Sulfadiazine Salicylaldehyde-Based Schiff Bases: Synthesis, Antimicrobial Activity and Cytotoxicity
Abstract
:1. Introduction
2. Results and Discussion
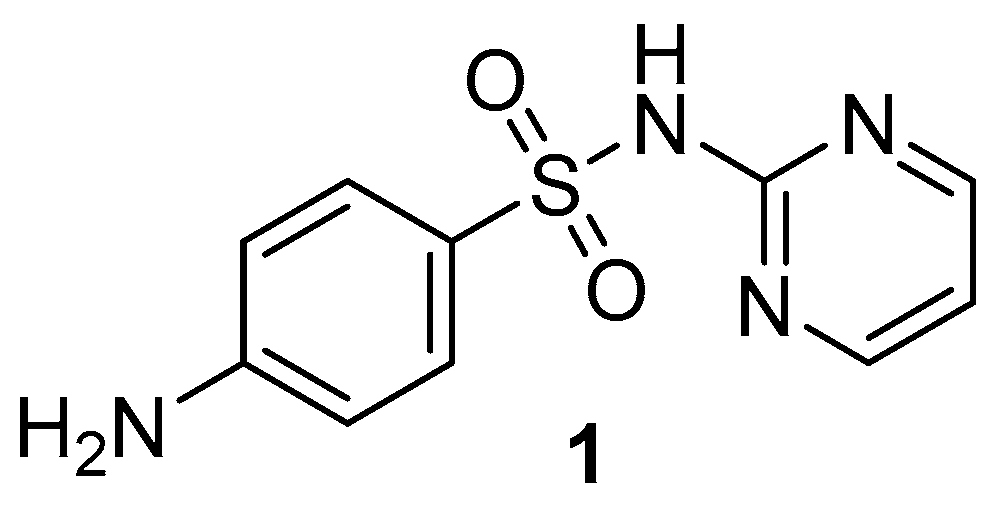
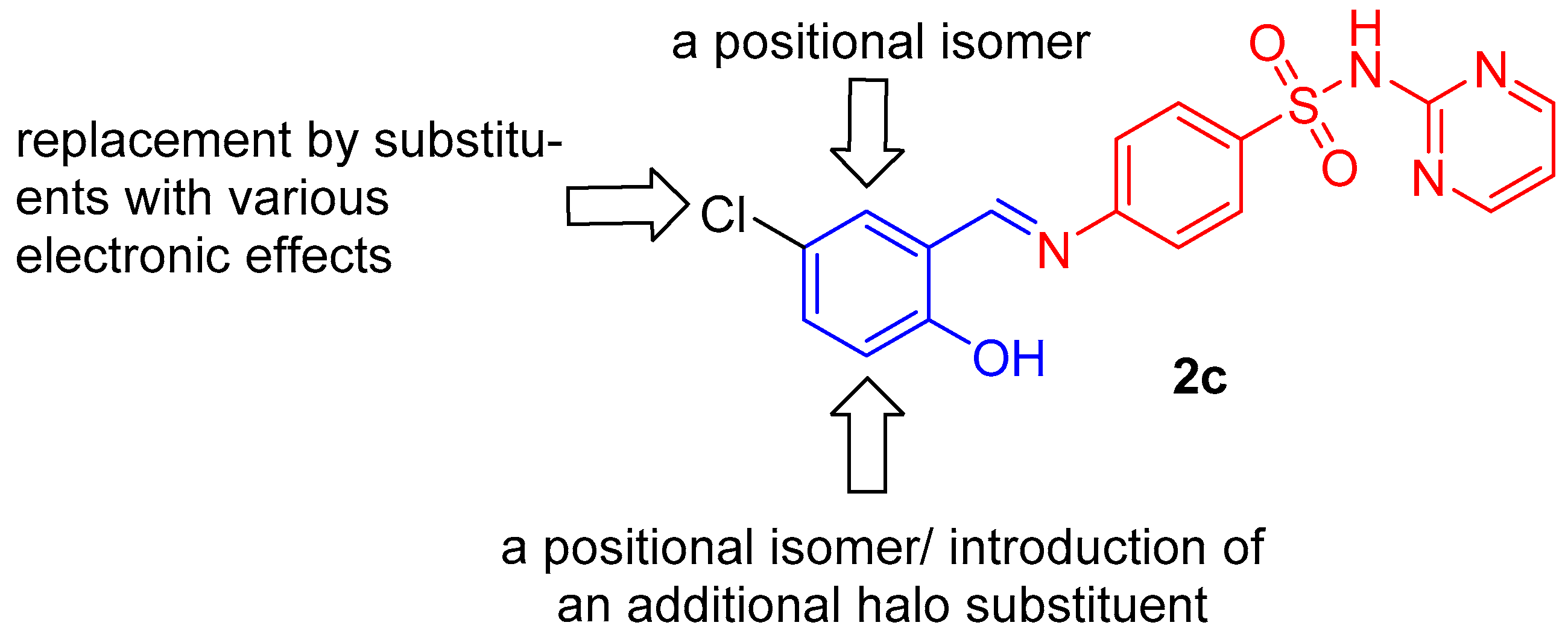
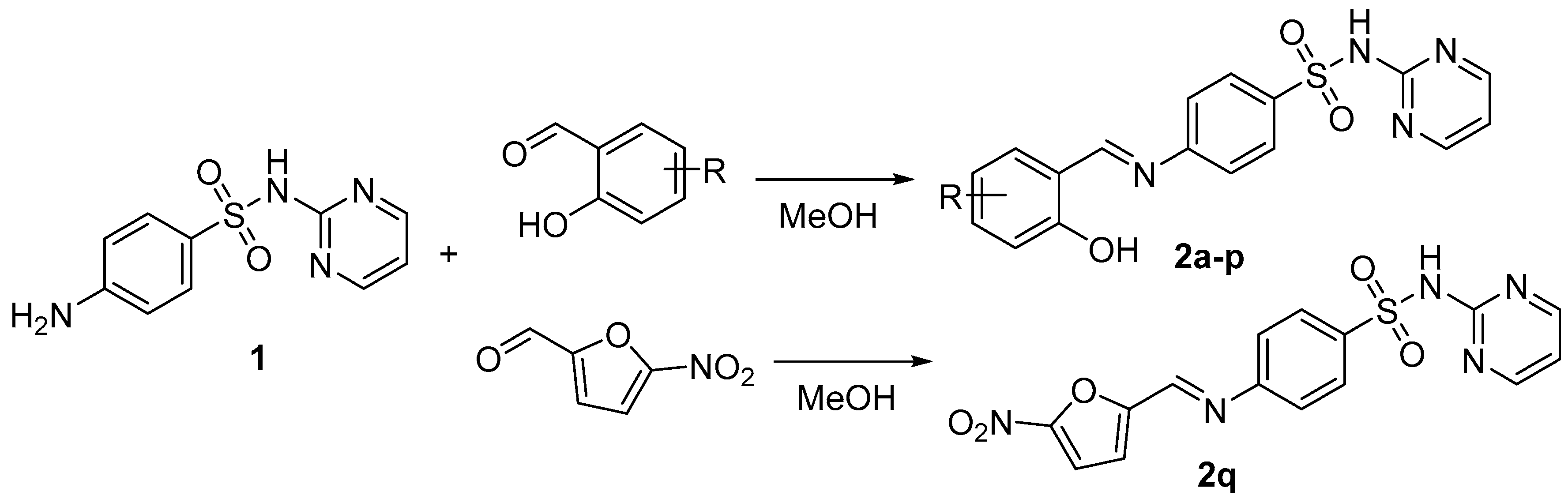
2.1. Chemistry
2.2. Antimicrobial Activity
2.3. Cytotoxicity and Selectivity
3. Materials and Methods
3.1. Chemistry
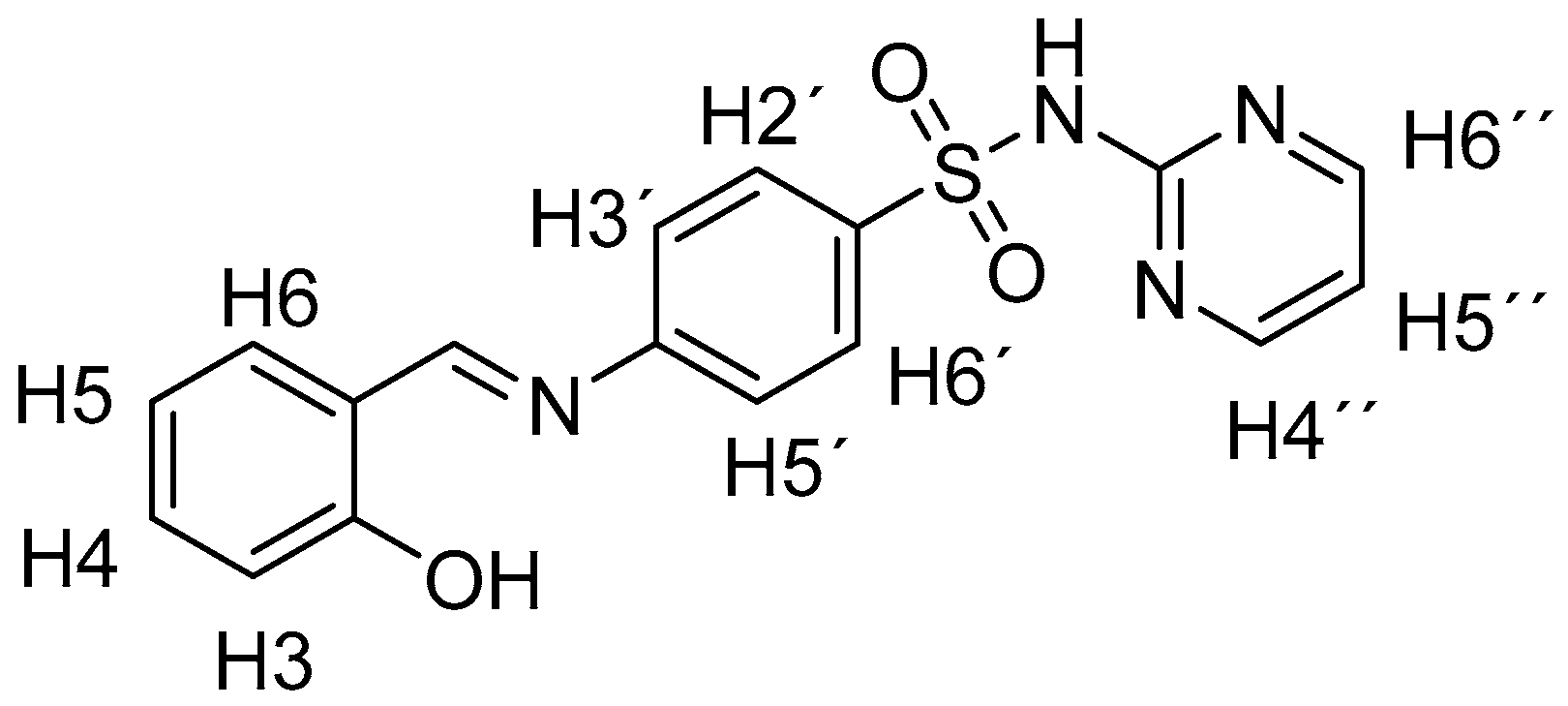
3.1.1. General Information
3.1.2. Synthesis
3.2. Antimicrobial Activity
3.2.1. In Vitro Antibacterial Activity
3.2.2. In Vitro Antimycobacterial Activity
3.2.3. In Vitro Antifungal Activity
3.3. Cytotoxicity Evaluation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Da Silva, C.M.; da Silva, D.L.; Modolo, L.V.; Alves, R.B.; de Resende, M.A.; Martins, C.V.B.; de Fátima, A. Schiff bases: A short review of their antimicrobial activities. J. Adv. Res. 2011, 2, 1–8. [Google Scholar] [CrossRef]
- Qin, W.; Long, S.; Panunzio, M.; Biondi, S. Schiff Bases: A short survey on an evergreen chemistry tool. Molecules 2013, 18, 12264–12289. [Google Scholar] [CrossRef] [PubMed]
- Kajal, A.; Bala, S.; Kamboj, S.; Sharma, N.; Saini, V. Schiff bases: A versatile pharmacophore. J. Catal. 2013, 2013. [Google Scholar] [CrossRef]
- Krátký, M.; Vinšová, J.; Volková, M.; Buchta, V.; Trejtnar, F.; Stolaříková, J. Antimicrobial activity of sulfonamides containing 5-chloro-2-hydroxybenzaldehyde and 5-chloro-2-hydroxybenzoic acid scaffold. Eur. J. Med. Chem. 2012, 50, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Yıldız, M.; Tan, E.; Demir, N.; Yıldırım, N.; Ünver, H.; Kiraz, A.; Mestav, B. Synthesis and spectral, antimicrobial, anion sensing, and DNA binding properties of Schiff base podands and their metal complexes. Russ. J. Gen. Chem. 2015, 85, 2149–2162. [Google Scholar] [CrossRef]
