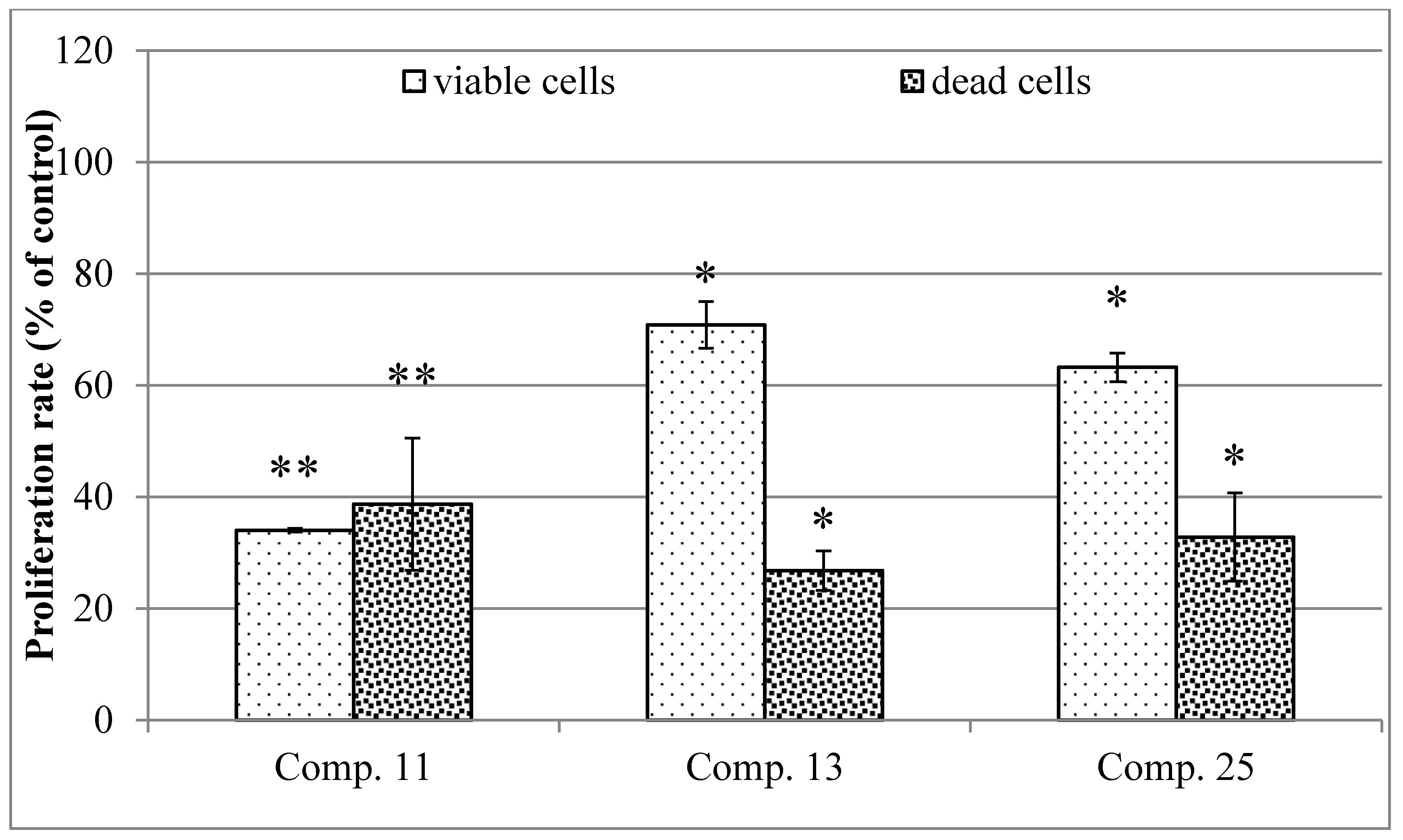
2.4. Cytotoxic Activity in HaCaT Cells
3. Materials and Methods
3.1. Chemistry
3.2. X-ray Crystalography
3.3. In Vitro Evaluation of Antimicrobial Activity
3.4. Cytotoxicity and Anti-HIV Assays
3.5. Antiproliferative Assays
3.6. Cytotoxic Activity in HaCaT Cells
3.6.1. Cell Culture: Conditions and Treatments
3.6.2. Cell Viability Assessment (Mitochondrial Function Assessment)
3.6.3. Lactate Dehydrogenase Release Assay (Cellular Membrane Integrity Assessment)
3.6.4. Proliferation Rate in Cultures of HaCaT Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bielenica, A.; Stefańska, J.; Stępień, K.; Napiórkowska, A.; Augustynowicz-Kopeć, E.; Sanna, G.; Madeddu, S.; Boi, S.; Giliberti, G.; Wrzosek, M.; et al. Synthesis, cytotoxicity and antimicrobial activity of thiourea derivatives incorporating 3-(trifluoromethyl)phenyl moiety. Eur. J. Med. Chem. 2015, 101, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Bielenica, A.; Stępień, K.; Napiórkowska, A.; Augustynowicz-Kopeć, E.; Krukowski, S.; Włodarczyk, M.; Struga, M. Synthesis and antimicrobial activity of 4-chloro-3-nitrophenylthiourea derivatives targeting bacterial type II topoisomerases. Chem. Biol. Drug Des. 2016, 87, 905–917. [Google Scholar] [CrossRef] [PubMed]
- Foley, T.L.; Rai, G.; Yasgar, A.; Daniel, T.; Baker, H.L.; Attene-Ramos, M.; Kosa, N.M.; Leister, W.; Burkart, M.D.; Jadhav, A.; et al. 4-(3-Chloro-5-(trifluoromethyl)pyridin-2-yl)-N-(4-methoxypyridin-2-yl)piperazine-1-carbothioamide (ML267), a potent inhibitor of bacterial phosphopantetheinyl transferase that attenuates secondary metabolism and thwarts bacterial growth. J. Med. Chem. 2014, 57, 1063–1078. [Google Scholar] [CrossRef] [PubMed]
- Madabhushi, S.; Mallu, K.K.; Vangipuram, V.S.; Kurva, S.; Poornachandra, Y.; Ganesh Kumar, C. Synthesis of novel benzimidazole functionalized chiral thioureas and evaluation of their antibacterial and anticancer activities. Bioorg. Med. Chem. Lett. 2014, 24, 4822–4825. [Google Scholar] [CrossRef] [PubMed]
- Ghorab, M.M.; Alsaid, M.S.; El-Gaby, M.S.A.; Elaasser, M.M.; Nissan, Y.M. Antimicrobial and anticancer activity of some novel fluorinated thiourea derivatives carrying sulfonamide moieties: Synthesis, biological evaluation and molecular docking. Chem. Cent. J. 2017, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ghorab, M.M.; Alsaid, M.S.; El-Gaby, M.S.A.; Safwat, N.A.; Elaasser, M.M.; Soliman, A.M. Biological evaluation of some new N-(2,6-dimethoxypyrimidinyl) thioureido benzenesulfonamide derivatives as potential antimicrobial and anticancer agents. Eur. J. Med. Chem. 2016, 124, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Medapi, B.; Renuka, J.; Saxena, S.; Sridevi, J.P.; Medishetti, R.; Kulkarni, P.; Yogeeswari, P.; Sriram, D. Design and synthesis of novel quinoline-aminopiperidine hybrid analogues as Mycobacterium tuberculosis DNA gyraseB inhibitors. Bioorg. Med. Chem. 2015, 23, 2062–2078. [Google Scholar] [CrossRef] [PubMed]
