A Comparative Pharmacokinetic Study by UHPLC-MS/MS of Main Active Compounds after Oral Administration of Zushima-Gancao Extract in Normal and Adjuvant-Induced Arthritis Rats
Abstract
:1. Introduction
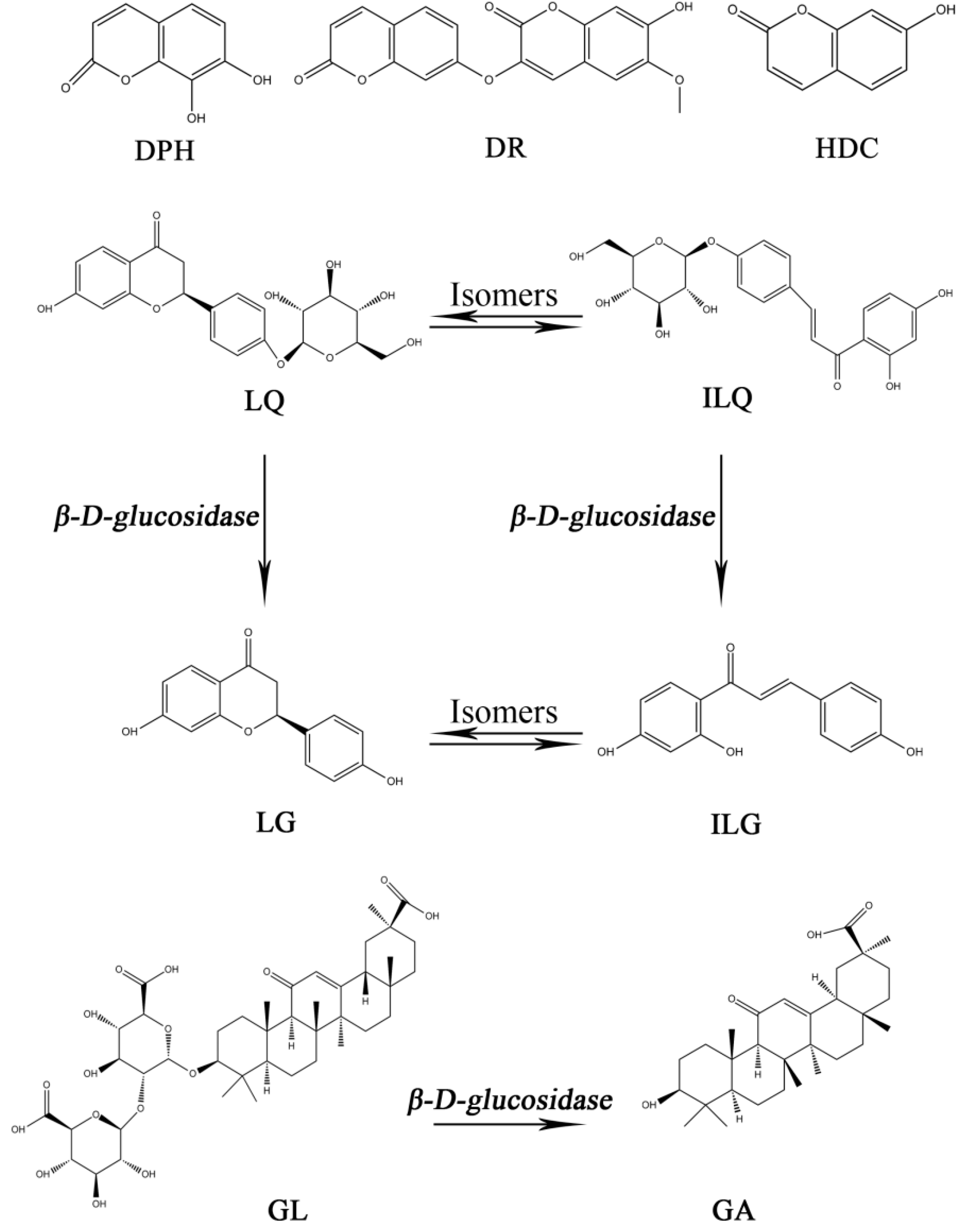
2. Results and Discussion
2.1. Method Validation
2.1.1. Specificity
2.1.2. Linearity and LLOQ
2.1.3. Accuracy and Precision
2.1.4. Recovery and Matrix Effect
2.1.5. Stability
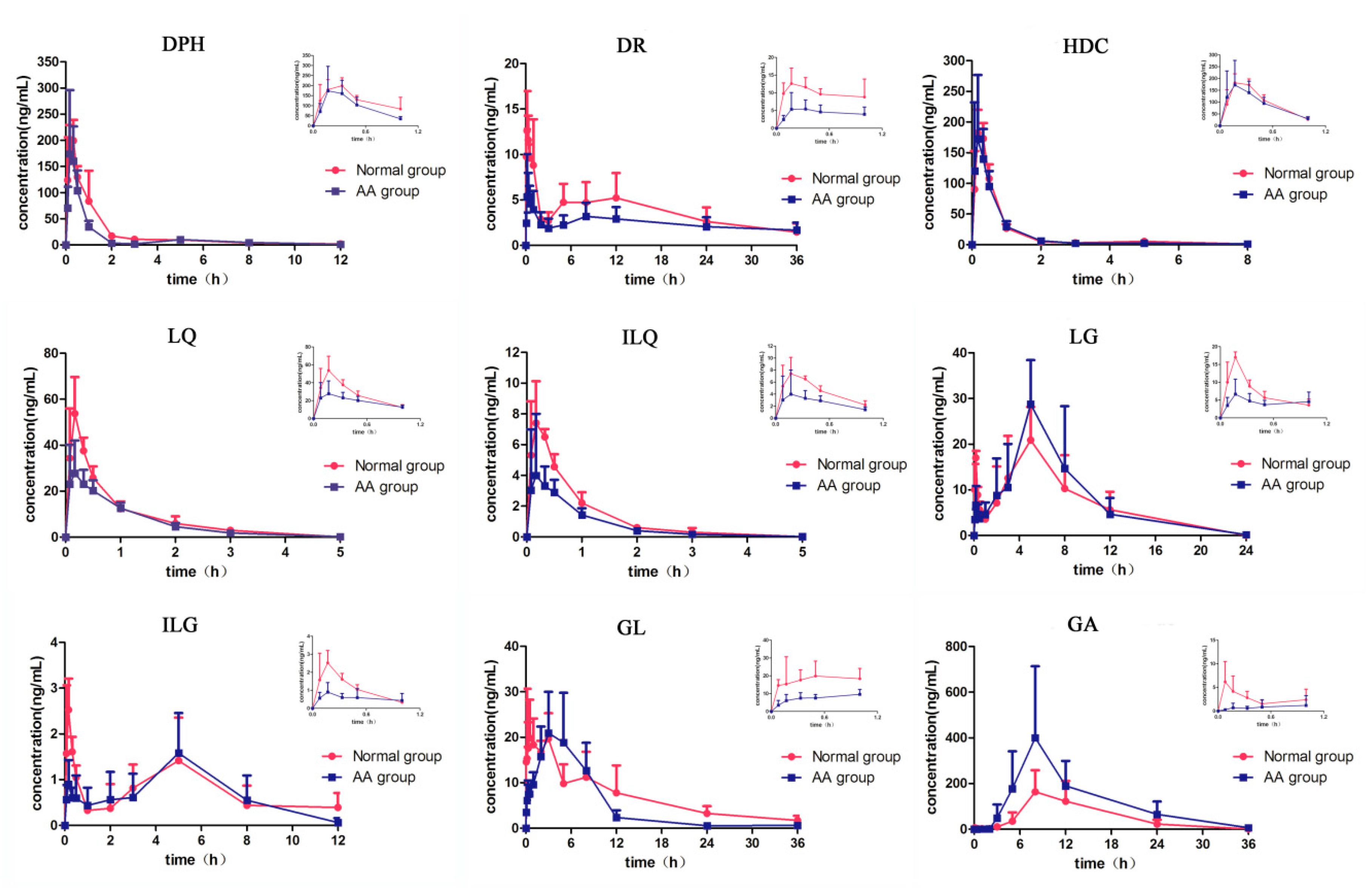
2.2. Pharmacokinetics
3. Materials and Methods
3.1. Materials and Reagents
3.2. Instrument and Analytical Conditions
3.3. Preparation of Zushima-Gancao Extract
3.4. Preparation of Standard Solutions and Quality Control (QC) Samples
3.5. Animals and Induction of FAC
3.6. Drug Administration and Blood Sampling
3.7. Plasma Sample Preparation
3.8. Method Validation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Qi, Y.; Li, S.; Pi, Z.; Song, F.; Lin, N.; Liu, S.; Liu, Z. Metabonomic study of wu-tou decoction in adjuvant-induced arthritis rat using ultra-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014, 953–954, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Van Vollenhoven, R.F. Treatment of rheumatoid arthritis: State of the art 2009. Nat. Rev. Rheumatol. 2009, 5, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Umekita, K.; Umeki, K.; Miyauchi, S.; Ueno, S.; Kubo, K.; Kusumoto, N.; Takajo, I.; Nagatomo, Y.; Okayama, A. Use of anti-tumor necrosis factor biologics in the treatment of rheumatoid arthritis does not change human t-lymphotropic virus type 1 markers: A case series. Mod. Rheumatol. 2015, 25, 794–797. [Google Scholar] [CrossRef] [PubMed]
- Cavagna, L.; Caporali, R.; Trifiro, G.; Arcoraci, V.; Rossi, S.; Montecucco, C. Overuse of prescription and otc non-steroidal anti-inflammatory drugs in patients with rheumatoid arthritis and osteoarthritis. Int. J. Immunopathol. Pharmacol. 2013, 26, 279–281. [Google Scholar] [CrossRef] [PubMed]
- Moller, B.; Pruijm, M.; Adler, S.; Scherer, A.; Villiger, P.M.; Finckh, A. Chronic nsaid use and long-term decline of renal function in a prospective rheumatoid arthritis cohort study. Ann. Rheum. Dis. 2015, 74, 718–723. [Google Scholar] [CrossRef] [PubMed]
- Tacheci, I.; Bradna, P.; Douda, T.; Bastecka, D.; Kopacova, M.; Rejchrt, S.; Lutonsky, M.; Soukup, T.; Bures, J. Small intestinal injury in NSAID users suffering from rheumatoid arthritis or osteoarthritis. Rheumatol. Int. 2016, 36, 1557–1561. [Google Scholar] [CrossRef] [PubMed]