- Shi, L.; Ge, M.M.; Tan, S.H.; Li, H.Q.; Song, Y.C.; Zhu, H.L.; Tan, R.X. Synthesis and antimicrobial activities of Schiff bases derived from 5-chloro-salicylaldehyde. Eur. J. Med. Chem. 2007, 42, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Govindaraj, V.; Ramanathan, S. Synthesis, spectral characterisation, electrochemical, and fluorescence studies of biologically active novel Schiff base complexes derived from E-4-(2-hydroxy-3-methoxybenzlideneamino)-N-(pyrimidin-2-yl)benzenesulfonamide. Turk. J. Chem. 2014, 38, 521–530. [Google Scholar] [CrossRef]
- Mondal, S.; Mandal, S.M.; Mondal, T.K.; Sinha, C. Spectroscopic characterization, antimicrobial activity, DFT computation and docking studies of sulfonamide Schiff bases. J. Mol. Struct. 2017, 1127, 557–567. [Google Scholar] [CrossRef]
- Chohan, Z.H.; Youssoufi, M.H.; Jarrahpour, A.; Hadda, T.B. Identification of antibacterial and antifungal pharmacophore sites for potent bacteria and fungi inhibition: Indolenyl sulfonamide derivatives. Eur. J. Med. Chem. 2010, 45, 1189–1199. [Google Scholar] [CrossRef] [PubMed]
- Ameen, S.M.; Drancourt, M. In Vitro susceptibility of Mycobacterium tuberculosis to trimethoprim and sulfonamides in France. Antimicrob. Agents Chemother. 2013, 57, 6370–6371. [Google Scholar] [CrossRef] [PubMed]
- Ameen, S.M.; Drancourt, M. In vitro susceptibility of Mycobacterium avium complex mycobacteria to trimethoprim and sulfonamides. Int. J. Antimicrob. Agents 2013, 42, 281–288. [Google Scholar] [CrossRef] [PubMed]
- El-Baradie, K.Y. Preparation and characterization of sulfadiazine Schiff base complexes of Co(II), Ni(II), Cu(II), and Mn(II). Monatshefte Chem. 2005, 136, 1139–1155. [Google Scholar] [CrossRef]
- Chohan, Z.H.; Shad, H.A.; Youssoufi, M.H.; Hadda, T.B. Some new biologically active metal-based sulphonamide. Eur. J. Med. Chem. 2010, 45, 2893–2901. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aal, W.S.; Hassan, H.Y.; Aboul-Fadl, T.; Youssef, A.F. Pharmacophoric model building for antitubercular activity of the individual Schiff bases of small combinatorial library. Eur. J. Med. Chem. 2010, 45, 1098–1106. [Google Scholar] [CrossRef] [PubMed]