- Sriram, D.; Yogeeswari, P.; Dinakaran, M.; Thirumurugan, R. Antimycobacterial activity of novel 1-(5-cyclobutyl-1,3-oxazol-2-yl)-3-(sub)phenyl/pyridylthiourea compounds endowed with high activity toward multidrug-resistant Mycobacterium tuberculosis. J. Antimicrob. Chemother. 2007, 59, 1194–1196. [Google Scholar] [CrossRef] [PubMed]
- Bielenica, A.; Sanna, G.; Madeddu, S.; Struga, M.; Jóźwiak, M.; Kozioł, A.E.; Sawczenko, A.; Materek, I.B.; Serra, A.; Giliberti, G. New thiourea and 1,3-thiazolidin-4-one derivatives effective on the HIV-1 virus. Chem. Biol. Drug Des. 2017, 90, 883–891. [Google Scholar] [CrossRef] [PubMed]
- D’Cruz, O.J.; Qazi, S.; Yiv, S.; Uckun, F.M. A novel vaginal microbicide containing the rationally designed anti-HIV compound HI-443 (N′-[2-(2-thiophene)ethyl]-N′-[2-(5-bromopyridyl)] thiourea]). Expert Opin. Investig. Drugs 2012, 21, 265–279. [Google Scholar] [CrossRef] [PubMed]
- Karakuş, S.; Güniz Küçükgüzel, S.; Küçükgüzel, I.; De Clercq, E.; Pannecouque, C.; Andrei, G.; Snoeck, R.; Sahin, F.; Bayrak, O.F. Synthesis, antiviral and anticancer activity of some novel thioureas derived from N-(4-nitro-2-phenoxyphenyl)-methanesulfonamide. Eur. J. Med. Chem. 2009, 44, 3591–3595. [Google Scholar] [CrossRef] [PubMed]
- Bloom, J.D.; Dushin, R.G.; Curran, K.J.; Donahue, F.; Norton, E.B.; Terefenko, E.; Jones, T.R.; Ross, A.A.; Feld, B.; Lang, S.A.; et al. Thiourea inhibitors of herpes viruses. Part 2: N-Benzyl-N′-arylthiourea inhibitors of CMV. Bioorg. Med. Chem. Lett. 2004, 14, 3401–3406. [Google Scholar] [CrossRef] [PubMed]
- Bielenica, A.; Kędzierska, E.; Koliński, M.; Kmiecik, S.; Koliński, A.; Fiorino, F.; Severino, B.; Magli, E.; Corvino, A.; Rossi, I.; et al. 5-HT2 receptor affinity, docking studies and pharmacological evaluation of a series of 1,3-disubstituted thiourea derivatives. Eur. J. Med. Chem. 2016, 116, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Stefanska, J.; Szulczyk, D.; Koziol, A.E.; Miroslaw, B.; Kedzierska, E.; Fidecka, S.; Busonera, B.; Sanna, G.; Giliberti, G.; La Colla, P.; et al. Disubstituted thiourea derivatives and their activity on CNS: Synthesis and biological evaluation. Eur. J. Med. Chem. 2012, 55, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Bielenica, A.; Kedzierska, E.; Fidecka, S.; Maluszynska, H.; Miroslaw, B.; Koziol, A.E.; Stefanska, J.; Madeddu, S.; Giliberti, G.; Sanna, G.; et al. Synthesis, Antimicrobial and Pharmacological Evaluation of Thiourea derivatives of 4H-1,2,4-triazole. Lett. Drug Discov. Dev. 2015, 12, 263–276. [Google Scholar] [CrossRef]