- Li, S.H.; Wu, L.J.; Yin, H.Y. Chemical and pharmacological advances of the study on zushima. China J. Chin. Mater. Med. 2002, 27, 401–403. [Google Scholar]
- Li, Y.; Wang, J.; Xiao, Y.; Wang, Y.; Chen, S.; Yang, Y.; Lu, A.; Zhang, S. A systems pharmacology approach to investigate the mechanisms of action of semen strychni and tripterygium wilfordii hook f for treatment of rheumatoid arthritis. J. Ethnopharmacol. 2015, 175, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.H.; Wang, L.L.; Feng, L.; Shi, S.L. Evaluation on pharmacodynamics of analgesia and anti-inflammation of zushima coumarins. Chin. Arch. Tradit. Chin. Med. 2015, 5, 1183–1185. [Google Scholar]
- Su, J.; Wu, Z.; Shen, Y.; Liu, R.; Zhang, C.; Li, H.; Zhang, W. Flavonoids from daphne giraldii nitsche. Nat. Prod. Res. 2008, 22, 1355–1358. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Wu, Z.J.; Zhang, W.D.; Zhang, C.; Li, H.L.; Liu, R.H.; Shen, Y.H. Two new bis-coumarin glycosides from daphne giraldii NITSCHE. Chem. Pharm. Bull. 2008, 56, 589–591. [Google Scholar] [CrossRef] [PubMed]
- Huyiligeqi; Dong, X.; Yang, C.; Xu, G.; Cao, S.; Fu, J.; Lin, L.; Ni, J. Chemical constituents from daphne giraldii Nitsche and their contents simultaneous determination by HPLC. Evid.-Based Complement. Altern. Med. 2016, 2016, 9492368. [Google Scholar]
- Alrushaid, S.; Davies, N.M.; Martinez, S.E.; Sayre, C.L. Pharmacological characterization of liquiritigenin, a chiral flavonoid in licorice. Res. Pharm. Sci. 2016, 11, 355–365. [Google Scholar] [PubMed]
- Ohno, H.; Araho, D.; Uesawa, Y.; Kagaya, H.; Ishihara, M.; Sakagami, H.; Yamamoto, M. Evaluation of cytotoxiciy and tumor-specificity of licorice flavonoids based on chemical structure. Anticancer Res. 2013, 33, 3061–3068. [Google Scholar] [PubMed]
- Mao, Y.C.; Peng, L.X.; Kang, A.; Xie, T.; Xu, J.Y.; Shen, C.S.; Ji, J.J.; Di, L.Q.; Wu, H.; Shan, J.J. Influence of jiegeng on pharmacokinetic properties of flavonoids and saponins in gancao. Molecules 2017, 22, 1587. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yin, X.J.; Guo, H.M.; Wang, R.L.; Song, R.; Tian, Y.; Zhang, Z.J. Identification and comparative analysis of the major chemical constituents in the extracts of single fuzi herb and fuzi-gancao herb-pair by UFLC-IT-TOF/MS. Chin. J. Nat. Med. 2014, 12, 542–553. [Google Scholar] [CrossRef]
- Meng, X.L.; Guo, X.H.; Zhang, S.S. Research on processing mechanism of zushima which was stir-fried with licorice based on tg-dtg. China J. Chin. Mater. Med. 2012, 37, 3558–3563. [Google Scholar]