- Zorzi, R.R.; Jorge, S.D.; Palace-Berl, F.; Pasqualoto, K.F.M.; de Sa Bortolozzo, L.; de Castro Siqueira, A.M.; Tavares, L.C. Exploring 5-nitrofuran derivatives against nosocomial pathogens: Synthesis, antimicrobial activity and chemometric analysis. Bioorg. Med. Chem. 2014, 22, 2844–2854. [Google Scholar] [CrossRef] [PubMed]
- Krátký, M.; Vinšová, J.; Novotná, E.; Mandíková, J.; Trejtnar, F.; Stolaříková, J. Antibacterial activity of salicylanilide 4-(Trifluoromethyl)benzoates. Molecules 2013, 18, 3674–3688. [Google Scholar] [CrossRef] [PubMed]
- Krátký, M.; Vinšová, J. Antifungal activity of salicylanilides and their esters with 4-(Trifluoromethyl)benzoic acid. Molecules 2012, 17, 9426–9442. [Google Scholar] [CrossRef] [PubMed]
- Sriram, D.; Yogeeswari, P.; Dhakla, P.; Senthilkumar, P.; Banerjee, D.; Manjashetty, T.H. 5-Nitrofuran-2-yl derivatives: Synthesis and inhibitory activities against growing and dormant mycobacterium species. Bioorg. Med. Chem. Lett. 2009, 19, 1152–1154. [Google Scholar] [CrossRef] [PubMed]
- Castle, R.N.; Witt, N.F.; Poe, C.F. The optical crystallographic properties of some sulfonamides and their derivatives. J. Am. Chem. Soc. 1949, 71, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, T.; Yuhi, K. Studies on halogenosalicylaldehyde. I halogenosalicylaldehyde as reagent for primary amines. Yakugaku Zasshi 1958, 78, 706–709. [Google Scholar] [CrossRef]
- Krátký, M.; Vinšová, J.; Novotná, E.; Wsól, V.; Ulmann, V.; Stolaříková, J.; Fernandes, S.; Bhat, S.; Liu, J.O. Salicylanilide derivatives block Mycobacterium tuberculosis through inhibition of isocitrate lyase and methionine aminopeptidase. Tuberculosis (Edinb.) 2012, 92, 434–439. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1, 2a–q are available from the authors. |
 | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Code | R | MIC (µM) | |||||||||||||||
| SA | MRSA | SE | EF | EC | KP | KP-E | PA | ||||||||||
| 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | ||
| 2a | H | 500 | 500 | 250 | 250 | 62.5 | 62.5 | 31.25 | 62.5 | >500 | >500 | >500 | >500 | >500 | >500 | 500 | 500 |
| 2b | 5-F | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 |
| 2c [4] | 5-Cl | 250 | 250 | 125 | 125 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 |
| 2d | 5-Br | 500 | 500 | 500 | 500 | 250 | 250 | >500 | >500 | 500 | 500 | 500 | 500 | 500 | 500 | 500 | 500 |
| 2e | 5-I | 250 | 250 | 250 | 250 | 62.5 | 125 | 250 | 500 | 250 | 250 | 500 | 500 | 500 | 500 | 500 | 500 |
| 2f | 5-NO2 | 250 | 250 | 250 | 250 | 500 | 500 | 500 | 500 | 250 | 250 | >500 | >500 | 500 | 500 | 500 | 500 |
| 2g | 5-CH3 | >250 | >250 | 125 | 125 | 125 | 125 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 |
| 2h | 5-CH3O | 500 | 500 | >500 | >500 | 62.5 | 62.5 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | 500 | 500 |
| 2i | 5-OH | 31.25 | 31.25 | 31.25 | 31.25 | 15.62 | 15.62 | 250 | 250 | 500 | 500 | >500 | >500 | >500 | >500 | 500 | 500 |
| 2j | 5-tert-Bu | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 |
| 2k | 6-Cl | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 |
| 2l | 3-Cl | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 |
| 2m | 3,5-Cl2 | 62.5 | 62.5 | 62.5 | 62.5 | 31.25 | 31.25 | >500 | >500 | >500 | >500 | >500 | >500 | 500 | 500 | >500 | >500 |
| 2n | 3-Br-5-Cl | 31.25 | 31.25 | 15.62 | 15.62 | 31.25 | 31.25 | >500 | >500 | 500 | 500 | >500 | >500 | 500 | 500 | >500 | >500 |
| 2o | 3-I-5-Cl | 15.62 | 15.62 | 15.62 | 15.62 | 31.25 | 31.25 | 250 | 250 | 250 | 250 | 500 | 500 | 500 | 500 | 500 | 500 |