- Karakus, S.; Koçyigit-Kaymakcioglu, B.; Toklu, H.Z.; Aricioglu, F.; Rollas, S. Synthesis and anticonvulsant activity of new N-(alkyl/sub-stituted aryl)-N′-[4-(5-cyclohexylamino)-1,3,4-thiadiazole-2-yl)phenyl]thioureas. Arch. Pharm. Weinheim 2009, 342, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Tokala, R.; Bale, S.; Janrao, I.P.; Vennela, A.; Kumar, N.P.; Senwar, K.R.; Godugu, C.; Shankaraiah, N. Synthesis of 1,2,4-triazole-linked urea/thiourea conjugates as cytotoxic and apoptosis inducing agents. Bioorg. Med. Chem. Lett. 2018, 28, 1919–1924. [Google Scholar] [CrossRef] [PubMed]
- Shankaraiah, N.; Kumar, N.P.; Amula, S.B.; Nekkanti, S.; Jeengar, M.K.; Naidu, V.G.; Reddy, T.S.; Kamal, A. One-pot synthesis of podophyllotoxin-thiourea congeners by employing NH2SO3H/NaI: Anticancer activity, DNA topoisomerase-II inhibition, and apoptosis inducing agents. Bioorg. Med. Chem. Lett. 2015, 25, 4239–4244. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Shan, Y.; Li, C.; Si, R.; Pan, X.; Wang, B.; Zhang, J. Discovery of novel anti-angiogenesis agents. Part 8: Diaryl thiourea bearing 1H-indazole-3-amine as multi-target RTKs inhibitors. Eur. J. Med. Chem. 2017, 141, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Turan-Zitouni, G.; Altıntop, M.D.; Özdemir, A.; Kaplancıklı, Z.A.; Çiftçi, G.A.; Temel, H.E. Synthesis and evaluation of bis-thiazole derivatives as new anticancer agents. Eur. J. Med. Chem. 2016, 107, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Koca, İ.; Özgür, A.; Er, M.; Gümüş, M.; Açikalin Coşkun, K.; Tutar, Y. Design and synthesis of pyrimidinyl acyl thioureas as novel Hsp90 inhibitors in invasive ductal breast cancer and its bone metastasis. Eur. J. Med. Chem. 2016, 122, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Mowafy, S.; Galanis, A.; Doctor, Z.M.; Paranal, R.M.; Lasheen, D.S.; Farag, N.A.; Jänne, P.A.; Abouzid, K.A. Toward discovery of mutant EGFR inhibitors; Design, synthesis and in vitro biological evaluation of potent 4-arylamino-6-ureido and thioureido-quinazoline derivatives. Bioorg. Med. Chem. 2016, 24, 3501–3512. [Google Scholar] [CrossRef] [PubMed]
- Hamed, M.M.; Darwish, S.S.; Herrmann, J.; Abadi, A.H.; Engel, M. First Bispecific Inhibitors of the Epidermal Growth Factor Receptor Kinase and the NF-κB Activity as Novel Anticancer Agents. J. Med. Chem. 2017, 60, 2853–2868. [Google Scholar] [CrossRef] [PubMed]
- Shing, J.C.; Choi, J.W.; Chapman, R.; Schroeder, M.A.; Sarkaria, J.N.; Fauq, A.; Bram, R.J. A novel synthetic 1,3-phenyl bis-thiourea compound targets microtubule polymerization to cause cancer cell death. Cancer Biol. Ther. 2014, 15, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.Y.; Zheng, Y.C.; Wang, S.Q.; Wang, B.; Wang, Z.R.; Pang, L.P.; Zhang, M.; Wang, J.W.; Ding, L.; Li, J.; et al. Design, synthesis, and structure-activity relationship of novel LSD1 inhibitors based on pyrimidine-thiourea hybrids as potent, orally active antitumor agents. J. Med. Chem. 2015, 58, 1705–1716. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Miyata, N. Lysine demethylases inhibitors. J. Med. Chem. 2011, 54, 8236–8250. [Google Scholar] [CrossRef] [PubMed]