- Zhang, W.; Di, L.Q.; Li, J.S.; Shan, J.J.; Kang, A.; Qian, S.; Chen, L.T. The effects of glycyrrhizae uralenis and its major bioactive components on pharmacokinetics of daphnetin in cortex daphnes in rats. J. Ethnopharmacol. 2014, 154, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Gong, L.; Zhou, L.L.; Shan, J.J.; Chen, L.T.; Xu, H.Q.; Di, L.Q. Effect of different compatibility of daphnes giraldii cortex and glycyrrhizae radix et rhizoma on adjuvant-induced arthritis in rats. Chin. Tradit. Herb. Drugs 2014, 45, 1418–1426. [Google Scholar]
- Peng, L.X.; Chen, L.H.; Di, L.Q.; Shan, J.J.; Xie, T.; Kang, A.; Xu, N.S. Plasma metabonomic study on Zushima Gancao Tablet in treatment of rheumatoid arthritis based on UPLC/LTQ-Orbitrap-MS. Chin. Tradit. Herb. Drugs 2017, 48, 1964–1970. [Google Scholar]
- Wang, P.; Liu, J.P.; Zhan, N.; Li, Y.P.; Lu, D. Progress in the research on chemical constituents and pharmacological activities of zushima. Spec. Wild Econ. Anim. Plant Res. 2011, 73–76. [Google Scholar]
- Shu, K.; Kuang, N.; Zhang, Z.; Hu, Z.; Zhang, Y.; Fu, Y.; Min, W. Therapeutic effect of daphnetin on the autoimmune arthritis through demethylation of proapoptotic genes in synovial cells. J. Transl. Med. 2014, 12, 287. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.F.; Feng, T.T.; Lu, Y.; Zhang, X.; Chen, L.Z.; Zhou, Y. Research on active of daphnoretin on inhibiting osteoclast differentiation and promoting osteoblast proliferation in vitro. Pharmacol. Clin. Chin. Mater. Med. 2016, 32, 69–72. [Google Scholar]
- Chen, L.H.; Shan, J.J.; Xie, T.; Di, L.Q. Influence of zushima combined with gancao on dissolution of their eight components by LC-MS /MS. Chin. Tradit. Pat. Med. 2014, 36, 965–969. [Google Scholar]
- Morgan, E.T. Regulation of cytochromes p450 during inflammation and infection. Drug Metab. Rev. 1997, 29, 1129–1188. [Google Scholar] [CrossRef] [PubMed]
- Cornelis, M.C.; Bae, S.C.; Kim, I.; El-Sohemy, A. Cyp1a2 genotype and rheumatoid arthritis in koreans. Rheumatol. Int. 2010, 30, 1349–1354. [Google Scholar] [CrossRef] [PubMed]