| 2p | 3,5-I2 | 7.81 | 7.81 | 7.81 | 7.81 | 7.81 | 7.81 | 125 | 125 | 125 | 125 | 125 | 125 | 125 | 125 | 500 | 500 |
| 2q | - | 125 | 125 | 125 | 125 | 31.25 | 31.25 | >500 | >500 | >500 | >500 | 500 | 500 | 500 | 500 | 250 | 250 |
| SDZ 1 | - | 500 | >500 | 500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 |
| BAC | - | 7.81 | 15.62 | 15.62 | 15.62 | 15.62 | 31.25 | 15.62 | 62.5 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 |
| Code | R | MIC (µM) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mtb. 331/88 | M. avium 330/88 | M. kansasii 235/80 | M. kansasii 6509/96 | ClogP | ||||||||
| 14 d | 21 d | 14 d | 21 d | 7 d | 14 d | 21 d | 7 d | 14 d | 21 d | |||
| 2a | H | 16 | 32 | 125 | 250 | 8 | 16 | 32 | 8 | 16 | 32 | 2.5 |
| 2b | 5-F | 125 | 125 | 500 | 1000 | 62.5 | 125 | 250 | 125 | 250 | 250 | 2.65 |
| 2c [4] | 5-Cl | 125 | 250 | 125 | 125 | 8 | 16 | 32 | 16 | 32 | 32 | 3.05 |
| 2d | 5-Br | 16 | 32 | 125 | 125 | 32 | 62.5 | 62.5 | 32 | 32 | 62.5 | 3.32 |
| 2e | 5-I | 62.5 | 125 | 125 | 250 | 32 | 62.5 | 125 | 32 | 32 | 62.5 | 3.85 |
| 2f | 5-NO2 | 250 | 250 | 500 | 1000 | 125 | 250 | 500 | 125 | 250 | 250 | 2.15 |
| 2g | 5-CH3 | 125 | 125 | 500 | 1000 | 125 | 125 | 250 | 62.5 | 62.5 | 125 | 2.98 |
| 2h | 5-CH3O | 32 | 62.5 | 125 | 125 | 16 | 32 | 62.5 | 16 | 16 | 32 | 2.37 |
| 2i | 5-OH | 125 | 125 | 500 | 1000 | 62.5 | 125 | 125 | 62.5 | 125 | 125 | 2.11 |
| 2j | 5-tert-Bu | 32 | 62.5 | 125 | 125 | 16 | 32 | 62.5 | 16 | 16 | 32 | 4.2 |
| 2k | 6-Cl | 125 | 125 | 250 | 250 | 62.5 | 125 | 250 | 62.5 | 125 | 125 | 3.05 |
| 2l | 3-Cl | 32 | 62.5 | 125 | 250 | 16 | 32 | 62.5 | 16 | 16 | 32 | 3.05 |
| 2m | 3,5-Cl2 | 125 | 125 | 250 | 500 | 125 | 250 | 500 | 62.5 | 125 | 250 | 3.61 |
| 2n | 3-Br-5-Cl | 125 | 125 | 250 | 500 | 125 | 125 | 250 | 62.5 | 125 | 125 | 3.88 |
| 2o | 3-I-5-Cl | 125 | 125 | 500 | 500 | 125 | 250 | 250 | 62.5 | 125 | 125 | 4.41 |