- Ghorab, M.M.; Alsaid, M.S.; Al-Dosary, M.S.; Nissan, Y.M.; Attia, S.M. Design, synthesis and anticancer activity of some novel thioureido-benzenesulfonamides incorporated biologically active moieties. Chem. Cent. J. 2016, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Lombardo, M.; Malkani, S.; Hale, J.J.; Mills, S.G.; Chapman, K.; Thompson, J.E.; Zhang, W.X.; Wang, R.; Cubbon, R.M.; et al. Novel 1-(2-aminopyrazin-3-yl)methyl-2-thioureas as potent inhibitors of mitogen-activated protein kinase-activated protein kinase 2 (MK-2). Bioorg. Med. Chem. Lett. 2009, 19, 3238–3242. [Google Scholar] [CrossRef] [PubMed]
- Kožurková, M.; Sabolová, D.; Kristian, P. A review on acridinylthioureas and its derivatives: Biological and cytotoxic activity. J. Appl. Toxicol. 2017, 37, 1132–1139. [Google Scholar] [CrossRef] [PubMed]
- Repich, H.H.; Orysyk, V.V.; Palchykovska, L.G.; Orysyk, S.I.; Zborovskii, Y.L.; Vasylchenko, O.V.; Storozhuk, O.V.; Biluk, A.A.; Nikulina, V.V.; Garmanchuk, L.V.; et al. Synthesis, spectral characterization of novel Pd(II), Pt(II) π-coordination compounds based on N-allylthioureas. Cytotoxic properties and DNA binding ability. J. Inorg. Biochem. 2017, 168, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Correa, R.S.; de Oliveira, K.M.; Delolo, F.G.; Alvarez, A.; Mocelo, R.; Plutin, A.M.; Cominetti, M.R.; Castellano, E.E.; Batista, A.A. Ru(II)-based complexes with N-(acyl)-N′,N′-(disubstituted)thiourea ligands: Synthesis, characterization, BSA- and DNA-binding studies of new cytotoxic agents against lung and prostate tumour cells. J. Inorg. Biochem. 2015, 150, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Plutín, A.M.; Mocelo, R.; Alvarez, A.; Ramos, R.; Castellano, E.E.; Cominetti, M.R.; Graminha, A.E.; Ferreira, A.G.; Batista, A.A. On the cytotoxic activity of Pd(II) complexes of N,N-disubstituted-N′-acyl thioureas. J. Inorg. Biochem. 2014, 134, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Louie, M.C.; Rajagopalan, V.; Zhou, G.; Ponce, E.; Nguyen, T.; Green, L. Synthesis and evaluation of the diarylthiourea analogs as novel anti-cancer agents. Bioorg. Med. Chem. Lett. 2015, 25, 1301–1305. [Google Scholar] [CrossRef] [PubMed]