- Sanada, H.; Sekimoto, M.; Kamoshita, A.; Degawa, M. Changes in expression of hepatic cytochrome p450 subfamily enzymes during development of adjuvant-induced arthritis in rats. J. Toxicol. Sci. 2011, 36, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Ploeger, B.; Mensinga, T.; Sips, A.; Seinen, W.; Meulenbelt, J.; DeJongh, J. The pharmacokinetics of glycyrrhizic acid evaluated by physiologically based pharmacokinetic modeling. Drug Metab. Rev. 2001, 33, 125–147. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, L.T.; Yun, F.; Di, L.Q.; Shan, J.J.; Zhao, X.L.; Cai, B.C. Research and thinking on characteristics and mechanisms of absorption and metabolism of glycyrrhiza radix et rhizoma and its compatible interactions with other herbs. Chin. Tradit. Herb. Drugs 2012, 43, 1443–1447. [Google Scholar]
- Chen, L.T.; Jing, Y.Y.; Di, L.Q.; Xu, H.Q.; Wu, H.; Shan, J.J. Study on the daphne giraldii nitsche. Effective parts of anti-rheumatoid arthritis. Res. Pract. Chin. Med. 2011, 36, 37–40. [Google Scholar]
- Xu, T.; Liu, S.; Zhao, J.; Feng, G.; Pi, Z.; Song, F.; Liu, Z. A study on the effective substance of the wu-tou formula based on the metabonomic method using UPLC-Q-TOF-HDMS. Mol. BioSyst. 2015, 11, 3081–3091. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Di, L.Q.; Shan, J.J.; Zhao, X.L.; Kang, A.; Bi, X.L.; Li, J.S. Studies on effects of achyranthes bidentata on tongsaimai pellets main active ingredients chlorogenic acid, isoliquiritin, harpagoside and glycyrrhizin in vivo pharmacokinetics. China J. Chin. Mater. Med. 2014, 39, 1502–1508. [Google Scholar]
- Akao, T. Effects of glycyrrhizin and glycyrrhetic acid on the growth, glycyrrhizin beta-d-glucuronidase and 3 beta-hydroxysteroid dehydrogenase of human intestinal bacteria. Biol. Pharm. Bull. 2000, 23, 104–107. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
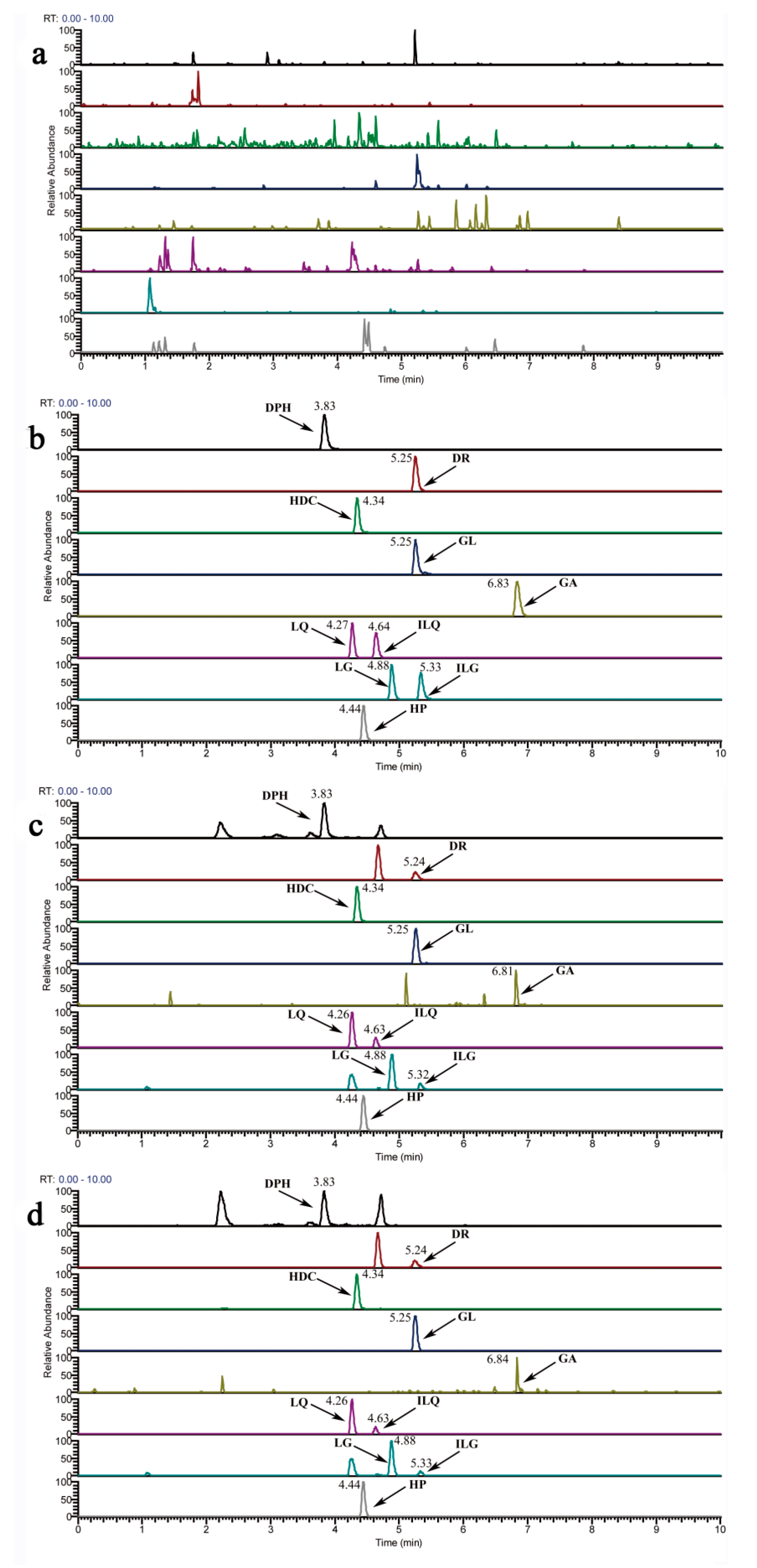
| Analytes | RT (min) | Calibration Curves | R2 | Liner Range (ng/mL) | LLOQ (ng/mL) |
|---|---|---|---|---|---|
| DPH | 3.83 | Y = 0.0058x − 0.0051 | 0.992 | 1.65–843.33 | 1.65 |
| DR | 5.24 | Y = 0.0985x + 0.0036 | 0.998 | 0.09–46.20 | 0.09 |
| HDC | 4.34 | Y = 0.0509x + 0.0189 | 0.995 | 0.80–410.00 | 0.80 |
| LQ | 4.26 | Y = 0.0349x + 0.0064 | 0.998 | 0.59–300.00 | 0.59 |
| ILQ | 4.63 | Y = 0.0408x − 0.0003 | 0.999 | 0.50–255.00 | 0.50 |
| LG | 4.88 | Y = 0.0420x + 0.0072 | 0.998 | 0.36–183.33 | 0.36 |
| ILG | 5.33 | Y = 0.0664x + 0.0010 | 0.998 | 0.34–175.00 | 0.34 |
| GL | 5.25 | Y = 0.0076x − 0.0032 | 0.997 | 1.06–540.00 | 1.06 |
| GA | 6.84 | Y = 0.0030x + 0.0016 | 0.986 | 4.92–2520.00 | 4.92 |
| Analytes | Spiked Conc. (Concentration) (ng/mL) | Intra-Batch | Inter-Batch | ||||
|---|---|---|---|---|---|---|---|