| 2p | 3,5-I2 | 250 | 250 | 250 | 500 | 125 | 250 | 250 | 62.5 | 125 | 250 | 5.21 |
| 2q | - | 125 | 125 | 500 | 500 | 125 | 250 | 250 | 62.5 | 62.5 | 125 | ND |
| SDZ 1 | - | 32 | 62.5 | 62.5 | 62.5 | 16 | 16 | 32 | 8 | 8 | 8 | 0.21 |
| INH | - | 0.5 | 1 | >250 | >250 | >250 | >250 | >250 | 8 | 8 | 8 | - |
| Code | R | MIC (µM) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CA | CT | CK | CG | TA | AF | LC | TI | ||||||||||
| 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 72 h | 120 h | ||
| 2a | H | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | 125 | 125 |
| 2b | 5-F | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 |
| 2c [4] | 5-Cl | 125 | 125 | >500 | >500 | >500 | >500 | 125 | 125 | 62.5 | 125 | >500 | >500 | >500 | >500 | >500 | >500 |
| 2d | 5-Br | 125 | 125 | 250 | 250 | 250 | 250 | 62.5 | 62.5 | 62.5 | 62.5 | 250 | 250 | >500 | >500 | 62.5 | 62.5 |
| 2e | 5-I | 62.5 | 125 | 125 | 125 | 62.5 | 125 | 62.5 | 62.5 | 125 | 125 | 250 | 250 | >500 | >500 | 31.25 | 31.25 |
| 2f | 5-NO2 | 500 | 500 | >500 | >500 | >500 | >500 | 250 | 250 | >500 | >500 | >500 | >500 | 500 | 500 | 62.5 | 62.5 |
| 2g | 5-CH3 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 |
| 2h | 5-CH3O | 500 | 500 | 500 | 500 | >500 | >500 | 500 | 500 | 500 | 500 | >500 | >500 | >500 | >500 | 62.5 | 62.5 |
| 2i | 5-OH | 15.62 | 15.62 | 15.62 | 15.62 | 31.25 | 31.25 | 15.62 | 15.62 | 15.62 | 15.62 | 125 | 125 | 500 | 500 | 1.95 | 1.95 |
| 2j | 5-tert-Bu | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | 62.5 | 62.5 |
| 2k | 6-Cl | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 |
| 2l | 3-Cl | 62.5 | 62.5 | 125 | 125 | 250 | 250 | 125 | 125 | 125 | 125 | 250 | 250 | >500 | >500 | 31.25 | 31.25 |
| 2m | 3,5-Cl2 | 62.5 | 62.5 | 125 | 125 | 62.5 | 62.5 | 31.25 | 31.25 | 62.5 | 62.5 | 250 | 250 | 500 | 500 | 125 | 125 |