- Rauf, M.K.; Zaib, S.; Talib, A.; Ebihara, M.; Badshah, A.; Bolte, M.; Iqbal, J. Solution-phase microwave assisted parallel synthesis, biological evaluation and in silico docking studies of N,N′-disubstituted thioureas derived from 3-chlorobenzoic acid. Bioorg. Med. Chem. 2016, 24, 4452–4463. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.J.; Yu, S.L.; Liu, Y.P.; Dai, X.J.; Wu, Y.; Li, R.J.; Tao, J.C. Synthesis, cytotoxic activity evaluation and HQSAR study of novel isosteviol derivatives as potential anticancer agents. Eur. J. Med. Chem. 2016, 115, 26–40. [Google Scholar] [CrossRef] [PubMed]
- El-Hossary, E.M.; Förstner, K.U.; François, P.; Baud, D.; Streker, K.; Schrenzel, J.; Ohlsen, K.; Holzgrabe, U. A Novel Mechanism of Inactivating Antibacterial Nitro Compounds in the Human Pathogen Staphylococcus aureus by Overexpression of a NADH-Dependent Flavin Nitroreductase. Antimicrob. Agents Chemother. 2018, 62, e01510–e01517. [Google Scholar] [CrossRef] [PubMed]
- Chu, P.S.; Lopez, M.I.; Abraham, A.; El Said, K.R.; Plakas, S.M. Residue depletion of nitrofuran drugs and their tissue-bound metabolites in channel catfish (Ictalurus punctatus) after oral dosing. J. Agric. Food Chem. 2008, 56, 8030–8034. [Google Scholar] [CrossRef] [PubMed]
- Bielenica, A.; Drzewiecka-Antonik, A.; Rejmak, P.; Stefańska, J.; Koliński, M.; Kmiecik, S.; Lesyng, B.; Włodarczyk, M.; Pietrzyk, P.; Struga, M. Synthesis, structural and antimicrobial studies of type II topoisomerase-targeted copper(II) complexes of 1,3-disubstituted thiourea ligands. J. Inorg. Biochem. 2018, 182, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard M7-A9; CLSI: Wayne, PA, USA, 2012. [Google Scholar]
- Clinical and Laboratory Standards Institute. Methods for Determining Bactericidal Activity of Antimicrobial Agents; Approved Guideline M26-A; CLSI: Wayne, PA, USA, 1999. [Google Scholar]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard M7-A7; CLSI: Wayne, PA, USA, 2006. [Google Scholar]
- Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standard. CLSI document M27-A3; CLSI: Wayne, PA, USA, 2008. [Google Scholar]
- Küçükgüzel, I.; Tatar, E.; Küçükgüzel, S.G.; Rollas, S.; De Clercq, E. Synthesis of some novel thiourea derivatives obtained from 5-[(4-aminophenoxy)methyl]-4-alkyl/aryl-2,4-dihydro-3H-1,2,4-triazole-3-thiones and evaluation as antiviral/anti-HIV and anti-tuberculosis agents. Eur. J. Med. Chem. 2008, 43, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hwang, H.-M.; Ekunwe, S. Comparing cytotoxicity and genotoxicity in HaCaT cells caused by 6-aminochrysene and 5,6-chrysenequinone under ultraviolet A irradiation. Environ. Toxicol. Chem. 2006, 25, 1920–1925. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-5. Part 5: Tests for In Vitro Cytotoxicity. In 2009 Biological Evaluation of Medical Devices; International Organization for Standardization: Geneva, Switzerland, 2009. [Google Scholar]
- Agilent Technologies, version 1.171.37.34; CrysAlisPro.: Oxfordshire, UK, 2014.