| Measured Conc. (ng/mL) | Accuracy (RE, %) | Precision (RSD, %) | Measured Conc. (ng/mL) | Accuracy (RE, %) | Precision (RSD, %) | ||
| DPH | 1.65 | 1.53 ± 0.22 | −6.92 | 14.24 | 1.76 ± 0.14 | 7.33 | 7.66 |
| 3.29 | 3.19 ± 0.46 | −3.17 | 14.42 | 3.24 ± 0.17 | −1.8 | 5.32 | |
| 52.71 | 60.34 ± 4.54 | 14.48 | 7.52 | 59.84 ± 3.31 | 13.52 | 5.53 | |
| 421.67 | 470.39 ± 51.80 | 11.56 | 11.01 | 467.53 ± 31.31 | 10.88 | 6.70 | |
| DR | 0.09 | 0.08 ± 0.01 | −12.68 | 13.76 | 0.09 ± 0.01 | −3.51 | 7.18 |
| 0.18 | 0.17 ± 0.02 | −6.5 | 12.22 | 0.16 ± 0.01 | −11.95 | 7.59 | |
| 2.89 | 3.17 ± 0.33 | 9.81 | 10.51 | 2.82 ± 0.33 | −2.32 | 11.69 | |
| 23.10 | 24.22 ± 3.05 | 4.86 | 12.57 | 20.18 ± 0.61 | −12.63 | 3.03 | |
| HDC | 0.80 | 0.73 ± 0.11 | −9.22 | 14.93 | 0.80 ± 0.09 | 0 | 10.90 |
| 1.60 | 1.50 ± 0.12 | −6.58 | 7.86 | 1.60 ± 0.08 | −0.28 | 4.89 | |
| 25.63 | 28.06 ± 0.94 | 9.49 | 3.34 | 27.33 ± 1.05 | 6.67 | 3.83 | |
| 205.00 | 225.27 ± 17.64 | 9.89 | 7.83 | 206.31 ± 5.42 | 0.64 | 2.63 | |
| LQ | 0.59 | 0.52 ± 0.06 | −10.76 | 11.20 | 0.52 ± 0.07 | −11.52 | 13.49 |
| 1.17 | 1.18 ± 0.08 | 0.23 | 7.11 | 1.23 ± 0.03 | 5.08 | 2.42 | |
| 18.75 | 20.80 ± 0.70 | 10.92 | 3.37 | 20.38 ± 0.39 | 8.67 | 1.92 | |
| 150 | 152.62 ± 4.09 | 1.75 | 2.68 | 148.79 ± 4.37 | −0.8 | 2.93 | |
| ILQ | 0.50 | 0.42 ± 0.02 | −14.96 | 4.59 | 0.41 ± 0.02 | −17.99 | 4.31 |
| 1.00 | 1.04 ± 0.11 | 4.31 | 10.46 | 1.10 ± 0.10 | 10.86 | 8.66 | |
| 15.94 | 16.71 ± 0.52 | 4.83 | 3.11 | 16.49 ± 0.42 | 3.47 | 2.57 | |
| 127.50 | 114.79 ± 2.43 | −9.97 | 2.12 | 114.25 ± 2.49 | −10.4 | 2.18 | |
| LG | 0.36 | 0.31 ± 0.04 | −12.56 | 13.15 | 0.31 ± 0.05 | −12.88 | 14.50 |
| 0.72 | 0.72 ± 0.09 | −0.03 | 12.41 | 0.67 ± 0.06 | −7 | 9.59 | |
| 11.46 | 12.45 ± 0.83 | 8.69 | 6.65 | 11.57 ± 0.32 | 1.01 | 2.75 | |
| 91.67 | 96.48 ± 6.61 | 5.25 | 6.85 | 89.12 ± 4.69 | −2.77 | 5.26 | |
| ILG | 0.34 | 0.33 ± 0.05 | −3.79 | 14.47 | 0.36 ± 0.02 | 6.48 | 5.18 |
| 0.68 | 0.75 ± 0.07 | 9.18 | 9.00 | 0.78 ± 0.05 | 14.44 | 6.02 | |
| 10.94 | 10.60 ± 1.55 | −3.05 | 14.60 | 12.24 ± 0.26 | 11.92 | 2.16 | |
| 87.50 | 75.49 ± 9.20 | −13.73 | 12.19 | 87.44 ± 4.65 | −0.06 | 5.31 | |
| GL | 1.06 | 1.02 ± 0.12 | −2.98 | 11.77 | 0.92 ± 0.13 | −13.03 | 13.63 |
| 2.11 | 2.19 ± 0.33 | 8.49 | 14.96 | 2.13 ± 0.17 | 5.45 | 7.96 | |
| 33.75 | 30.74 ± 1.75 | −8.91 | 5.69 | 28.94 ± 1.34 | −14.25 | 4.62 | |
| 270.00 | 230.24 ± 20.08 | −14.73 | 8.72 | 241.57 ± 29.73 | −10.53 | 12.31 | |
| GA | 4.92 | 4.26 ± 0.42 | −13.55 | 9.76 | 4.37 ± 0.55 | −11.17 | 12.60 |