| 2n | 3-Br-5-Cl | 31.25 | 31.25 | 62.5 | 62.5 | 62.5 | 62.5 | 31.25 | 31.25 | 62.5 | 62.5 | 250 | 250 | 500 | 500 | 62.5 | 62.5 |
| 2o | 3-I-5-Cl | 7.81 | 7.81 | 7.81 | 7.81 | 7.81 | 7.81 | 7.81 | 7.81 | 7.81 | 7.81 | 62.5 | 62.5 | 250 | 250 | 1.95 | 1.95 |
| 2p | 3,5-I2 | 3.9 | 3.9 | 7.81 | 7.81 | 7.81 | 7.81 | 3.9 | 3.9 | 3.9 | 3.9 | 62.5 | 62.5 | 62.5 | 62.5 | 15.62 | 15.62 |
| 2q | - | 250 | 250 | 250 | 250 | 250 | 250 | 250 | 250 | 500 | 500 | 500 | 500 | >500 | >500 | 250 | 250 |
| FLU | - | 0.24 | 0.24 | >500 | >500 | 125 | 250 | 31.25 | 500 | 250 | 500 | >500 | >500 | >500 | >500 | 7.81 | 125 |
| Strain | Improving Activity | Decreasing Activity |
|---|---|---|
| M. tuberculosis | H, 5-Br, 5-CH3O, 5-t-Bu, 3-Cl | 5-Cl, 5-NO2, 3,5-I2 |
| M. avium | H, 5-Cl, 5-Br, 5-I, 5-CH3O, 5-t-Bu, 3-Cl | 5-F, 5-NO2, 5-CH3, 5-OH, 3-I-5-Cl, 5-NO2-furylidene |
| M. kansasii | H, 5-Cl, 5-Br, 5-CH3O, 5-t-Bu, 3-Cl | 5-F, 5-NO2, 5-CH3, 3,5-X2, 3-X-5-Cl, 5-NO2-furylidene |
| Code | R | IC50 (µM) | Range of Concentrations Tested | SI for Staphylococci (MRSA/SE) | SI for Candida sp. | SI for TI | SI for Mtb. | SI for M. kansasii |
|---|---|---|---|---|---|---|---|---|
| 2a | H | >500 * | 1–500 | >2/>8 | inactive | >4 | >15.63 | >15.63 |
| 2b | 5-F | >250 * | 1–250 | inactive | inactive | inactive | >2 | >1 |
| 2c | 5-Cl | NT | NT | NT | NT | inactive | NT | NT |
| 2d | 5-Br | 159.1 | 1–500 | 0.32/0.64 | 0.64–2.55 | 2.55 | 4.98–9.94 | 2.55–4.97 |
| 2e | 5-I | 156.0 | 1–500 | 0.62/1.25–2.50 | 1.25–2.50 | 4.99 | 1.25–2.50 | 1.25–4.88 |
| 2f | 5-NO2 | 140.5 | 1–500 | 0.56/0.28 | ≤0.56 | 2.25 | 0.56 | 0.28–1.12 |
| 2g | 5-CH3 | 569.5 ** | 1–500 | 4.56 | inactive | inactive | 4.56 | 2.28–9.11 |
| 2h | 5-CH3O | 351.1 | 1–500 | inactive/5.62 | ≤0.70 | 5.62 | 5.62–11.24 | 5.62–21.94 |
| 2i | 5-OH | >500 | 1–500 | >16/>32 | >16 | >256.41 | >4 | >4 |