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of compound 1–30 are available from the authors. |

| Comp. | R | MT-4 * 1 CC50 [µM] | HIV-1IIIB * 2 EC50 [µM] |
|---|---|---|---|
| 1 | 2-chlorophenyl | 8.8 | >8.8 |
| 2 | 3-chlorophenyl | 7.0 | >7.0 |
| 3 | 4-chlorophenyl | 7.0 | >7.0 |
| 4 | 2-bromophenyl | 9.6 | >9.7 |
| 5 | 3-bromophenyl | 8.0 | >8.0 |
| 6 | 4-bromophenyl | 6.6 | >6.6 |
| 7 | 2-fluorophenyl | 13.0 | >13.0 |
| 8 | 3-fluorophenyl | 9.0 | >9.0 |
| 9 | 4-fluorophenyl | 7.0 | >7.0 |
| 10 | 3-chloro-4-fluorophenyl | 7.3 | >7.3 |
| 11 | 3,4-dichlorophenyl | 7.9 | >7.9 |
| 12 | 2,4-dichlorophenyl | 8.0 | >8.0 |
| 13 | 3-chloro-4-methylphenyl | 8.0 | >8.0 |
| 14 | 5-chloro-2-methylphenyl | 8.2 | >8.2 |
| 15 | 3-(trifluoromethyl)phenyl | 8.0 | >8.0 |
| 16 | 4-(trifluoromethyl)phenyl | 7.0 | >7.0 |
| 17 | 4-iodophenyl | 7.8 | >7.8 |
| 18 | 4-nitrophenyl | 7.0 | >7.0 |
| 19 | 4-cyanophenyl | 8.2 | >8.2 |
| 20 | 4-methylphenyl | 14.6 | >14.6 |
| 21 | 4-methoxyphenyl | 35.0 | >35.0 |
| 22 | 4-butyl-2-methylphenyl | 18.7 | >18.7 |
| 23 | phenyl | 23.0 | >23.0 |
| 24 | bicyclo[2.2.1]hept-2-yl | 16.0 | >16.0 |
| 25 | benzyl | 36.7 | >36.7 |
| 26 | 1-phenylethyl | 16.7 | >16.7 |
| 27 | (2-methyl)prop-2-en-1-yl | 15.5 | >15.5 |
| 28 | prop-2-en-1-yl | 43.0 | 43.0 |
| 29 | 3-(methylsulfanyl)propyl | 33.7 | >33.7 |
| 30 | furan-2-ylmethyl | 25.6 | >25.6 |
| Comp. | S. aureus NCTC 4163 | S. aureus ATCC 25923 | S. aureus ATCC 6538 | S. aureus ATCC 29213 | S. epidermidis ATCC 35984 | S. epidermidis ATCC 12228 | B. subtilis ATCC 6633 | B. cereus ATCC 11778 | E. faecalis ATCC 29212 | M. luteus ATCC 9341 | M. luteus ATCC 10240 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 20 | 2 | 2 | 4 | 2 | 4 | 4 | 4 | 4 | ‒ | 4 | 4 |
| 22 | 4 | 2 | 2 | 2 | 4 | 4 | 4 | 4 | ‒ | 4 | 2 |
| 23 | 16 | 16 | 16 | 16 | 16 | 16 | 8 | 8 | 16 | 4 | 8 |
| 24 | 8 | 8 | 8 | 8 | 16 | 16 | 8 | 8 | ‒ | 32 | 16 |
| 27 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 8 | 64 | 16 | 32 |
| 28 | 64 | 32 | 32 | 64 | 32 | 32 | 32 | 32 | 64 | 32 | 32 |
| 29 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 8 | 64 | 16 | 32 |
| 30 | 16 | 8 | 16 | 16 | 32 | 32 | 16 | 16 | 32 | 16 | 16 |
| * Ref. | 0.25 | 0.5 | 0.25 | 0.5 | 0.25 | 0.25 | <0.12 | 0.25 | 1 | 1 | 2 |
| Comp. | S. epidermidis MRSE 403/11 | S. epidermidis MRSE 404/11 | S. epidermidis MRSE 405/11 | S. epidermidis MRSE 406/11 | S. epidermidis MRSE 407/11 | S. epidermidis MRSE 409/11 | S. epidermidis MRSE 411/11 | S. epidermidis MRSE 412/11 | S. epidermidis MRSE 413/11 | S. epidermidis MRSE 422/11 |
|---|---|---|---|---|---|---|---|---|---|---|