| 9.84 | 8.76 ± 0.94 | −11.02 | 10.75 | 8.49 ± 0.87 | −13.77 | 10.24 | |
| 157.50 | 153.85 ± 18.33 | −2.31 | 11.92 | 177.70 ± 6.92 | 12.83 | 3.90 | |
| 1260.00 | 1390.83 ± 80.9 | 10.38 | 5.81 | 1413.66 ± 76.5 | 12.20 | 5.41 | |
| Analytes | Spiked Conc. (ng/mL) | Extraction Recovery | Matrix Effect | ||
|---|---|---|---|---|---|
| Accuracy(%) | RSD (%) | Accuracy (%) | RSD (%) | ||
| DPH | 3.29 | 69.56 | 12.53 | 109.47 | 11.90 |
| 52.71 | 72.51 | 5.00 | 113.45 | 10.59 | |
| 421.67 | 79.09 | 7.37 | 114.66 | 2.36 | |
| DR | 0.18 | 89.54 | 4.81 | 112.16 | 1.69 |
| 2.89 | 82.73 | 10.52 | 105.43 | 3.01 | |
| 23.10 | 76.71 | 3.60 | 99.00 | 1.52 | |
| HDC | 1.60 | 86.79 | 3.62 | 107.81 | 8.83 |
| 25.63 | 84.35 | 3.14 | 106.78 | 1.43 | |
| 205.00 | 84.53 | 2.67 | 101.20 | 2.64 | |
| LQ | 1.17 | 64.58 | 2.49 | 99.95 | 6.24 |
| 18.75 | 61.50 | 2.53 | 102.39 | 1.89 | |
| 150.00 | 61.91 | 2.16 | 92.07 | 3.26 | |
| ILQ | 1.00 | 74.46 | 10.65 | 96.94 | 7.39 |
| 15.94 | 68.70 | 2.25 | 100.51 | 3.06 | |
| 127.50 | 60.50 | 1.68 | 97.23 | 0.80 | |
| LG | 0.72 | 79.71 | 6.76 | 113.61 | 13.08 |
| 11.46 | 82.75 | 2.55 | 106.29 | 2.28 | |
| 91.67 | 84.13 | 6.64 | 99.74 | 0.97 | |
| ILG | 0.68 | 60.88 | 4.65 | 86.52 | 4.67 |
| 10.94 | 60.11 | 1.89 | 88.90 | 3.69 | |
| 87.50 | 64.45 | 3.45 | 85.54 | 1.48 | |
| GL | 2.11 | 78.43 | 9.73 | 113.73 | 8.27 |
| 33.75 | 71.89 | 3.78 | 105.21 | 5.49 | |
| 270.00 | 72.50 | 11.11 | 97.11 | 7.39 | |
| GA | 9.84 | 63.06 | 6.59 | 112.46 | 9.39 |
| 157.50 | 62.43 | 2.51 | 85.65 | 4.21 | |
| 1260.00 | 62.59 | 6.21 | 86.18 | 5.93 | |
| HP | 130.00 | 56.53 | 1.81 | 96.23 | 1.67 |
| Analytes | Spiked Conc. (ng/mL) | 24 h Stability | Short-Term Stability | Long-Term Stability | Freeze-Thaw Stability | ||||
|---|---|---|---|---|---|---|---|---|---|
| RE (%) | RSD (%) | RE (%) | RSD (%) | RE (%) | RSD (%) | RE (%) | RSD (%) | ||
| DPH | 3.29 | −13.85 | 5.82 | −7.83 | 2.46 | −12.07 | 12.53 | −6.29 | 3.04 |
| 52.71 | −13.73 | 2.46 | −12.68 | 2.48 | −15 | 1.05 | −8.25 | 9.42 | |
| 421.67 | −11.8 | 1.01 | −12.42 | 4.59 | −12.23 | 4.78 | −14.04 | 8.97 | |
| DR | 0.18 | −11.79 | 2.82 | −13.63 | 13.16 | −12.79 | 7.07 | 0.05 | 7.16 |
| 2.89 | −11.33 | 1.64 | −13.99 | 1.55 | −14.41 | 2.08 | −14.22 | 2.82 | |
| 23.10 | −6.11 | 2.00 | −5.38 | 1.15 | −13.85 | 0.91 | −9.71 | 4.68 | |
| HDC | 1.60 | −1.43 | 8.39 | −13.86 | 11.16 | −10.07 | 1.85 | −13.48 | 10.06 |