| 2j | 5-tert-Bu | 45.6 | 1–500 | inactive | inactive | 0.73 | 0.73–1.46 | 0.73–2.85 |
| 2k | 6-Cl | 102.20 | 1–500 | inactive | inactive | inactive | 0.82 | 0.41–1.64 |
| 2l | 3-Cl | 160.5 | 1–500 | inactive | 0.64–2.57 | 5.14 | 2.57–5.14 | 2.57–10.03 |
| 2m | 3,5-Cl2 | 114.30 | 1–500 | 1.83/3.66 | 0.91–3.66 | 0.91 | 0.91 | 0.23–1.83 |
| 2n | 3-Br-5-Cl | 92.94 | 1–500 | 5.95/2.98 | 1.49–2.97 | 1.49 | 0.74 | 0.37–1.49 |
| 2o | 3-I-5-Cl | 59.23 | 1–500 | 3.79/1.90 | 7.58 | 30.37 | 0.47 | 0.24–0.95 |
| 2p | 3,5-I2 | 38.0 | 1–500 | 4.87 | 4.87–9.73 | 2.43 | 0.15 | 0.15–0.61 |
| 2q | - | 16.3 | 1–500 | 0.13/0.52 | 0.07 | 0.07 | 0.13 | 0.07–0.26 |
| SDZ 1 | - | >1500 | 1–1500 | >3 (24 h) | inactive | inactive | >24 | >46.88 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krátký, M.; Dzurková, M.; Janoušek, J.; Konečná, K.; Trejtnar, F.; Stolaříková, J.; Vinšová, J. Sulfadiazine Salicylaldehyde-Based Schiff Bases: Synthesis, Antimicrobial Activity and Cytotoxicity. Molecules 2017, 22, 1573. https://doi.org/10.3390/molecules22091573
Krátký M, Dzurková M, Janoušek J, Konečná K, Trejtnar F, Stolaříková J, Vinšová J. Sulfadiazine Salicylaldehyde-Based Schiff Bases: Synthesis, Antimicrobial Activity and Cytotoxicity. Molecules. 2017; 22(9):1573. https://doi.org/10.3390/molecules22091573
Chicago/Turabian StyleKrátký, Martin, Magdaléna Dzurková, Jiří Janoušek, Klára Konečná, František Trejtnar, Jiřina Stolaříková, and Jarmila Vinšová. 2017. "Sulfadiazine Salicylaldehyde-Based Schiff Bases: Synthesis, Antimicrobial Activity and Cytotoxicity" Molecules 22, no. 9: 1573. https://doi.org/10.3390/molecules22091573
APA StyleKrátký, M., Dzurková, M., Janoušek, J., Konečná, K., Trejtnar, F., Stolaříková, J., & Vinšová, J. (2017). Sulfadiazine Salicylaldehyde-Based Schiff Bases: Synthesis, Antimicrobial Activity and Cytotoxicity. Molecules, 22(9), 1573. https://doi.org/10.3390/molecules22091573