| 20 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 32 | 16 | 32 |
| 22 | 4 | 8 | 4 | 8 | 4 | 4 | 4 | 8 | 8 | 8 |
| 23 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 32 | 16 | 16 |
| 24 | 4 | 4 | 4 | 8 | 4 | 4 | 4 | 4 | 4 | 4 |
| 27 | 32 | 32 | 32 | 32 | 32 | 32 | 32 | 64 | 32 | 64 |
| 28 | 64 | 64 | 64 | 64 | 64 | 64 | 64 | 128 | 64 | 128 |
| 29 | 32 | 32 | 32 | 32 | 32 | 32 | 32 | 32 | 32 | 32 |
| 30 | 32 | 32 | 32 | 64 | 32 | 32 | 32 | 32 | 32 | 32 |
| * Ref. | 16 | 64 | 4 | 0.5 | 64 | 2 | 4 | 64 | 32 | 4 |
| Comp. | MT-4 | CCRF-CEM | WIL-2NS | CCRF-SB | SK-MEL-28 | DU145 | CRL7065 |
|---|---|---|---|---|---|---|---|
| 1 CC50 | |||||||
| 1 | 8.8 | 13 | 14 | 14 | 8.7 | 7.6 | 18 |
| 4 | 9.6 | 22 | 30 | 22 | 15 | 8.0 | 20 |
| 6 | 6.6 | 6.0 | 7.0 | 8.0 | 6.0 | 6.0 | 30 |
| 9 | 7.0 | 9.1 | 11 | 9.4 | 20 | 8.4 | 22 |
| 10 | 7.3 | 5.0 | 7.0 | 8.8 | 6.6 | 6.5 | 16 |
| 11 | 7.9 | 8.0 | 9.9 | 8.6 | 8.5 | 6.0 | 16 |
| 13 | 8.0 | 4.0 | 7.6 | 6.7 | 6.0 | 7.0 | 16 |
| 14 | 8.2 | 9.4 | 10 | 9.8 | 9.0 | 7.8 | 18 |
| 17 | 7.8 | 6.0 | 7.0 | 8.3 | 6.0 | 11 | 30 |
| 19 | 8.2 | 9.1 | 10 | 9.2 | 9.0 | 8.0 | 20 |
| * Ref | 0.004 | 0.003 | 0.005 | 0.004 | 0.07 | 0.08 | 0.3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bielenica, A.; Sanna, G.; Madeddu, S.; Giliberti, G.; Stefańska, J.; Kozioł, A.E.; Savchenko, O.; Strzyga-Łach, P.; Chrzanowska, A.; Kubiak-Tomaszewska, G.; et al. Disubstituted 4-Chloro-3-nitrophenylthiourea Derivatives: Antimicrobial and Cytotoxic Studies. Molecules 2018, 23, 2428. https://doi.org/10.3390/molecules23102428
Bielenica A, Sanna G, Madeddu S, Giliberti G, Stefańska J, Kozioł AE, Savchenko O, Strzyga-Łach P, Chrzanowska A, Kubiak-Tomaszewska G, et al. Disubstituted 4-Chloro-3-nitrophenylthiourea Derivatives: Antimicrobial and Cytotoxic Studies. Molecules. 2018; 23(10):2428. https://doi.org/10.3390/molecules23102428
Chicago/Turabian StyleBielenica, Anna, Giuseppina Sanna, Silvia Madeddu, Gabriele Giliberti, Joanna Stefańska, Anna E. Kozioł, Oleksandra Savchenko, Paulina Strzyga-Łach, Alicja Chrzanowska, Grażyna Kubiak-Tomaszewska, and et al. 2018. "Disubstituted 4-Chloro-3-nitrophenylthiourea Derivatives: Antimicrobial and Cytotoxic Studies" Molecules 23, no. 10: 2428. https://doi.org/10.3390/molecules23102428
APA StyleBielenica, A., Sanna, G., Madeddu, S., Giliberti, G., Stefańska, J., Kozioł, A. E., Savchenko, O., Strzyga-Łach, P., Chrzanowska, A., Kubiak-Tomaszewska, G., & Struga, M. (2018). Disubstituted 4-Chloro-3-nitrophenylthiourea Derivatives: Antimicrobial and Cytotoxic Studies. Molecules, 23(10), 2428. https://doi.org/10.3390/molecules23102428