| 25.63 | −0.85 | 0.41 | −5.72 | 1.54 | −1.8 | 0.62 | −1.27 | 3.13 | |
| 205.00 | 1.39 | 1.03 | 3.09 | 4.68 | −0.05 | 2.08 | 2.64 | 2.00 | |
| LQ | 1.17 | 2.03 | 7.34 | −9.95 | 12.36 | 8.14 | 7.75 | 13.76 | 6.71 |
| 18.75 | 3.31 | 6.83 | 0.14 | 4.83 | −0.13 | 4.22 | −5.95 | 3.17 | |
| 150.00 | 4.89 | 0.74 | 5.23 | 0.98 | 7.57 | 1.46 | 7.92 | 3.61 | |
| ILQ | 1.00 | 4.99 | 8.75 | 9.08 | 2.98 | 7.49 | 1.72 | −2.51 | 10.17 |
| 15.94 | 5.33 | 5.05 | 12.35 | 3.29 | 5.37 | 5.51 | −6.3 | 12.35 | |
| 127.50 | 6.64 | 1.21 | 3.29 | 1.67 | 2.66 | 0.63 | 4.51 | 4.30 | |
| LG | 0.72 | 6.72 | 2.25 | 9.76 | 4.54 | 2.87 | 1.91 | 7.22 | 1.47 |
| 11.46 | 8.15 | 10.56 | 11.16 | 8.65 | 0.58 | 3.69 | 7.96 | 7.41 | |
| 91.67 | 8.56 | 5.59 | 1.5 | 3.74 | 3.89 | 4.49 | 6.75 | 8.16 | |
| ILG | 0.68 | 8.75 | 4.32 | −3.23 | 9.06 | −11.29 | 7.39 | 2.53 | 13.94 |
| 10.94 | 10.08 | 0.72 | 11.76 | 0.61 | 12.68 | 2.32 | 12.3 | 6.33 | |
| 87.50 | 10.13 | 1.01 | 12.09 | 5.32 | 13.23 | 4.15 | 7.59 | 2.24 | |
| GL | 2.11 | 11.79 | 3.24 | 10.26 | 0.34 | 11.48 | 2.49 | 10.89 | 4.87 |
| 33.75 | 11.8 | 2.74 | 10.68 | 2.60 | 9.96 | 4.18 | 14.3 | 3.73 | |
| 270.00 | 13.22 | 3.31 | 13.85 | 1.02 | 9.2 | 2.20 | 14.36 | 2.99 | |
| GA | 9.84 | 13.49 | 10.22 | 2.08 | 1.00 | 0.04 | 6.85 | −14.06 | 5.84 |
| 157.50 | 14.1 | 11.46 | −3.38 | 5.79 | 5.44 | 3.31 | 4.21 | 11.57 | |
| 1260.00 | 14.35 | 0.98 | 13.05 | 2.43 | 9.57 | 2.15 | 13.8 | 2.27 | |
| Analytes | Group | Cmax (ng/mL) | Tmax (h) | AUC(0–t) (ng/mL·h) | AUC(0–∞) (ng/mL·h) | t1/2 (h) |
|---|---|---|---|---|---|---|
| DPH | Normal | 214.45 ± 32.55 | 0.24 ± 0.11 | 245.51 ± 53.86 | 255.68 ± 54.77 | 3.40 ± 0.96 |
| AA | 200.83 ± 108.24 | 0.20 ± 0.07 | 164.51 ± 49.38 | 167.32 ± 49.04 * | 2.06 ± 0.26 * | |
| DR | Normal | 14.40 ± 3.31 | 0.39 ± 0.31 | 131.35 ± 58.12 | 163.26 ± 78.67 | 14.53 ± 2.91 |
| AA | 7.06 ± 3.82 * | 0.42 ± 0.31 | 86.17 ± 33.84 | 153.80 ± 79.83 | 28.32 ± 9.39 | |
| HDC | Normal | 193.15 ± 29.30 | 0.22 ± 0.08 | 141.91 ± 24.42 | 147.92 ± 24.89 | 2.86 ± 1.94 |
| AA | 179.33 ± 98.00 | 0.22 ± 0.08 | 127.35 ± 26.80 | 130.73 ± 29.06 | 2.52 ± 1.82 | |
| LQ | Normal | 58.75 ± 12.54 | 0.17 ± 0.09 | 45.45 ± 3.89 | 45.55 ± 3.90 | 0.47 ± 0.15 |
| AA | 30.11 ± 13.83 ** | 0.21 ± 0.10 | 33.12 ± 6.80 ** | 34.36 ± 8.89 * | 0.75 ± 0.65 | |
| ILQ | Normal | 8.92 ± 1.68 | 0.17 ± 0.09 | 6.72 ± 0.60 | 6.74 ± 0.60 | 0.52 ± 0.20 |
| AA | 4.63 ± 3.64 * | 0.36 ± 0.13 * | 4.04 ± 1.20 * | 4.11 ± 1.12 ** | 0.94 ± 0.83 | |
| LG | Normal | 23.95 ± 3.72 | 3.86 ± 1.98 | 169.46 ± 62.38 | 169.58 ± 62.49 | 1.66 ± 0.44 |
| AA | 29.69 ± 10.69 | 4.70 ± 2.52 | 192.60 ± 83.23 | 193.82 ± 84.43 | 2.30 ± 0.95 | |
| ILG | Normal | 2.77 ± 0.83 | 0.14 ± 0.05 | 8.89 ± 3.12 | 12.38 ± 6.97 | 1.08 ± 0.67 |
| AA | 1.92 ± 0.55 | 3.70 ± 2.10 ** | 8.25 ± 2.90 | 8.48 ± 3.04 | 1.39 ± 0.65 | |
| GL | Normal | 29.02 ± 10.49 | 0.58 ± 0.35 | 248.04 ± 47.75 | 266.68 ± 49.71 | 8.72 ± 4.81 |
| AA | 24.95 ± 7.81 | 4.83 ± 2.64 * | 183.66 ± 61.69 | 186.96 ± 64.62 * | 4.88 ± 3.04 | |
| GA | Normal | 167.00 ± 92.99 | 8.67 ± 1.63 | 1938.01 ± 1244.25 | 1959.74 ± 1261.64 | 3.88 ± 1.29 |
| AA | 417.84 ± 296.20 | 9.33 ± 2.07 | 4258.35 ± 1862.33 * | 4331.78 ± 1862.81 * | 4.94 ± 2.43 |
| Analytes | M.W. | m/z | Collision Energy (V) | S-Lens |
|---|---|---|---|---|
| DPH | 178.14 | 176.9→121.0 | 25 | 75 |
| DR | 352.29 | 351.0→162.9 | 39 | 102 |
| HDC | 162.14 | 160.9→133.0 | 21 | 77 |
| GL | 822.93 | 821.1→350.8 | 39 | 160 |
| GA | 470.68 | 469.1→425.2 | 37 | 160 |
| LQ(ILQ) | 418.39 | 417.1→255.0 | 22 | 92 |
| LG(ILG) | 256.25 | 255.0→135.0 | 18 | 65 |
| HP | 610.56 | 609.2→301.1 | 27 | 160 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shan, J.; Qian, W.; Peng, L.; Chen, L.; Kang, A.; Xie, T.; Di, L. A Comparative Pharmacokinetic Study by UHPLC-MS/MS of Main Active Compounds after Oral Administration of Zushima-Gancao Extract in Normal and Adjuvant-Induced Arthritis Rats. Molecules 2018, 23, 227. https://doi.org/10.3390/molecules23010227
Shan J, Qian W, Peng L, Chen L, Kang A, Xie T, Di L. A Comparative Pharmacokinetic Study by UHPLC-MS/MS of Main Active Compounds after Oral Administration of Zushima-Gancao Extract in Normal and Adjuvant-Induced Arthritis Rats. Molecules. 2018; 23(1):227. https://doi.org/10.3390/molecules23010227
Chicago/Turabian StyleShan, Jinjun, Wenjuan Qian, Linxiu Peng, Lianghui Chen, An Kang, Tong Xie, and Liuqing Di. 2018. "A Comparative Pharmacokinetic Study by UHPLC-MS/MS of Main Active Compounds after Oral Administration of Zushima-Gancao Extract in Normal and Adjuvant-Induced Arthritis Rats" Molecules 23, no. 1: 227. https://doi.org/10.3390/molecules23010227
APA StyleShan, J., Qian, W., Peng, L., Chen, L., Kang, A., Xie, T., & Di, L. (2018). A Comparative Pharmacokinetic Study by UHPLC-MS/MS of Main Active Compounds after Oral Administration of Zushima-Gancao Extract in Normal and Adjuvant-Induced Arthritis Rats. Molecules, 23(1), 227. https://doi.org/10.3390/molecules23010227




